Abstract
Introduction
This study examined the efficacy and tolerability of a once-daily regimen of a 3% salicylic acid treatment gel containing turmeric and a low concentration of salicylic acid plus shea butter exfoliating moisturizer when used as monotherapy or in as an adjunct to other Rx psoriasis medications.
Patients and Methods
This single-site 12-week study enrolled 20 subjects >18 years of age with mild-to-moderate psoriasis involving <10% body surface area. Assessments were performed at baseline and Weeks 4, 8, and 12 using a 5-point scale (0 = none to 4 = severe). The investigator-assessed efficacy (changes from baseline for erythema, desquamation, induration, and overall global assessment [IGA]) and tolerability (irritation and edema). Study subjects assessed efficacy parameters of redness, scaling, and overall skin problems along with tolerability parameters of stinging, burning, itching, and irritation. Subjects applied the turmeric and salicylic acid treatment gel along with a moisturizer once daily to all affected areas.
Results
Half (50%) of the subjects were using concomitant Rx psoriasis treatments, while the other 50% received the study psoriasis treatment regimen as monotherapy. Investigator assessments of erythema, desquamation, induration, and IGA scores showed significant reductions from baseline (P ≤ 0.021) at Weeks 4, 8, and 12. At Week 12, these reductions reached 48%, 46%, 51%, and 48%, for these parameters, respectively. The investigator observed no irritation or edema at any time point. Subject assessments of redness, scaling, and overall improvement demonstrated significant reductions in 8 of 9 assessments (P ≤ 0.037). The subjects reported mild irritation at Weeks 4, 8, and 12. No treatment compliance issues or adverse events related to study product occurred during the study.
Conclusion
A once-daily over-the-counter (OTC) turmeric/salicylic acid gel followed by a shea butter/salicylic acid exfoliating moisturizer demonstrated excellent tolerability and efficacy in plaque-type psoriasis after 12 weeks of once-daily use.
Keywords: psoriasis, salicylic acid, shea butter, turmeric, curcumin
Introduction
Psoriasis is a common, chronic skin disease affecting over 65 million people worldwide.1 In the United States, estimates in 2020 show the disease affects more than 7.55 million adults, representing a prevalence of 3.0%.2 Frequently employed treatments include prescription topical corticosteroids and injectable biologics, among others. Yet, even with prescription intervention, stubborn plaques on the hands, elbows, knees, neck, and scalp can persist. In these cases, OTC products can be considered to provide the necessary extra therapeutics to achieve clearing. Further, in persons with more mild localized disease, OTC products might also be an easily accessible inexpensive option.
This research was undertaken to assess the ability of an OTC formulation to improve psoriasis in subjects who used the OTC product as sole treatment and subjects who were using concomitant topical prescription corticosteroids and biologics. This design was chosen to represent a real-world setting of patient types seen by clinicians. The treatment regimen consisted of a lipophilic aqueous 3% salicylic acid and turmeric gel followed by an anhydrous shea butter and salicylic acid moisturizer with the combination applied daily.3 This treatment regimen combined anti-inflammatory activity along with moisturization and exfoliation of psoriatic scale in an aesthetically elegant formulation containing antioxidants.4–8 The two-step psoriasis treatment system was developed to maximize the efficacy, tolerability, and cosmetic elegance of topical salicylic acid.
Salicylic acid is an effective agent used for the treatment of psoriasis.5 With its combined anti-inflammatory and exfoliating effects, it effectively reduces scale, induration, itching, and erythema.9 Salicylic acid is also antiproliferative, improving the onset of action of drugs by enhancing skin penetration of topical medications.
