Abstract
The efficacies of orally (p.o.) dosed linezolid and intravenously (i.v.) dosed vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) in rabbits with experimental aortic-valve endocarditis were investigated. After endocarditis was established with a recent clinical MRSA isolate, rabbits were dosed for 5 days with linezolid (p.o., three times a day) at either 25, 50, or 75 mg/kg of body weight or vancomycin (i.v., twice a day) at 25 mg/kg. The 25-mg/kg linezolid group had a high mortality rate and bacterial counts in the valve vegetations that were not different from those of the controls. Linezolid dosed p.o. at 50 and 75 mg/kg and i.v. vancomycin produced statistically significant reductions in bacterial counts compared to those of the untreated controls. The reduced bacterial counts and culture-negative valve rates for the animals treated with linezolid at 75 mg/kg were similar to those for the vancomycin-treated animals. Concentrations of linezolid in plasma were determined at several points in the dosing regimen. These results suggest that the efficacy of linezolid in this infection model is related to trough levels in plasma that remain above the MIC for this microorganism. At the ineffective dose of linezolid (25 mg/kg) the concentration at sacrifice was 0.045 times the MIC, whereas the concentrations of linezolid in plasma in the 50- and 75-mg/kg groups were 2 and 5 times the MIC at sacrifice, respectively. The results from this experimental model suggest that the oxazolidinone linezolid may be effective for the treatment of serious staphylococcal infections when resistance to other antimicrobials is present.
In recent years the effectiveness of new antimicrobials has been routinely evaluated in experimental bacterial endocarditis models. Bacterial endocarditis is considered to be a subacute to chronic, serious infection that requires maintenance of bactericidal levels of antibiotics for prolonged periods of time to result in culture-negative status. The use of the rabbit endocarditis model allows several aspects of antimicrobial efficacy to be explored (1, 5).
Oxazolidinones are a new class of antimicrobials with a unique mechanism of action. This class of bacterial protein synthesis inhibitors functions by binding to the 50S ribosomal subunit; this binding prevents formation of a functional initiation complex in bacterial translation systems (20). Linezolid is an oxazolidinone that has been approved by the Food and Drug Administration for use in treating infections caused by gram-positive microorganisms. Linezolid's use in treating staphylococcal infections has been well documented (2, 6). Major advantages of linezolid are the lack of inherent cross-resistance to other antibiotic classes and the lack of rapid in vitro resistance development. The excellent bioavailability of linezolid in humans allows the drug to be administered intravenously (i.v.) or orally, providing an added benefit of this drug compared to other antibiotics with similar antimicrobial spectra. Previously, our group has shown that when trough levels of linezolid are maintained above the MIC, orally administered linezolid is as effective as vancomycin in the treatment of the methicillin-sensitive Staphylococcus aureus in the rabbit endocarditis model (16).
The increasing prevalence of methicillin-resistant S. aureus (MRSA) has become a major therapeutic challenge to the hospital infection community. Nosocomial MRSA infections are associated with longer hospital stays and increased hospital costs (4). Risk factors for developing postoperative infections caused by MRSA include previous antimicrobial therapy, prolonged hospitalization, severe underlying disease, old age, and multiple invasive procedures. Typically these nosocomial MRSA strains are multidrug resistant, making vancomycin the most frequently used therapy for MRSA infections (4). The increase in vancomycin utilization for these serious infections has recently led to a new concern. Institutions in the United States, Europe, Japan, and Guatemala have documented the existence of MRSA strains that have decreased sensitivities to vancomycin (10, 17, 19; C. R. Mejia, G. Martinez, M. R. Gordillo, T. Nagatake, C. A. Ramirez, and M. A. Aguilera, 37th Infect. Dis. Soc. Am. session 31, abstr. 2, 1999). Linezolid has been the subject of few clinical reports of resistance and may provide a needed alternative to vancomycin therapy in these multidrug-resistant serious staphylococcal infections (L. D. Dresser, M. C. Birmingham, A. W. Karchmer, C. R. Rayner, S. M. Flavin, and J. J. Schentag, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2239, 2000).
