Abstract
Escherichia coli (E. coli) is a zoonotic pathogen that showed growing resistance to antibiotics. No descriptive analysis highlights the threat of antimicrobial-resistant (AMR) of E. coli among livestock in the United Arab Emirates (UAE). Herein, we conducted phenotypic and genotypic resistance studies on E. coli isolates from livestock samples in the Emirate of Abu Dhabi based on routine diagnosis between the periods 2014–2019. Bacterial culture and disk diffusion methods were used for bacterial isolation and phenotypic resistance analysis. Resistance mechanism was studied by PCR targeting the most commonly resistance genes: ampicillin (blaSHV, blaCMY, and blaTEM-1B), tetracyclines (tetA and tetB), co-trimoxazole [sulfamethoxazole (sul1, sul2, and sul3) + trimethoprim (dfrA1 and dfrA17)], aminoglycosides [aph(3”)-Ia, aph(6)-Id, and aac(3)-IV], and fluoroquinolones (qnrA and aac(6')-Ib-cr). Analysis of 165 E. coli isolates showed resistant to ampicillin, tetracycline, co-trimoxazole, gentamicin, and enrofloxacin by 157/165 (95.4%), 154/165 (93.6%), 141/165 (86%), 139/165 (85%), and 135/165 (82.7%), respectively. Predominant resistance gene/s detected by PCR were blaCMY (119/160, 72%) and blaTEM-1B (154/160, 96.3%) for ampicillin; tetA (162/164, 98.8%) and tetB (112/164, 68.3%) for tetracyclines; sul2 (156/164, 95%), sul3 (138/164, 84%), and dfra17 (74/164, 44.5%) for co-trimoxazole; aph(3”)-Ia (134/164, 82.1%) and aph(6)-Id (161/164, 98.2%) for aminoglycosides; and aac(6')-Ib-cr (61/61, 100%) for enrofloxacin. Both phenotypic and genotypic analyses revealed that all E. coli isolates were multidrug-resistant (resistance to 3, 4, and 5 antibiotics classes by 3.6%, 57.6%, and 38.8%, respectively) carrying one or more resistance gene/s for the same antibiotic. PCR profiling confirmed the presence of resistance genes corresponding to their antibiotic profile. Results of the study will highlight the knowledge based on E. coli AMR related to livestock in UAE that may call for interventions.
1. Introduction
Escherichia coli (E. coli) is a Gram-negative rod bacterium taxonomized within the family of Enterobacteriaceae. E. coli is a ubiquitous bacterium isolated from humans, animals, birds, and different environments. In humans and animals, E. coli normally exists in the intestine as microflora and plays an important role in digestion and absorption [1]. On the contrary, E. coli can be pathogenic or zoonotic, causing serious diseases such as diarrhea, hemorrhagic colitis (HC), and hemolytic uremic syndrome (HUS) in both humans and animals [2, 3].
Antimicrobials are normally used in livestock to treat diseases caused by E. coli, such as diarrhea, gastrointestinal infections, mastitis, and urinary tract infection (UTI). Nevertheless, the uses of antimicrobials in the treatment of livestock or as a growth promoter have been linked to the development of multidrug-resistant E. coli, a threat to public health [4–6]. Indeed, E. coli isolated from livestock samples has been reported to show different resistances to several antimicrobials, including erythromycin, tetracycline, ampicillin, gentamicin, sulfamethoxazole/trimethoprim, chloramphenicol, kanamycin, and streptomycin [7–13]. This resistance phenomenon is also an economic burden for farmers because of costs incurred in treatment failure and a prolonged period of treatment of the bacterial infections [14]. In UAE, the common antibiotics used to treat animals include tetracycline, oxytetracycline, penicillin, ampicillin, amoxicillin, oxacillin and cefoxitin, gentamicin, sulfamethoxazole/trimethoprim (co-trimoxazole), enrofloxacin, and ceftiofur. However, the current knowledge based on E. coli AMR against these antibiotics concerning livestock in UAE is unclear.
The detection of antimicrobial resistance was mostly done phenotypically assessing the sensitivity to these antibiotics as recommended by the Clinical and Laboratory Standards Institute (CLSI) and/or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [15]. The molecular detection based on polymerase chain reaction (PCR) has been widely used to detect the genes responsible for the resistance against several antibiotics [11].
Antimicrobial-resistant bacteria have been detected in a variety of domestic animals and environments [16, 17]. Studies from the Middle East highlight the spread of resistant organisms in hospitals and to a lesser extent in livestock and the environment [18]. In UAE, most of AMR studies in E. coli are related to humans [19–21]. Yet, E. coli-based AMR surveillance in animals in UAE is lacking.
Based on the samples that were analyzed and diagnosed in our laboratories for bacterial infection, we found a considered number of E. coli isolates showed resistance to several antibiotics commonly used for animal treatment by the veterinarians in Abu Dhabi Agriculture and Food Safety Authority (ADAFSA), Abu Dhabi, UAE. Therefore, we aimed to study the AMR in E. coli isolated from the Emirate of Abu Dhabi livestock to understand the resistance level in the region that may pose a risk to public health. We applied the antibiotic susceptibility test and PCR to screen the antibiotic resistance in E. coli and explore the potential resistance mechanisms. To our knowledge, this is the first comprehensive study that provides useful information for veterinary practices and public health concerns in UAE.
2. Materials and Methods
2.1. Sampling, Bacterial Isolation, and Identification
A total of 165 samples from different regions of the Emirate of Abu Dhabi [Al Ain = 108/165 (65.5%), Al Dhafra = 20/165 (12.1%), and Abu Dhabi = 37/165 (22.4%) samples] were received by our laboratory from January 2014 to May 2019 for bacterial infection investigation. The samples include organs (liver, kidney, heart, lungs, spleen, intestine, and lymph nodes), blood, milk, urine, pus, uterine discharges, and fecal swabs from different animals (camel = 42/165 (25.5%), sheep = 42/165 (25.5%), goat = 54/165 (32.7%), and poultry = 27/165 (16.4%)). The animal samples cultured onto a range of general and selective bacterial culture media (purchased from Pharmatrade, Dubai, UAE), including blood agar (incubated aerobically and anaerobically), MacConkey, Xylose Lysine Deoxycholate agar (XLD), Eosin methylene blue (EMB), Brilliant Green Agar (BG) and nutrient agar (NA). The cultured media were incubated at 37°C for 24 hours aerobically and 48 hours anaerobically. Bacterial identification was done using the VITEK II identification system (bioMérieux). For this study, each isolate was harvested into a microbial cryopreservation beads system (Mast Cryobank) according to the manufacturer's instruction and stored at −80°C.