Turmeric (Curcuma longa, curcumin) is a spice, food preservative, and traditional medicine used commonly in China and Southeast Asia.10 It possesses immune-modulating, anti-inflammatory, antibacterial, and antioxidant properties. The functionality of the compound in psoriasis stems from its ability to modulate TH-17 functions.8 Specifically, turmeric has been shown to inhibit inflammatory mediators such as TNF-alpha, IL-17, IL-21, IL-22, IL 12/23, PDE1, PDE4, IL-1, IL-6, IL-8, JAK/STAT, ROR gamma, MMP9, PGE2, COX −2, NFkappa B, PHK, MAPK, NO, MMP-9, PI3K/Akt, and INF gamma.4,7,11 The potential multiple targets of curcumin in the IL-23/IL-17A axis are highlighted in Figure 1. It may also exhibit inhibitory activity on potassium channels in T cells,12 which are important in the pathogenesis of psoriasis.13,14
Figure 1.
The potential targets of curcumin in the IL-23/IL-17A axis. From: Sun et al.8 doi:10.1371/journal.pone.0067078.g005.
Abbreviations: CCR6, chemokine receptor 6; CUR, curcumin; IL, interleukin; IMG, imiquimod; MAPK, mitogen-activated protein kinase; IRAK, interleukin-1 receptor-associated kinase 4; NF-κB, NF-kappaB, nuclear factor kappa-light-chain-enhancer of activated B cells; STAT, signal transducer and activators of transcription; TLR, toll-like receptor.
Aligned with these findings, the recently published psoriasis treatment guidelines suggest the usefulness of salicylic acid and turmeric as adjuvant therapies for the treatment of psoriasis.5 Currently, clinical studies on the effects of topical turmeric and curcumin treatment for psoriasis are lacking.15,16 The current study examined the efficacy and tolerability of a once-daily treatment gel and salicylic acid moisturizer regimen for treating psoriasis when used as monotherapy or as an adjunct to other prescription psoriasis medications.
Materials and Methods
This single-site 12-week open-label study enrolled 20 subjects with mild-to-moderate plaque-type psoriasis. Subjects signed informed and photography consents prior to undergoing any study procedures. The Allendale Institutional Review Board approved the study, which was conducted in accordance with the tenets of the Declaration of Helsinki. There were no prescription drugs in the turmeric/salicylic acid product used in this study. Further, salicylic acid is a monographed ingredient for psoriasis and is considered an over-the-counter drug. The concentration of salicylic acid is 3% in the treatment gel.
An abbreviated medical history, including baseline medications, was performed. All oral and topical prescription medications remained unchanged during the course of the study. The subjects applied the study treatment gel and moisturizer once daily to all psoriasis-affected areas.
Key Inclusion Criteria
The study included female and male subjects >18 years of age with Fitzpatrick skin types I–VI and visible mild-to-moderate psoriasis of a body surface area (BSA) less than 10%. Women of childbearing potential used an acceptable form of birth control during the study.
Key Exclusion Criteria
Subjects were excluded for any dermatological disorder, which in the investigator’s opinion, could interfere with the accurate evaluation of the subject’s skin characteristics, except for psoriasis; a demonstrated hypersensitivity reaction to any of the ingredients of the study products; a clinically significant unstable medical disorder; or participation in another clinical study in the past 30 days.
Endpoints
The primary efficacy endpoint was the decrease in investigator global assessment score after 12 weeks of study product use. The secondary efficacy endpoint measured the decrease in subject-assessed overall psoriasis skin problems. The safety endpoint encompasses the overall incidence of all adverse events reported during the study.
Outcome Measures
Assessments were performed at baseline, Week 4, Week 8, and Week 12 on a 5-point ordinal scale (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe) for investigator tolerability parameters (irritation and edema), investigator observed efficacy parameters (change from baseline for erythema, desquamation, induration, and IGA), subject tolerability parameters (stinging, burning, itching, and irritation), and subject efficacy parameters (redness, scaling, and overall psoriasis skin problems). Efficacy was calculated by taking the mean scores for each parameter and calculating the percent reduction from the mean baseline values.
High-quality digital photographs of target sites were taken at baseline and Week 12. Each subject maintained a compliance diary.
