Abstract
Over the last decade, nanotechnology has widely addressed many nanomaterials in the biomedical area with an opportunity to achieve better-targeted delivery, effective treatment, and an improved safety profile. Nanocarriers have the potential property to protect the active molecule during drug delivery. Depending on the employing nanosystem, the delivery of drugs and genes has enhanced the bioavailability of the molecule at the disease site and exercised an excellent control of the molecule release. Herein, the chapter discusses various advanced nanomaterials designed to develop better nanocarrier systems used to face different diseases such as cancer, heart failure, and malaria. Furthermore, we demonstrate the great attention to the promising role of nanocarriers in ease diagnostic and biodistribution for successful clinical cancer therapy.
Keywords: Cancer therapy, Drug delivery, Heart diseases, Nanomaterials, Optical properties, Gene therapy
Introduction
Nanomedicine generates a start-up in a new discipline that bodes well for the twenty-first century by using nanotechnology to improve healthcare and pharmaceutical products (Langer and Weissleder 2015; Hamimed et al. 2022a). Nanomedicine recognizes the site of human disease and delivers medications, diagnostics, and therapeutics to the target biological cell. Initially, the diagnosis was founded on the notion of cell theory, but research has progressed to the atomic and molecular levels (Jackson et al. 2021). Thus, the nanoscale dimension defines new properties of materials such as structure, shape, and high surface area, which improve their therapeutic and diagnostics forms (Satalkar et al. 2016; Bakir et al. 2021). Hence, the main objective of nanomaterials in delivery applications is to carry the desired molecules to their target sites with minimizing the side effects and maximizing their therapeutic effects (El-Say and El-Sawy 2017; Hamimed and Chatti 2022).
In drug delivery systems, nanomaterials play a crucial role in improving the stability and solubility of drugs, controlling their release, minimizing their toxicity, and giving higher therapeutic effects (Patra et al. 2018). Many nanocarrier systems have been developed, such as polymeric nanoparticles, inorganic nanoparticles, and nanohydrogels (Jacob et al. 2018). Understanding their interactions with targeted cells, administration method, bioavailability, and biodistribution are also essential (Jahangirian et al. 2017; Kthiri et al. 2021). On the other hand, gene delivery offers new perspectives for treating diseases by introducing new genetic materials into cells via ex vivo and/or in vivo administrations (Mali 2013). Different vectors deliver DNA chemical transduction and transfection using lipids and calcium phosphate, which ensure the gene transferring process (Candiani et al. 2010).
Generally, these systems have more advantages for treating different diseases, especially cancer, known as the second most significant cause of mortality in 2020, after heart disease leading to death worldwide about 10 million, estimated one in six deaths. (WHO 2022). As well as, their effective investigations proved against several viral infections such as coronavirus, Ebola virus, and malaria (Nasrollahzadeh et al. 2020). In addition, several studies proved their safety, non-viral methods, biodegradability, versatility of structural conformations, ability to deliver high amounts of therapeutic agents (Fornaguera et al. 2015; Jahangirian et al. 2017).
Drug delivery
During the past decade, nanotechnology-based drug delivery has shown significant interest where studies have enhanced the administration and the efficacy of active molecules (Kanwar et al. 2019; Hamimed and Chatti 2022). By improving the solubility, stability and minimizing the toxicity of drug molecules, researchers investigated the use of chemical and biological approaches giving high clinical benefits. However, most related research is still in the preclinical stage, and safety assessment remains a difficult task. Therefore, future research should concentrate on therapeutic nanomedicine’s performance modification, molecular mechanism, and potential toxicity (Morton et al. 2018). As a result, it was established that various nanocarriers offer numerous advantages such as (i) avoiding drug concentration fluctuation while maintaining the constant dose and the specific target, (ii) giving ultimate therapeutic effects and minimizing side effects and toxicity risks, and (iii) protecting drugs from enzymatic catalysis (De Jong and Ja Borm 2008). Figure 1 illustrates different nanocarriers used in biomedical applications, discussed in the following sections.
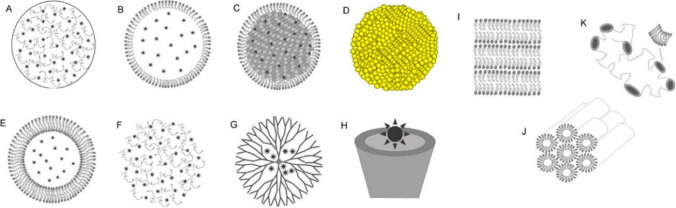
Fig. 1.
Different nanocarriers used in drug delivery system. (A) Polymeric nanoparticles; (B) nanostructured lipid carriers; (C) solid lipid nanoparticles; (D) metallic nanoparticles; (E) liposomes; (F) nanohydrogels; (G) dendrimers; (H) cyclodextrin; and liquid crystalline system ((I) lamellar; (J) hexagonal; (K) cubic). Copyright 2016, MDPI and ACS Style. (Calixto et al. 2016)
Metallic nanoparticles
Metallic nanoparticles have fascinated scientists in the biomedical field due to their unique physicochemical properties and broad functional groups, allowing the binding with different drug molecules (Mody et al. 2010). Interestingly, the size, shape, and composition of metal nanoparticles (silver (Ag) and gold (Au)), as well as metals (Titanium (Ti), zinc (Zn), and iron (Fe)), offer potential use in drug delivery system.
Gold nanoparticles
Gold nanoparticles (AuNPs) are the most functionalized metallic nanoparticles in drug delivery due to the strong bond mechanisms via covalent and non-covalent conjugation while maintaining high stability in the release of the drug from AuNPs (Su et al. 2013). Many methods are applied to synthesize these nanoparticles, such as the colloidal approach, and the obtained NPs have variable shapes and sizes (3–200 nm) (Zhao et al. 2013). Generally, in drug carriers, the AuNPs are synthesized by reducing gold precursors with sodium borohydride or sodium citrate in the bottom-up method (Duncan et al. 2010). The obtained AuNPs are functionalized by molecules such as drugs, enzymes, or plant extract, then the capping agents like transferrin, tannic acid, polyethylene glycol (PEG), porphyrin, etc. enhance the potential delivery and the therapeutic effects (Li et al. 2016a, b) (Fig. 2A).
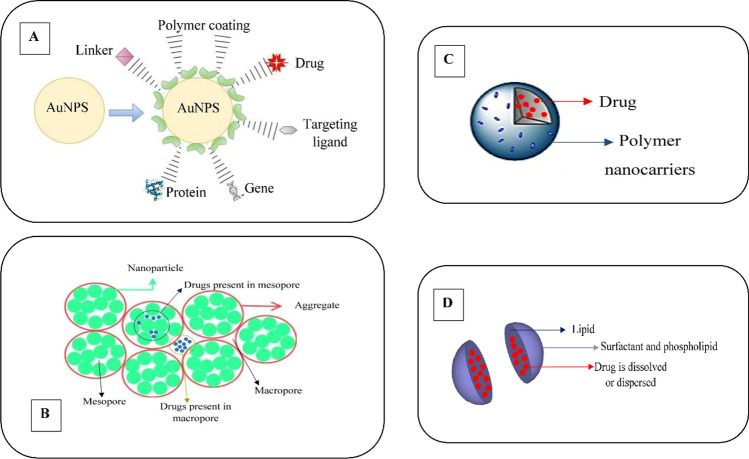
Fig. 2.
Numerous incorporations of nanocarriers with drug molecule. (A) Functionalization of gold metallic nanoparticles (AuNPs) by interacting on the surface with drug. (B) The nanohydrogels formed an aggregation of mesopore and macropore nanoparticles trapping the drug molecule in which the release occurs under absorption of water. (C) Polymeric nanocarriers exhibited nanoencapsulation of the drug molecule. (D) High dispersion of the drug molecule into the solid lipid nanocarriers
The functional AuNPs have shown good use in cancer therapy via the delivery of anticancer drugs. As a result of tumour growth in a xenograft mouse model, the morin drug encapsulated by AuNPs promoted tumour apoptosis by regulating signal crosstalk and enhancing the production of reactive oxygen species (Ding et al. 2020). The 5-fluorouracil carried on AuNPS functionalized by casein was a promising nanocarrier to minimize the high toxicity of 5-fluorouracil while treating breast cancer (Ganeshkumar et al. 2013). Similarly, Akinyelu and Singh (2019) studied the potent delivery of 5-fluorouracil-based AuNPS to various tumour cells and revealed an excellent delivery for cancer management. Another study has developed effective methotrexate-conjugated AuNPs, which demonstrated higher cytotoxicity towards the human choriocarcinoma cell lines than the free methotrexate (Tran et al. 2013).
Furthermore, doxorubicin (Dox), often used as a model in cancer therapy, when bound AuNPs capped with PEG, demonstrated toxicity against the multidrug-resistant MCF-7/ADR cancer cells (Wang et al. 2011). Cui et al. (2017) constructed an enhanced Dox carrier conjugated with AuNPs-lipoic acid-modified PEG, which showed stability to deliver the Dox into the nucleus of the human hepatocellular liver carcinoma cell line. As a treatment for malignant skin tumours, Dox- conjugated to glutathione-stabilized AuNPs showed a potential delivery system for feline injection-site sarcomas (Zabielska-Koczywąs et al. 2017). In a recent study, AuNPs capped with different biopolymers and conjugated with Dox demonstrated promising results in treating colorectal cancer cell lines (DLD-1 and HCT-116) (Hung et al. 2019). Additionally, Coelho et al. (2019) studied the efficient conjugation of Dox and varlitinib within AuNPs-PEG, which revealed the inhibition of pancreatic cancer cell lines (S2-013 s) while minimizing the side effects on normal cells. Also, hesperidin (Hsp) loaded with AuNPs via the chemical method was effective against the human breast cancer cell line (MDA-MB-231), enhanced the production of macrophages, and inhibited the production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF) (Sulaiman et al. 2020).
All these materials can have great potential as alternatives to traditional photothermal with high control, less toxicity, and more stability in drug release.
The large surface area-to-volume ratio, bio-inert, and low immunogenicity of AuNPs offer wide use in cardiovascular diseases where their incorporation within coiled fibre scaffolds provides a quick and robust contraction and relaxation of the myocardium (Fleischer et al. 2014). (Ravichandran et al. 2014) developed a hybrid scaffold formed of AuNP-loaded bovine serum albumin (BSA)/polyvinyl alcohol (PVA) nanofibers that enhance the cardiomyogenic differentiation. In order to manage cardiovascular diseases in diabetic patients, AuNPs showed efficient delivery of miR155 into macrophages that improve cardiac function (Jia et al. 2017).
Moreover, the delivery of the antibiotic levofloxacin within bromelain-capped AuNPs showed high control and localization of the target site while enhancing the antimicrobial activity compared to free levofloxacin (Bagga et al. 2016). As well as, gentamicin was conjugated with AuNPs for the delivery and enhancement of severe microbial infection (Ahangari et al. 2013). Apart from chemotherapeutic drugs, peptide-drug-conjugates with AuNPs have good chemical and biological performance while improving the target efficacy (Kalimuthu et al. 2018).