A written consent, which was included in the sample request form, approved by ADAFSA research ethics committee for the use of the samples for publication, was obtained from the animal's owners before inclusion in the study.
2.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was performed against the most commonly used antibiotics for animal treatment in UAE tetracycline, oxytetracycline, penicillin, ampicillin, amoxicillin, oxacillin and cefoxitin, gentamicin, co-trimoxazole, enrofloxacin, and ceftiofur using disk diffusion method according to the guidelines of Clinical and Laboratory Standards Institute (CLSI-VET01 5th Edition/VET08 4th Edition). A panel of five antibiotics disks was used, which include, ampicillin (10 μg), enrofloxacin (5 μg), gentamicin (10 μg), tetracycline (30 μg), and co-trimoxazole (25 μg) and a combination of trimethoprim and sulfamethoxazole 1.25/23.75 μg antibiotics.
2.3. Detection of AMR Genes by PCR in E. coli-Resistant Isolates
2.3.1. DNA Extraction
From each cryovial (representing one isolate), a single bead was removed aseptically with a sterile needle, streaked over a plate of blood agar, and incubated overnight at 37°C. Then, for genomic DNA extraction, 3–5 isolated colonies were suspended in 200 μL phosphate-buffered saline (PBS) for manual extraction with QIAamp® DNA Mini Kit (Qiagen) following the manufacturer's instructions. The DNA was quantified by Nanodrop 2000 (Thermo Fisher Scientific, USA) and used as a template for PCR.
2.3.2. Primers and PCR Analysis
Based on antibiotic-resistant phenotype of E. coli and previous studies, we selected common resistant gene/s per antibiotic to be tested by PCR [11, 12, 22]. These genes were as follows: ampicillin (blaSHV, blaCMY, and blaTEM-1B), tetracycline (tetA and tetB), trimethoprim (dfrA1 and dfra17), sulfamethoxazole (sul1, sul2, and sul3), gentamicin [aac(3)-IV, aph(3”)-Ia, and aph(6)-Id], and fluoroquinolones (qnrA and aac(6')-Ib-cr).
The primers used the genes (blaSHV, blaCMY,tetA, tetB, dfrA1, sul1, aac(3)-IV, and qnrA) were previously described [11, 23–28]. Other genes including blaTEM-1B, dfra17, sul2, sul3, aph(3”)-Ia, aph(6)-Id, and aac(6')-Ib-cr were designed in this study based on the sequence of the AMR genes predicted from the whole-genome sequencing of 10 isolates (data not shown). The information about the primers used is given in Table 1.
Table 1.
List of primers used in the study.
| Antibiotic | Gene | DNA sequence (5'-3') | Annealing temperature (°C) | Product (bp) | Reference |
|---|---|---|---|---|---|
| Gentamicin | aph(6)-Id | F: ATCGTCAAGGGATTGAAACC R: GGATCGTAGAACATATTGGC |
50 | 509 | [25] |
| aph(3')-Ia | F: ATGGGCTCGCGATAATGTCG R: AGAAAAACTCATCGAGCATC |
60 | 734 | [23] | |
| aac(3)-IV | F: CTTCAGGATGGCAAGTTGGT R: TCATCTCGTTCTCCGCTCAT |
55 | 286 | [28] | |
| Ampicillin | bla SHV | F: TCGCCTGTGTATTATCTCCC R: CGCAGATAAATCACCACAATG |
52 | 768 | |
| blaCMY | F: TGGCCAGAACTGACAGGCAAA R: TTTCTCCTGAACGTGGCTGGC |
47 | 462 | ||
| blaTEM-1B | F: TCCTTGAGAGTTTTCGCCCC R: TGACTCCCCGTCGTGTAGAT |
60 | 634 | This study | |
| Sulfamethoxazole | sul1 | F: TTCGGCATTCTGAATCTCAC R: ATGATCTAACCCTCGGTCTC |
47 | 822 | [28] |
| Sul2 | F: CACATTGCGGCGTTCTTTGA R: TTGCGGTTTCTTTTAGCGCC |
60 | 241 | This study | |
| Sul3 | F: AGTGGGCGTTGTGGAAGAAA R: AGTAGCTGCACCAATTCGCT |
60 | 361 | ||
| Trimethoprim | dfrA1 | F: GGAGTGCCAAAGGTGAACAGC R: GAGGCGAAGTCTTGGGTAAAAAC |
45 | 367 | [27] |
| dfrA17 | F: GGCGTAATCGGTAGTGGTCC R: CCCCCGCCAGAGACATATAC |
60 | 263 | This study | |
| Fluoroquinolone | qnrA | F: GGGTATGGATATTATTGATAAAG R: CTAATCCGGCAGCACTATTTA |
50 | 670 | [24] |
| aac(6')-Ib-cr | F: GCGATGCTCTATGAGTGGCT R: TTTCTTCTTCCCACCGTCCG |
60 | 220 | This study | |
| Tetracycline | tet(A) | F: GGTTCACTCGAACGACGTCA R: CTGTCCGACAAGTTGCATGA |
57 | 577 | [26] |
| tet(B) | F: CCTCAGCTTCTCAACGCGTG R: GCACCTTGCTGATGACTCTT |
56 | 634 |
For the PCR, we used AmpliTaq® GoldTM 360 Master Mix Kit (Applied Biosystems) in a total volume of 25 µL, including 12.5 µL Mix, 8.5 µL water, 1 µL enhancer, 1 µL each primer (10 pmol/µL), and 1 µL DNA template (50–150 ng/µL). Amplification reactions were carried out using a DNA Thermocycler (Eppendorf Mastercycler, Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany) with suitable annealing temperatures shown in Table 1. We included positive and negative controls in each reaction. Immediately after electrophoresis in agarose gel (1.5%), the PCR products and DNA marker (100 bp, NEB) were viewed under UV transilluminator, and the gel image was documented.
