Abstract
Objectives
To perform a survey among all European Society of Breast Imaging (EUSOBI) radiologist members to gather representative data regarding the clinical use of breast DWI.
Methods
An online questionnaire was developed by two board-certified radiologists, reviewed by the EUSOBI board and committees, and finally distributed among EUSOBI active and associated (not based in Europe) radiologist members. The questionnaire included 20 questions pertaining to technical preferences (acquisition time, magnet strength, breast coils, number of b values), clinical indications, imaging evaluation, and reporting. Data were analyzed using descriptive statistics, the Chi-square test of independence, and Fisher’s exact test.
Results
Of 1411 EUSOBI radiologist members, 275/1411 (19.5%) responded. Most (222/275, 81%) reported using DWI as part of their routine protocol. Common indications for DWI include lesion characterization (using an ADC threshold of 1.2–1.3 × 10−3 mm2/s) and prediction of response to chemotherapy. Members most commonly acquire two separate b values (114/217, 53%), with b value = 800 s/mm2 being the preferred value for appraisal among those acquiring more than two b values (71/171, 42%). Most did not use synthetic b values (169/217, 78%). While most mention hindered diffusion in the MRI report (161/213, 76%), only 142/217 (57%) report ADC values.
Conclusion
The utilization of DWI in clinical practice among EUSOBI radiologists who responded to the survey is generally in line with international recommendations, with the main application being the differentiation of benign and malignant enhancing lesions, treatment response assessment, and prediction of response to chemotherapy. Report integration of qualitative and quantitative DWI data is not uniform.
Key Points
• Clinical performance of breast DWI is in good agreement with the current recommendations of the EUSOBI International Breast DWI working group.
• Breast DWI applications in clinical practice include the differentiation of benign and malignant enhancing, treatment response assessment, and prediction of response to chemotherapy.
• Report integration of DWI results is not uniform.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-022-08833-0.
Keywords: Breast neoplasms, Magnetic resonance imaging, Surveys and questionnaires
Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is the most sensitive test for breast cancer detection, with reported sensitivities ranging from 81 to 100% [1]. However, the positive predictive value of MRI-induced biopsy ranges between 20 and 40%, which implies that many women are still subjected to invasive procedures for benign breast disease detected at MRI. In this context, diffusion-weighted imaging (DWI) has emerged as a key imaging technique to complement DCE-MRI, specifically to improve the specificity of the breast MRI examination. DWI improves lesion characterization and can reduce the number of unnecessary biopsy recommendations [2–16]. Other possible indications for DWI include assessment and prediction of response to neoadjuvant treatment, and stratification of in situ from invasive disease [17–23]. Currently, DWI is also being explored as a promising technique for non-contrast breast screening [24].
The European Society of Breast Radiology (EUSOBI)’s International Breast DWI working group consists of several breast MRI experts, MRI physicists, and representatives from large vendor companies with proven expertise in breast MRI and DWI. This working group considers DWI an essential part of the multiparametric breast MRI protocol. The mission of this working group is not only to encourage the use of DWI in multiparametric breast MRI protocols, but also to find consensus on optimal methods for DWI image processing/analysis, visualization, and interpretation, and to improve breast DWI sequences by working side-by-side with system vendors.
The first consensus and mission statement from the working group provided basic requirements for the routine clinical application of breast DWI, including recommendations on b values, fat saturation, spatial resolution, and other sequence parameters [25]. To enable successful clinical implementation and widespread use of DWI, additional factors including other technical preferences (acquisition time, magnet strength, breast coils, number of b values), clinical applications, imaging evaluation, and reporting must also be addressed. To this end, the EUSOBI International Breast DWI working group performed a survey among all EUSOBI members to gather representative data regarding these additional considerations, the results of which are reported in this paper.
Materials and methods
Survey design
Two board-certified radiologists, each with over 10 years of experience in breast imaging and breast MRI, developed a questionnaire which included 20 questions pertaining to technical preferences (acquisition time, magnet strength, breast coils, number of b values), clinical indications, imaging evaluation, and reporting. The full questionnaire is available online as well as provided in the Electronic Supplementary Material.
After review and approval from the EUSOBI executive board, the questionnaire was made available online on a dedicated software platform (Google forms, Google). EUSOBI active and associated (not based in Europe) radiologist members were sent an invitation email from the central EUSOBI office to respond anonymously to the questionnaire, the link of which was provided in the body of the email.
The self-administered questionnaire was available online for a period of 45 weeks (starting from January 20, 2020). With the ensuing COVID-19 pandemic, it was felt that responses could have been impeded; and thus, the survey was kept open longer than originally planned to give respondents ample time. Two reminder emails were sent and reminders on the EUSOBI Facebook page were given within this timeframe.