This study investigated the efficacy of oral linezolid at three different doses for the treatment of rabbit experimental aortic-valve endocarditis. Bacterial counts in the valve vegetation and kidney were compared to those in the untreated control and vancomycin-treated animals. Concentrations of linezolid in plasma were determined at several points in the dosing regimen. Finally, reductions in bacterial density of the valve vegetations were correlated to levels of linezolid in plasma.
(This work was presented in part at the 100th General Meeting of the American Society for Microbiology, Los Angeles, Calif., May 2000 [C. F. Dailey, M. P. Oramas-Shirey, L. V. Buchanan, C. L. Dileto-Fang, R. J. Lemay, R. J. Zielinski, M. T. Kuo, C. W. Ford, D. H. Batts, and J. K. Gibson, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. A-21, 2000].)
MATERIALS AND METHODS
Bacterial strain and in vitro susceptibility.
The MRSA strain used in this study was obtained from a patient with endocarditis in January 1999. An initial panel of in vitro antibiotic susceptibility tests was run using Sensititer plates (AccuMed International, Inc., Westlake, Ohio) for aerobic bacteria. Methicillin resistance was determined using the agar dilution method with Mueller-Hinton agar plates (Difco Laboratories, Detroit, Mich.) supplemented with 2% NaCl and oxacillin. The MICs and minimum bactericidal concentrations of linezolid and vancomycin (Sigma, St. Louis, Mo.) were determined in Mueller-Hinton broth as recommended by National Committee for Clinical Laboratory Standards publication M7-A4 (15).
Experimental endocarditis.
All procedures in these studies were in compliance with the Animal Welfare Act Regulations (Code of Federal Regulation parts 1, 2, and 3). Left-sided endocarditis was induced in the aortic valves of male New Zealand White rabbits (2 to 2.5 kg) (Covance, Kalamazoo, Mich.) by a catheter method described elsewhere (8). Briefly, using sterile surgical techniques, a polyethylene catheter (PE-50; Becton Dickinson, Sparks, Md.) was advanced retrograde through the right carotid artery into the left ventricle. The catheter was securely tied in place and remained in place throughout the study. Twenty-four hours after catheter insertion, the animals were challenged via an ear vein with approximately 2.25 × 106 CFU of MRSA in 1 ml of sterile saline.
Eighteen hours after bacterial challenge, all animals had blood drawn from an ear vein for culture. After blood samples were obtained, animals were randomized into the following treatment groups: untreated controls (n = 12), animals treated with linezolid at one of three different doses, or animals treated with vancomycin. Linezolid-treated animals received either 25 mg/kg of body weight (n = 15), 50 mg/kg (n = 14), or 75 mg/kg (n = 13) orally in a 0.25% methylcellulose vehicle three times daily (at 8-h intervals). The oral doses of linezolid were adjusted based on an approximate 30% bioavailability in rabbits, compared to almost 100% in humans (R. J. Zielinski and M. T. Kuo, unpublished data). Vancomycin-treated animals (n = 13) received 25 mg/kg i.v. in sterile saline twice a day. All antimicrobials were administered for 5 days.
Preliminary kinetic studies with this strain determined a high degree of virulence, with peak bacterial levels in the valve vegetation measured at 18 h. Death due to bacteremia was observed beginning at 24 h postinfection. Survival of the rabbits required antimicrobial treatment to begin at 18 h postinfection. Therefore, untreated control animals were sacrificed 18 h after inoculation. Treated animals were sacrificed 8 h after the final dose of linezolid or 12 h after the final dose of vancomycin using a 1-ml (200-mg/kg) rapid i.v. injection of sodium pentobarbital. Blood samples were collected, and the heart was clamped at the ascending aorta and removed. The left ventricle was dissected to expose the aortic valve and to confirm that the catheter tip extended into the left ventricle. Only animals with properly placed catheters (completely across the aortic valve) were further evaluated. The right kidney and the vegetation associated with the aortic valve were excised and weighed prior to being homogenized in an appropriate volume of brain heart infusion broth (Difco Laboratories). The blood samples and tissue homogenates were serially diluted and plated on brain heart infusion agar to quantitate surviving bacteria at 20 to 24 h after plating. The limit of detectable bacteria, in CFU per gram of tissue or milliliter of blood, was determined by calculating the result for one observed bacterial colony in an undiluted sample. The lower limit of detection for the blood was determined to be 1.3 CFU/ml of blood, for the valve vegetation the average lower limit of detection was 2.9 CFU/g of tissue, and for the kidney the average lower limit of detection was 1.8 CFU/g of tissue. Tissue homogenates or blood samples in which no bacterial colonies were detected (culture negative) were assigned the value of one observed colony.