Silver nanoparticles
Silver nanoparticles (AgNPs) are tailored in drug delivery due to their electrical conductivity, broad antimicrobial activity, and localized surface plasmon resonance effect (Ocsoy et al. 2018). Generally, the drug molecules interact with AgNPs via multiple bonds such as sulphide/thiol, amine/carboxylic, azide-alkyne bio-conjugation (Prasher et al. 2020). Liu et al. (2012) developed a conjugate cell-penetrating peptide (TAT) using AgNPs for multidrug-resistant cancer treatment; this drug delivery system showed unusual antitumour activity. The AgNPs were used as a nanocarrier for Dox and alendronate (Ald) for cancer therapy, where they improve the drug delivery and anticancer activity (Benyettou et al. 2015). Interestingly, Shao et al. (2016) used a practical approach to deliver Dox within a nanocarrier formed of Janus AgNPs, where the delivery system was found to be efficient for cancer theranostic with less toxic effects. Capanema et al. (2019) designed a hybrid material of AgNPs embedded in the carboxymethylcellulose (CMC) as a nanocarrier for Dox by using a green process. This nanocarrier showed potent anticancer and antibacterial activities against skin cancer. The high conjugation properties of AgNPs with curcumin demonstrate the potential delivery for cancer therapy; they also showed less haemolytic toxicity than free curcumin (Gang and Gang 2018). The biocompatibility (AgNPs-NGR-graphene oxide (GO) showed excellent delivery of Dox to tumour cells with high targeting properties and great potential cancer therapy (Shi et al. 2014). In an attempt to enhance the delivery and the biosensing of Dox, the surfactant-free AgNPs coated with nanoGO served as potent nanocarrier and showed great theragnostic for anticancer effect (Zeng et al. 2018). The conjugation of camptothecin via an acid-labile β—this propionate on the surface of AgNPs improves the delivery and allows tracking the mechanism “on”/ “off” of the release process in the tumour cells (Qiu et al. 2017).
Therefore, the AgNPs demonstrated an essential ability to carry vast amounts of anaesthetic drug tetracaine hydrochloride by using Gemini surfactant aggregation. Furthermore, these results showed that the mole fraction controlled the aggregate size of the nanocarrier (Srivastava et al. 2019). Other reports revealed the excellent delivery of antibiotics via conjugating the ciprofloxacin within a composite of AgNPs-GO-cobalt ferrite (Kooti et al. 2018). Furthermore, incorporating rifampicin within AgNPs entrapped on amphiphilic chitosan-grafted-(cetyl alcohol-maleic anhydride-pyrazinamide) enhanced cellular uptake, biocompatibility and showed an immediate effect against Mycobacterium tuberculosis (Amarnath Praphakar et al. 2018). In addition, the AgNPs conjugated with antimalarial drugs, such as chloroquine and fosmidomycin, exhibited active and passive targeting delivery (Rai et al. 2017).
Titanium, magnesium, iron, and zinc nanoparticles
It is widely known that titanium oxide nanoparticles (TiO2NPs) may significantly enhance the performance of drug delivery systems. Generally, the incorporation of the drug molecule within TiO2NPs requires two approaches: (i) soaking TiO2NPs in an aqueous drug solution or (ii) pipetting a volume of drug solution on the surface of TiO2NPs (Chennell et al. 2013). For example, Ren et al. (2013) have successfully conjugated Dox with TiNPs via electrostatic interactions and confirmed the improvement of Dox delivery into the intracellular cytoplasm with better anticancer activity against the multidrug resistance MCF-7/ADM cells. Similarly, Mund et al. (2014) used the TiO2NPs to deliver the anticancer drug paclitaxel (PTX) into breast cancer cells and found that PTX-TiO2NPs have better anticancer activity than free PTX. As shown in Table 1, erlotinib (ERL) and vorinostat (SAHA) drugs were loaded in TiO2NPs for the treatment of the breast cancer cells (MDA-MB-231 and MCF-7) and human cancerous amniotic cells (WISH) (Abdel-Ghany et al. 2020). The results showed that the hybrid nanocarrier could upregulate the cancer cells by arresting them at the G2/M phase.
Table 1.
Anti-tumour applications of different nanocarriers in drug delivery
| Nanocarriers | Size (nm) | Cell Lines | Ref |
|---|---|---|---|
| MTX-AuNPs | 3–20 | Human choriocarcinoma cell lines (JAR) | (Tran et al. 2013) |
| MTX- Folic acid-conjugated magnetic nanoparticles | 50–150 | HeLa cells | (Madeeha et al. 2022) |
| Mor-AuNPs | 20–48 | MCF-7 cells | (Kondath et al. 2014) |
| 5-FU-AuNPs | 31–33 | Human breast adenocarcinoma (MCF-7) Hepatocellular carcinoma (HepG2) and Kidney (HEK293) cells | (Akinyelu and Singh 2019) |
| Dox-AuNPs | 10 | Human hepatocellular liver carcinoma cell line (HepG2) | (Wu et al. 2018) |
| Dox-AuPtNPs | 37–72 | Human cancer cells A549 (lung) and MCF-7 (breast) | (Oladipo et al. 2020) |
| Hsp-AuNPs | 15–30 | Human breast cancer cell line (MDA-MB-231) | (Sulaiman et al. 2020) |
| PDCs-AuNPs | 20–40 | Human HL-60, NB4, and murine A20 leukemic cells | (Kalimuthu et al. 2018) |
| Dox-Ag-MSNs | 2 | Human hepatocellular liver carcinoma cell line (HepG2) Lung cancer (A549) Breast cancer (MCF-7) | (Shao et al. 2016) |
| Dox- Ag–In–Zn–S quantum dots nanocrystals modified with 11-mercaptoundecanoic acid (MUA), L-cysteine, and lipoic acid decorated with folic acid (FA) | 11–19 | Adenocarcinomic human alveolar basal epithelial cells (A549) | (Ruzycka-Ayoush et al. 2021) |
| PTX-TiO2NPs | 30–40 | Mammalian breast cancer cell line (MCF-7) | (Mund et al. 2014) |
| ERL-SAHA-TiO2NPs | 5–25 | Breast cancer cells (MDA-MB-231 and MCF-7) and human cancerous amniotic cells (WISH) | (Abdel-Ghany et al. 2020) |
| Dox-ZnONPs | 476 | Mammalian breast cancer cell line (MCF-7) | (Sharma et al. 2014) |
| Taxifolin-ZnONPs | 70–80 | Human breast cancer cell (MCF-7) | (Sundraraman and Jayakumari 2020) |
| Curcumin-ZnONPS-PEG-beta cyclodextrin | 26 | Human breast cancer cell (MCF-7) | (Sawant and Bamane 2018) |
| Dox-(p(HEMA)-b-p(His) NPs | 100–120 | Human colon tumour 116 human colon carcinoma cell line | (Johnson et al. 2012) |
| Dox- iRGD-PEG- p(His)@IO NPs | 210–219 | PC3MM2 human prostate cancer cells | (Herranz-Blanco et al. 2016) |
| Dox-sorafenib-PEG-PLGA | 177 | Human cancer cell line HT-29 | (Babos et al. 2018) |
| Dox- collagen-PAPBA NPs | 81.3 | Ovarian cancer A2780 cells | (Jiang et al. 2020) |
| MTX- PHLNPs | 173.51–233.37 | U-87 MG glioma cells | (Bhattacharya 2021) |
| GmcH-SLNPs | 103–228 | Lung adenocarcinoma epithelial A-549cell line | (Soni et al. 2016) |
| GmcH-metal-doped boron nitride nanostructure | 125–500 | Cancerous cells | (Bibi et al. 2022) |
| Resveratrol-SLNPs | 168 | Human breast cancer cells (MDA-MB-231) | (Wang et al. 2017) |
| Crucumin-SLNPs | 40 | Human breast cancer cells (SKBR3) | (Wang et al. 2018) |
| Dox-NLCs | 100 | Breast cancer cells ( MCF-7 ADR) | (Li et al. 2018) |
| Resveratrol-NLCs | 88 | Human breast cancer cell (MCF-7) | (Poonia et al. 2019) |
| Dox-GEM-VCR-NLCs | 112 | Human Burkitt’s lymphoma cell line | (Ni et al. 2017) |
| Dox-β-elemene-NLCs | 190 | Lung cancer cells (A549) | (Cao et al. 2019a) |
| Dox-Liposome | 60 | Human breast cancer cell (MCF-7/MX cells) | (Tahover et al. 2015) |
| PD-1-Liposome-DOX | 85 | Mouse breast tumour cell line (4T1-fLuc) | (Du et al. 2017) |
| Dox-Liposome enrobed (PLGA-PEG-PLGA) | 75 | Murine breast cancer cell line (4T1) | (Cao et al. 2019b) |
| Curcumin-antiSTAT3 siRNA- Cationic Liposome | 276 | Mouse melanoma cells (B16F10) | (Jose et al. 2017) |
| TPGS-transferrin-Liposome | 200 | Brain cancer | (Sonali et al. 2016) |
| Dox-PAMAM | 10 | Human lung adenocarcinoma cells (A549) and murine fibroblast cell line cell line (NIH/3T3) | (Almuqbil et al. 2020) |
| Dox-ß cyclodextrins-PEG-folic acid | 30–60 | Human hepatocellular carcinoma cells (HepG2) | (Fan et al. 2019) |
| Dox-ß cyclodextrins | 17 | Human hepatocellular carcinoma cells (HepG2) | (Yang et al. 2019) |
| Dox-Mesoporous structured UiO-66 MOFs- carboxymethylcellulose | 2.6 | Lung Carcinoma Cell Line of A549 Cells | (Xie et al. 2021) |
MTX, methotrexate; AuNPs, gold nanoparticles; PHLNPs, polymeric lipid hybrid nanoparticles; Mor, morin; 5-FU, 5-fluorouracil; Dox, doxorubicin; AuPtNPs, gold-platinum nanoparticles; Hsp, hesperidin; PDCs, peptide-drug-conjugates; Ag-MSNs, janus silver-mesoporous silica nanocarriers; PTX, paclitaxel; ERL, erlotinib; SAHA, vorinostat; TiO2NPs, titanium oxide nanoparticles; (p(HEMA)-b-p(His) NPs, poly(2-hydroxyethyl methacrylate)-b-poly(l-histidine) nanoparticles; PEG- p(His) NPs, poly(ethylene glycol)-block-poly(histidine) nanoparticles; IO NPs, iron oxide nanoparticles; iRGD, tumour homing peptide; PLGA, poly-lactic-co-glycolic acid; collagen-PAPBA NPs, collagen-poly (3-acrylamidophenylboronic acid) nanoparticles; GmcH, gemcitabine; SLNPs, solid lipid nanoparticles; NLCs, nanostructured lipid carriers; GEM, gemcitabine; VCR, vincristine; PD-1, programmed cell death-1; STAT3, signal transducer and activator of transcription 3; TPGS, theranostic D-alpha-tocopheryl polyethylene glycol 1000 succinate mono-ester; PAMAM, poly (amidoamine) dendrimers
Moreover, the chitosan/cobalt ferrite/TiO2 nanofibers conjugated with Dox via electrospinning process treated the melanoma cancer B16F10 cell lines. These nanocomposites showed the fastest release of Dox from the nanofibers and high-localized cancer therapy (Radmansouri et al. 2018). At the same time, mesoporous TiO2@ zinc oxide-GO nanocarriers conjugated with curcumin revealed significant anticancer activity and promising candidates to deliver drugs for colon cancer (Zamani et al. 2017). Another recent study demonstrated that the magnetic nanoparticles (Fe3O4NPs) functionalized with (3-aminopropyl)triethoxysilane and coated by chitosan and tragacanth gum were able to deliver curcumin with the best-recorded release of curcumin (60% within 120 h) (Shafiee et al. 2019).
Nowadays, various green synthetic methods have been developed to minimize the use of toxic solvents and control the physicochemical properties of the obtained metallic nanoparticles (Hamimed et al. 2020, 2021a, b). For example, magnesium oxide nanoparticles (MgONPs) synthesized through the green approach can conjugate with Dox, leading to high control drug release (Somanathan et al. 2016). Besides, safer molecules (e.g. phenolic compounds) from natural sources gained much interest in recent studies (Hamimed and Kthiri 2022; Hamimed et al. 2022b).