Statistical analysis
Descriptive statistics were reported as frequencies and percentages. The Chi-square test of independence or Fisher’s exact test was used to evaluate associations between responses regarding DWI reporting, DWI technical preferences, awareness of the EUSOBI International Breast Diffusion-Weighted Imaging working group, willingness to join the working group, and awareness of the working group’s consensus and mission statement. All statistical analyses were conducted using R 3.6.3. Type I error rate was set to 0.05 (α). Since this was an exploratory study, we did not adjust for multiple comparisons.
Results
Of 1411 EUSOBI radiologist members, 275/1411 (19.5%) responded to the survey, although not every respondent answered all questions provided within the questionnaire. Among the 275 respondents, 222/275 (81%) used DWI as part of their clinical MRI protocol whereas the remaining 53/275 (19%) did not use DWI as part of their routine clinical MRI protocol.
DWI technique
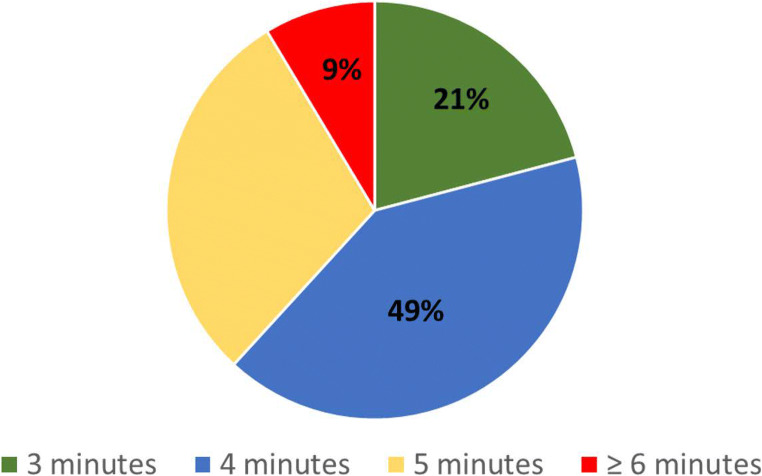
Regarding acquisition time, of 220 respondents, 90/220 (49%) responded that 4 min is a clinically acceptable acquisition time for a DWI sequence, followed by 65/220 (30%) who responded that 5 min is acceptable, 46/220 (21%) that 3 min is acceptable, and 19/220 (9%) that ≥ 6 min is acceptable (Fig. 1).
Fig. 1.
Pie chart of clinically acceptable acquisition time for a DWI as declared by 220 respondents
Regarding magnet strength, of 206 respondents, 113/206 (55%) reported working with 1.5-T scanners only, 49 (24%) reported working with 3-T scanners only, and 44 (21%) reported working with both 1.5- and 3-T scanners.
Regarding the number of channels in breast coils that are used clinically, the most common response was 16 channels (120/195 respondents, 61.5%), followed by eight channels (71/195 respondents, 36%) and four channels (17/195 respondents, 9%).
Regarding b values, the majority of respondents (114/217, 53%) reported acquiring two separate b values, while 59/217 (27%) reported acquiring three b values, 32/217 (15%) reported acquiring four b values, and 12/217 (5%) reported acquiring ≥ five b values. Among the respondents who reported acquiring > two b values, the preferred b value for the assessment of diffusion hindrance within contrast-enhancing lesion was 800 (71/171; 42%), followed by 1000 (55/171; 32%), 1500 (24/171; 14%), and 1200 (18/171; 11%) (Fig. 2). The EUSOBI International Breast DWI working group recommends the use of b values 0 and 800. There was, however, no significant correlation between responders who were aware of the EUSOBI working group and the selection of b values (p = 0.9) Only 48/217 (22%) of respondents frequently use synthetic b values in their clinical protocol.
Fig. 2.
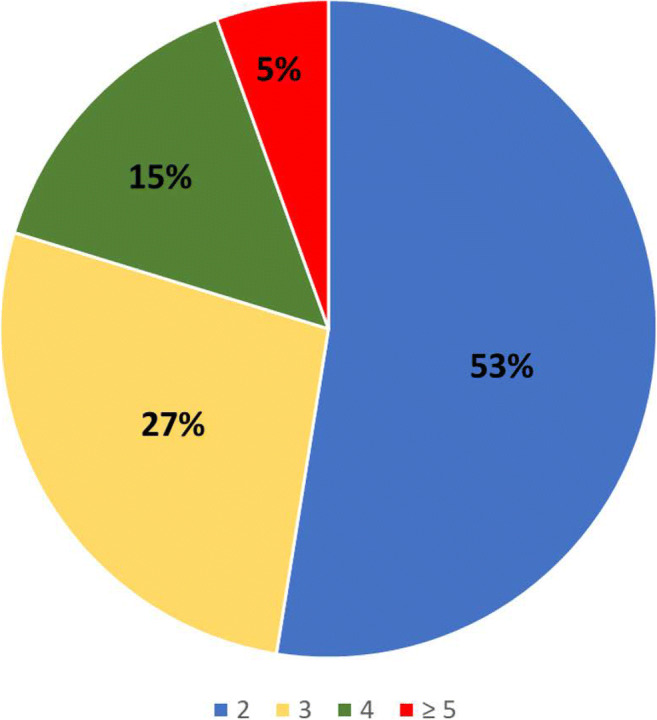
Pie chart of number of acquired b values as declared by 217 respondents
Applications for breast DWI
The most common indication for breast DWI among 217 respondents was the differentiation of benign and malignant enhancing lesions (204/217; 94%), followed by the assessment of response to neoadjuvant chemotherapy (146/217; 67%), the pre-treatment prediction of response to neoadjuvant chemotherapy (59/214; 27%), and non-contrast screening/research (38/217; 18%). One respondent reported using DWI for the differentiation between high grade in situ and (micro)invasive carcinoma in cases of dense breast with non-mass enhancement. One other respondent reported using DWI to guide biopsy procedures.