3. Results
3.1. Bacterial Isolation and Phenotypic Characterization of AMR
From three different regions of the Emirate of Abu Dhabi, 165 E. coli were isolated from different animal species (camel = 42, sheep = 42, goat = 54, and poultry = 27).
Phenotypic analysis by the disk diffusion method showed that all the E. coli isolates are multidrug-resistant (MDR) since they showed resistance to 2, 3, 4, and even five classes of antibiotics by 5/165(3%), 17/165(10.3%), 47/165(28.5%), and 96/165 (58.2%), respectively (Table 2). Regardless of the source of E. coli isolates, the isolates were resistant to ampicillin, tetracycline, co-trimoxazole, gentamicin, and enrofloxacin by 157 (95.4%), 154 (93.6%), 141 (86%), 139 (85%), and 135 (82.7%), respectively (Figure 1(a)). All E. coli isolates from different animals were shown considerable resistance phenomena to all antibiotics tested (Figure 1(b)).
Table 2.
The main phenotypic multidrug-resistant patterns of 165 E. coli strains were isolated from different livestock samples of the Emirate of Abu Dhabi.
| No. of classes of drugs | Patterns of multidrug resistance (no. of isolates in this pattern) | No. of total isolates (%) (n = 165) |
|---|---|---|
| 2 | Ampicillin-enrofloxacin (n = 4) | 5 (3%) |
| Ampicillin-gentamicin (n = 1) | ||
| 3 | Tetracycline-gentamicin-enrofloxacin (n = 4) | 17 (10.3%) |
| Tetracycline-co-trimoxazole-gentamicin (n = 1) | ||
| Ampicillin-tetracycline-gentamicin (n = 2) | ||
| Ampicillin-tetracycline-co-trimoxazole (n = 6) | ||
| Ampicillin-co-trimoxazole-gentamicin (n = 1) | ||
| Ampicillin-tetracycline-enrofloxacin (n = 2) | ||
| Ampicillin-gentamicin-enrofloxacin (n = 1) | ||
| 4 | Tetracycline-co-trimoxazole-gentamicin-enrofloxacin (n = 2) | 47 (28.5%) |
| Ampicillin-co-trimoxazole-gentamicin-enrofloxacin (n = 3) | ||
| Ampicillin-tetracycline-gentamicin-enrofloxacin (n = 11) | ||
| Ampicillin-tetracycline-co-trimoxazole-enrofloxacin (n = 14) | ||
| Ampicillin-tetracycline-co-trimoxazole-gentamicin- (n = 17) | ||
| 5 | Ampicillin-tetracycline-co-trimoxazole-gentamicin-enrofloxacin (n = 96) | 96 (58.2%) |
Figure 1.
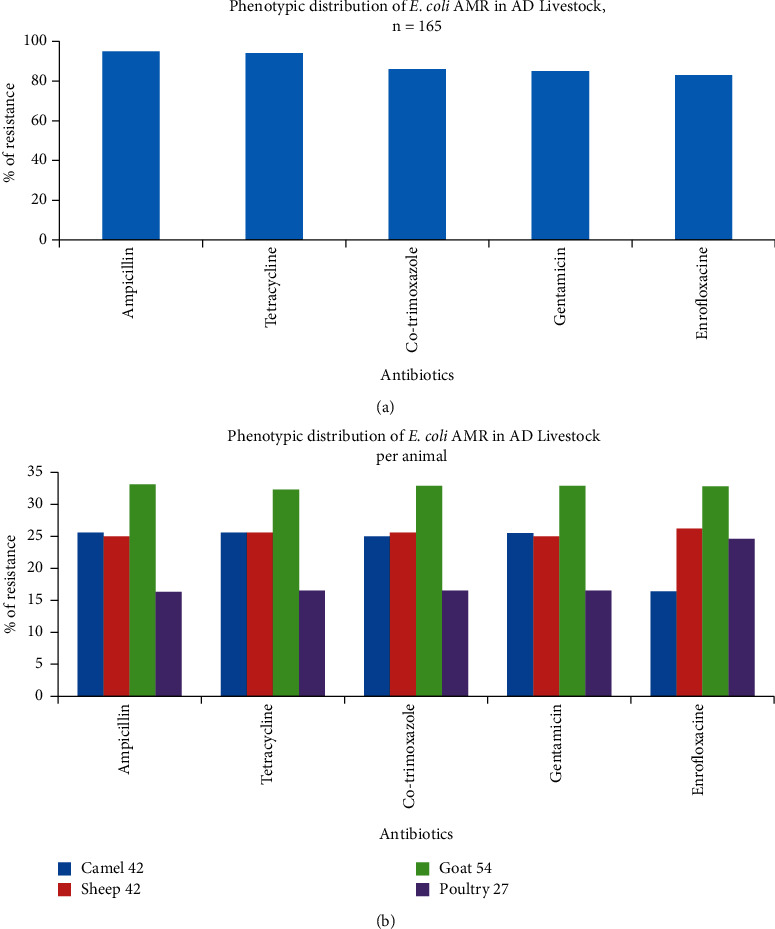
Antimicrobial resistance of E. coli isolates in the Emirate of Abu Dhabi livestock. (a) Per 165 E. coli isolates. (b) Per animal species (camel, sheep, goat, and poultry). A total number of animals are indicated.
Furthermore, the patterns of “ampicillin-tetracycline-co-trimoxazole-gentamicin-enrofloxacin” (n = 96, 58.2%) and “ampicillin-tetracycline-co-trimoxazole-gentamicin” (n = 17, 10%) were the most frequent resistance profiles observed across all E. coli isolates (Table 2).