In addition, the zinc oxide nanoparticles (ZnONPs) were found to be promising for Dox delivery, where the nanocarrier exhibited high chemotherapeutic effect and low toxicity toward normal cells (Sharma et al. 2014). Similarly, Liu et al. (2016) improved the Dox delivery through ZnONPs by increasing cell uptake and decreasing cell efflux in human breast cancer cells (MCF-7 cells). Results revealed the dual roles of ZnONPs by overcoming drug resistance and probing the intracellular drug release. In an attempt to enhance anticancer drug delivery, Sadhukhan et al. (2019) fabricated phenylboronic acid (PBA) conjugated with ZnONPs to deliver quercetin. Researchers indicated that the PBA-ZnO-quercetin improved apoptotic cell death in MCF-7 cells via enhanced oxidative stress and mitochondrial damage. Therefore, Taxifolin, one of the flavanols used for cancer therapy loaded with ZnONPs, demonstrated a potential anticancer activity against MCF-7 cells and high drug release (Sundraraman and Jayakumari 2020). Another study revealed the better conjugation of ZnONPs with A. socotrina extract, giving a potent antibacterial activity in the urinary tract infection (Fahimmnisha et al. 2020).
Polymeric nanoparticles and nanohydrogels
Polymeric drug nanocarriers (PNCs) are formed from natural or synthetic polymers such as PEG, chitosan, and collagen, which seal the drug molecule on a spherical bilayer shape or distributed on micelles (Fig. 2C). The polymer usually interacts with the drug via carboxyl, amine, and hydroxyl groups (Ciejka et al. 2017). The Dox delivery through pH-sensitive polymeric nanoparticles can retain the Dox during circulation while controlling the Dox release at the tumour site (Meng et al. 2014). (Hu et al. 2018) developed poly (ε-caprolactone-co-lactide)-b-PEG-b-poly (ε-caprolactone-co-lactide) for the treatment of breast cancer. They synthesized Dox theranostic nanoparticles with high stability, biocompatibility, and antitumour activity. Carborane-conjugated amphiphilic copolymer nanoparticles were fabricated to deliver anticancer drugs by Xiong et al. (2015); they showed that PEG-b-poly(L-lactide-co-2-methyl-2(2-dicarba-closo-dodecarborane) propyloxycarbonyl-propyne carbonate) (PLMB) enhanced the delivery of boron atoms and Dox to the tumour sites. It was suggested that these PLMB nanoparticles are promising for combining chemotherapy and boron neutron capture therapy. To enhance the oral bioavailability of Dox, Ahmad et al. (2018) prepared PEGylated-Dox-loaded-poly-lactic-co-glycolic acid (PLGA)-nanoparticles using a single emulsion/solvent evaporation method. Results showed that PEGylated-Dox-PLGA-NPs improved the Dox oral delivery, which provides an alternative to intravenous therapy for better patient care. Herranz-Blanco et al. (2016) fabricated polymeric-drug conjugate solid nanoparticles containing encapsulated superparamagnetic iron oxide nanoparticles (IO NPs) and decorated with a tumour homing peptide (iRGD) using the nanoprecipitation technique. This strategy showed potential tumour therapy with reduced cytotoxicity and haemolytic effects. In addition, the conjugation of Dox within the poly(ethylene glycol)-block-poly(histidine) @ IO NPs- iRGD offers a potential anticancer activity while improving intracellular delivery. Babos et al. (2018) reported an efficient encapsulation of Sorafenib and Dox using PLGA and PEG-PLGA nanoparticles assessed for anticancer activity against hepatocellular carcinoma (HT-29). The polymeric nanocarriers displayed higher cellular uptake with high toxicity toward tumoural cells (Babos et al. 2018). In addition, the copolymer D-α-tocopheryl polyethylene glycol 1000-block-poly (b-amino ester) (TPGS-PAE) was synthesized for the co-delivery of Dox and curcumin. It showed an efficient drug release in acidic pH and effective inhibition of human hepatocellular carcinoma (Zhang et al. 2017). Also, biopolymer such as chitosan for drug delivery remains an interesting way for green nanocarriers. Deng et al. (2014) developed nanocarriers based on Dox and miR-34a co-encapsulated into hyaluronic acid (HA)-chitosan nanoparticles to enhance the drug resistance in cancer cells and minimize side effects. Their results showed that co-delivery enhanced the suppressive breast cancer cells. Recently, findings on the delivery of Dox using biopolymer-based of collagen-poly (3-acrylamidophenylboronic acid) nanoparticles (collagen-PAPBA NPs) proved their efficiency against ovarian cancer A2780 cells (Jiang et al. 2020). Furthermore, donepezil, rivastigmine, and galantamine delivery by using chitosan nanoparticles to treat Alzheimer’s disease showed the potential delivery system via intranasal administration and better targeting efficiency to the brain (Fazil et al. 2012; Bhavna et al. 2014; Hanafy et al. 2015).
Nanohydrogels are systems of nanocarriers that are recognized for their ability to absorb water, better dispersion, and release of hydrophilic drug molecules (Fig. 2B). Nanohydrogels such as poly (vinylcaprolactam) cross-linked with poly (ethylene glycol) diacrylate modified with lysine loaded with Dox have shown a high cellular uptake and potential anticancer activity against MCF-7 cell line (Farjadian et al. 2019). In addition, chitosan hydrogel exhibited excellent properties in anticancer drug delivery due to its high biocompatibility, bioadhesion, biodegradability, and cationic character. Hence, many studies investigated the use of chitosan hydrogel incorporated with poly (acrylic acid-N-isopropyl acrylamide) nanoparticles (Ghaem et al. 2020), de-esterified tragacanth nanoparticles (Sadrjavadi et al. 2018), or porous silicon nanoparticles@Au (Xia et al. 2019) for the delivery of Dox and Methotrexate. They found that the chitosan hydrogel presented higher absorption capacity, better localization of tumoural cells, and slower sustained release of anticancer drugs. Recently, George et al. (2020) developed a co-delivery of naringenin, quercetin, and curcumin drugs using functionalized nanohybrid hydrogel-based L-histidine, conjugated chitosan, phyto-synthesised ZnONPs and dialdehyde cellulose. The nanohydrogel significantly killed human skin carcinoma cell lines (A431).
Moreover, nanohydrogels based on silver nanoparticles are promising in drug delivery applications. Prusty and Swain (2018) synthesized polyacrylamide/dextran nanohydrogels using in situ polymerization technique incorporating reduced nanosilver and showed an in vitro control of ornidazole release within 6 h with potent antibacterial activity. Gulsonbi et al. (2016) designed polymeric nanohydrogels based on carboxymethylcellulose–poly (acrylamide) conjugated with AgNPs and revealed good use in drug delivery systems. Furthermore, PVA containing copper oxide nanoparticle hydrogel was successfully loaded with ibuprofen drug and results indicated a higher rate of drug release from hydrogel than from nanocomposite form (Ahmadian et al. 2018).
Furthermore, the primary application of clinical nanotechnology is expected to be in pharmaceutical development within a short period. Preclinical investigation of the epidermal photosensitizer protoporphyrin IX (PpIX) production may predict clinical efficacy accurately (Wu et al. 2013). Schmitz et al. (2016) developed a human ex vivo model suitable to explore drug permeation in human skin for epidermal neoplasia disease in clinical practice (10 patients) using different drug formulations. After the clinical trial, the nanoemulsion formulation (BF-200 ALA “5-aminolevulinic acid”) led to a more than threefold higher distribution of (PpIX) than the 20% ALA cream formulation, which is frequently used in clinical practice. In similar work, Morton et al. (2018) showed that a phase III trial on 138 patients using BF-200 ALA gel-photodynamic therapy (PDT) was highly effective with slightly lower recurrence compared to MAL (a cream containing methyl-aminolevulinate) after 1 year. Thus, the nanoemulsion ALA proves its stability to enhance epidermal penetration compared to other clinical drugs used (Schmitz et al. 2016). The nanoemulsion-based 10% BF-200 ALA was recently tested in 2 maximal usage pharmacokinetic trials (MUsTs) in patients severely affected with actinic keratosis, where MUsTs were used to assess baseline-adjusted plasma concentration–time curves for three tubes of BF-200 ALA and PpIX after a single PDT treatment (Novak et al. 2022). Based on the obtained MUsTs, ALA plasma concentrations were increased to a concentration at about 2.5 to 3.3 times above endogenous baseline at 3 h after dosing and then were subsequently returned to baseline within 10 h. Overall, no safety concern due to high PpIX exposure is apparent upon application of up to 6 g of BF-200 ALA for PDT in treating actinic keratosis (Novak et al. 2022).
Lipid-based nanocarriers
It is known that some polymeric nanoparticles present some disadvantages, such as cytotoxicity and difficulty to manufacture at a large scale. Hence, solid lipid nanoparticles (SLNPs) are developed as substitute systems of colloidal drug delivery because they are biocompatible, cost-effective, and can incorporate drugs molecules (Mishra et al. 2018) (Fig. 2D). SLNPs have multiple uses in drug delivery, especially in cancer therapy. Surface-modified SLNPs can be done to effectively deliver gemcitabine for targeting lung cancer cells treatment (Soni et al. 2016). Wang et al. (2017) developed SLNPs containing resveratrol that is more efficient for inhibiting invasion and migration of human breast cancer cells (MDA-MB-231). These researchers also proved the higher delivery and potent activity of curcumin loaded in SLNPs to treat human breast cancer cells (SKBR3) (Wang et al. 2018). Using IONPs loaded with SLNPs to target Dox delivery (Shen et al. 2019) found that the nanocarriers presented chemo/magnetothermal combination therapy against colon cancer cells line (CT26, 4T1, and A549). Moreover, the SLNPs improved the delivery of resveratrol for Alzheimer’s treatment after intravenous injection (Loureiro et al. 2017). Also, nicotinamide-loaded SLNPs and functionalized with polysorbate 80 and phosphatidylserine showed a potential brain delivery via oral administration, preserving the neuronal cells and improving cognition (Vakilinezhad et al. 2018). Dara et al. (2019) developed erythropoietin-loaded SLNPs by using a double emulsion solvent evaporation method. The nanocarrier showed a reduction of oxidative stress, beta-amyloid plaque deposition, and ADP/ATP ratio in-patient with Alzheimer’s disease. As well, SLNPs proved their efficiency to deliver antimicrobial molecules such as polymyxin (Severino et al. 2017), Eugenia caryophyllata essential oil (Fazly Bazzaz et al. 2018), and carvacrol (He et al. 2019) for effective elimination of infections.