DWI evaluation
Qualitative evaluation
A total of 151/217 (70%) respondents reported performing qualitative evaluation of DWI using the high b value acquisitions. In addition, 123/215 (58%) respondents agreed that qualitative Breast Imaging Data and Reporting System (BI-RADS) descriptors could be applied to DWI images, including descriptors pertaining to the evaluation of internal characteristics (89/123; 72%), distribution (70/123; 57%), shape (64/123; 52%), and margins (51/123; 41%).
Quantitative evaluation
Regarding how the apparent diffusion coefficient (ADC) value is measured, 161/213 (76%) respondents reported using a focused 2D region of interest (ROI) selecting the lowest ADC value within the enhancing lesion, 36/213 (17%) respondents reported using a whole-lesion 2D ROI, 9/213 (4%) reported using a focused 3D ROI selecting the lowest ADC value within the enhancing lesion, and 7/213 (3%) reported using a whole-lesion 3D ROI. The recommended measurement of the ADC value according to the EUSOBI working group is by means of a focused 2D ROI selecting the lowest ADC value within the enhancing lesion. There was no significant correlation between responders who were aware of the EUSOBI International Breast DWI working group and the means of ADC value measurement (p = 0.5).
The most common ADC threshold used to differentiate benign from malignant lesions was 1.2 × 10−3 mm2/s (75/204, 37%), followed by 1.3 × 10−3 mm2/s (41/204, 20%), 1.4 × 10−3 mm2/s (38/204, 19%), and 1.25 × 10−3 mm2/s (20/204, 10%). The EUSOBI working group recommends using an ADC cutoff of 1.3 × 10−3 mm2/s. There was no significant correlation between responders who were aware of the EUSOBI International Breast DWI working group and the ADC threshold used (p = 0.3).
DWI reporting
Of 216 respondents, 165/216 (76%) reported mentioning DWI in the imaging technique section of the report and 22/216 (10%) reported not mentioning DWI, while 29/216 (13%) were unsure. Hindered diffusion on DWI was more commonly reported than actual ADC values: 164/216 (76%) respondents reported mentioning restricted diffusion of lesions in the body of the MRI report, whereas 124/217 (57%) respondents reported mentioning ADC values in the body of the report.
EUSOBI International Breast Diffusion-Weighted Imaging working group
Of 271 respondents, 184/271 (68%) were aware of the EUSOBI International Breast DWI working group, 37/271 (14%) were already members of the EUSOBI International Breast DWI working group, and 159/271 (59%) were aware of the consensus and mission statement of the working group. Of 270 respondents, 168/270 (62%) expressed interest in becoming part of the working group, 65/270 (24%) of responders were undecided, and 37/270 (14%) were not interested.
Tables 1, 2, 3, and 4 summarize the results from the analysis that assessed the relationship between the respondents’ current use of DWI in the clinic and either their knowledge of the working group or their willingness to join the working group. Respondents who reported mentioning hindered diffusion of lesions in their reports were more likely to express interest in joining the EUSOBI working group (p = 0.018). Respondents who reported working with both 1.5- and 3-T scanners or 3-T scanners only were both more likely to be familiar with the EUSOBI working group (p = 0.001) and the working group’s mission statement (p = 0.001), as well as more willing to join the working group (p = 0.015), compared with radiologists who reported working with 1.5-T scanners only.
Table 1.
Relationship between the respondents’ current mentioning restricted diffusion of lesions and their interest to join the working group
| Q17: Are you interested in becoming part of the EUSOBI IBDWI working group? | |||||
|---|---|---|---|---|---|
| Characteristic | Overall, N = 270 | Maybe, N = 65 | No, N = 37 | Yes, N = 168 | p-value1 |
| Q16 Do you mention restricted diffusivity, n (%) | 0.018 | ||||
| Maybe | 34 (100%) | 12 (35%) | 3 (8.8%) | 19 (56%) | |
| No | 17 (100%) | 6 (35%) | 0 (0%) | 11 (65%) | |
| Yes | 163 (100%) | 24 (15%) | 21 (13%) | 118 (72%) | |
| Unknown | 56 | 23 | 13 | 20 | |
Table 2.