Statistics
Statistical significance was defined as P < 0.05. A Mann–Whitney two-tailed paired test was used to analyze the nonparametric data, which included subject assessments, investigator assessments, and the investigator global assessment (IGA).
Results
The mean age of the subjects was 57.1 years (Table 1). Most subjects were female and Caucasian. Half of the subjects were taking treatments for their psoriasis at baseline. Topical corticosteroids were the most common concomitant therapies, accounting for 30% of the subjects, with 15% of the subjects using injectable biologics. All subjects in the study had psoriasis for over 2 years. The mean durations of treatment at baseline were 12 months for biologics and 8.7 months for corticosteroids.
Table 1.
Subject Demographics
| Parameter | |
|---|---|
| Age (years) | |
| Mean ± Std. Dev. | 57.1 ± 15.6 |
| Gender | |
| Females/Males, n (%) | 12/8 (60/40) |
| Race, n (%) | |
| Caucasian | 15 (75) |
| African American | 4 (20) |
| Hispanic | 1 (5) |
| Concomitant Treatment, n (%) | |
| None | 10 (50) |
| Treatment | 10 (50) |
| Topical Corticosteroid | 6 (30) |
| Biologic | 3 (15) |
| Other | 1 (5) |
| Treatment Duration (months) | |
| Mean ± Std. Dev. | |
| Topical Corticosteroid | 8.7 (2.6) |
| Biologic | 12 (0) |
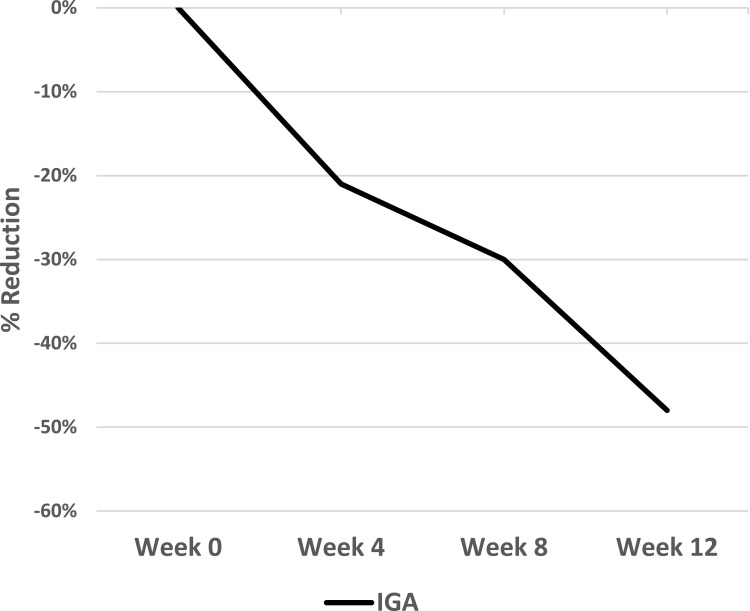
Investigator assessments of erythema, desquamation, induration, and investigator global assessment, the primary endpoint) scores demonstrated significant reductions from baseline (P ≤ 0.021) at all time points (Weeks 4, 8, and 12) (Figures 2 and 3). At the Week 12 study conclusion, there was a 48% reduction in erythema (P = 0.002), a 46% reduction in desquamation (P = 0.003), a 51% reduction in induration (P = 0.002,) and a 48% reduction in IGA (P = 0.003).
Figure 2.
Investigator-assessed IGA of an OTC psoriasis treatment regimen. Values are shown as percent reduction from baseline.
Abbreviations: IGA, investigator global assessment; OTC, over-the-counter.
Figure 3.
Investigator-assessed efficacy of an OTC psoriasis treatment regimen. Values are shown as percent reduction from baseline.
Abbreviation: OTC, over-the-counter.