The SLNPs presented limitations, such as insufficient space for drug encapsulation, low crystallization, and interactions with lipid melt. Thus, nanostructured lipid carriers (NLCs) were developed to overcome these drawbacks due to their drug overload, non-ideal crystalline structure, and preventing solid lipid crystallization (Mishra et al. 2018). Czajkowska-Kośnik et al. (2018) paid attention to the excellent use of NLCs loaded with different drug molecules, such as econazole, artemether, spironolactone, methotrexate, flurbiprofen, ibuprofen, and meloxicam for dermal and transdermal applications. To enhance the drug loading for brain disease, Wavikar et al. (2017) developed NLC-loaded rivastigmine. They revealed that this nanocarrier’s intravenous and intranasal administration exhibited an excellent regain of memory without signs of inflammation or toxicity. As well as, Tapeinos et al. (2017) showed the high efficiency of NLCs to deliver multiple drugs such as apomorphine for Parkinson’s disease, huperzine for Alzheimer’s disease, and vinpocetine for ischemic stroke. Moreover, NLCs revealed good biocompatibility for anticancer drug therapy. Olerile et al. (2017) developed a theragnostic system based on co-loaded quantum dots (CdTe/CdS/ZnS) and paclitaxel with NLCs, which are found as splendid parenteral drug delivery system for cancer theragnostic against human hepatocellular carcinoma cells (HepG2). Li et al. (2018) prepared NLC-loaded Dox by using the melted ultrasonic dispersion method to treat breast cancer. Results revealed an enhanced delivery of Dox with potent anticancer effects. Similarly, the use of NLCs for the delivery of resveratrol demonstrated high photostability and anticancer treatment against human breast cancer cells (MCF-7) (Poonia et al. 2019). Kamel et al. (2019) investigated the efficacy of curcumin incorporated in NLCs for photodynamic therapy and showed that the nanocarrier enhanced the curcumin penetration into cells and improved the anticancer activity in dark/light conditions. The use of drugs Dox, gemcitabine (GEM), and vincristine (VCR) loaded on NLCs exhibited a high antitumour effect against human lymphoma cells compared to single drug-loaded (Ni et al. 2017). Another study revealed the potent delivery of dual drugs Dox and β-elemene within NLCs in inhibiting lung tumour cells growth (Cao et al. 2019a).
Although the possible use of NLCs in mRNA COVID-19 vaccines has gained much interest due to the pandemic with rapidly mutating viruses (Verbeke et al. 2021), NLCs are able for excellent encapsulation and protection of mRNA (Granados-Riveron and Aquino-Jarquin 2021; Papi et al. 2022). The nucleoside-modified mRNA /NLCs COVID-19 vaccines developed by BioNTech/Pfizer and Moderna encode the viral spike (S) glycoprotein of SARS-CoV-2 that includes two proline substitutions (K986P and V987P mutations) on the one hand, and on the other hand neutral phospholipid, cholesterol, a polyethene-glycol (PEG)-lipid, and an ionizable cationic lipid; these components have an average size of 75 nm (McKay et al. 2020). To enhance the storage at ultra-low temperatures while maintaining the stability of mRNA/NLCs molecules, Schoenmaker et al. (2021) mentioned that the subcutaneous injection of BNT162b2/Comirnaty-Onpattro patisiran vaccine (Pfizer) induced an active immunization of 98% after the first dose. Furthermore, the immune response can also be evaluated using 3D bioprinting tissues, which could expedite vaccine availability faster than in animal models (Papi et al. 2022).
In an attempt to establish more controllable drug delivery, spherical bilayered nanometer phospholipid vesicles dispersed in an aqueous medium are getting more attention. According to their structure and modifications, different types of these vesicles (liposomes) like ethosomes, transfersomes, and phytosomes are obtained (He et al. 2018). For instance, the liposomes can encapsulate the hydrophilic and lipophilic drug molecule, which improves the therapeutic effect. Many researchers have demonstrated the active role of liposomes in target cancer therapy due to their flexibility, biocompatibility, and biodegradability (Table 1). Tahover et al. (2015) investigated the efficiency of Dox incorporated in liposomes and showed potent anticancer activity against breast cancer cell line (MCF-7/MX cells). Later, Du et al. (2017) developed high sensitivity programmed cell death-1 antibody based on liposome loaded with Dox and conjugated with a hybrid of IRDye800CW and 64Cu. They visualized the tumour successfully via NIRF/PET imaging and exhibited potential inhibition of breast cancer. However, enzymes, macrophages, pH, and ions could affect drug delivery. Thus, using multibranched gold nanoantennas with thermosensitive liposomes loaded with Dox could induce the delivery via photothermal actuators (Ou et al. 2016). The authors suggested that the low-temperature-sensitive liposome showed higher delivery (25 times) than the non-temperature-sensitive liposome (Ou et al. 2016). A recent study enhanced the nanocarrier based on liposome-Dox through enrobing with triblock copolymers (PLGA-PEG-PLGA) to remove breast cancer with minimal side effects (Cao et al. 2019b). Therefore, Caddeo et al. (2016) evaluated novel co-delivery of bioactive compounds, such as quercetin and resveratrol, within liposomes for skin lesion therapy. They found an excellent restoration of tissue, significant reduction of oedema, and leukocyte infiltration. The combination of curcumin and anti-(signal transducer and activator of transcription 3) siRNA using cationic liposomes was also reported to be effective in co-delivery against skin cancer (Jose et al. 2017). Moreover, liposomes proved their potent nanodelivery of theranostic D-alpha-tocopheryl polyethylene glycol 1000 succinate mono-ester via crossing the blood–brain barrier for imaging and treating brain cancer (Sonali et al. 2016).
Dendrimers and cyclodextrin
Dendrimers are classified as artificial polymers, defined by nanodimension size and have been widely used in drug delivery systems (Huang and Wu 2018). They interact with drug molecules via two reactions, where the first consists of the formation of non-covalent interactions and the second of nanoconstruct bonds through covalent interactions (Chauhan 2018). Generally, poly (amidoamine) (PAMAM) dendrimers are the most used in drug delivery. Zhang et al. (2018) investigated their unique features to conjugate and delivered the Dox and showed high targeting anticancer activity.
Similarly, Almuqbil et al. (2020) enhanced the Dox delivery by conjugating with 4-succinamic PAMAM via enzyme-liable tetrapeptide. They noticed an efficient delivery of Dox through dendrimers threefold greater than free Dox. In another study, Zhu et al. (2014) developed novel theranostic nanocarriers based on PAMAM functionalized with alpha-tocopheryl succinate, entrapped with AuNPs, and conjugated with Dox. The Dox delivery system exhibited faster release of the drug under pH acid with specific chemotherapy and computed tomography imaging. Furthermore, the PAMAM was very promising in the co-delivery of cisplatin drug conjugated with Dox using an intravenous way. The chemotherapy combination exhibited potent anticancer activity against breast cancer cells (MCF-7 and MDA-MB-231) (Guo et al. 2019). Therefore, another type of dendrimers like poly (propylene imine) has presented an excellent use for the delivery of maltose-histidine shell (G4HisMal) with promising results in Alzheimer’s disease prevention via synapse protection (Aso et al. 2019).
Cyclodextrins are recognized as natural cyclic oligosaccharides with numerous characteristics and applications. They present a unique ability to form hydro-soluble inclusion with many poorly organic and inorganic lipophilic compounds (Shelley and Babu 2018). The ß cyclodextrins are commonly used in drug delivery. Previous studies showed that ß cyclodextrins-PEG-folic acid nanoparticles loaded with Dox are efficient against breast and liver cancer (Hyun et al. 2018; Fan et al. 2019). A recent study demonstrated that the reducing size of the ß cyclodextrins-Dox nanocarriers to 17 nm gave excellent stability, pH sensitivity, and enhanced activity for liver cancer therapy (Yang et al. 2019).
Liquid crystalline systems
Finally, the liquid crystalline systems are extensively used in drug delivery due to their thermodynamically stability, non-toxicity, photo-degradation, and high control release. They are categorized as hexagonal, cubic, or lamellar mesophases (Aida et al. 2018). Their application is preliminary and limited for some chemopreventive treatments by conjugating with Celecoxib. In vitro experiments showed that cubic liquid crystalline mesophases enhanced the delivery and the release of celecoxib against skin cancer (Dante et al. 2018). Therefore, the lamellar and hexagonal liquid crystalline mesophases demonstrated great ability to conjugate with the peptide p1025. This peptidic drug is responsible for inhibiting the formation of dental biofilm by Streptococcus mutans. The drug delivery system displayed high p1025 delivery with enhanced antibiofilm activity (Calixto et al. 2017).
Gene delivery
The efficient delivery of the nucleic acid gained attention to its target tissue and nanocarriers. Generally, the exogenous genetic material must be delivered to the nucleus of the targeted cells, where they manufacture the protein products of the introduced gene. The ideal vector transfers a precise amount of genetic material into a specific cell type that achieves the level and duration of transgene expression sufficient to correct the defect and be non-immunogenic and harmless, allowing expression of the gene product without causing toxicity (Shillitoe 2009).
Organic nanocarriers
Polymeric nanocarriers
In materials, sciences offer biodegradable products and environmentally friendly, biocompatible, and highly novel polymeric systems for targeted delivery. Gene-loaded polymeric nanocarriers (PNCs) have been showing as a novel promising strategy for the treatment of cancer, as they may not only improve the pharmacokinetics of the drug but also as an additional response to the permeation and retention effects, which improve the accumulation of drugs at the site of the tumour during cancer treatment (Kapoor et al. 2015). The use of biodegradable PNCs such as chitosan, dextran, gelatin, pullulan, and synthetic analogues with sophisticated characteristics like guanidinylated bio-reducible polymers has emerged a crucial role in gene therapy due to their facile synthesis and flexible properties (Rai et al. 2019). As shown in Fig. 3, cationic polymers showed high performance for non-viral gene delivery systems by conjugating via electrostatic bonds at physiological pH (Samal et al. 2012). In addition, biodegradable poly(beta-amino ester) (PBAE) nanoparticles were also described as biodegradable cationic polymers for treating pediatric central nervous system (CNS) malignancies. The PBAE conjugated with HSVtk suicide gene (Plasmid DNA) designed for intracellular gene delivery to orthotopic tumour xenografts revealed significant survival of mice and excellent therapeutic effects (Choi et al. 2020). The PBAE demonstrated exemplary performance in the siRNA and miRNA delivery, offering effective nanocarriers for pediatric malignant CNS tumours (Kozielski et al. 2014; Lopez-Bertoni et al. 2018).
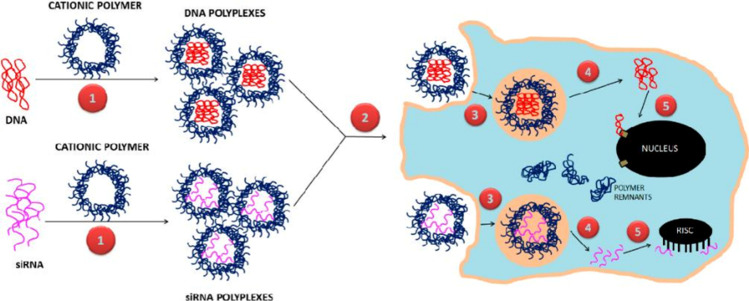
Fig. 3.
Design of polymeric gene delivery process. Polymeric nanocarriers for DNA and siRNA delivery: (1) polyplexes are formed by combining anionic DNA and siRNA with cationic polymers. (2) cellular uptake of polyplexes via various endocytic routes, (3) enclosure and subsequent release of polyplexes from endo-lysosomal compartments, (4) release of free DNA and siRNA from polyplexes leaving behind polymer remnants, and (5) transfer of DNA to the nucleus for expression by nuclear membrane transport proteins and binding of siRNA by the RNA-induced silencing complex (RISC). Copyright 2019, MDPI and ACS Style. (Rai et al. 2019)
Therefore, the polyethylenimine polymeric particles conjugated with extracellular vesicles and complexed with siRNAs or antimiRs showed dose-dependent inhibition of miRNAs and a decrease in xenographts size after 12 days (Zhupanyn et al. 2020). Zhang et al. (2019) developed hyaluronic acid–modified chitosan nanoparticles labelled with cyanine 3 (Cy3) to deliver the gene BCL2 siRNA to human lung cancer cells (A549), which induced inhibition of cell proliferation via BCL2 downregulation. The curdlan polymer loaded with FITC siRNA enhanced the endosomal escape and inhibited the Hela and HepG2 cells through DNA damage (Su et al. 2020). The alkylation procedure improves the STAT3 siRNA delivery to human HepG2 and a murine B16 cell line with a high anticancer effect (Erdene-Ochir et al. 2020).