Relationship between the respondents’ clinical working with both 1.5- and 3-T scanners or 3-T scanners only and their awareness of the EUSOBI working group
| Q18: Are you aware of the EUSOBI IBDWI working group | ||||
|---|---|---|---|---|
| Characteristic | Overall, N = 271 | No, N = 87 | Yes, N = 184 | p-value1 |
| Q4: Do you work with 3T or 1.5T MRI scanners?, n (%) | 0.001 | |||
| 1.5T | 111 (100%) | 44 (40%) | 67 (60%) | |
| 3T | 48 (100%) | 11 (23%) | 37 (77%) | |
| Both | 44 (100%) | 5 (11%) | 39 (89%) | |
| Unknown | 68 | 27 | 41 | |
Table 3.
Relationship between the respondents’ clinical working with both 1.5- and 3-T scanners or 3-T scanners only and their awareness of the working group’s mission statement
| Q19: Are you aware of the consensus and mission statement from EUSOBI | ||||
|---|---|---|---|---|
| Characteristic | Overall, N = 270 | No, N = 111 | Yes, N = 159 | p-value1 |
| Q4: Do you work with 3T or 1.5T MRI scanners?, n (%) | 0.001 | |||
| 1.5T | 110 (100%) | 53 (48%) | 57 (52%) | |
| 3T | 48 (100%) | 13 (27%) | 35 (73%) | |
| Both | 44 (100%) | 9 (20%) | 35 (80%) | |
| Unknown | 68 | 36 | 32 | |
Table 4.
Relationship between the respondents’ clinical working with both 1.5- and 3-T scanners or 3-T scanners only and the willingness to join the working group
| Q20: Are you interested in becoming part of the EUSOBI IBDWI working group? | |||||
|---|---|---|---|---|---|
| Characteristic | Overall, N = 270 | Maybe, N = 65 | No, N = 37 | Yes, N = 168 | p-value1 |
| Q4: Do you work with 3T or 1.5T MRI scanners?, n (%) | 0.015 | ||||
| 1.5T | 111 (100%) | 30 (27%) | 16 (14%) | 65 (59%) | |
| 3T | 47 (100%) | 6 (13%) | 7 (15%) | 34 (72%) | |
| Both | 44 (100%) | 6 (14%) | 1 (2.3%) | 37 (84%) | |
| Unknown | 68 | 23 | 13 | 32 | |
Discussion
This survey provides data about the clinical use of breast DWI among radiologist members of EUSOBI. There is general agreement that DWI is a valuable technique, with the majority of respondents reporting that they use DWI as part of their routine multiparametric diagnostic protocol whereas only 19% of respondents reported that they do not use DWI. While DWI is widely used, there are differences in utilization with respect to technique, clinical applications, evaluation, and reporting.
The response to the survey was relatively low at 19%, compared to a recent survey among EUSOBI radiologist members on their awareness, reporting, and action regarding breast arterial calcifications found on mammography (34.9%) [26]. While the COVID-19 pandemic would have negatively affected the rate of response to the current survey, it is also likely that the low response rate might be driven by the limited implementation of DWI in MRI protocols or the limited use of MRI in general among EUSOBI radiologist members. Indeed, in a previous survey by EUSOBI on the clinical utilization of breast MRI in general (i.e., not specifically focusing on DWI) [27], the response rate which was not affected by the COVID-19 pandemic was also low at 27.4%.
Technique
Minimal technical recommendations for breast DWI were previously established by the EUSOBI International Breast DWI working group [25]. According to the responses to the survey in this study, the clinical use of DWI is in line with these recommendations. Over half of the respondents reported using a 1.5-T magnet only, which is reflective of the available scanner landscape, and most used a 16-channel breast coil. The majority of respondents indicated that the acquisition time of DWI should not exceed 5 min, with 62% of respondents indicating that the best clinically acceptable acquisition time of DWI within a multiparametric framework is from 3 to 4 min; on the other hand, 8% of responders indicated that an acquisition time exceeding 5 min is clinically acceptable. With continuous advances in software and hardware, different DWI acquisition techniques could be used [28] and it is expected that acquisition will become faster in the future [29], which would facilitate the further adoption of DWI in routine clinical protocols. With the introduction and clinical use of abbreviated DCE-MRI protocols that reduce examination, the saved time may be invested in the DWI acquisition without increasing the examination time over a current full DCE-MRI protocol. Another consideration is to tailor the DWI technique used and thereby the afforded DWI acquisition time used, i.e., a simple monoexponential signal decay model vs. more advanced techniques such as advanced DWI (IVIM, non-Gaussian diffusion), to the respective clinical application.