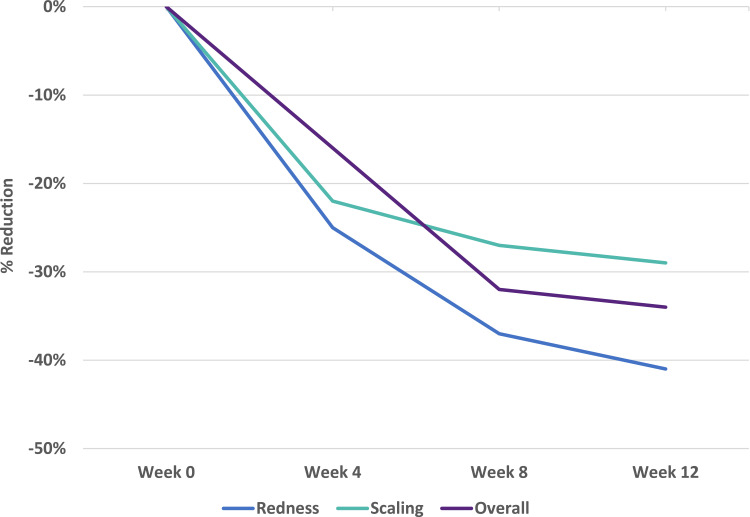
Subject assessments of redness, scaling, and overall improvement demonstrated significant reductions from baseline in eight of nine assessments (P ≤ 0.037) (Figure 4). The Week 4 overall assessment showed a 16% reduction compared with baseline (P = 0.098). At the Week 12 study conclusion, there was a 41% reduction in redness (P = 0.003), a 29% reduction in scaling (P = 0.013), and an overall improvement of 34% (P = 0.005).
Figure 4.
Subject-assessed efficacy of an OTC psoriasis treatment regimen. Values are shown as percent reduction from baseline.
Abbreviation: OTC, over-the-counter.
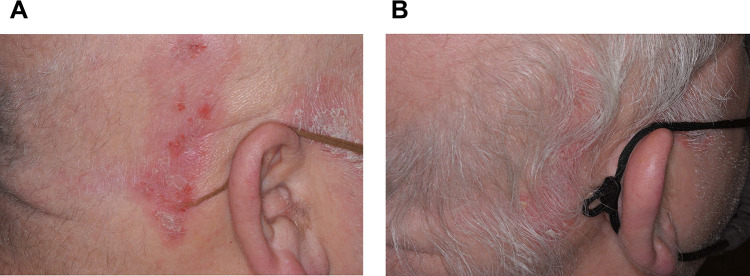
Regarding the tolerability of the treatment regimen, mild irritation was reported by the subjects at Weeks 4, 8, and 12. No compliance issues or adverse events occurred during the course of the study. Representative images before and after treatment are presented in Figures 5A, B and 6A, B.
Figure 5.
(A) Image of psoriasis on the scalp of a 58-year-old male subject who was not showing an adequate response to topical corticosteroid treatment at baseline. (B) Image of psoriasis improvement on the scalp of the same subject after 12 weeks of treatment with a once-daily regimen of a salicylic acid treatment gel containing turmeric and a salicylic acid plus shea butter exfoliating moisturizer added as adjunctive therapy to a topical corticosteroid.
Figure 6.
(A) Image of psoriasis on the knee of a 76-year-old male subject at baseline before any form of treatment. (B) Image of psoriasis improvement on the knee of the same subject after 12 weeks of treatment with a once-daily monotherapy regimen of a salicylic acid treatment gel containing turmeric and a salicylic acid plus shea butter exfoliating moisturizer.
Discussion
The current study reported statistically significant efficacy and favorable tolerability with a once-daily OTC regimen consisting of a salicylic acid gel containing turmeric plus a salicylic acid and shea butter exfoliating moisturizer as monotherapy and as adjunctive therapy with a prescription psoriasis treatment, such as a corticosteroid or biologic. Investigator assessments of erythema, desquamation, induration, and IGA scores showed approximately 50% reductions from baseline to Week 12. Subject assessments of redness, scaling, and overall improvement from baseline to Week 12 showed improvements of 29% to 41%. In addition, no adverse events related to study product occurred and the investigator observed no irritation or edema at any time point. The subjects reported mild irritation, which was likely due to the salicylic acid in the treatment formulation.