3.2. Detection of Antibiotic Resistance Genes by PCR
The PCR was used to test the presence or absence of the resistance genes to understand the molecular basis of the resistance observed in E. coli. The following AMR genes were targeted: blaCMY and blaTEM-1B (for ampicillin); tetA and tetB (for tetracycline); sul1, sul2, and sul3 (for sulfamethoxazole); dfrA1 and dfrA17 (for trimethoprim) (sulfamethoxazole and trimethoprim are components of co-trimoxazole); aph(3”)-Ia, aph(6)-Id, and aac(3)-IV (for gentamicin); and qnrA and aac(6')-Ib-cr (for fluoroquinolones).
For ampicillin, blaTEM-1B showed a higher frequency (96.3%) than blaCMY (72%) in the isolates (Figure 2(a)). About 50/160 (31.2%) isolates carry a single gene, either blaCMY or blaTEM-1B, while 110/160 (68.8%) carry both genes. However, the blaSHV gene was not detected.
Figure 2.
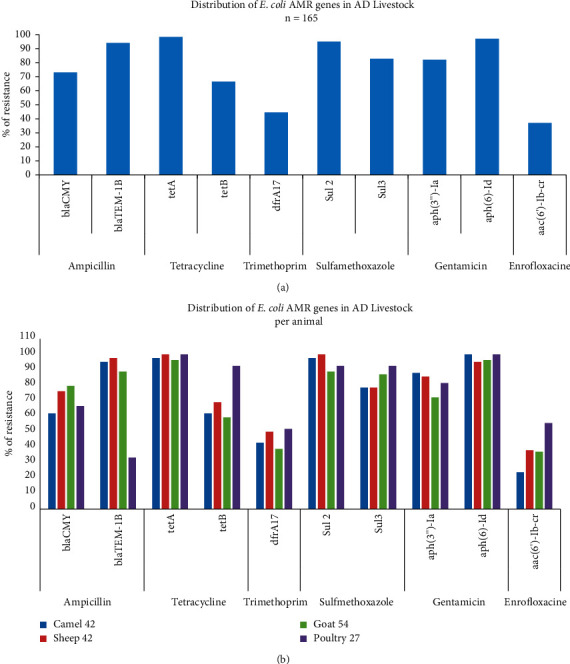
PCR detection of antimicrobial-resistant genes of E. coli isolated from livestock in the Emirate of Abu Dhabi. (a) Per 165 E. coli isolates and (b) per animal species (camel, sheep, goat, and poultry).
For tetracycline, we detected the tetA and tetB in 162/164 (98.8%) and 112/164 (68.3%) of the isolates, respectively (Figure 2(a)). About 61/162 (37.7%) carry either tetA or tetB, and 101/162(62.3%) carry both genes.
Among the 164 cotrimoxazole-resistant E. coli isolates, the dfra17, sul2, and sul3 genes were detected in 74 (44.5%), 156 (95%), and 138 (84%) isolates, respectively (Figure 2(a)). For sulfamethoxazole alone, one isolate 1/164 (0.6%) carried neither sul2 nor sul3. Thirty-four (20.7%) isolates carried either sul2 or sul3, while 130 (79.3%) carried both genes. Generally, in co-trimoxazole-resistant isolates, about 64/164 (39%) of the isolates carried the three genes (dfra17, sul2, and sul3). The genes dfrA1 and sul1 were not detected in our isolates.
The aph(3”)-Ia and aph(6)-Id genes were detected in 134 (82.1%) and 136 (98.2%) isolates, respectively, among the 164 gentamicin-resistant isolates (Figure 2(a)). Twenty-eight (17.1%) carried either aph(3”)-Ia or aph(6)-Id and 136 (82.9%) carried both genes. We could not detect aac(3)-IV gene in our isolates. All the 61 enrofloxacin-resistant isolates harbored the aac(6')-Ib-cr gene; however, qnrA gene was not detected. The detailed detection of the antibiotic resistance genes across different animals is shown in Figure 2(b).
3.3. Phenotypic: Genotypic Resistance Compatibility
Some E. coli isolates showed phenotypic resistance, but the resistance genes were not detected (mainly in gentamicin and enrofloxacin); the vice situation also exists. The overall phenotypic and genotypic compatibilities are 156/165 (94.5%), 153/165 (92.7%), 138/165 (83.6%), 137/165 (83%), and 73/165 (44.2%) for ampicillin, tetracycline, co-trimoxazole, gentamicin, and enrofloxacin, respectively, as detailed in Table 3.
Table 3.
Comparison of AMR in E. coli isolates according to phenotypic and genotypic testing.
| Antibiotic | Phenotype | Genotype | % of dis-agreement | % of agreement |
|---|---|---|---|---|
| Ampicillin | P− (n = 5) | G+ (n = 5) | 9/165 (5.5%) | 156/165 (94.5%) |
| P+ (n = 4) | G− (n = 4) | |||
| Tetracycline | P− (n = 11) | G+ (n = 11) | 12/165 (7.3%) | 153/165 (92.7%) |
| P+ (n = 1) | G− (n = 1) | |||
| Co-trimoxazole | P− (n = 26) | G+ (n = 26) | 27/165 (16.4%) | 138/165 (83.6%) |
| P+ (n = 1) | G− (n = 1) | |||
| Gentamicin | P− (n = 28) | G+ (n = 28) | 28/165 (17%) | 137/165 (83%) |
| Enrofloxacin | P− (n = 10) | G+ (n = 10) | 92/165 (55.8%) | 73/165 (44.2%) |
| P+ (n = 82) | G− (n = 82) |
P+/G− = Phenotypic resistant with no resistance gene identified. P−/G+ = Phenotypic susceptible with resistance gene identified.
4. Discussion
Currently, AMR is becoming a global concern requiring collaborated efforts in all fields, including humans, animals, and the environment, to identify its epidemiological patterns and apply proper approaches to improve treatment efficiency.