Most respondents reported acquiring 2 or 3 b values; for those respondents who acquire more than 2 b values, the preferred b value for clinical assessment is 800. It should be noted that acquiring more b values, especially those higher than 800, may increase tumor conspicuity while obscuring benign lesions and breast tissue parenchyma, but this increases the acquisition time and decreases the signal-to-noise ratio [30–34]. This problem can be somewhat mitigated by using synthetic b values, which are b values that are mathematically calculated in a voxel-wise manner from two DWI acquisitions with different b values by applying a monoexponential signal decay model [31, 35–37]. While such an extrapolation of low b value data does not reproduce true diffusion restriction at high b values, it can provide practical conspicuity advantages. Indeed, the use of synthetic b values has been shown in several studies to improve tumor-to-tissue contrast, lesion visibility, and image quality of breast DWI, while avoiding the disadvantages of performing DWI at very high b values [35, 37]. However, available data for the use of synthetic b values in the breast are still scarce and of a preliminary nature. It remains unclear whether there is truly added value (it does not increase the amount of information) and the optimal synthetic b value for the detection of malignant breast tumors is uncertain, especially for smaller size lesions and non-mass enhancements. This is reflected by the fact that only 22% of respondents reported using synthetic b values for clinical evaluation.
Applications
Results from the survey indicate that respondents consider breast DWI an important addition to DCE-MRI for lesion characterization and treatment response assessment. The most common application for breast DWI was for the differentiation of benign and malignant enhancing lesions (94%), followed by assessment of response to neoadjuvant chemotherapy (67%) and pre-treatment prediction of response to neoadjuvant chemotherapy (27%).
At present, respondents agree that there is not yet enough evidence to justify the incorporation of DWI in breast cancer screening; only 18% of respondents reported using DWI for non-contrast screening and research including non-contrast screening as well as other research (unspecified). This is likely related to the relatively low image quality of DWI images compared to that of dynamic T1-weighted images. Detailed information on the intended screening population (high vs. greater than average vs. average risk) was not collected in this survey. The use of DWI for non-contrast screening remains an active area of research, with prospective multi-center trials underway [29, 38], the number of which will likely increase with ongoing advancements in techniques involving improved image quality and lesion conspicuity.
DWI evaluation
Results from the survey indicate that although the spatial resolution of DWI can be a limiting factor for the evaluation of lesions in the breast, lesion location, and descriptors such as size and morphology (shape, internal signal pattern, and distribution) may yet be evaluated on DWI in a similar fashion as on DCE-MRI. Indeed, 70% of respondents reported performing qualitative evaluation of DWI, and 58% agreed that qualitative BI-RADS descriptors could be applied to the evaluation of DWI images. A lexicon for the qualitative assessment of DWI acquisitions may thus be created using terminology with which most breast radiologists are already familiar, and then the next step would be to include this lexicon in the BI-RADS lexicon as has been done for the PI-RADS classification of prostate lesions. This will likely facilitate its acceptability in clinical practice. It is expected that continuous advances in software and hardware will also lead to improved spatial resolution and image quality. This will further improve morphologic DWI assessment and thus facilitate the identification of lesions directly on DWI, which is a prerequisite for the use of DWI as a stand-alone technique. Whether and how qualitative descriptors on DWI complement the quantitative ADC assessment alone for eventual lesion classification demands further research [39, 40].
For quantitative lesion evaluation, the EUSOBI International Breast DWI working group previously recommended that the ADC value should be measured by selecting the lowest value within the lesion and that the ROI should fall completely contrast-enhancing part of the lesion and contain at least 3 voxels. Responses to the questionnaire are in line with these recommendations; 79% of respondents reported measuring the lowest ADC value within the enhancing lesion using a 2D or 3D ROI, although the remaining 21% of respondents reported measuring ADC using a whole-lesion ROI. The most used ADC threshold to differentiate benign from malignant lesions was 1.2 × 10−3 mm2/s (37%), followed by 1.3 × 10−3 mm2/s (20%); the latter value separates low from intermediate diffusion levels. Recent data shows that an ADC threshold of 1.5 × 10−3 mm2/s allows downgrading of lesions classified as suspicious on breast CE-MRI and thus aids in obviating unnecessary biopsies [3, 41]. ROI selection approaches for some respondents may also have been influenced by treatment response monitoring or prediction tasks.
Reporting
The reporting of DWI of the breast in clinical practice is a challenging task. According to the responses to our survey, most respondents currently mention hindered diffusivity in the body of the imaging report while only 57% of respondents report actual ADC values. Despite DWI with ADC mapping’s being considered a valuable imaging biomarker with several relevant indications, it is only recommended as optional in BI-RADS [42] and not formally integrated with defined qualitative and quantitative descriptors as has been done for the Prostate Imaging Reporting & Data System (PI-RADS). While the value of ADC will be indicated and discussed in the upcoming revised BI-RADS MRI atlas, reporting of DWI of the breast will remain optional, such that no formal reporting guidelines will be issued and it remains at the discretion of the reporting radiologist if and how DWI information will be integrated into the imaging report. The EUSOBI recommends that if DWI was contributory to the final assessment category, then the radiologist should include the diffusion level of the respective enhancing lesion, i.e., very low (≤ 0.9 × 10−3 mm2/s), low to intermediate (0.9–1.3 × 10−3 mm2/s), high = normal (1.3–1.7 × 10−3 mm2/s), and very high (> 2.1 × 10−3 mm2/s) in the report [25].