Curcumin, a compound derived from turmeric, has beneficial effects in the treatment of psoriasis.17,18 The interleukin-23 (IL-23)/IL-17A cytokine pathway plays a critical role in the pathogenesis of psoriasis.8 Sun et al8 found that mRNA levels of IL-17A, IL-17F, IL-22, IL-1beta, IL-6 and TNF-alpha cytokines were decreased significantly after curcumin treatment in a imiquimod-induced psoriasis-like inflammatory mouse model. Another molecule, progranulin, has been implicated in the etiology of psoriasis.17 A recent study by Zhou et al17 employed a mouse model of psoriatic skin lesions using topical application of imiquimod (IMQ) in both wild type and progranulin (PGRN)-knockout mice to evaluate the efficacy of curcumin. They found the direct regulation of PGRN in keratinocyte proliferation and differentiation in psoriatic lesions and showed a protective effect for curcumin on PGRN deficiency-induced psoriatic skin lesion exacerbation. A prospective, randomized, study of 34 subjects with mild-to-moderate plaque psoriasis demonstrated improvements for clinical and quality of life parameters in treated lesions in comparison with untreated lesions (P < 0.05).4 The current study corroborates these findings with reductions in IGA, erythema, desquamation, induration, redness, and scaling with a once-daily regimen containing turmeric.
Salicylic acid is a keratolytic, which promotes stratum corneum desquamation.5 This agent is thought to work by digesting the skin keratin and opening up water-binding sites, which allows the skin scale to desquamate properly.19 Salicylic acid has been used alone and in combination with other medications, such as topical corticosteroids, since the 1950s.20–32 This regimen utilized 3% salicylic acid gel, which can be an irritant causing skin barrier disruption. However, salicylic acid was better tolerated due to the presence of petrolatum, shea butter, and paraffin, all of which are occlusive agents that enhance the retention of water in the skin, in the exfoliating moisturizer.33,34 This moisturizing formulation placed on top of the salicylic acid gel created an environment optimal for barrier repair, minimizing the irritant effects of the salicylic acid.
Shea butter, also known as Butyrospermum parkii, is a remarkably stable moisturizing ingredient due to its 7–10% unsaponifiable lipid content making it high in bioactive triterpene esters, such as lupeol, alpha and beta-amyrin, and butyrospermol.35 Shea butter comes from sub-Saharan Africa and is extracted from the fruit kernels of the shea tree.36 Its main fatty acids are oleic and stearic. The triterpene lupeol inhibits lipopolysaccharide-induced iNOS, COX-2, TNF-alpha, IL-1beta and IL-12 mRNA expression.37,38 It may be through these anti-inflammatory effects that shea butter assists in psoriasis healing.
Limitations of this study include its open-label design. No placebo comparison group was used and the number of subjects was low for conducting meaningful statistical analysis of two study populations (half of the study population were being treated at baseline and the other half were naïve to treatment).
This clinical pilot study was designed to assess the efficacy and tolerability of an over-the-counter salicylic acid, curcumin, and shea butter regimen for the treatment of mild-to-moderate psoriasis when used as monotherapy or as adjunct treatment with Rx psoriasis treatments. A larger scale vehicle-controlled study is necessary to corroborate the current findings.
Conclusion
A once-daily OTC salicylic acid/turmeric gel plus a shea butter/salicylic acid exfoliating moisturizer demonstrated significant efficacy in improving psoriasis either alone or as adjunctive therapy with topical corticosteroids or injectable biologics.
Acknowledgments
The author would like to thank Julie Crider, PhD, for medical writing/editing contributions.
Funding Statement
This study was supported by Nuvothera Inc.
Disclosure
Dr Zoe Diana Draelos reports grants from Prosoria, during the conduct of the study. The author reports no other conflicts of interest in this work.
