ABSTRACT
This brief communication demonstrates the correlation of persistent respiratory symptoms with functional, tomographic, and transbronchial pulmonary biopsy findings in patients with COVID-19 who had a long follow-up period. We report a series of six COVID-19 patients with pulmonary involvement who presented with persistent dyspnea within 4-15 months of discharge. We performed transbronchial biopsies, and the histopathological pattern consistently demonstrated peribronchial remodeling with interstitial pulmonary fibrosis. Therefore, lung biopsy may be useful in the approach of patients with long COVID-19, although the type of procedure, its precise indication, and the moment to perform it are yet to be clarified.
(Brazilian Registry of Clinical Trials-ReBEC; identifier: RBR-8j9kqy [http://www.ensaiosclinicos.gov.br])
Keywords: COVID-19, COVID-19/pathology, Pulmonary fibrosis, Respiratory function tests, Biopsy
RESUMO
Esta comunicação breve demonstra a correlação de sintomas respiratórios persistentes com achados funcionais, tomográficos e de biópsia pulmonar transbrônquica em pacientes com COVID-19 que tiveram um longo período de acompanhamento. Relatamos uma série de seis pacientes com COVID-19 com acometimento pulmonar que apresentavam dispneia persistente após 4-15 meses da alta. Realizamos biópsias transbrônquicas, e o padrão histopatológico consistentemente demonstrou remodelação peribrônquica com fibrose pulmonar intersticial. Portanto, a biópsia pulmonar pode ser útil na abordagem de pacientes com COVID-19 prolongada, embora o tipo de procedimento, suas indicações precisas e o momento de sua realização ainda não estejam esclarecidos.
(Registro Brasileiro de Ensaios Clínicos - ReBEC; número de identificação: RBR-8j9kqy [http://www.ensaiosclinicos.gov.br])
Descritores: COVID-19, COVID-19/patologia, Fibrose pulmonar, Testes de função respiratória, Biópsia
The prevalence of pulmonary involvement in severe acute COVID-19 is high, and there is a concern regarding the occurrence of lung sequelae in the long term. 1 , 2 However, the risk, prevalence, and severity of post-COVID-19 pulmonary fibrosis over time are still uncertain. 1 , 3 There is still a low number of histopathological reports regarding pulmonary lesions, and they are mostly based on explants and autopsies. 4 - 10 Additionally, pathological reports of interstitial lung disease secondary to COVID-19 in the long term are even scarcer.
Patients with COVID-19 may present three major lung histopathological patterns: epithelial lesions and diffuse alveolar damage (DAD); vascular injuries; and interstitial fibrosis. These patterns may coexist in the same patient during the natural history of the disease. 9 , 11 The most common histopathological pattern described in acute and severe cases is DAD. Progressive phases of DAD include an early exudative pattern with edema and hyaline membrane formation, a transition to an organizing phase, followed by a fibrosing stage later. 9 , 12
A better understanding of the pulmonary sequelae of the lung lesions in the long term is warranted for determining a more targeted and optimized therapeutic proposal. The aim of this study was to report pulmonary histopathological findings obtained from transbronchial biopsy in a series of COVID-19 patients who had long follow-up periods.
We included six patients with a confirmed diagnosis of COVID-19 by a positive RT-PCR from a nasal swab specimen who presented with persistent respiratory symptoms and interstitial lung abnormalities on CT scans within 4 months of discharge at least and suspected of having post-COVID-19 pulmonary fibrosis. All patients were followed at the Hospital das Clínicas of the Universidade Estadual Paulista (UNESP, São Paulo State University).
This study was approved by the institutional research ethics committee (CAAE n. 31258820.5.1001.5411) and registered with the Brazilian Registry of Clinical Trials (identifier, RBR-8j9kqy). Bronchoscopy with transbronchial biopsy 13 was performed after a multidisciplinary discussion based on the presence of symptoms or lung function impairment and persistence of interstitial lung abnormalities on CT scans. The lung samples (average size = 3.8 mm2) were evaluated by an experienced pulmonary pathologist. The final diagnosis was defined by a multidisciplinary team, mainly based on the combination of clinical, tomographic, and histopathological features. We selected patients who were neither on immunosuppressive treatment nor using systemic corticosteroids after discharge. The following variables were collected from all of the patients before the biopsy procedure: baseline dyspnea index (BDI); post-bronchodilator spirometry; DlCO; TLC; PaO2 on room air; SpO2 on room air; six-minute walk distance (6MWD); and Saint George’s Respiratory Questionnaire (SGRQ) score. All patients signed the informed consent form.