Working group
The results of this survey showed that most respondents are either interested (62%) or may be interested (24%) in becoming part of the EUSOBI International Breast DWI working group. This is encouraging and reflects the steadily growing interest in DWI among members of the EUSOBI community. A previous survey on utilization of breast MRI in clinical practice among EUSOBI members found that only slightly more than half of survey respondents regularly applied DWI [27]. In this survey, 81% of responders now use DWI, which may be indicative of an increasing favorable opinion towards the importance of DWI for image interpretation among EUSOBI members.
Limitations, next steps, and conclusion
Limitations of this survey include a selection bias as only radiologist members (active and affiliated) of EUSOBI were asked to participate, and it can be assumed that they already have a special interest in breast imaging and breast imaging research. The high rate of 222/275 respondents routinely using breast DWI also indicates that those not interested in DWI or not using it may not have responded to the survey. The survey was anonymous and the institutional affiliation of each responder was not noted in the survey; this is a limitation as answers of individuals coming from the same institution may have biased the results, particularly in terms of technique. Another limitation is that technically challenging and detailed questions were avoided to keep the time required to answer the questions within reasonable limits and to achieve higher response rates. Future surveys could be conducted for in-depth analysis of points of interest found in the current survey.
This first survey on the dedicated clinical use of DWI of the breast among members of EUSOBI shows that (1) DWI of the breast is mainly performed in agreement with the current recommendations of the EUSOBI International Breast DWI working group, although there was no significant difference in responses between responders who were aware of the EUSOBI International Breast DWI working group and those who were not, (2) common clinical applications of DWI of the breast include the differentiation of benign and malignant enhancing lesions, followed by assessment of response to neoadjuvant chemotherapy and prediction of response to neoadjuvant chemotherapy, (3) quantitative assessment of DWI with ADC mapping is performed in line with current recommendations of the EUSOBI International Breast DWI working group, although thresholds for each clinical task (malignancy, treatment monitoring, response predictions) have not yet reached consensus, and (4) incorporation of DWI results in the imaging report is not uniform across radiologist members and further recommendations will be necessary to encourage standardization in reporting and actual clinical use. The next necessary step is to provide a standardized reporting system for DWI with ADC mapping that it can be easily and formally integrated into the radiology report similar to BI-RADS. This need has been recognized by the EUSOBI International Breast DWI working group and is an ongoing project. In the future, qualitative DWI assessment with descriptors similar to BI-RADS descriptors used for DCE-MRI assessment could be useful. While some radiologists may refrain from providing qualitative lesion assessment with DWI high b value images, there is potential for the use of synthetic b values that can improve image quality, increase lesion conspicuity, and improve lesion visibility, without adding scan times.
In conclusion, the data presented in this study allows for a better understanding of the current use and the necessary future steps for clinical implementation and standardization of DWI in clinical MRI of the breast.
Supplementary information
(PDF 58 kb)
Acknowledgements
The authors would like to thank Joanne Chin, MFA, ELS, for manuscript editing.
Abbreviations
- ADC
Apparent diffusion coefficient
- BI-RADS
Breast Imaging Data and Reporting System
- DCE-MRI
Dynamic contrast-enhanced magnetic resonance imaging
- DWI
Diffusion-weighted imaging
- EUSOBI
European Society of Breast Imaging
- ROI
Region of interest
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Katja Pinker, MD, PhD, at Memorial Sloan Kettering Center (pinkerdk@mskcc.org).
Conflict of interest
Paola Clausa and Katja Pinker serve on the Scientific Editorial Board of European Radiology. Ritse M Mann serves on Advisory Editorial Board of European Radiology. As such, they had no role in the manuscript handling and decision processes.
The authors of this manuscript declare relationships with the following companies: Katja Pinker is supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and a grant from the Breast Cancer Research Foundation. Katja Pinker received payment for activities not related to the present article including lectures including service on speakers bureaus and for travel/accommodations/meeting expenses unrelated to activities listed the European Society of Breast Imaging (MRI educational course, annual scientific meeting) and Siemens Healthineers. She is serving as a consultant for Vara Advisory Council/Merantix Healthcare GmbH (non-monetary) and AURA Health Technologies GmbH. Fiona Gilbert has research support from GE Healthcare & Bayer. Gilbert is supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the author and not necessarily those of the NIHR or the UK Department of Health and Social Care. For the remaining authors, none was declared.