Lipid-based nanocarriers
Lipid-based nanocarriers (NLCs) are efficient in delivering vectors for gene therapy. In recent years, LNPs have been widely used to encapsulate RNA within particles (Francia et al. 2020). Lipid vectors are generated by a combination of plasmid DNA and a lipid solution, resulting in a liposome nanocarrier that can be merged with cellular membranes of various types of cells (Mali 2013). The cationic solid lipid nanoparticles (SLNPs) have gained attention in gene delivery due to their excellent physical stability and biocompatibility (Jin and Kim 2014). Positively charged nanoparticles are most likely to be captured by cells via electrostatic interaction with negatively charged cell membranes (Remaut et al. 2014). However, cationic NLCs should be able to complex RNA similarly to protect RNA from degradation by complexation and condensation (Démoulins et al. 2016). Recent research confirmed that lipoplexes are mainly associated with the periphery of tumour spheroids, possibly resulting in their positive surface charge, leading to fusion with the cells at the spheroid surface or aggregation (Niora et al. 2020).
Over the past two decades, optimizing NLC formulation for nucleic acid delivery has led to establishing a body of knowledge, which resulted in the first RNA interference therapy using NLC technology (Cullis and Hope 2017). Onpattro® Apolipoprotein E is among proteins that can be adsorbed at the surface of NLCs, allowing specific targeting for hepatocytes. In addition, this work demonstrated the possible use of NLCs for biomolecular corona targeting (Francia et al. 2020).
In recent times, various therapeutic agents targeting several types of diseases have reached different stages of clinical trials. The NLCs were developed by Alnylam Pharmaceuticals Company using enhanced stabilization chemistry-GalNAc conjugate delivery technology. For example, ALN-TTRsc (targeting TTR for the treatment of transthyretin-mediated amyloidosis) and ALN-PCS02 (targeting proprotein convertase subtilisin/Kexin type 9 (PCSK9) for the treatment of hypercholesterolemia) are being used in clinical trials. At the same time, ALN-TTR02 is known as newly published clinical NLCs for the sustained reduction of serum Transthyrétine protein (96%) (Fitzgerald et al. 2014).
Inorganic nanoparticles
Inorganic nanomaterials have recently emerged as robust and versatile nanocarriers for efficient gene delivery applications. Moreover, inorganic nanomaterials offer an attractive set for practical applications, including scalability in synthesis and simple functionalization with silane or thiol groups leading to enhance interaction with biomolecules via chemical and thermal stability (Markman et al. 2013). Generally, there are three strategies for modifying inorganic nanoparticles for gene delivery: (i) the use of inorganic nanoparticles positively charged to form a complex with the negatively charged genetic material, (ii) the direct conjugation of the genetic material on the inorganic nanoparticles with a reactive linker, and (iii) the use of a cationic amphiphilic polymer derived from the nanoparticles to induce the complexation of the inorganic nanoparticles and the genetic material (Loh et al. 2016).
Gold nanoparticles
AuNPs have been widely studied as nanocarriers of multifunctional genes due to their facile synthesis, excellent biocompatibility, well-defined surface chemistry, and easy molecular imaging (Yeo et al. 2018). Peng et al. (2016) demonstrated AuNPs for simultaneous gene and antimicrobial therapy by conjugating antimicrobial peptides with cationic AuNPs for gene delivery to mesenchymal stem cells. The typical methodology for the AuNP-based gene delivery is the functionalization on the surface of AuNPs with positively charged molecules, such as amino acids, cationic peptides, and molecules containing tertiary amines (Ye and Loh 2013; Ye et al. 2015). AuNPs conjugated with oligonucleotides have proved their practical application in gene therapy (Mendes et al. 2017). AuNPs functionalized with an antisense oligonucleotide against BCR-ABL mRNA, which is translated to give active tyrosine kinase, induced an effective silencing and increased in K562 cell death (leukemogenesis) (Vinhas et al. 2017).
Moreover, the Au-nanobeacons demonstrated their efficiency for in vivo silencing fli-enhanced green fluorescence protein (fli-EGFP) transgenic zebrafish embryos (Cordeiro et al. 2017). While Abrica-González et al. (2019) functionalized AuNPs with chitosan oligosaccharide for higher delivery of DNA transfection in HEK-293 cells.
Diverse AuNPs have been developed for gene delivery, such as mixed monolayer-protected AuNPs, complexes of polymer and AuNPs, double-stranded DNA-functionalized AuNPs, and single-stranded DNA-functionalized AuNPs (Agbasi-Porter et al. 2006; Rosi et al. 2006). These nanocarriers demonstrated greater gene expression, higher binding affinity for target DNA, higher nuclease immunity, and lower cellular toxicity than antisense DNA delivered by lipofectamine or cytofectin (Loh et al. 2016).
Iron nanoparticles
Iron oxide nanoparticles (IO NPs) and superparamagnetic iron oxide nanoparticles (SPIO NPs) present an essential type of inorganic nanoparticles used in gene delivery due to their low toxicity, efficient biodegradability, low cost of production, and ease of surface modification (Liu et al. 2011). Furthermore, magnetic nanoparticles can ameliorate gene transfection of viral vectors and non-viral vectors (McBain et al. 2008). The gene delivery vehicle was constructed with a core of IO NPs and a shell of alkylated polyethyleneimine where the siRNA is attached to IO NPs, and the delivery to targeted cells has been achieved through the use of high field/high gradient magnets (Liu et al. 2011). Results showed an excellent efficiency of the siRNA-loaded nanocarriers for the downregulation of luciferase (fluc-4T1). In addition, Shakil et al. (2019) successfully conducted IO NPs as theranostic agents for breast cancer gene therapy. It was demonstrated that the efficacy of DNA transfer increases by using a magnetic field leading to an increase in the delivery into the cellular compartments. Another study (Jin et al. 2019) proved that IO NPs could potentiate the gene silencing effect via targeting B-cell lymphoma-2 (BCL2) in Ca9-22 oral cancer cells. Interestingly, SPIO NPs delivered siRNA against HIV‐1 nef (anti‐nef siRNA) into two cell lines, HEK293 and macrophage RAW 264.7 (Kamalzare et al. 2019). They showed that the coating of nanocarriers with carboxymethyl dextran improves the uptake of siRNA into both cells and reduces the expression of HIV-1 nef.
Silica nanoparticles
Silica-based vectors have deflected some attention from viral and non-viral vectors due to the presence of the silanol group on the surface of nanoparticles, which provide a positive charge for functionalization with nucleic acids (Kamegawa et al. 2018). However, the silanol groups can interact with cell membrane components leading to membranolysis (Narayan et al. 2018). Silica-based vectors with smaller molecular weight (Namgung et al. 2011) or with modified hyaluronic (Li et al. 2016a, b) achieved excellent results in transfection. The application of silica nanoparticles in gene delivery systems using in vitro cellular models facilitated the integration of genetic material and raised the sedimentation of nanoparticles (Carvalho et al. 2020). At the same time, the functionalization of silica nanoparticles with 3-aminopropyltrimethoxysilane leads to binding electrostatically with plasmid DNA to deliver in vitro models like COS-7 and 293 T cell lines (Bhakta et al. 2011). The combination of silica with other materials, like polymers, lipids, or inorganic particles, improved its nanocarrier characteristics.
Carbon nanotubes
Carbon nanotubes (CNTs) have shown promising delivery of vectors due to their high aspect ratio and capacity to translocate through plasma membranes (Wen et al. 2014). In addition, their nanoneedle properties allow their diffusion into the cytosol and protect the delivered gene from enzyme degradation (Mu et al. 2009). Based on these features, CNTs with different diameters have been coated with polyallylamine leading to a positively charged nanocarrier to conjugate with GFP plasmid, while results showed potent delivery to 3T3 cells (Cifuentes-Rius et al. 2017).
Moreover, Ohta et al. (2016) developed a nanocarrier system based on single-walled CNTs designed of polycationic and amphiphilic peptides modified by PEG. The cellular uptake of CNTs-peptide-PEG by A549 human lung adenocarcinoma epithelial cells showed the potential functional complex as an attractive candidate for anticancer activity. Similarly, Taghavi et al. (2016) fabricated single-walled CNTs loaded with PEG and polyethylenimine (PEI) modified by alkylcarboxylation to increase lipophilicity for vector delivery. Results demonstrated that the nanocarrier could condense DNA into a size of 150 nm and improve the gene delivery of sh-RNA to MCF7 cells. Another study enhanced the co-delivery by using single-walled CNTs-PEG-PEI conjugated with Bcl-xL-specific shRNA and shallow content of Dox for an effective and simultaneous intrinsic apoptotic against AGS and L929 cancer cells (Taghavi et al. 2017).
Protein-based nanoparticles
Protein-based nanoparticles (PNPs) are getting attention in gene delivery vectors because of their biocompatibility, biodegradability, minimal toxicity, and amphiphilic nature (Lohcharoenkal et al. 2014). Many different proteins are used for gene delivery, such as ferritin, gelatin, albumin, heat shock protein 16.5 (Hsp 16.5), and Silk (Riley and Vermerris 2017; Faria et al. 2018). Ferritin is the most used PNPs for chemotherapeutics delivery where the encapsulation depends on siRNA sequence and high intrinsic immune-activated T- and B-cells (Li et al. 2016a, b, c). In addition, albumin was often utilized to assist other molecules in delivering their gene cargo and is usually obtained from bovine serum albumin or human serum albumin (Lohcharoenkal et al. 2014). Even Karimi et al. (2013) revealed the potential co-delivery of genetic material by using nanocarrier-based albumin-chitosan. Similarly, Kumari et al. (2018) enhanced the albumin properties through binding with a natural oligosaccharide, oligochitosan, for an efficient non-viral gene delivery vector. Results showed the protection of plasmid DNA from enzymes with good stability and high transfect ability.
Different deposit challenges of nanocarriersS
Over the past few decades, the global efforts for cancer treatment have increased by developing plenty of active targets (Morales-Cruz et al. 2019). Thus, nanomedicine established multiple types of nanosystems to enhance cancer therapy by facilitating the extravasation of nanocarriers into the targeted cells (El-Say and El-Sawy 2017). Metallic nanoparticles, especially AuNPs, are broadly used in drug and gene delivery systems due to their biocompatibility, straightforward functionalization, and variety in shapes and size (Kumar et al. 2019; Ding et al. 2020). Notably, the AuNPs can conjugate surfaces with different drug molecules, peptides, and vectors simultaneously (Singh et al. 2018). In addition, cationic lipids as liposome and polymer nanoparticles have shown an exciting transport of hydrophobic and hydrophilic molecules and intracellular nucleic acid delivery due to their biodegradability, low toxicity, and high toxicity tissue penetration in cancer therapy, and large-scale production (Pala et al. 2020). However, their low targeting capability and low storage stability lead to moderate crystallization after an extended period, and non-degradable polymers cause allergic reactions, limiting their applications in clinical trials (Garcia-Pinel et al. 2019; Rai et al. 2019). Therefore, protein-based nanoparticles revealed significant delivery of vectors by reducing the immune response and protecting gene material from enzymatic degradation, but they limited transfection efficiency and required more in vivo studies (Gaber et al. 2017).