Antibiotic concentration in plasma.
Blood samples were taken at four time points, three times during the dosing regimen and at sacrifice, to determine the antimicrobial concentration in the plasma. Initial samples (day 1) were drawn 1 h after the first dose of drug (peak), and samples to determine trough levels in blood were drawn during the same dose interval just prior to the second dose (8 h). Blood samples were drawn again on the final day (day 5) of treatment 1 h (peak) after the 13th (linezolid) or 9th (vancomycin) dose and at sacrifice (trough). Samples were spun for 1 min in a microcentrifuge, and plasma aliquots were stored at −20°C.
The concentrations of linezolid and vancomycin in the plasma samples were measured by high-pressure liquid chromatography (HPLC)/mass spectrometry using a PE SCIEX API 3000 triple quadrupole mass spectrometer with a heated nebulizer ion source and a Hewlett-Packard 1100 HPLC as the solvent delivery injection system. Mass spectrometry data were acquired in the SRM scan mode with a dwell time of 250 ms, a scan speed of 0.51 s, and a pause time of 5.0 ms. The SRM ion pair for linezolid was 338.1 (Q1) and 296.2 (Q3). The internal standard SRM ion pair was 319.0 (Q1) and 216.0 (Q3). The HPLC and mass spectrometer were controlled by PE SCIEX API MassChrom software (version 1.1). HPLC mobile-phase solutions were prepared according to standard practices.
The typical range of standards for linezolid in rabbit plasma was from 0.00372 to 244 μg/ml. The dose-response curve for linezolid over this range was linear for all assays, with ±20% being the typical range for individual standard points on the line. Quality control standards were run at four or more levels ranging throughout the standard curve, and typical acceptability criteria were ±20% of the expected quality control value. The lower limit of quantitation for all analyses was set at 80% of the lowest standard analyzed for each individual assay. The lower limits of quantitation for linezolid and vancomycin were 0.004 and 1 μg/ml, respectively.
Susceptibility testing.
Bacterial colonies recovered from linezolid-treated animals were retested for linezolid susceptibility by redetermining the MIC of linezolid. The linezolid MICs were determined using MicroScan plates (Dade Behring, Inc., West Sacramento, Calif.) that included a linezolid panel.
Statistical testing.
All results are reported as means ± standard deviations. Kruskal-Wallis analysis of variance was used to compare differences between staphylococcal densities in blood, kidney, and valve vegetation. A P value of less than 0.05 at the 95% confidence interval was considered statistically significant.
RESULTS
In vitro testing of UC-15209.
The strain of S. aureus used in these studies was determined to be class 3 heterogenous methicillin resistant, and the MIC for this strain was 128 μg/ml. The vast majority of the antibiotic or antimicrobial agents tested scored in the resistant range for the given agent with the exception of the following: linezolid, tetracycline, gentamicin, nitrofurantoin, trimethoprim-sulfamethoxazole, and vancomycin. This MRSA strain has been catalogued in the Pharmacia and Upjohn Culture Collection and designated UC-15209. The MICs and the minimum bactericidal concentrations for UC-15209 were 2 and >64 μg/ml for linezolid and 1 and >16 μg/ml for vancomycin, respectively.
Mortality and blood cultures.
The mean quantitative blood bacterial culture from the 18-h untreated controls was 2.41 ± 0.83 log10 CFU/ml, with 10 of 12 animals having positive cultures. In the treatment groups, only the linezolid 25-mg/kg group had animals with detectable bacteremia at sacrifice. This dose group had a high associated mortality rate, 73% (11 of 15). Three out of four surviving animals in this group had positive blood cultures, with a mean bacterial count of 2.00 ± 0.96 log10 CFU/ml. At 50 mg/kg, the mortality rate was 14% (2 of 14), and no deaths were observed in the 75-mg/kg group. All animals from these two linezolid dose groups had culture-negative blood by the end of dosing. The mortality rate in the vancomycin treatment group was 15% (2 of 13), and the remaining animals had negative blood cultures.