Epidemiological studies describing the dissemination of MDR in animals and the environment in the Middle East countries were conducted in only six out of the 15 countries [29]. In the present study, we analyzed the phenotypic and genotypic patterns of 165 MDR E. coli isolated from livestock in the Emirate of Abu Dhabi, UAE (based on samples received in the laboratory for bacterial investigation between 2014 and 2019) to provide an insight into this matter of concern. To the best of our knowledge, this is the first comprehensive study in this field.
Based on our results, all isolates 165/165 (100%) were phenotypically and genotypically multidrug-resistant (MDR) carry one or more resistant determinants (Tables 2 and 3). The most dominant antimicrobials with high resistance were ampicillin, tetracycline, and co-trimoxazole, and the lowest resistance is enrofloxacin, which is consistent with a previously published study in another region [30].
The genetic analysis in this study revealed that two AMR genes, namely, blaTEM-1B and blaCMY, are responsible for developing the resistance against ampicillin in the region. Similar results have been reported by other researchers [31, 32].
Generally, the resistance mechanisms for the tetracyclines fall in three categories: efflux pumps, ribosomal protection proteins (RPPs), or enzymatic inactivation. Over 40 different acquired tetracycline resistance determinants are recognized, that is, 38 tet (tetracycline resistance) [33]: 25 of the tet code for efflux pumps, 10 tet code for an RPP, and 3 tet genes for enzymatic inactivation mechanism. This study detected two tetracycline efflux genes tetA and tetB by 98.8% and 68.3%, respectively. Both determinants were among the most widespread tet genes found in enterobacteria [28, 34]. However, tetA marker is most common (67%) compared with tetB (31%) [8], consistent with our observations. By contrast, other studies have found that humans and ruminants carry less tetA and tetB [35].
For sulfamethoxazole (dihydropteroate synthetase)-resistant E. coli, it is generally attributed to the presence of sul1, sul2, and/or sul3 genes [36], which are known to be associated with class 1 integrons [37]. In this study, we frequently detected sul2 and sul3 AMR genes. However, sul2 was detected in 90% of the isolates, which is expected since it is the most frequent resistance mechanism to sulfamethoxazole in E. coli isolates [38, 39]. By contrast, other studies showed that sul1, sul2, and sul3 have equal importance for sulfamethoxazole resistance in E. coli strains from food-producing animals in China [40]. Our PCR could not detect sul1 gene, which indicates the absence or low abundance of class 1 integrons carry sul1 gene [41] or even PCR failure, which needs to be investigated.
Trimethoprim inhibits the enzyme dihydrofolate reductase (DHFR) by competitively binding to its active site [42]. Acquired resistance to trimethoprim could be conferred by dfrA and dfrB gene families [43]. However, PCR only detected the dfrA17 gene in 44.5% of the E. coli isolates. This distribution of the dfrA17 gene could be attributed to their association with class 1 and class 2 integrons and plasmids [44, 45].
High rates of quinolones resistance (18.2%–92.5%) in E. coli from animal origins were observed worldwide [46], consistent with our phenotypic data (85% resistance). It is known that quinolone mediates resistance by four mechanisms: chromosome-encoded resistance, overexpression of naturally occurring efflux pumps, mutations of the molecular targets DNA gyrase and topoisomerase IV [47], and plasmid-mediated resistance genes, such as plasmids encodes a qnr determinant that protects DNA gyrase and type IV topoisomerase from quinolone inhibition [48] or plasmid encodes quinolone-resistant genes such as aac(6')-Ib-cr [49]. In the present study, we detected the aac(6')-Ib-cr gene in 37% of the resistant isolates indicating a plasmid-mediated determinant. However, this percentage of genes detected is less than phenotypic data, which suggests the involvement of other mechanisms since resistance to these drugs is derived from DNA gyrase mutations rather than by specific resistance genes and enzymes, which requires further studies.
The resistance phenotype and genotype were found not entirely consistent. Some isolates are phenotypically resistant, but no AMR genes were identified, and those are phenotypically susceptible but carrying resistance genes. For example, gentamicin genes were highly detected in the isolates (99.4%), while some strains were susceptible to most of the gentamicin that were tested (only 85% were resistant). In addition, the isolates were resistant to enrofloxacin (82.7%), while we detected only 37% of the determinants. The overall agreement between resistances is 94.5%, 92.7%, 83.6%, 83%, and 44.2% for ampicillin, tetracycline, co-trimoxazole, gentamicin, and enrofloxacin, respectively, as shown in Table 3. The apparent contradiction of susceptible isolates carrying resistance genes can be attributed to different possible reasons. The resistance genes may be unexpressed if they are distant from or associated with a weak promoter in an integron. Moreover, gene cassettes were known to confer resistance to most classes of antibiotics including that containing β-lactams, aminoglycosides, trimethoprim, and quinolones [50, 51]. However, gene cassettes are generally lack promoters and requires to be incorporated into the integron to be expressed (integron's promoter is required for the expression) [41, 52]. Thus, free gene cassettes (not integrated into an integron) are silent and confer no resistance [32]. Also, low minimum inhibitory concentration (MIC) test sensitivity provides an alternative explanation. Isolates could be falsely categorized as susceptible if the MIC breakpoint is higher than the resistance conveyed by the gene [43, 53]. By contrast, some resistant isolates did not carry any of the tested AMR genes, indicating other AMR genes (e.g., tetD, tetE, or tetM for tetracycline, and blaCMY and blaTEM for ampicillin) might be present, or other novel genetic resistant determinants exist [54]. Furthermore, factors such as enzymatic inactivation, target modification, or decreased outer membrane permeability may also be involved [55, 56]. Interestingly, at least one of the AMR genes was detected in most AMR in E. coli isolates (e.g., tetA or tetB for tetracycline) that encodes resistant phenotypes to the tested antibiotics in this study. Those isolates that were phenotypically resistant, but PCR negative should be screened for additional resistance determinants such as other possible genes, plasmid, transposons, and integrons.