Statistics and biometry
One of the authors (Varadan Sevilimedu, MBBS, DrPH, at Memorial Sloan Kettering Cancer Center) has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because no human subjects were involved in the generation of this paper.
Ethical approval
Institutional Review Board approval was not required because this is a study surveying the opinion of the ESUOBI member who participated on a voluntary basis.
Methodology
• retrospective
• observational
• multicenter study
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura Martincich and Katja Pinker contributed equally to this work.
References
- 1.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. 2019;50:377–390. doi: 10.1002/jmri.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinker K, Bickel H, Helbich TH, et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the "Breast Imaging Reporting and Data System" for multiparametric 3-T imaging of breast lesions. Eur Radiol. 2013;23:1791–1802. doi: 10.1007/s00330-013-2771-8. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar H, Zhang Z, Chenevert TL, et al. Utility of diffusion-weighted imaging to decrease unnecessary biopsies prompted by breast MRI: a trial of the ECOG-ACRIN Cancer Research Group (A6702) Clin Cancer Res. 2019;25:1756–1765. doi: 10.1158/1078-0432.CCR-18-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spick C, Pinker-Domenig K, Rudas M, Helbich TH, Baltzer PA. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol. 2014;24:1204–1210. doi: 10.1007/s00330-014-3153-6. [DOI] [PubMed] [Google Scholar]
- 5.Baltzer A, Dietzel M, Kaiser CG, Baltzer PA. Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol. 2016;26:884–891. doi: 10.1007/s00330-015-3886-x. [DOI] [PubMed] [Google Scholar]
- 6.Woodhams R, Matsunaga K, Iwabuchi K, et al. Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr. 2005;29:644–649. doi: 10.1097/01.rct.0000171913.74086.1b. [DOI] [PubMed] [Google Scholar]
- 7.Woodhams R, Matsunaga K, Kan S, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005;4:35–42. doi: 10.2463/mrms.4.35. [DOI] [PubMed] [Google Scholar]
- 8.Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24:319–324. doi: 10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 9.Wenkel E, Geppert C, Schulz-Wendtland R, et al. Diffusion weighted imaging in breast MRI: comparison of two different pulse sequences. Acad Radiol. 2007;14:1077–1083. doi: 10.1016/j.acra.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Baltzer PA, Renz DM, Herrmann KH, et al. Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol. 2009;19:1612–1620. doi: 10.1007/s00330-009-1326-5. [DOI] [PubMed] [Google Scholar]
- 11.Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193:1716–1722. doi: 10.2214/AJR.08.2139. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. doi: 10.1186/1471-2407-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol. 2014;24:2835–2847. doi: 10.1007/s00330-014-3338-z. [DOI] [PubMed] [Google Scholar]
- 14.Shi RY, Yao QY, Wu LM, Xu JR. Breast lesions: diagnosis using diffusion weighted imaging at 1.5T and 3.0T-systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e305–e320. doi: 10.1016/j.clbc.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Iima M, Kataoka M, Kanao S, et al. Intravoxel incoherent motion and quantitative non-Gaussian diffusion MR imaging: evaluation of the diagnostic and prognostic value of several markers of malignant and benign breast lesions. Radiology. 2018;287:432–441. doi: 10.1148/radiol.2017162853. [DOI] [PubMed] [Google Scholar]
- 16.Iima M, Yano K, Kataoka M, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol. 2015;50:205–211. doi: 10.1097/RLI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 17.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Richard R, Thomassin I, Chapellier M, et al. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2013;23:2420–2431. doi: 10.1007/s00330-013-2850-x. [DOI] [PubMed] [Google Scholar]
- 19.Galbán CJ, Ma B, Malyarenko D, et al. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One. 2015;10:e0122151. doi: 10.1371/journal.pone.0122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Abramson RG, Arlinghaus LR, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50:195–204. doi: 10.1097/RLI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong KM, Lau P, Ramadan S. Utilisation of MR spectroscopy and diffusion weighted imaging in predicting and monitoring of breast cancer response to chemotherapy. J Med Imaging Radiat Oncol. 2015;59:268–277. doi: 10.1111/1754-9485.12310. [DOI] [PubMed] [Google Scholar]