Kidney and bacterial valve vegetation counts.
Bacterial counts and culture-negative rates for each treatment group are shown in Table 1. In the kidney, untreated controls had a mean bacterial count of 5.72 ± 0.78 log10 CFU/g, with 12 of 12 animals having bacteria present in the kidney. Treatment with linezolid at 25 mg/kg reduced the mean bacterial count in the kidney to 4.57 ± 1.31 log10 CFU/g. Significant decreases in mean bacterial counts were seen in the kidneys from animals in the 50-mg/kg group, with a mean bacterial count of 2.63 ± 1.33 log10 CFU/g and a culture negativity rate of 58.3%. In the linezolid 75-mg/kg group all of the animals were able to clear the kidney infection (100% culture negative). The vancomycin dose group had one treatment failure (6.4 log10 CFU/g) in the kidney, with a mean bacterial count of 2.36 ± 1.33 log10 CFU/g.
TABLE 1.
Outcome of 5-day treatment of experimental endocarditis caused by MRSA
| Drug and dose (mg/kg) | No. culture negative/total
|
Mean bacterial count (log10 CFU/g) ± SD
|
||
|---|---|---|---|---|
| Valve | Kidney | Valve | Kidney | |
| No drug | 0/12 | 0/12 | 9.26 ± 0.79 | 5.72 ± 0.78 |
| Vancomycin,a 25 | 11/11 | 10/11 | 2.99 ± 0.14b | 2.36 ± 1.33b |
| Linezolidc | ||||
| 25 | 0/4 | 0/4 | 7.85 ± 0.66d | 4.57 ± 1.31 |
| 50 | 2/12 | 7/12 | 4.84 ± 1.28bd | 2.63 ± 1.33b |
| 75 | 10/13 | 13/13 | 3.20 ± 0.47b | 2.03 ± 0.04b |
Administered i.v. twice daily.
P < 0.05 versus controls.
Administered orally three times daily.
P < 0.05 versus vancomycin treatment group.
Valve vegetations in untreated controls had a mean bacterial count of 9.26 ± 0.79 log10 CFU/g, with 12 of 12 animals having bacteria present in the vegetation. Similar to our previous study with endocarditis caused by methicillin-susceptible S. aureus (16), there was a clear stepwise decrease in the mean bacterial valve vegetation counts as the linezolid dose increased, dropping from 7.85 ± 0.66 log10 CFU/g at 25 mg/kg to 4.84 ± 1.28 and 3.20 ± 0.47 log10 CFU/g at 50 and 75 mg/kg, respectively. Compared to the value for untreated controls, the decrease in valve vegetation counts was statistically significant at both 50 and 75 mg/kg. The percentage of culture-negative valves also increased as the dose increased, increasing from 0% at 25 mg/kg to 17 and 77% at 50 and 75 mg/kg, respectively. The vancomycin-treated animals also showed a significant decrease in valve vegetation counts; all animals had bacterial valve vegetation counts below the limit of detection at the end of the study.
Concentrations of linezolid and vancomycin in plasma.
The mean concentrations of linezolid in plasma for the 25-, 50-, and 75-mg/kg groups are shown in Table 2. For each dose group the peak linezolid concentration was above the MIC for the test organism (2 μg/ml). The average day 5 peak concentration in plasma for each group also showed drug accumulation compared to the day 1 peaks. The day 5 peak concentrations in plasma at 25, 50, and 75 mg/kg showed average increases of 1.9-, 2.2-, and 2.9-fold, respectively. The day 1 troughs of all linezolid doses were significantly below the MIC for UC-15209; however, at the end of the dosing interval, the 50- and 75-mg/kg groups had average concentrations in plasma that were two and five times the MIC for UC-15209, respectively.
TABLE 2.