Conclusion
The greatest challenge in nanodelivery is selecting the appropriate nanocarriers for targeted sites, whatever tumoural or viral cells. In this regard, AuNPs offer clear advantages as a nanocarrier for drug and gene delivery by increasing the retention time, improving the therapeutic effects, minimizing the side effects, protecting the delivery molecule, and holding high specific targeting. Nonetheless, it is essential to understand the involving mechanisms in the delivery to avoid any related circumstances. In addition, other promising nanocarriers, including lipid-based and polymeric nanoparticles, demonstrated another aspect of nanodelivery in the future. However, the challenge remains to precise the adequate formula for better material release. Furthermore, it appears that these nanocarriers hold wide-open prospects in nanomedicine.
Abbreviations
- AgNPs
Silver nanoparticles
- AuNPs
Gold nanoparticles
- BSA
Serum albumin
- CNTs
Carbon nanotubes
- Dox
Doxorubicin
- GO
Graphene oxide
- IO NPs
Iron oxide nanoparticles
- MgONPs
Magnesium oxide nanoparticles
- NLCs
Nanostructured lipid carriers
- PAMAM
Poly (amidoamine)
- PEG
Polyethylene glycol
- PLGA
Poly-lactic-co-glycolic acid
- PLMB
Propyloxycarbonyl-propyne carbonate)
- PNCs
Polymeric nanocarriers
- PNPs
Protein-based nanoparticles
- PVA
Polyvinyl alcohol
- SLNPs
Solid lipid nanoparticles
- TiO2NPs
Titanium oxide nanoparticles
- ZnONPs
Zinc oxide nanoparticles
Author contribution
S.H.: conceptualization, article idea, data research and collection, writing—review and editing. M.J.: data research and article writing. A.C.: supervision, critically revised the paper. All authors approved the manuscript and all data were generated in-house and that no paper mill was used.
Data availability
No datasets were generated or analyzed during this study.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Yes.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Ghany S, Raslan S, Tombuloglu H, Shamseddin A, Cevik E, Said OA, Madyan EF, Senel M, Bozkurt A, Rehman S, Sabit H (2020) Vorinostat-loaded titanium oxide nanoparticles (anatase) induce G2/M cell cycle arrest in breast cancer cells via PALB2 upregulation. 3 Biotech 10(9). 10.1007/s13205-020-02391-2 [DOI] [PMC free article] [PubMed]
- Abrica-González P, Zamora-Justo JA, Sotelo-López A, Vázquez-Martínez GR, Balderas-López JA, Muñoz-Diosdado A, Ibáñez-Hernández M. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res Lett. 2019;14(1):258. doi: 10.1186/s11671-019-3083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbasi-Porter C, Ryman-Rasmussen J, Franzen S, Feldheim D (2006) DJBc: Transcription inhibition using oligonucleotide-modified gold nanoparticles. 17(5):1178–1183. 10.1021/bc060100f [DOI] [PubMed]
- Ahangari A, Salouti M, Heidari Z, Kazemizadeh AR, Safari AA. Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv. 2013;20:34–39. doi: 10.3109/10717544.2012.746402. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Ahmad R, Alam MA, Ahmad FJ (2018) Enhancement of oral bioavailability of doxorubicin through surface modified biodegradable polymeric nanoparticles. Chem Cent J 12(1). 10.1186/s13065-018-0434-1 [DOI] [PMC free article] [PubMed]
- Ahmadian Y, Bakravi A, Hashemi H, Namazi H. Synthesis of polyvinyl alcohol/CuO nanocomposite hydrogel and its application as drug delivery agent. Polym Bull. 2018 doi: 10.1007/s00289-018-2477-9. [DOI] [Google Scholar]
- Aida K, Kreling P, Caiaffa K, Calixto G, Chorilli M, Spolidorio D, SantosFilho NA, Cilli EM, Duque C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int J Nanomed. 2018;13:3081–3091. doi: 10.2147/IJN.S155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyelu J, Singh M. Folate-tagged chitosan-functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl Nanosci. 2019;9:7–17. doi: 10.1007/s13204-018-0896-4. [DOI] [Google Scholar]
- Almuqbil RM, Heyder RS, Bielski ER, Durymanov M, Reineke JJ, da Rocha SRP. Dendrimer Conjugation enhances tumor penetration and efficacy of doxorubicin in extracellular matrix-expressing 3D lung cancer models. Mol Pharm. 2020;17(5):1648–1662. doi: 10.1021/acs.molpharmaceut.0c00083. [DOI] [PubMed] [Google Scholar]
- Amarnath Praphakar R, Jeyaraj M, Ahmed M, Suresh Kumar S, Rajan M. Silver nanoparticle functionalized CS-g-(CA-MA-PZA) carrier for sustainable anti-tuberculosis drug delivery. Int J Biol Macromol. 2018;118:1627–1638. doi: 10.1016/j.ijbiomac.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Aso E, Martinsson I, Appelhans D, Effenberg C, Benseny-Cases N, Cladera J, Gouras G, Ferrer I, Klementieva O. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed Nanotechnol Biol Med. 2019;17:198–209. doi: 10.1016/j.nano.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Babos G, Biró E, Meiczinger M, Feczkó T. Dual drug delivery of sorafenib and doxorubicin from PLGA and PEG-PLGA polymeric nanoparticles. Polymers. 2018;10(8):895. doi: 10.3390/polym10080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga P, Ansari TM, Siddiqui HH, Syed A, Bahkali AH, Rahman MA, Khan MS. Bromelain capped gold nanoparticles as the novel drug delivery carriers to aggrandize effect of the antibiotic levofloxacin. EXCLI J. 2016;15:772. doi: 10.17179/excli2016-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir A, Hamimed S, Landoulsi A, Chatti A. Nano-structures differentiation of biosynthesis zinc oxide under musical sounds. Int J Nanotechnol Nanomed. 2021;6(2):59–67. [Google Scholar]
- Benyettou F, Rezgui R, Ravaux F, Jaber T, Blumer K, Jouiad M, Motte L, Olsen CJ-C, Platas-Iglesias M, Trabolski Magzoub A. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J Mat Chem b. 2015;3:7237–7245. doi: 10.1039/C5TB00994D. [DOI] [PubMed] [Google Scholar]
- Bhakta G, Sharma RK, Gupta N, Cool S, Nurcombe V, Maitra A. Multifunctional silica nanoparticles with potentials of imaging and gene delivery. Nanomed Nanotechnol Biol Med. 2011;7(4):472–479. doi: 10.1016/j.nano.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. Methotrexate-loaded polymeric lipid hybrid nanoparticles (PLHNPs): a reliable drug delivery system for the treatment of glioblastoma. J Exp Nanosci. 2021;16(1):344–367. doi: 10.1080/17458080.2021.1983172. [DOI] [Google Scholar]
- Bhavna, Md S, Ali M, Ali R, Bhatnagar A, Baboota S, Ali J. Donepezil nanosuspension intended for nose to brain targeting: in vitro and in vivo safety evaluation. Int J Biol Macromol. 2014;67:418–425. doi: 10.1016/j.ijbiomac.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Bibi S, Ur-rehman S, Khalida L, Bhattia IA, Bhattia HN, Iqbal N, Hong-Xing Z. Investigation of the adsorption properties of gemcitabine anticancer drug with metal-doped boron nitride fullerenes as a drug-delivery carrier: a DFT study. RSC Adv. 2022;12:2873–2887. doi: 10.1039/D1RA09319C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddeo C, Nacher A, Vassallo A, Armentano MF, Pons R, Fernàndez-Busquets X, Carbone C, Valenti D, Fadda AM, Manconi M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int J Pharm. 2016;513(1–2):153–163. doi: 10.1016/j.ijpharm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Calixto G, Duque C, Aida K, Rodrigues dos Santos V, Massunari L, Chorilli M. Development and characterization of p1025-loaded bioadhesive liquid-crystalline system for the prevention of Streptococcus mutans biofilms. Int J Nanomed. 2017;13:31–41. doi: 10.2147/IJN.S147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto GMF, Bernegossi J, De Freitas LM, Fontana CR, Chorilli M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review. Molecules. 2016;21:342. doi: 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiani G, Pezzoli D, Ciani L, Chiesa R, Ristori SJPO. Bioreducible Liposomes for Gene Delivery: from the Formulation to the Mechanism of Action. PLoS One. 2010;5(10):e13430. doi: 10.1371/journal.pone.0013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Wang Q, Liu Y. Lung cancer combination therapy: doxorubicin and β-elemene co-loaded, pH-sensitive nanostructured lipid carriers. Drug Des Dev Ther. 2019;13:1087–1098. doi: 10.2147/DDDT.S198003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Zhang X, Akabar MD, Luo Y, Wu H, Ke X, Ci T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif Cells Nanomed Biotechnol. 2019;47:181–191. doi: 10.1080/21691401.2018.1548470. [DOI] [PubMed] [Google Scholar]
- Capanema NSV, Carvalho IC, Mansur AAP, de Carvalho SM, Lage AP, Mansur HS. Hybrid hydrogel composed of carboxymethylcellulose-silver nanoparticles-doxorubicin for anticancer and antibacterial therapies against melanoma skin cancer cells. ACS Appl Nano Mater. 2019;2(11):7393–7408. doi: 10.1021/acsanm.9b01924. [DOI] [Google Scholar]
- Carvalho AM, Cordeiro RA, Faneca HJP. Silica-Based Gene Delivery Systems: from Design to Therapeutic Applications. Pharmaceutics. 2020;12(7):649. doi: 10.3390/pharmaceutics12070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. Dendrimers for drug delivery. Molecules. 2018;23(4):938. doi: 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennell P, Feschet-Chassot E, Devers T, Awitor KO, Descamps S, Sautou V. In vitro evaluation of TiO2 nanotubes as cefuroxime carriers on orthopaedic implants for the prevention of periprosthetic joint infections. Int J Pharm. 2013;455:298–305. doi: 10.1016/j.ijpharm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Choi J, Rui Y, Kim J, Gorelick N, Wilson DR, Kozielski K, Mangraviti A, Sankey E, Brem H. Tyler B et al Nonviral polymeric nanoparticles for gene therapy in pediatric CNS malignancies. Nanomed Nanotechnol Biol Med. 2020;23:102115. doi: 10.1016/j.nano.2019.102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejka J, Wolski K, Nowakowska M, Pyrc K, Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater Sci Eng C Mater Biol Appl. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Rius A, Boase NRB, Font I, Coronas N, Ramos-Perez V, Thurecht KJ, Borrós S. In vivo fate of carbon nanotubes with different physicochemical properties for gene delivery applications. ACS Appl Mater Interfaces. 2017;9(13):11461–11471. doi: 10.1021/acsami.7b00677. [DOI] [PubMed] [Google Scholar]
- Coelho SC, Reis DP, Pereira MC, Coelho MAN. Doxorubicin and varlitinib delivery by functionalized gold nanoparticles against human pancreatic adenocarcinoma. Pharmaceutics. 2019;11(11):551. doi: 10.3390/pharmaceutics11110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro M, Carvalho L, Silva J, Fernandes AR, Baptista PVJN. Gold Nanobeacons for Tracking Gene Silencing in Zebrafish. Nanomaterials (Basel) 2017;7(1):10. doi: 10.3390/nano7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui T, Liang J-J, Chen H, Geng D-D, Jiao L, Yang J-Y, Zhang., C., Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: regulation of drug location. ACS Appl Mater Interfaces. 2017;9(10):8569–8580. doi: 10.1021/acsami.6b16669. [DOI] [PubMed] [Google Scholar]