Figure 1 shows CT scans of the study subjects before and after discharge, whereas Figure 2 shows the histopathological findings from the biopsy samples of the patients.
Figure 1. Chest CT scans of the patients studied. Patient 1: in A, a scan during the acute phase showing bilateral ground-glass opacities (GGO), consolidations, and parenchymal bands; in B, a scan after 15 months of follow-up showing subtle peripheral and posterior GGO. Patient 2: in C, a scan during the acute phase showing bilateral and peripheral GGO; in D, a scan after 7 months of follow-up showing subtle GGO with subpleural curvilinear lines and small dilated bronchioles in the right lower lobe. Patient 3: in E, a scan during the acute phase showing bilateral GGO and crazy-paving pattern; in F, a scan after 6 months of follow-up showing subtle scattered GGO. Patient 4: a scan during the acute phase showing bilateral and peripheral GGO and consolidations; in H, a scan after 4 months of follow-up showing subtle bilateral and peripheral GGO. Patient 5: in I, a scan during the acute phase showing bilateral GGO; in J, a scan after 10 months of follow-up showing subtle GGO and mosaic attenuation in the lung parenchyma. Patient 6: in K, a scan during the acute phase showing bilateral GGO and consolidations; in L, a scan after 7 months of follow-up showing bilateral GGO with some dilated bronchioles.
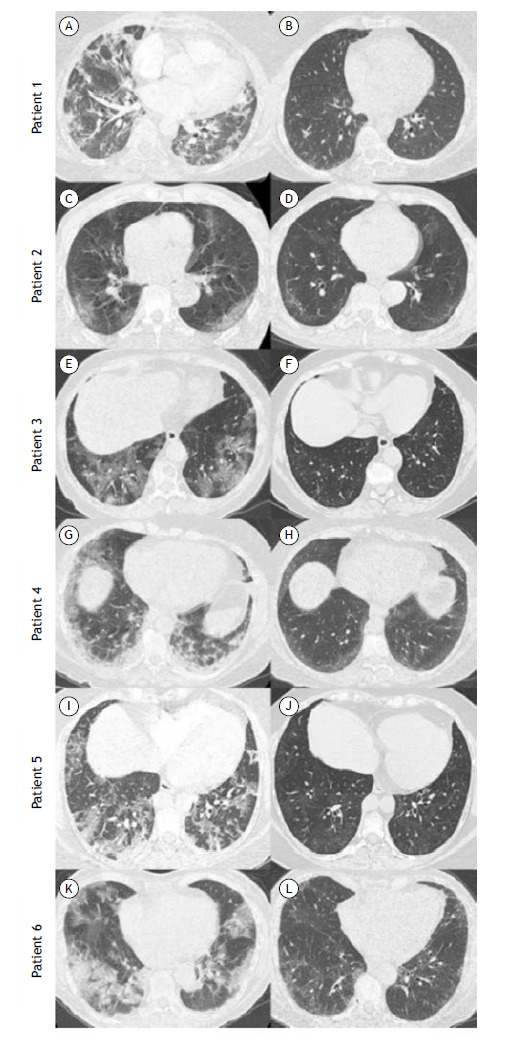
Figure 2. Histopathological panel of transbronchial biopsy samples collected from the patients studied (H&E; low power field, ×4, and high power field, ×40). All of the patients showed hyaline peribronchial remodeling with septal extension. Patient 1: focal septal thickening by prominent extracellular matrix deposition (blue dashed ellipse in A) associated with architectural distortion of the bronchial smooth muscle layer (blue arrows in B). Patient 2: mild hyaline peribronchial remodeling with septal extension (red arrow in C). Note the focal septal thickening by prominent extracellular matrix deposition (red dashed ellipse in D). Patient 3: prominent peribronchial remodeling with extensive extracellular matrix deposition (double green arrow in E). The architectural distortion of the bronchial smooth muscle layer is highlighted (green arrow in F). Patient 4: the architectural distortion around hyaline peribronchial remodeling promoted focal simile-desquamative reaction (area enclosed by blue dashed line in G). Note the disarray and hypertrophy of the bronchial smooth muscle layer (double blue arrow in H). Patient 5: prominent peribronchial remodeling (red arrow in I) with extensive extracellular matrix deposition (double red arrow in J) and small calcification. Patient 6: prominent peribronchial remodeling (green arrow in K) with extensive extracellular matrix deposition (green arrow in L).