Our data showed that E. coli from camel, sheep, goat, and poultry share a similar resistance gene pool for the different antibiotics tested. Regardless of animal species and number, the MDR pattern of E. coli has been observed in the three regions in which the isolated found resistant to all classes of antibiotics tested in this study. This resistance profile observed in the three regions of the Emirate of Abu Dhabi, possibly reflecting the same improper practice of antibiotic usage in animal husbandry. This study's result does not reflect a complete picture of E. coli AMR in the emirate of Abu Dhabi, because as mentioned early in this paper, the sample source of this study is the routine diagnostic samples received by our laboratory. Some limitations in our study have been observed. For example, only a few common resistance genes per antibiotic were investigated. Future studies in this region should consider additional genes for screening, especially for trimethoprim and enrofloxacin resistance, as these were poorly explained. Also, other animals like cattle should be included in our future screenings. The overall observed MDR E. coli isolated from livestock in the Emirate of Abu Dhabi, UAE, is far above previously reported figures of 20% in Finland, and it is similar to the 100% level reported for China [57].
Although the study did not include human samples, linking our results with previous human hospital-based data on E. coli AMR in the region is crucial. In UAE, in human, the E. coli was reported to have a high resistance to ampicillin in 1994 with the rates varying between 58 and 89%, and the resistance was increased for the two hospitals (Al-Ain and Tawam) in 2005. Similar resist pattern was observed for gentamycin and cotrimoxazole (Al-Ain Hospital) [19]. In another study, the extended-spectrum beta-lactamase (ESBL)-producing isolates found increased significantly over the period 2004–08 [21], and E. coli is one of the bacteria contributing to this phenomenon in UAE (from 7.0% to 22.3%) [21]. A third study in UAE has identified 53 (41%) of the 130 isolates as having ESBL phenotype; of these, 32 (60%) were E. coli [58]. Thus, understanding the interplay between humans and livestock in the context of AMR of E. coli based on a one-health approach is recommended.
In conclusion, the study generated preliminary data on the resistance of E. coli isolated from livestock in the Emirate of Abu Dhabi across five years and highlights the possible molecular mechanisms of the resistance. Furthermore, it suggests the improper use of antimicrobials in veterinary practice in the region. Thus, a comprehensive AMR survey system on a large scale should be initiated and E. coli could be one of the choices for the surveillance as other countries did [34, 59, 60]. Furthermore, the governmental agencies in UAE should critically supervise antimicrobial usage to reduce the threat to public health through the food chain and to avoid dissemination of antibiotic resistance in humans, animals, and the environment.
Acknowledgments
This work was supported by Abu Dhabi Agriculture and Food Safety Authority (ADAFSA), Abu Dhabi, UAE.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
A written consent that was included in the sample request form approved by the ADAFSA research ethics committee for the use of samples for publication was obtained from the animal's owners before inclusion in the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
GEA contributed to bacteriology and manuscript editing. HZA. I contributed to PCR, analyzed the data, and drafted the manuscript. ZM. A, MFBMY, SM. A, and SS. A participated in PCR. S. M. A, M. S. A, S. S. A, A. MA, F. H. A, and I. A. S. A were contributed equally in bacteriology. MAA. A participated in conception and design of the study, revised, and edited the manuscript. SSM. A revised and approved the final version of the manuscript to be submitted.
References
- 1.Verraes C., Van Boxstael S., Van Meervenne E., et al. Antimicrobial resistance in the food chain: a review. International Journal of Environmental Research and Public Health . 2013;10:2643–2669. doi: 10.3390/ijerph10072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dho-Moulin M., Fairbrother J. M. Avian pathogenic Escherichia coli (APEC) Veterinary Research . 1999;30:299–316. [PubMed] [Google Scholar]
- 3.Kaper J. B., Nataro J. P., Mobley H. L. T. Pathogenic escherichia coli. Nature Reviews Microbiology . 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 4.Hoelzer K., Wong N., Thomas J., Talkington K., Jungman E., Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Veterinary Research . 2017;13(1):p. 211. doi: 10.1186/s12917-017-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall B. M., Levy S. B. Food animals and antimicrobials: impacts on human health. Clinical Microbiology Reviews . 2011;24(4):718–733. doi: 10.1128/cmr.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouiche M. M. M., Moffo F., Akoachere J.-F. T. K., et al. Antimicrobial resistance from a one health perspective in Cameroon: a systematic review and meta-analysis. BMC Public Health . 2019;19(1):p. 1135. doi: 10.1186/s12889-019-7450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbassi M., Kilani H., Zouari M., Mansouri R., Oussama E. Antimicrobial resistance in Escherichia coli isolates from healthy poultry, bovine and ovine in Tunisia: a real animal and human health threat. J Clin Microbiol Biochem Technol . 2017;3:019–023. doi: 10.17352/jcmbt.000021. [DOI] [Google Scholar]
- 8.Karczmarczyk M., Walsh C., Slowey R., Leonard N., Fanning S. Molecular characterization of multidrug-resistant Escherichia coli isolates from Irish cattle farms. Applied and Environmental Microbiology . 2011;77(20):7121–7127. doi: 10.1128/aem.00601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazurek J., Pusz P., Bok E., Stosik M., Baldy-Chudzik K. The phenotypic and genotypic characteristics of antibiotic resistance in Escherichia coli populations isolated from farm animals with different exposure to antimicrobial agents. Polish Journal of Microbiology . 2013;62(2):173–179. doi: 10.33073/pjm-2013-022. [DOI] [PubMed] [Google Scholar]