References
- 1.AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59(5):566–571. doi: 10.1111/ijd.14864 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–946. doi: 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draelos ZD. Tolerability and efficacy of an over-the-counter psoriasis treatment regimen. In: Fall Clinical Dermatology Conference 2021; October 21–24, 2021; Las Vegas, NV. [Google Scholar]
- 4.Sarafian G, Afshar M, Mansouri P, Asgarpanah J, Raoufinejad K, Rajabi M. Topical turmeric microemulgel in the management of plaque psoriasis; A clinical evaluation. Iran J Pharm Res. 2015;14(3):865–876. [PMC free article] [PubMed] [Google Scholar]
- 5.Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi: 10.1016/j.jaad.2020.07.087 [DOI] [PubMed] [Google Scholar]
- 6.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104 [DOI] [PubMed] [Google Scholar]
- 7.Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res. 2016;30(8):1243–1264. doi: 10.1002/ptr.5640 [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):e67078. doi: 10.1371/journal.pone.0067078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torsekar R, Gautam MM. Topical therapies in psoriasis. Indian Dermatol Online J. 2017;8(4):235–245. doi: 10.4103/2229-5178.209622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad S, Aggarwal BB. Turmeric, the golden spice: from traditional medicine to modern medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton (FL); 2011. [PubMed] [Google Scholar]
- 11.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39(1):41–47. doi: 10.1006/phrs.1998.0404 [DOI] [PubMed] [Google Scholar]
- 12.Lian YT, Yang XF, Wang ZH, et al. Curcumin serves as a human kv1.3 blocker to inhibit effector memory T lymphocyte activities. Phytother Res. 2013;27(9):1321–1327. doi: 10.1002/ptr.4863 [DOI] [PubMed] [Google Scholar]
- 13.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9(4):302–309. doi: 10.1038/cmi.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karczewski J, Dobrowolska A, Rychlewska-Hanczewska A, Adamski Z. New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity. 2016;49(7):435–450. doi: 10.3109/08916934.2016.1166214 [DOI] [PubMed] [Google Scholar]
- 15.Di Nardo V, Gianfaldoni S, Tchernev G, et al. Use of curcumin in psoriasis. Open Access Maced J Med Sci. 2018;6:218–220. doi: 10.3889/oamjms.2018.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann J, Gendrisch F, Schempp CM, Wolfle U. New herbal biomedicines for the topical treatment of dermatological disorders. Biomedicines. 2020;8:2. doi: 10.3390/biomedicines8020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou T, Zhang S, Zhou Y, et al. Curcumin alleviates imiquimod-induced psoriasis in progranulin-knockout mice. Eur J Pharmacol. 2021;909:174431. doi: 10.1016/j.ejphar.2021.174431 [DOI] [PubMed] [Google Scholar]
- 18.Nardo VD, Gianfaldoni S, Tchernev G, et al. Use of curcumin in psoriasis. Open Access Maced J Med Sci. 2018;6(1):218–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salicylic acid for Psoriasis. Available from: https://www.mypsoriasisteam.com/treatments/salicylic-acid. Accessed September 14, 2021.