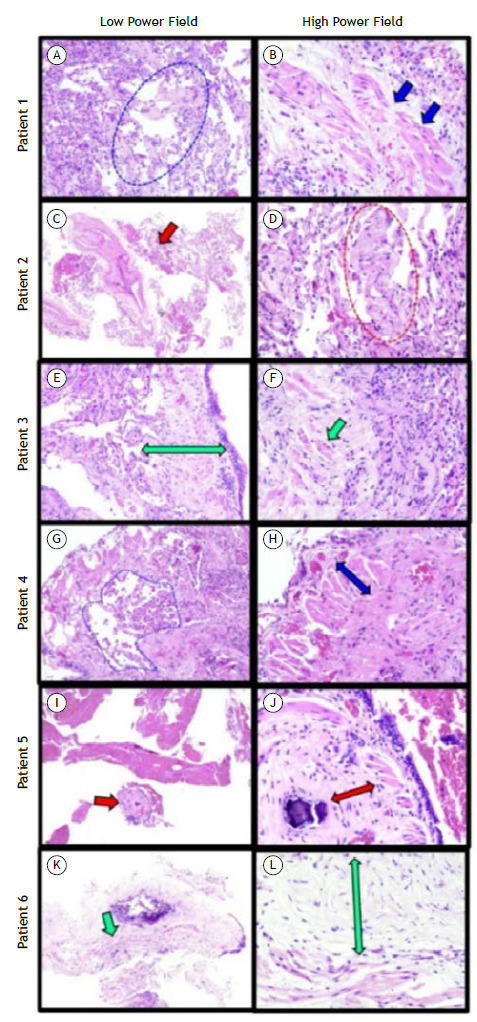
Patient 1: a 62-year-old female, former smoker with diabetes mellitus experienced COVID-19 symptoms for 15 days before hospitalization and was discharged 25 days later without oxygen supplementation. Maximal oxygen supplementation (MOS) during the use of mechanical ventilation was FiO2 = 90% and PaO2/FiO2 < 100. Fifteen months after discharge, she presented with dyspnea (BDI = 7); SGRQ score = 32.2; and reduced DlCO (57%). Spirometry, SpO2, PaO2, and 6MWD (440 m) were unremarkable. There was improvement from chest CT results obtained at hospital admission (Figure 1A) to those obtained at 15 months after discharge (Figure 1B), demonstrating subtle peripheral and posterior ground-glass opacities (GGO). Histopathological analysis of biopsy samples showed focal septal thickening by prominent extracellular matrix deposition associated with architectural distortion of the bronchial smooth muscle layer (Figures 2A and 2B).
Patient 2: a 69-year-old female with systemic arterial hypertension (SAH), diabetes mellitus, and dementia experienced COVID-19 symptoms for 15 days before hospitalization and was discharged 9 days later. MOS flow with nasal prong was 4 L/min. Seven months after discharge, the patient reported persistent dyspnea (BDI = 7). Post-bronchodilator spirometry, SpO2, and PaO2 were unremarkable. However, TLC (75%), DlCO (52%), 6MWD (389 m/78.8% of predicted), and SGRQ score (15.7) were reduced. CT scans at hospital admission and at 7 months after discharge are presented in Figures 1C and 1D, respectively, and there was tomographic improvement in the follow-up, although there were residual pulmonary abnormalities. Pulmonary histopathological analysis demonstrated mild hyaline peribronchial remodeling with septal extension and focal septal thickening by prominent extracellular matrix deposition (Figures 2C and 2D).
Patient 3: a 65-year-old female with hypothyroidism experienced COVID-19 symptoms for 14 days before hospitalization and was discharged 8 days later. She used a nasal prong (O2 flow = 4 L/min). Six months after discharge, she still reported dyspnea (BDI = 9), and 6MWD was 502 m. Spirometry, SpO2, and PaO2 were unremarkable. DlCO was mildly reduced (62%), and the SGRQ score was 30.8. Comparing CT scans at hospital admission and at 6 months after discharge, there were improvements, demonstrating subtle scattered GGO (Figures 1E and 1F, respectively). Pulmonary histopathological findings highlighted prominent peribronchial remodeling with extensive extracellular matrix deposition (Figure 2E). The architectural distortion of the bronchial smooth muscle layer was highlighted (Figure 2F).