- 10.Medina A., Horcajo P., Jurado S., et al. Phenotypic and genotypic characterization of antimicrobial resistance in enterohemorrhagic Escherichia coli and atypical enteropathogenic E. coli strains from ruminants. Journal of Veterinary Diagnostic Investigation . 2011;23(1):91–95. doi: 10.1177/104063871102300114. [DOI] [PubMed] [Google Scholar]
- 11.Momtaz H., Rahimi E., Moshkelani S. Molecular detection of antimicrobial resistance genes in E. coli isolated from slaughtered commercial chickens in Iran. Veterinarni Medicina . 2012;57(4):193–197. doi: 10.17221/5916-vetmed. [DOI] [Google Scholar]
- 12.Okubo T., Yossapol M., Maruyama F., et al. Phenotypic and genotypic analyses of antimicrobial resistant bacteria in livestock in Uganda. Transbound Emerg Dis . 2019;66(1):317–326. doi: 10.1111/tbed.13024. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S., Nakano M., Kitagawa W., et al. Characterization of multi-antibiotic-resistant Escherichia coli isolated from beef cattle in Japan. Microbes and Environments . 2014;29(2):136–144. doi: 10.1264/jsme2.me13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengtsson B., Greko C. Antibiotic resistance—consequences for animal health, welfare, and food production. Upsala Journal of Medical Sciences . 2014;119(2):96–102. doi: 10.3109/03009734.2014.901445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals; Approved Standard . 5. Wayne, PA, USA: Clinical laboratory Standardas Institute; 2018. [Google Scholar]
- 16.Chen B., Zheng W., Yu Y., et al. Class 1 integrons, selected virulence genes, and antibiotic resistance in Escherichia coli isolates from the Minjiang River, Fujian Province, China. Applied and Environmental Microbiology . 2011;77:148–155. doi: 10.1128/aem.01676-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez S., Martínez R., García A., et al. Longitudinal study of Shiga toxin-producing Escherichia coli shedding in sheep feces: persistence of specific clones in sheep flocks. Applied and Environmental Microbiology . 2009;75(6):1769–1773. doi: 10.1128/aem.02043-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Quesne W. J., Baker-Austin C., Verner-Jeffreys D. W., Al-Sarawi H. A., Balkhy H. H., Lyons B. P. Antimicrobial resistance in the Gulf Cooperation Council region: a proposed framework to assess threats, impacts and mitigation measures associated with AMR in the marine and aquatic environment. Environment International . 2018;121:1003–1010. doi: 10.1016/j.envint.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Al-Dhaheri A. S., Al-Niyadi M. S., Al-Dhaheri A. D., Bastaki S. M. Resistance patterns of bacterial isolates to antimicrobials from 3 hospitals in the United Arab Emirates. Saudi Medical Journal . 2009;30:618–623. [PubMed] [Google Scholar]
- 20.Alfaresi M., Kim Sing G., Senok A. First report of blaCTX-M-28 in Enterobacteriaceae isolates in the United Arab Emirates. Journal of Pathogens . 2018;2018:5. doi: 10.1155/2018/1304793.1304793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kaabi M., Wu T., Hassanein A. Rising bacterial resistance to common antibiotics in Al Ain, United Arab Emirates. Eastern Mediterranean Health Journal . 2011;17(06):479–484. doi: 10.26719/2011.17.6.479. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z., Wang J., Ho H., Wang Y., Huang S., Han R. Prevalence and antimicrobial-resistance phenotypes and genotypes of Escherichia coli isolated from raw milk samples from mastitis cases in four regions of China. Journal of Global Antimicrobial Resistance . 2020;22:94–101. doi: 10.1016/j.jgar.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Kim H. C., Jang J.-H., Kim H., Kim Y.-J., Lee K.-R., Kim Y.-T. Multiplex PCR for simultaneous detection of aminoglycoside resistance genes in Escherichia coli and Klebsiella pneumoniae. Korean J Clin Lab Sci . 2012;44:155–165. [Google Scholar]
- 24.Mammeri H., Van De Loo M., Poirel L., Martinez-Martinez L., Nordmann P. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrobial Agents and Chemotherapy . 2005;49(1):71–76. doi: 10.1128/aac.49.1.71-76.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka Y., Ohkusu K., Kawamura Y., Baba S., Ezaki T., Kimura S. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagnostic Microbiology and Infectious Disease . 2006;54(2):109–114. doi: 10.1016/j.diagmicrobio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Randall L. P., Cooles S., Osborn M., Piddock L., Woodward M. J. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. Journal of Antimicrobial Chemotherapy . 2004;53(2):208–216. doi: 10.1093/jac/dkh070. [DOI] [PubMed] [Google Scholar]
- 27.Toro C., Farfán M., Contreras I., et al. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiology and Infection . 2005;133(1):81–86. doi: 10.1017/s0950268804003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van T. T. H., Chin J., Chapman T., Tran L. T., Coloe P. J. Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. International Journal of Food Microbiology . 2008;124(3):217–223. doi: 10.1016/j.ijfoodmicro.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Dandachi I., Chaddad A., Hanna J., Matta J., Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Frontiers in Microbiology . 2019;10:p. 1941. doi: 10.3389/fmicb.2019.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayah R. S., Kaneene J. B., Johnson Y., Miller R. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic-and wild-animal fecal samples, human septage, and surface water. Applied and Environmental Microbiology . 2005;71(3):1394–1404. doi: 10.1128/aem.71.3.1394-1404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anjum M. F., Choudhary S., Morrison V., et al. Identifying antimicrobial resistance genes of human clinical relevance within Salmonella isolated from food animals in Great Britain. Journal of Antimicrobial Chemotherapy . 2011;66(3):550–559. doi: 10.1093/jac/dkq498. [DOI] [PubMed] [Google Scholar]
- 32.Rosengren L. B., Waldner C. L., Reid-Smith R. J. Associations between antimicrobial resistance phenotypes, antimicrobial resistance genes, and virulence genes of fecal Escherichia coli isolates from healthy grow-finish pigs. Applied and Environmental Microbiology . 2009;75(5):1373–1380. doi: 10.1128/aem.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts M. C. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiology Reviews . 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 34.Lanz R., Kuhnert P., Boerlin P. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Veterinary Microbiology . 2003;91(1):73–84. doi: 10.1016/s0378-1135(02)00263-8. [DOI] [PubMed] [Google Scholar]