- 22.Partridge SC, Zhang Z, Newitt DC, et al. Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: The ACRIN 6698 Multicenter Trial. Radiology. 2018;289:618–627. doi: 10.1148/radiol.2018180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newitt DC, Zhang Z, Gibbs JE, et al. Test-retest repeatability and reproducibility of ADC measures by breast DWI: results from the ACRIN 6698 trial. J Magn Reson Imaging. 2019;49:1617–1628. doi: 10.1002/jmri.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amornsiripanitch N, Bickelhaupt S, Shin HJ, et al. Diffusion-weighted MRI for unenhanced breast cancer screening. Radiology. 2019;293:504–520. doi: 10.1148/radiol.2019182789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltzer P, Mann RM, Iima M, et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol. 2020;30:1436–1450. doi: 10.1007/s00330-019-06510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trimboli RM, Capra D, Codari M, Cozzi A, Di Leo G, Sardanelli F. Breast arterial calcifications as a biomarker of cardiovascular risk: radiologists' awareness, reporting, and action. A survey among the EUSOBI members. Eur Radiol. 2021;31:958–966. doi: 10.1007/s00330-020-07136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clauser P, Mann R, Athanasiou A, et al. A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol. 2018;28:1909–1918. doi: 10.1007/s00330-017-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter GC, Graves MJ, Gilbert FJ, Patterson AJ. A meta-analysis of the diagnostic performance of diffusion MRI for breast lesion characterization. Radiology. 2019;291:632–641. doi: 10.1148/radiol.2019182510. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves BA, Daniel B. Abbreviated non-contrast-enhanced mri for breast cancer screening (R01CA249893) National Cancer Institute, Stanford University; 2021. [Google Scholar]
- 30.Tamura T, Murakami S, Naito K, Yamada T, Fujimoto T, Kikkawa T. Investigation of the optimal b-value to detect breast tumors with diffusion weighted imaging by 1.5-T MRI. Cancer Imaging. 2014;14:11. doi: 10.1186/1470-7330-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Yun B, Jang M, et al. Comparison of the diagnostic performance of synthetic versus acquired high b-value (1500 s/mm(2) ) diffusion-weighted MRI in women with breast cancers. J Magn Reson Imaging. 2019;49:857–863. doi: 10.1002/jmri.26259. [DOI] [PubMed] [Google Scholar]
- 32.Bickelhaupt S, Laun FB, Tesdorff J, et al. Fast and noninvasive characterization of suspicious lesions detected at breast cancer X-ray screening: capability of diffusion-weighted MR imaging with MIPs. Radiology. 2016;278:689–697. doi: 10.1148/radiol.2015150425. [DOI] [PubMed] [Google Scholar]
- 33.Bickelhaupt S, Paech D, Kickingereder P, et al. Prediction of malignancy by a radiomic signature from contrast agent-free diffusion MRI in suspicious breast lesions found on screening mammography. J Magn Reson Imaging. 2017;46:604–616. doi: 10.1002/jmri.25606. [DOI] [PubMed] [Google Scholar]
- 34.Stadlbauer A, Bernt R, Gruber S, et al. Diffusion-weighted MR imaging with background body signal suppression (DWIBS) for the diagnosis of malignant and benign breast lesions. Eur Radiol. 2009;19:2349–2356. doi: 10.1007/s00330-009-1426-2. [DOI] [PubMed] [Google Scholar]
- 35.Daimiel Naranjo I, Lo Gullo R, Saccarelli C, et al. Diagnostic value of diffusion-weighted imaging with synthetic b-values in breast tumors: comparison with dynamic contrast-enhanced and multiparametric MRI. Eur Radiol. 2021;31:356–367. doi: 10.1007/s00330-020-07094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi BH, Baek HJ, Ha JY, et al. Feasibility study of synthetic diffusion-weighted MRI in patients with breast cancer in comparison with conventional diffusion-weighted MRI. Korean J Radiol. 2020;21:1036–1044. doi: 10.3348/kjr.2019.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bickel H, Polanec SH, Wengert G, et al. Diffusion-weighted MRI of breast cancer: improved lesion visibility and image quality using synthetic b-values. J Magn Reson Imaging. 2019;50:1754–1761. doi: 10.1002/jmri.26809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin HJ, Lee SH, Park VY, et al. Diffusion-weighted magnetic resonance imaging for breast cancer screening in high-risk women: design and imaging protocol of a prospective multicenter study in Korea. J Breast Cancer. 2021;24:218–228. doi: 10.4048/jbc.2021.24.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kul S, Metin Y, Kul M, Metin N, Eyuboglu I, Ozdemir O. Assessment of breast mass morphology with diffusion-weighted MRI: beyond apparent diffusion coefficient. J Magn Reson Imaging. 2018;48:1668–1677. doi: 10.1002/jmri.26175. [DOI] [PubMed] [Google Scholar]
- 40.Radovic N, Ivanac G, Divjak E, Biondic I, Bulum A, Brkljacic B. Evaluation of breast cancer morphology using diffusion-weighted and dynamic contrast-enhanced MRI: intermethod and interobserver agreement. J Magn Reson Imaging. 2019;49:1381–1390. doi: 10.1002/jmri.26332. [DOI] [PubMed] [Google Scholar]
- 41.Clauser P, Krug B, Bickel H, et al. Diffusion-weighted imaging allows for downgrading MR BI-RADS 4 lesions in contrast-enhanced MRI of the breast to avoid unnecessary biopsy. Clin Cancer Res. 2021;27:1941–1948. doi: 10.1158/1078-0432.CCR-20-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 58 kb)