Patient 4: a 44-year-old female with asthma and SAH started having COVID-19 symptoms 7 days before hospitalization and was discharged 14 days later. She used a non-rebreathing mask with a maximum O2 flow of 6 L/min. Four months after discharge, she presented with dyspnea (BDI = 8) and an SGRQ score of 40.3. Spirometry, TLC, DlCO (76%), SpO2, and PaO2 were unremarkable. In comparison with CT scans obtained at hospital admission (Figure 1G), only residual lesions were identified on CT obtained at 4 months after discharge (Figure 1H). Histopathologically, the architectural distortion around hyaline peribronchial remodeling promoted focal simile-desquamative reaction (Figure 2G). Note the disarray and hypertrophy of the bronchial smooth muscle layer (Figure 2H).
Patient 5: an 85-year-old male with SAH experience COVID-19 symptoms for 7 days before hospitalization and was discharged 16 days later. He used a nasal prong with MOS of 2 L/min. Ten months after discharge he still presented with reduced quality of life (SGRQ score = 62.0) and 6MWD (226 m/43.4% of predicted). He was unable to perform spirometry. SpO2 and PaO2 were normal. The comparison of CT scans at admission and at 10 months after discharge revealed improvements, the latter showing residual pulmonary lesions only (Figures 1I and 1J, respectively). Histopathological analysis was compatible with prominent peribronchial remodeling with extensive extracellular matrix deposition and small calcification (Figures 2I and 2J).
Patient 6: a 44-year-old female with a history of hysterectomy presented with COVID-19 symptoms for 7 days before hospitalization and was discharged 21 days later. MOS with non-rebreathing mask was 10 L/min. Seven months after discharge, she reported persistent dyspnea (BDI = 7), and her SGRQ score was 41.7. Spirometry suggested a restrictive pattern. SpO2 and PaO2 were normal, and 6MWD was reduced (375 m/69.0% of predicted). Follow-up CT scanning demonstrated an improvement in comparison with that at hospital admission (Figures 1K and 1L, respectively), showing residual pulmonary lesions only. Pulmonary histopathological analysis was compatible with peribronchial remodeling with extensive extracellular matrix deposition (Figures 2K and 2L).
Few studies have described pulmonary histopathological findings in patients with COVID-19 after a long follow-up period. Most case series have detailed features during the acute phase and based their findings on lung tissue from autopsy or transplant. 4 - 10 To our knowledge, this is the first study that evaluated histopathological features obtained from transbronchial biopsies during the late stage of pulmonary involvement by COVID-19, 4-15 months after the acute infection. The main finding of our study was that the histological analysis of the six patients with long COVID-19 and persistent pulmonary abnormalities on HRCT demonstrated signs of bronchiolocentric interstitial pneumonia, most of them presenting with architectural distortion and peribronchial remodeling with extracellular matrix deposition.
Although all of the patients in our series showed tomographic improvements, dyspnea and pulmonary tomographic abnormalities still remained. Only one patient met the criteria for ARDS. However, the late functional and tomographic findings of this patient with ARDS were similar to that of the others. It is not completely clear, however, when the presence of irreversible post-COVID-19 pulmonary fibrosis is defined, and which patients may present tomographic and functional improvement over time. Additionally, it remains uncertain in which scenario after COVID-19 lung biopsy should be considered for histopathological analysis. 1 , 3 , 14 , 15
Previous descriptions of pulmonary histopathological characterization of COVID-19 were mostly obtained from autopsy and explanted lungs. 1 , 5 - 7 , 9 , 10 The main patterns identified alone or in combination were exudative and organizing DAD, hemorrhage, thrombosis, intra-alveolar fibrin deposition, lymphoid infiltrates, and organizing pneumonia. However, in the fibrosing DAD phase, our group has already demonstrated a fibrotic phenotype with excessive extracellular matrix and collagen deposition and lung architectural distortion, corroborating other studies. 7 - 12 Likewise, some autopsy and explanted lung cases demonstrated interstitial and bronchiolar fibrosis, with collagen deposition, bronchial metaplasia, and pulmonary vascular remodeling. Some cases have demonstrated areas of microscopic honeycombing. 5 - 9 A recent case series reinforced the presence of diffuse interstitial fibrosis and areas of microscopic honeycombing in patients after a 4-month follow-up period. 8 Our findings demonstrated some similarities, as we predominantly identified bronchiolocentric interstitial pneumonitis. However, to our knowledge, no study has described histological features and their patterns after COVID-19 with pulmonary involvement during a long follow-up period, which would add for a better understanding of this process.