- 20.Chandra A, Aggarwal G, Manchanda S, Narula A. Development of topical gel of methotrexate incorporated ethosomes and salicylic acid for the treatment of psoriasis. Pharm Nanotechnol. 2019;7(5):362–374. doi: 10.2174/2211738507666190906123643 [DOI] [PubMed] [Google Scholar]
- 21.Ma S, Gobis K, Swindell WR, Chaudhuri R, Bojanowski R, Bojanowski K. Synthesis and activity of the salicylic acid ester of bakuchiol in psoriasis-surrogate keratinocytes and skin substitutes. Clin Exp Dermatol. 2017;42(3):251–260. doi: 10.1111/ced.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nast A. An open label prospective randomized trial to compare the efficacy of coal tar-salicylic acid ointment versus calcipotriol/betamethasone dipropionate ointment in the treatment of limited chronic plaque psoriasis. Indian J Dermatol. 2015;60(2):198. doi: 10.4103/0019-5154.152531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khandpur S, Sahni K. An open label prospective randomized trial to compare the efficacy of coal tar-salicylic acid ointment versus calcipotriol/betamethasone dipropionate ointment in the treatment of limited chronic plaque psoriasis. Indian J Dermatol. 2014;59(6):579–583. doi: 10.4103/0019-5154.143523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh P, Gupta S, Abidi A, Krishna A. Comparative evaluation of topical calcipotriol versus coal tar and salicylic acid ointment in chronic plaque psoriasis. J Drugs Dermatol. 2013;12(8):868–873. [PubMed] [Google Scholar]
- 25.Kircik L. Salicylic acid 6% in an ammonium lactate emollient foam vehicle in the treatment of mild-to-moderate scalp psoriasis. J Drugs Dermatol. 2011;10(3):270–273. [PubMed] [Google Scholar]
- 26.Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(8):905–912. doi: 10.1111/j.1468-3083.2009.03214.x [DOI] [PubMed] [Google Scholar]
- 27.Lebwohl M. The role of salicylic acid in the treatment of psoriasis. Int J Dermatol. 1999;38(1):16–24. doi: 10.1046/j.1365-4362.1999.00500.x [DOI] [PubMed] [Google Scholar]
- 28.Going SM, Guyer BM, Jarvie DR, Hunter JA. Salicylic acid gel for scalp psoriasis. Clin Exp Dermatol. 1986;11(3):260–262. doi: 10.1111/j.1365-2230.1986.tb00457.x [DOI] [PubMed] [Google Scholar]
- 29.Hillstrom L. Comparison of topical treatment with desoxymethasone solution 0.25% with salicylic acid 1% and betamethasone valerate solution 0.1% in patients with psoriasis of the scalp. J Int Med Res. 1984;12(3):170–173. doi: 10.1177/030006058401200306 [DOI] [PubMed] [Google Scholar]
- 30.Hovding G. Treatment of psoriasis of the scalp with betamethasone 17, 21-dipropionate plus salicylic acid lotion (‘Diprosalic’). Pharmatherapeutica. 1981;3(1):61–66. [PubMed] [Google Scholar]
- 31.Pullmann H. [Salicylic acid in the treatment of psoriasis]. Z Hautkr. 1976;51(6):219–222. Polish. [PubMed] [Google Scholar]
- 32.Cimitan O, Pacchiani G. [Parenteral administration of associated salicylic acid-resorcinol and antipyrine in the treatment of psoriasis]. G Sci Mediche. 1951;6(5):93–96. Italian. [PubMed] [Google Scholar]
- 33.Purnamawati S, Indrastuti N, Danarti R, Saefudin T. The role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15(3–4):75–87. doi: 10.3121/cmr.2017.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26(3 Pt 2):387–396. doi: 10.1016/0190-9622(92)70060-S [DOI] [PubMed] [Google Scholar]
- 35.Akihisa T, Kojima N, Katoh N, et al. Triacylglycerol and triterpene ester composition of shea nuts from seven African countries. J Oleo Sci. 2011;60(8):385–391. doi: 10.5650/jos.60.385 [DOI] [PubMed] [Google Scholar]
- 36.Nde DB, Boldor D, Astete C, Muley P, Xu Z. Oil extraction from sheanut (Vitellaria paradoxa Gaertn C.F.) kernels assisted by microwaves. J Food Sci Technol. 2016;53(3):1424–1434. doi: 10.1007/s13197-015-2160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez MA, de las Heras B, Garcia MD, Saenz MT, Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J Pharm Pharmacol. 2001;53(11):1533–1539. doi: 10.1211/0022357011777909 [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Li X, Chen J, et al. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2016;30:74–84. doi: 10.1016/j.intimp.2015.11.031 [DOI] [PubMed] [Google Scholar]