- 35.Bryan A., Shapir N., Sadowsky M. J. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Applied and Environmental Microbiology . 2004;70(4):2503–2507. doi: 10.1128/aem.70.4.2503-2507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammerum A. M., Sandvang D., Andersen S. R., et al. Detection of sul1, sul2 and sul3 in sulphonamide resistant Escherichia coli isolates obtained from healthy humans, pork and pigs in Denmark. International Journal of Food Microbiology . 2006;106(2):235–237. doi: 10.1016/j.ijfoodmicro.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Deekshit V., Kumar B., Rai P., Srikumar S., Karunasagar I., Karunasagar I. Detection of class 1 integrons in Salmonella Weltevreden and silent antibiotic resistance genes in some seafood-associated nontyphoidal isolates of Salmonella in south‐west coast of India. Journal of Applied Microbiology . 2012;112(6):1113–1122. doi: 10.1111/j.1365-2672.2012.05290.x. [DOI] [PubMed] [Google Scholar]
- 38.Enne V. I., Livermore D. M., Stephens P., Hall L. M. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. The Lancet . 2001;357(9265):1325–1328. doi: 10.1016/s0140-6736(00)04519-0. [DOI] [PubMed] [Google Scholar]
- 39.Kerrn M. B., Klemmensen T., Frimodt-Møller N., Espersen F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. Journal of Antimicrobial Chemotherapy . 2002;50(4):513–516. doi: 10.1093/jac/dkf164. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T., Wang C., Zhong X. Survey on sulfonamide antibiotic-resistant genotype and phenotype of avian Escherichia coli in North China. Poultry Science . 2012;91(4):884–887. doi: 10.3382/ps.2011-01960. [DOI] [PubMed] [Google Scholar]
- 41.Carattoli A. Importance of integrons in the diffusion of resistance. Veterinary Research . 2001;32(3/4):243–259. doi: 10.1051/vetres:2001122. [DOI] [PubMed] [Google Scholar]
- 42.Skold O. Resistance to trimethoprim and sulfonamides. Veterinary Research . 2001;32(3/4):261–273. doi: 10.1051/vetres:2001123. [DOI] [PubMed] [Google Scholar]
- 43.Howell E. E. Searching sequence space: two different approaches to dihydrofolate reductase catalysis. ChemBioChem . 2005;6(4):590–600. doi: 10.1002/cbic.200400237. [DOI] [PubMed] [Google Scholar]
- 44.Kadlec K., Schwarz S. Analysis and distribution of class 1 and class 2 integrons and associated gene cassettes among Escherichia coli isolates from swine, horses, cats and dogs collected in the BfT-GermVet monitoring study. Journal of Antimicrobial Chemotherapy . 2008;62(3):469–473. doi: 10.1093/jac/dkn233. [DOI] [PubMed] [Google Scholar]
- 45.Vinué L., Saenz Y., Somalo S., et al. Prevalence and diversity of integrons and associated resistance genes in faecal Escherichia coli isolates of healthy humans in Spain. Journal of Antimicrobial Chemotherapy . 2008;62(5):934–937. doi: 10.1093/jac/dkn331. [DOI] [PubMed] [Google Scholar]
- 46.Lei T., Tian W., He L., et al. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. Veterinary Microbiology . 2010;146(1-2):85–89. doi: 10.1016/j.vetmic.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Jacoby G. A. Mechanisms of resistance to quinolones. Clinical Infectious Diseases . 2005;41(Supplement_2):S120–S126. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- 48.Courvalin P. Plasmid-mediated 4-quinolone resistance: a real or apparent absence? Antimicrobial Agents and Chemotherapy . 1990;34(5):681–684. doi: 10.1128/aac.34.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park C. H., Robicsek A., Jacoby G. A., Sahm D., Hooper D. C. Prevalence in the United States of aac (6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrobial Agents and Chemotherapy . 2006;50(11):3953–3955. doi: 10.1128/aac.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Y., Bao X., Ji L., et al. Resistance integrons: class 1, 2 and 3 integrons. Annals of Clinical Microbiology and Antimicrobials . 2015;14:45–11. doi: 10.1186/s12941-015-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lomniczi B., Wehmann E., Herczeg J., et al. Newcastle disease outbreaks in recent years in western Europe were caused by an old (VI) and a novel genotype (VII) Archives of Virology . 1998;143(1):49–64. doi: 10.1007/s007050050267. [DOI] [PubMed] [Google Scholar]
- 52.Enne V. I., Delsol A. A., Roe J. M., Bennett P. M. Evidence of antibiotic resistance gene silencing in Escherichia coli. Antimicrobial Agents and Chemotherapy . 2006;50(9):3003–3010. doi: 10.1128/aac.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sunde M., Norström M. The genetic background for streptomycin resistance in Escherichia coli influences the distribution of MICs. Journal of Antimicrobial Chemotherapy . 2005;56(1):87–90. doi: 10.1093/jac/dki150. [DOI] [PubMed] [Google Scholar]
- 54.Tuckman M., Petersen P. J., Howe A. Y. M., et al. Occurrence of tetracycline resistance genes among Escherichia coli isolates from the phase 3 clinical trials for tigecycline. Antimicrobial Agents and Chemotherapy . 2007;51(9):3205–3211. doi: 10.1128/aac.00625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chopra I., Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews . 2001;65(2):232–260. doi: 10.1128/mmbr.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniels C., Ramos J. Adaptive drug resistance mediated by root–nodulation–cell division efflux pumps. Clinical Microbiology and Infections . 2009;15:32–36. doi: 10.1111/j.1469-0691.2008.02693.x. [DOI] [PubMed] [Google Scholar]
- 57.Memon J., Kashif J., Yaqoob M., Liping W., Yang Y., Hongjie F. Molecular characterization and antimicrobial sensitivity of pathogens from sub-clinical and clinical mastitis in Eastern China. Prevalence . 2012;33:170–174. [Google Scholar]
- 58.Al-Zarouni M., Senok A., Rashid F., Al-Jesmi S. M., Panigrahi D. Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Medical Principles and Practice . 2008;17(1):32–36. doi: 10.1159/000109587. [DOI] [PubMed] [Google Scholar]
- 59.Isaacson R. E., Torrence M. E. The Role of Antibiotics in Agriculture . Washington, DC, USA: Amer Acad Microbiol; 2002. [PubMed] [Google Scholar]
- 60.Schroeder C. M., Zhao C., DebRoy C., et al. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Applied and Environmental Microbiology . 2002;68(2):576–581. doi: 10.1128/aem.68.2.576-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