Patients with interstitial lung disease secondary to COVID-19 might need to be monitored for a longer time, preferably through a multidisciplinary approach. 3 Pulmonary lesions may persist in the long term after COVID-19, although a significant proportion of patients may present progressive functional and tomographic improvement during follow-up. 15
Our study has limitations. First, there were a small number of patients included for robust conclusions. Second, transbronchial biopsy does not allow assessment of peripheral lesions, which may have histological patterns that are different from those identified. Third, all patients showed tomographic improvements at follow-up, and we cannot conclude that the pattern found here would be the same as those found in patients who remain stable or deteriorate during follow-up.
In conclusion, findings compatible with bronchiolocentric interstitial pneumonia were identified from transbronchial biopsies in patients with long COVID-19. Patients with pulmonary involvement secondary to COVID-19 that require biopsy during the follow-up period need to be better defined. Based on our series, transbronchial biopsy may be an initial step in the assessment of patients with post-COVID-19 pulmonary fibrosis in the presence of symptoms or lung function impairment and persistence of interstitial lung abnormalities on CT scans. Further studies are warranted to determine the histopathological patterns in a larger number of lung tissue samples obtained from transbronchial cryobiopsy or surgical biopsy, as well as in patients who remain stable or present worsening of pulmonary involvement associated with COVID-19. It will also be important to assess the indications for and responses to drug treatment in such scenarios, including the use of corticosteroids and antifibrotic drugs.
ACKNOWLEDGEMENTS
We acknowledge all patients and their families who contributed to the present article.
Footnotes
Study carried out at the Hospital das Clínicas, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu (SP) Brasil.
Financial support: None.
REFERENCES
- 1.Tanni SE, Fabro AT, de Albuquerque A, Ferreira EVM, Verrastro CGY, Sawamura MVY, et al. Pulmonary fibrosis secondary to COVID-19: a narrative review. Expert Rev Respir Med. 2021;15(6):791–803. doi: 10.1080/17476348.2021.1916472. [DOI] [PubMed] [Google Scholar]
- 2.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19 the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi BG, Tanni SE. Pulmonary fibrosis and follow-up of COVID-19 survivors an urgent need for clarification. J Bras Pneumol. 2021;47(4):e20210213. doi: 10.36416/1806-3756/e20210213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doglioni C, Ravaglia C, Chilosi M, Rossi G, Dubini A, Pedica F, et al. Covid-19 Interstitial Pneumonia: Histological and Immunohistochemical Features on Cryobiopsies. Respiration. 2021;100(6):488–498. doi: 10.1159/000514822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574) doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XJ, Li K, Xu L, Yu YJ, Wu B, He YL. Novel insight from the first lung transplant of a COVID-19 patient. Eur J Clin Invest. 2021;51(1):e13443. doi: 10.1111/eci.13443. [DOI] [PubMed] [Google Scholar]
- 7.Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 8.Aesif SW, Bribriesco AC, Yadav R, Nugent SL, Zubkus D, Tan CD. Pulmonary Pathology of COVID-19 Following 8 Weeks to 4 Months of Severe Disease A Report of Three Cases, Including One With Bilateral Lung Transplantation. Am J Clin Pathol. 2021;155(4):506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wu J, Wang S, Li X, Zhou J, Huang B. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2021;78(4):542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, et al. Late histopathologic characteristics of critically ill COVID-19 patients Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19 a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson AG, Osborn M, Devaraj A, Wells AU. COVID-19 related lung pathology: old patterns in new clothing? Histopathology. 2020;77(2):169–172. doi: 10.1111/his.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults accredited by NICE. Thorax. 2013;68(1):i1–i44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Shen C, Wang L, Majumder S, Zhang D, Deen MJ. Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia a cohort study. Respir Res. 2021;22(1):203–203. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