Mean concentrations of linezolid in plasma for peak and trough samples
| Linezolid dose (mg/kg) (no. of animals) | Mean day 1 concn (μg/ml) in plasma ± SD at:
|
Mean day 5 concn (μg/ml) in plasma ± SD at:
|
||
|---|---|---|---|---|
| 1 h (peak) | 8 h (trough) | 1 h (peak) | 8 h (trough) | |
| 25 (4) | 4.7 ± 4.0 | 0.28 ± 0.42 | 9.0 ± 3.9 | 0.09 ± 0.07 |
| 50 (12) | 11.7 ± 10.9 | 0.23 ± 0.52 | 25.8 ± 11.5 | 4.13 ± 5.95 |
| 75 (13) | 18.5 ± 15.0 | 0.23 ± 0.26 | 54.8 ± 23.2 | 10.8 ± 5.0 |
The concentrations of vancomycin in plasma for the vancomycin-treated animals were also determined. Previous studies using identical doses and dosing intervals of vancomycin in a similar rabbit model of endocarditis reported a typical 1-h postdose peak level of vancomycin in plasma between 33 and 48 μg/ml (5, 7, 12). The mean peak levels of vancomycin in plasma, 36.0 ± 5.9 μg/ml after the first dose and 38.4 ± 12.5 μg/ml after the last dose, are within the reported range for peak levels of vancomycin in plasma. Trough vancomycin concentrations in plasma were below our assay's lower limit of quantitation (1 μg/ml).
Susceptibility testing.
There was no change in the MIC of linezolid for any bacterial colonies that survived linezolid treatment.
DISCUSSION
The purpose of this study was to compare the therapeutic efficacy of linezolid to that of vancomycin using an in vivo model of a serious MRSA infection. In this endocarditis study, 5 days of therapy with either oral linezolid (50 or 75 mg/kg) or i.v. vancomycin (25 mg/kg) resulted in significant decreases in the bacterial valve vegetation counts compared to those of the controls. The efficacy of linezolid at 75 mg/kg in terms of culture negative rate and bacterial valve vegetation counts was similar to that of vancomycin.
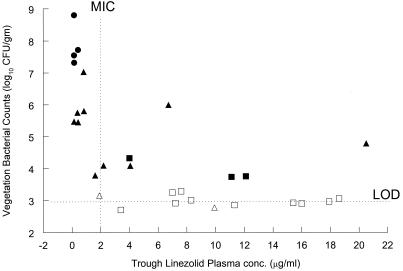
Previous studies in our laboratory with endocarditis caused by methicillin-susceptible S. aureus have demonstrated that the therapeutic efficacy of linezolid in this model requires trough levels in plasma to be above the MIC at the end of the treatment period (16). Although the primary goal of this study was not to study pharmacokinetics, a similar observation was made in the present study with endocarditis caused by MRSA. In general, in the animals treated with linezolid at 50 and 75 mg/kg, significant reductions in bacterial CFU per gram of vegetation were associated with trough linezolid concentrations greater than the MIC at the end of the final dosing interval (Fig. 1). Although the peak level of linezolid in plasma after 5 days of oral dosing with 25, 50, or 75 mg/kg was 4.5, 12, or 27 times the MIC, respectively, successful therapy required the trough level of linezolid to equal or exceed the linezolid MIC for the isolate at the end of the therapy. High peak levels in plasma alone did not reduce the bacterial valve vegetation CFU. Previous studies of experimental endocarditis with β-lactams have also demonstrated that successful therapeutic doses were related to maintenance of levels in plasma above the MIC during the entire dosing interval (3).
FIG. 1.
Bacterial vegetation counts versus trough (day 5) linezolid concentrations in plasma. The trough linezolid concentrations in plasma and valve vegetation bacterial counts were determined after 5 days of treatment. The symbols represent animals treated with linezolid in doses of 25 mg/kg (circles), 50 mg/kg (triangles), and 75 mg/kg (squares). The closed symbols represent culture-positive vegetations, and the open symbols represent culture-negative valvular vegetations (bacterial counts below the limit of detection [LOD]). The linezolid MIC for this strain of MRSA is 2 μg/ml, and the LOD is 2.9 to 3.2 log10 CFU/g.
Examination of the linezolid concentrations in plasma in individual animals with culture-positive vegetations treated with linezolid at 50 or 75 mg/kg revealed that four animals had trough levels in plasma on day 5 which were well above (i.e., 6.7, 11.1, 12.1, and 20.5 μg/ml) the MIC for the S. aureus strain used in this study. However, further examination of the data also revealed that these animals had low trough levels in plasma (i.e., 0.02, 0.7, 0.8, and 0.2 μg/ml) on the first day of dosing compared to the other animals in the 50- or 75-mg/kg treatment groups. These data suggest that a combination of linezolid levels at or above the MIC in plasma and a minimum number of treatment days are required for the therapeutic efficacy of linezolid in this model. This conclusion is also supported by preliminary, unpublished experiments we have conducted comparing 5- and 7-day dosing regimens with linezolid which also suggest that there was a time-dependent increase in efficacy as the length of time that the concentration was above the MIC was increased. While the time that the concentration is above the MIC is an important parameter for efficacy in this model, further studies will be necessary to address the significance of other pharmacokinetic parameters.
Traditional in vitro time-kill experiments have demonstrated linezolid to be a bacteriostatic antimicrobial agent against staphylococci (18). While the use of bacteriostatic agents for therapy in serious infections has been questioned in the past, in this study linezolid functioned as an in vivo bactericidal drug (4- to 5-log reduction in valvular bacterial counts) over 5 days of therapy. In addition, a recent review of clinical trials with linezolid has suggested clinical bactericidal activity with linezolid against endocarditis caused by vancomycin-resistant enterococci (G. A. Noskin, Guest commentary, Drugs 59:828, 2000). Moreover, other factors may contribute to the success of linezolid in this in vivo deep-seated infection model. Successful treatment of chronic infections such as endocarditis is dependent on tissue penetration by the drug. While tissue penetration by linezolid into the bacterial vegetation was not measured in the present study, a recent report of successful linezolid therapy for bacteremia due to an infected central vein thrombus after the failure of quinupristin-dalfopristin therapy was attributed to better penetration of the clot by linezolid (13). Therefore, linezolid levels in valvular vegetation must be determined in future endocarditis studies.
An important consideration in direct comparisons between the linezolid and vancomycin results obtained in this study is the difference in the route of antimicrobial administration and bioavailability of these compounds. Vancomycin treatments were limited to i.v. administration. Linezolid, which can be given either i.v. or orally, was administered orally in this study. The increase in trough levels between the first and last dose of linezolid in each linezolid treatment group suggests that drug accumulation occurred over the 5-day dosing interval. This was not seen with the vancomycin-treated animals. This demonstrates the importance of pharmacokinetic analyses of antimicrobial agents in multiple-dose studies to determine the efficacy of a given compound.
Increases in MICs over time are essential considerations in the treatment of chronic infections. Repeat MIC testing of microorganisms obtained from linezolid-treated animals that remained infected demonstrated that the linezolid MIC did not change over this relatively short (5-day) treatment period. This may offer an advantage over other antimicrobial compounds since many of the newer fluoroquinolones exhibit resistance after even shorter dosing periods (11). Cross-resistance to linezolid and to antimicrobial agents having similar modes of action (50S ribosomal protein inhibitors) from the macrolide, lincosamide, and streptogramin B group has not been reported to date (9). Cross-resistance was not evident in this study given that this strain of MRSA was resistant in vitro to erythromycin, clarithromycin, and clindamycin.
In summary, linezolid, when orally administered to rabbits with experimental aortic-valve endocarditis, significantly reduced bacterial vegetation densities. Limited pharmacokinetic sampling suggested that maintaining trough levels in plasma above the MIC was necessary in order to achieve therapeutic efficacy in this model. The therapeutic effects of linezolid appear to be dependent on both the maintenance of levels above the MIC in plasma during the dosing interval and a minimal duration of treatment for optimum antibacterial activity in this model. This suggests that the use of linezolid in cases of reduced sensitivity to vancomycin, such as that demonstrated by vancomycin-intermediate S. aureus and vancomycin-resistant enterococci, may be a valid approach in humans (14; Noskin, guest commentary). In addition, the benefit of linezolid as both an i.v. and an oral drug may reduce the hospital costs associated with long-term i.v. catheter dosing (4).
ACKNOWLEDGMENTS
We acknowledge Ming T. Kuo and Ray Zielinski for expert assistance in measuring the linezolid and vancomycin concentrations in plasma. We also acknowledge Gary Zurenko and Betty Yagi for help with the in vitro assays and Richelle LeMay and Mark Shattuck for assistance with animal surgery and dosing.
REFERENCES
- 1.Backo M, Gaenger E, Burkart A, Chai Y L, Bayer A S. Treatment of experimental staphylococcal endocarditis due to a strain with reduced susceptibility in vitro to vancomycin: efficacy of ampicillin-sulbactam. 1999 Antimicrob. Agents Chemother. 1999;43:2565–2568. doi: 10.1128/aac.43.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmingham M C, Craig R R, Hafkin B, Todd W M, Flavin S M, Root J D, Zimmer G S, Batts D H, Schentag J J. Critical care patients with significant, resistant, gram-positive infections enrolled in the linezolid compassionate use protocol. Crit Care Med. 1999;27(12)(Suppl. S):42. [Google Scholar]
- 3.Carbon C. Impact of the antibiotic dosage schedule on efficacy in experimental endocarditis. Scand J Infect Dis. 1991;74:163–172. [PubMed] [Google Scholar]
- 4.Carbon C. Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J Antimicrob Chemother. 1999;44:31–36. doi: 10.1093/jac/44.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 5.Chambers H F. Studies of RP 59500 in vitro and in a rabbit model of aortic valve endocarditis caused by methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1992;30(Suppl. A):117–122. doi: 10.1093/jac/30.suppl_a.117. [DOI] [PubMed] [Google Scholar]
- 6.Chien J W, Kucia M L, Salata R A. Use of linezolid, an oxazolidinone in the treatment of multidrug-resistant gram-positive bacterial infections. Clin Infect Dis. 2000;30:146–151. doi: 10.1086/313597. [DOI] [PubMed] [Google Scholar]
- 7.de Górgolas M, Avilés P, Verdejo C, Fendández Guerrero M L. Treatment of experimental endocarditis due to methicillin-susceptible or methicillin-resistant Staphylococcus aureus with trimethoprim-sulfamethoxazole and antibiotics that inhibit cell wall synthesis. Antimicrob Agents Chemother. 1995;39:953–957. doi: 10.1128/aac.39.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Fines M, Leclercq R. Activity of linezolid against Gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J Antimicrob Chemother. 2000;45:797–802. doi: 10.1093/jac/45.6.797. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Jones M E, Visser M R, Klootwijk M, Heisig P, Verhoef J, Schmitz F-J. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, oflaxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid [sic], quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob Agents Chemother. 1999;43:421–423. doi: 10.1128/aac.43.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maserati R, Cagni A E, Segu C. Sparfloxacin therapy for experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Chemotherapy. 1996;42:133–139. doi: 10.1159/000239432. [DOI] [PubMed] [Google Scholar]
- 13.McNeil A, Clark N M, Chandrasekar P H, Kauffman C A. Successful treatment of vancomycin-resistant Enterococcus faecium bacteremia with linezolid after failure of treatment with Synercid (quinupristin/dalfopristin) Clin Infect Dis. 2000;30:403–404. doi: 10.1086/313669. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki S, Ishii Y, Ohno A, Furuya N, Matsumoto T, Tateda K, Yamaguchi K. In-vitro activities of 11 antibiotics against vancomycin-resistant enterococci isolated in Japan. J Antimicrob Chemother. 1999;44:415–420. doi: 10.1093/jac/44.3.415. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.Oramas-Shirey M P, Buchanan L V, Dileto-Fang C L, Dailey C F, Ford C W, Batts D H, Gibson J K. Efficacy of linezolid in a staphylococcal endocarditis rabbit model. J Antimicrob Chemother. 2001;47:349–352. doi: 10.1093/jac/47.3.349. [DOI] [PubMed] [Google Scholar]
- 17.Rotun S S, McMath V, Schoonamaker D J, et al. Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg Infect Dis. 1999;5:147–149. doi: 10.3201/eid0501.990118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak M J, Cappelletty D M, Moldovan T, Aeschlimann J R, Kaatz G W. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob Agents Chemother. 1998;42:721–724. doi: 10.1128/aac.42.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith T L, Pearson M L, Wilcox K R, et al. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 20.Swaney S M, Aoki H, Ganoza M C, Shinabarger D L. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother. 1998;42:3251–3255. doi: 10.1128/aac.42.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]