- Cullis PR, Hope MJ. Hope MJJMT: Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowska-Kośnik A, Szekalska M, Winnicka K. Nanostructured lipid carriers: a potential use for skin drug delivery systems. Pharmacol Rep. 2018;71:156–166. doi: 10.1016/j.pharep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- de Dante MCL, Borgheti-Cardoso LN, de Fantini MCA, Praça FSG, Medina WSG, Pierre MBR, Lara MG. Liquid crystalline systems based on glyceryl monooleate and penetration enhancers for skin delivery of celecoxib: characterization, in vitro drug release, and in vivo studies. J Pharm Sci. 2018;107(3):870–878. doi: 10.1016/j.xphs.2017.10.039. [DOI] [PubMed] [Google Scholar]
- Dara T, Vatanara A, Sharifzadeh M, Khani S, Alsadat Vakilinezhad M, Vakhshiteh F, Meybodi MN, Malvajerd SS, Hassani S, Hossein Mosaddegh M. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol Learn Mem. 2019;166:107082. doi: 10.1016/j.nlm.2019.107082. [DOI] [PubMed] [Google Scholar]
- De Jong WH, Ja Borm P. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démoulins T, Milona P, Englezou PC, Ebensen T, Schulze K, Suter R, Pichon C, Midoux P, Guzmán CA, Ruggli N. Biology et al Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine. 2016;12(3):711–722. doi: 10.1016/j.nano.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Xiao X, Yang Y, Sheng W, Wu Y, Zeng Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35(14):4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Ding X, Yin C, Zhang W, Sun Y, Zhang Z, Yang E, Sun D, Wang W. Designing aptamer-gold nanoparticle-loaded pH-sensitive liposomes encapsulate morin for treating cancer. Nanoscale Res Lett. 2020;15:1–17. doi: 10.1186/s11671-020-03297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Liang X, Li Y, Sun T, Jin Z, Xue H, Tian J. Nuclear and fluorescent labeled PD-1-Liposome-DOX-64Cu/IRDye800CW allows improved breast tumor targeted imaging and therapy. Mol Pharm. 2017;14(11):3978–3986. doi: 10.1021/acs.molpharmaceut.7b00649. [DOI] [PubMed] [Google Scholar]
- Duncan B, Kim C, Rotello VM. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J Control Release. 2010;148:122–127. doi: 10.1016/j.jconrel.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Say KM, El-Sawy HS. Polymeric nanoparticles: promising platform for drug delivery. Int J Pharm. 2017;528(1):675–691. doi: 10.1016/j.ijpharm.2017.06.052. [DOI] [PubMed] [Google Scholar]
- Erdene-Ochir T, Ganbold T, Zandan J, Han S, Borjihan G, Baigude H. Alkylation enhances biocompatibility and sirna delivery efficiency of cationic curdlan nanoparticles. Int J Biol Macromol. 2020;143:118–125. doi: 10.1016/j.ijbiomac.2019.12.048. [DOI] [PubMed] [Google Scholar]
- Fahimmnisha B, Ishwarya R, AlSalhi MS, Devanesan S, Govindarajan M, Vaseeharan B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: a novel drug delivery approach. J Drug Deliv Sci Technol. 2020;55:101465. doi: 10.1016/j.jddst.2019.101465. [DOI] [Google Scholar]
- Fan W, Xu Y, Li Z, Li Q (2019) Folic acid-modified β-cyclodextrin nanoparticles as drug delivery to load DOX for liver cancer therapeutics. Soft Mater 1–11. 10.1080/1539445X.2019.1624265
- Faria M, Björnmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR, Boyd BJ, et al. Minimum information reporting in bio-nano experimental literature. Nat Nanotechnol. 2018;13(9):777–785. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjadian F, Rezaeifard S, Naeimi M, Ghasemi S, Mohammadi-Samani S, Welland ME, Tayebi L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam) Int J Nanomed. 2019;14:6901–6915. doi: 10.2147/IJN.S214467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni J, kaur, & Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47(1):6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Fazly Bazzaz BS, Khameneh B, Namazi N, Iranshahi M, Davoodi D, Golmohammadzadeh S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: the novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett Appl Microbiol. 2018;66(6):506–513. doi: 10.1111/lam.12886. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, Hutabarat RM, Clausen VA, Karsten V, Cehelsky J, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet (london, England) 2014;383(9911):60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Shevach M, Feiner R, Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale. 2014;6(16):9410–9414. doi: 10.1039/C4NR00300D. [DOI] [PubMed] [Google Scholar]
- Fornaguera C, Grijalvo S, Galán M, Fuentes-Paniagua E, de la Mata FJ, Gómez R, Eritja R, Calderó G, Solans C. Novel non-viral gene delivery systems composed of carbosilane dendron functionalized nanoparticles prepared from nano-emulsions as non-viral carriers for antisense oligonucleotides. Int J Pharm. 2015;478(1):113–123. doi: 10.1016/j.ijpharm.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Francia V, Schiffelers R, Cullis P, Witzigmann D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug Chem. 2020;31(9):2046–2059. doi: 10.1021/acs.bioconjchem.0c00366. [DOI] [PubMed] [Google Scholar]
- Gaber M, Medhat W, Hany M, Saher N, Fang J-Y, Elzoghby A. Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: challenges and outcomes. J Control Release. 2017;254:75–91. doi: 10.1016/j.jconrel.2017.03.392. [DOI] [PubMed] [Google Scholar]
- Ganeshkumar M, Sathishkumar M, Ponrasu T, Dinesh MG, Suguna L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf B. 2013;106:208–216. doi: 10.1016/j.colsurfb.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Gang S, Gang A. Encapsulation of curcumin in silver nanoparticle for enhancement of anticancer drug delivery. Int J Pharm Sci Res. 2018;9:1160–1166. [Google Scholar]
- Garcia-Pinel B, Porras-Alcala C, Ortega-Rodriguez A, Sarabia F, Prados J, Melguizo C, Lopez-Romero JM. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials. 2019;9:638. doi: 10.3390/nano9040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D, Maheswari PU, Begum KMMS. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: kinetics and in-vitro biological studies. Carbohyd Polym. 2020;236:116101. doi: 10.1016/j.carbpol.2020.116101. [DOI] [PubMed] [Google Scholar]
- Ghaem B, Sadeghi M, Bardajee GR. Synthesis of nano-polymer supported on nano-hydrogel chitosan base and its application for DOX delivery. J Polym Environ. 2020 doi: 10.1007/s10924-020-01775-y. [DOI] [Google Scholar]
- Granados-Riveron JT, Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother. 2021;142:111953. doi: 10.1016/j.biopha.2021.111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsonbi M, Parthasarathy S, Bharat Raj K, Jaisankar V. Green synthesis, characterization and drug delivery applications of a novel silver/carboxymethylcellulose – poly(acrylamide) hydrogel nanocomposite. Ecotoxicol Environ Saf. 2016;134:421–426. doi: 10.1016/j.ecoenv.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Guo X-L, Kang X-X, Wang Y-Q, Zhang X-J, Li C-J, Liu Y, Du L-B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomaterialia. 2019;84:367–377. doi: 10.1016/j.actbio.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Hamimed S, Abdeljelil N, Landoulsi A, Chatti A, Aljabali A AA, Barhoum A (2022a) Bacterial cellulose nanofibers: biosynthesis, unique properties, modification, and emerging applications. In book: Handbook of Nanocelluloses Edition: First Edition Publisher: Springer, Cham 10.1007/978-3-030-62976-2_15-1
- Hamimed S, Barkaoui T, Trabelsi I, Landoulsi A, Chatti A (2021b) High-performance biological treatment of tuna wash processing wastewater using Yarrowia lipolytica. Environ Sci Pollut Res 28(2):1545–1554. 10.1007/s11356-020-10586-6 [DOI] [PubMed]
- Hamimed S, Chatti A (2022) Chapter 8: Photocatalytic metal bionanocomposites for biomedical applications. Barhoum (Ed) - Bionanotechnology: Emerging Applications of Bionanomaterials. Elsevier.B.V. 10.1016/B978-0-12-823915-5.00010-1
- Hamimed S, Gamraoui A, Landoulsi A, Chatti A (2022b) Bio-nanocrystallization of NaCl using saline wastewaters through biological treatment by Yarrowia lipolytica. Environ Technol Innov 26C:102338. 10.1016/j.eti.2022b.102338
- Hamimed S, Jebli N, Sellami H, Landoulsi A, Chatti A. Dual valorization of olive mill wastewater by bio-nanosynthesis of magnesium oxide and Yarrowia lipolytica biomass production. Chem Biodivers. 2020;17:e1900608. doi: 10.1002/cbdv.201900608. [DOI] [PubMed] [Google Scholar]
- Hamimed S, Kthiri A. Potential valorization of polyphenols from olive mill wastewater on sheep rumen function. Int J Environ Sci Technol. 2022 doi: 10.1007/s13762-022-04120-z. [DOI] [Google Scholar]
- Hamimed S, Landoulsi A, Chatti A (2021a) The bright side of olive mill wastewater: valuables bioproducts after bioremediation. Int J Environ Sci Technol 18:4053–4074. 10.1007/s13762-021-03145-0
- Hanafy AS, Farid RM, ElGamal SS. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: preparation and detection in rat brain. Drug Dev Ind Pharm. 2015;41(12):2055–2068. doi: 10.3109/03639045.2015.1062897. [DOI] [PubMed] [Google Scholar]
- He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharmaceutica Sinica b. 2018;9:36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Huang S, Sun X, Han L, Chang C, Zhang W, Zhong Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: preparation, characterization, and synergistic antimicrobial activity. Nanomaterials. 2019;9(8):1162. doi: 10.3390/nano9081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz-Blanco B, Shahbazi M-A, Correia AR, Balasubramanian V, Kohout T, Hirvonen J, Santos HA. pH-switch nanoprecipitation of polymeric nanoparticles for multimodal cancer targeting and intracellular triggered delivery of doxorubicin. Adv Healthcare Mater. 2016;5(15):1904–1916. doi: 10.1002/adhm.201600160. [DOI] [PubMed] [Google Scholar]
- Hu D, Chen L, Qu Y, Peng J, Chu B, Shi K, Hao Y, Zhon L, Wang M, Qian Z. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics. 2018;8(6):1558–1574. doi: 10.7150/thno.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C. 2018;90:713–727. doi: 10.1016/j.msec.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Hung W-H, Zheng J-H, Lee K-C, Cho E-C. Doxorubicin conjugated AuNP/biopolymer composites facilitate cell cycle regulation and exhibit superior tumor suppression potential in KRAS mutant colorectal cancer. J Biotechnol. 2019;306:149–158. doi: 10.1016/j.jbiotec.2019.09.015. [DOI] [PubMed] [Google Scholar]
- Hyun H, Lee S, Lim W, Jo D, Jung JS, Jo G, Kim SY, Lee DW, Um S, Yang DH, Chun HJ. Engineered beta-cyclodextrin-based carrier for targeted doxorubicin delivery in breast cancer therapy in vivo. J Ind Eng Chem. 2018;70:145–151. doi: 10.1016/j.jiec.2018.09.052. [DOI] [Google Scholar]
- Liu J, Zhao Y, Guo Q, Wang Z, Wang H, Yang Y, Huang Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials. 2012;33:6155–6161. doi: 10.1016/j.biomaterials.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Jackson TC, Obiakor NM, Iheanyichukwu IN, Ita OO, Ucheokoro AS. Biotechnology and nanotechnology drug delivery: a review (March 24. J Pharm Pharmacol. 2021;9(2021):127–132. doi: 10.17265/2328-2150/2021.04.001. [DOI] [Google Scholar]
- Jacob J, Haponiuk JT, Thomas S, Gopi S. Biopolymer based nanomaterials in drug delivery systems: a review. Mater Today Chem. 2018;9:43–55. doi: 10.1016/j.mtchem.2018.05.002. [DOI] [Google Scholar]
- Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017;12:2957–2978. doi: 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Chen H, Wei M, et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomedicine. 2017;12:4963–4979. doi: 10.2147/IJN.S138400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liang G, Dai M, Dong Y, Wu Y, Zhang L, Qi X, Qi L. Preparation of doxorubicin-loaded collagen-PAPBA nanoparticles and their anticancer efficacy in ovarian cancer. Ann Transl Med. 2020;8(14):880–880. doi: 10.21037/atm-20-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Wang Q, Chen J, Wang Z, Xin H, Zhang D (2019) Efficient delivery of therapeutic siRNA by Fe(3)O(4) magnetic nanoparticles into oral cancer cells. Pharmaceutics 11(11):615. 10.3390/pharmaceutics11110615 [DOI] [PMC free article] [PubMed]
- Jin S-E, Kim C-K. Charge-Mediated Topical Delivery of Plasmid DNA with Cationic Lipid Nanoparticles to the Skin. Colloids Surf B Biointerfaces. 2014;116:582–590. doi: 10.1016/j.colsurfb.2014.01.053. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Jeong Y-I, Choi E, et al. Biocompatible poly(2-hydroxyethyl methacrylate)-b-poly(L-histidine) hybrid materials for pH-sensitive intracellular anticancer drug delivery. Adv Func Mater. 2012;22(5):1058–1068. doi: 10.1002/adfm.201102756. [DOI] [Google Scholar]
- Jose A, Labala S, Ninave KM, Gade SK, Venuganti VVK. Effective skin cancer treatment by topical co-delivery of curcumin and STAT3 siRNA using cationic liposomes. AAPS PharmSciTech. 2017;19(1):166–175. doi: 10.1208/s12249-017-0833-y. [DOI] [PubMed] [Google Scholar]
- Kalimuthu K, Lubin B.-C, Bazylevich A, Gellerman G, Shpilberg O, Luboshits G, Firer MA (2018) Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J Nanobiotechnol 16(1):34. 10.1186/s12951-018-0362-1 [DOI] [PMC free article] [PubMed]
- Kamalzare S, Noormohammadi Z, Rahimi P, Atyabi F, Irani S, Tekie FSM, Mottaghitalab F. Carboxymethyl dextran-trimethyl chitosan coated superparamagnetic iron oxide nanoparticles: an effective siRNA delivery system for HIV-1 Nef. J Cell Physiol. 2019;234(11):20554–20565. doi: 10.1002/jcp.28655. [DOI] [PubMed] [Google Scholar]
- Kamegawa R, Naito M, Miyata K. Functionalization of silica nanoparticles for nucleic acid delivery. Nano Res. 2018;11(10):5219–5239. doi: 10.1007/s12274-018-2116-7. [DOI] [Google Scholar]
- Kamel AE, Fadel M, Louis D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: development and application in breast cancer cell line. Int J Nanomed. 2019;14:5073–5085. doi: 10.2147/IJN.S210484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar R, Rathee J, Salunke DB, Mehta SK. Green nanotechnology-driven drug delivery assemblies. ACS Omega. 2019;4(5):8804–8815. doi: 10.1021/acsomega.9b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6(1):41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- Karimi M, Avci P, Mobasseri R, Hamblin MR, Naderi-Manesh H. The novel albumin-chitosan core-shell nanoparticles for gene delivery: preparation, optimization and cell uptake investigation. J Nanoparticle Res Interdiscip Forum Nanoscale Sci Technol. 2013;15(4):1651. doi: 10.1007/s11051-013-1651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kthiri A, Hamimed S, Othmani A, et al. Novel static magnetic field effects on green chemistry biosynthesis of silver nanoparticles in Saccharomyces cerevisiae. Sci Rep. 2021;11:20078. doi: 10.1038/s41598-021-99487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondath S, Srinivas Raghavan B, Anantanarayanan R, Rajaram R. Synthesis and characterisation of morin reduced gold nanoparticles and its cytotoxicity in MCF-7 cells. Chem Biol Interact. 2014;224:78–88. doi: 10.1016/j.cbi.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Kooti M, Sedeh AN, Motamedi H, Rezatofighi SE. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl Microbiol Biotechnol. 2018;102(8):3607–3621. doi: 10.1007/s00253-018-8880-1. [DOI] [PubMed] [Google Scholar]
- Kozielski KL, Tzeng SY, Hurtado De Mendoza BA, Green JJ. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano. 2014;8(4):3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Diwan A, Singh P, Gulati S, Choudhary D, Mongia A, Shukla S, Gupta A. Functionalized gold nanostructures: promising gene delivery vehicles in cancer treatment. RSC Adv. 2019;9(41):23894–23907. doi: 10.1039/C9RA03608C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Liu C-H, Wu W-C. Efficient gene delivery by oligochitosan conjugated serum albumin: facile synthesis, polyplex stability, and transfection. Carbohyd Polym. 2018;183:37–49. doi: 10.1016/j.carbpol.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Langer R, Weissleder R. Scientific Discovery and the Future of Medicine. JAMA. 2015;313:135–136. doi: 10.1001/jama.2014.16315. [DOI] [PubMed] [Google Scholar]
- Li L, Muñoz-Culla M, Carmona U, Lopez MP, Yang F, Trigueros C, Otaegui D, Zhang L, Knez M. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials. 2016;98:143–151. doi: 10.1016/j.biomaterials.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Li R, Liu T, Wang K (2016c) Hyaluronic modified and amine-functionalized silica nanoparticles as intracellular siRNA delivery carriers in lung. Cancer Gene Ther 9(6):10191–10200
- Li W, Zhao X, Du B, Li X, Liu S, Yang X.-Y, Ding H, Yang W, Pan F, Wu X (2016b) Gold nanoparticle–mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci Rep 6:30619. 10.1038/srep30619 [DOI] [PMC free article] [PubMed]
- Li X, Jia X, Niu H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int J Nanomed. 2018;13:4107–4119. doi: 10.2147/IJN.S163929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Xie J, Zhang F, Wang Z, Luo K, Zhu L, Quan Q, Niu G, Lee S, Ai H. N-Alkyl-PEI-functionalized iron oxide nanoclusters for efficient siRNA delivery. Small. 2011;7(19):2742–2749. doi: 10.1002/smll.201100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ma X, Jin S, Xue X, Zhang C, Wei T, Guo W, Liang X-J. Zinc oxide nanoparticles as adjuvant to facilitate doxorubicin intracellular accumulation and visualize pH-responsive release for overcoming drug resistance. Mol Pharm. 2016;13(5):1723–1730. doi: 10.1021/acs.molpharmaceut.6b00311. [DOI] [PubMed] [Google Scholar]
- Loh XJ, Lee T-C, Dou Q, Deen GR. Utilising inorganic nanocarriers for gene delivery. Biomaterials Science. 2016;4(1):70–86. doi: 10.1039/C5BM00277J. [DOI] [PubMed] [Google Scholar]
- Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bertoni H, Kozielski KL, Rui Y, Lal B, Vaughan H, Wilson DR, Mihelson N, Eberhart CG, Laterra J, Green JJ. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18(7):4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Andrade S, Duarte A, Neves A, Queiroz J, Nunes C, Seven E, Fenart L, Gosselet E, Coelho MAN, Pereira M. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22(2):277. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi MS, Khalid F, Khan MT, Samra ZQ, Muhammad S, Zhang Y-J, Mou K. A novel method of magnetic nanoparticles functionalized with anti-folate receptor antibody and methotrexate for antibody mediated targeted drug delivery. Molecules. 2022;27(1):261. doi: 10.3390/molecules27010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali S. Delivery systems for gene therapy. Indian J Hum Genet. 2013;19(1):3–8. doi: 10.4103/0971-6866.112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65(13):1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomed. 2008;3(2):169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay PF, Hu K, Blakney AK, Samnuan K, Brown JC, Penn R, Zhou J, Bouton CR, Rogers P, Polra K, Lin PJC, Barbosa C, Tam YK, Barclay WS, Shattock RJ. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice Nat. Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes R, Fernandes AR, Baptista PV (2017) Gold nanoparticle approach to the selective delivery of gene silencing in cancer-the case for combined delivery? Genes 8(3):94. 10.3390/genes8030094 [DOI] [PMC free article] [PubMed]
- Meng F, Zhong Y, Cheng R, Deng C, Zhong Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomedicine. 2014;9(3):487–499. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- Mishra V, Bansal K, Verma A, Yadav N, Thakur S, Sudhakar K, Rosenholm J. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10(4):191. doi: 10.3390/pharmaceutics10040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody V, Siwale R, Singh A, Mody H. Introduction to metallic nanoparticles. J Pharm Bioall Sci. 2010;2(4):282. doi: 10.4103/0975-7406.72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Cruz M, Delgado Y, Castillo B, Figueroa CM, Molina A, Torres A, Griebenow K. Smart targeting to improve cancer therapeutics. Drug Des Dev Ther. 2019;13:3753–3772. doi: 10.2147/dddt.s219489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton CA, Dominicus R, Radny P, Dirschka T, Hauschild A, Reinhold U, Szeimies R-M. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018 doi: 10.1111/bjd.16441. [DOI] [PubMed] [Google Scholar]
- Mu Q, Broughton DL, Yan B. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett. 2009;9(12):4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund R, Panda N, Nimesh S, Biswas A (2014) Novel titanium oxide nanoparticles for effective delivery of paclitaxel to human breast cancer cells. J Nanoparticle Res 16(12). 10.1007/s11051-014-2739-x
- Namgung R, Zhang Y, Fang QL, Singha K, Lee HJ, Kwon IK, Jeong YY, Park IK, Son SJ, Kim WJ. Multifunctional silica nanotubes for dual-modality gene delivery and MR imaging. Biomaterials. 2011;32(11):3042–3052. doi: 10.1016/j.biomaterials.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10(3):118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Soufi GJ, Iravani S, Varma RS. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials. 2020;10:1072. doi: 10.3390/nano10061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Qiu L, Zhang G, Zhou H, Han Y. Lymph cancer chemotherapy: delivery of doxorubicin-gemcitabine prodrug and vincristine by nanostructured lipid carriers. Int J Nanomed. 2017;12:1565–1576. doi: 10.2147/IJN.S120685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niora M, Pedersbæk D, Münter R, Weywadt M, Farhangibarooji Y, Andresen T, Simonsen J, Jauffred L. Head-to-head comparison of the penetration efficiency of lipid-based nanoparticles into tumor spheroids. ACS Omega. 2020;5(33):21162–21171. doi: 10.1021/acsomega.0c02879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak B, DuBois J, Chahrour O, Papusha T, Hirt S, Philippi T, Zogel C, Osenberg K, Schmitz B, Lübbert H. Clinical pharmacokinetics and safety of a 10% aminolevulinic acid hydrochloride nanoemulsion gel (BF-200 ALA) in photodynamic therapy of patients extensively affected with actinic keratosis: results of 2 maximal usage pharmacokinetic trials. Clini Pharmacol Drug Dev. 2022;11:535–550. doi: 10.1002/cpdd.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocsoy I, Tasdemir D, Mazicioglu S, Celik C, Katı A, Ulgen F. Biomolecules incorporated metallic nanoparticles synthesis and their biomedical applications. Mater Lett. 2018;212:45–50. doi: 10.1016/j.matlet.2017.10.068. [DOI] [Google Scholar]
- Ohta T, Hashida Y, Yamashita F, Hashida M. Development of novel drug and gene delivery carriers composed of single-walled carbon nanotubes and designed peptides with PEGylation. J Pharm Sci. 2016;105(9):2815–2824. doi: 10.1016/j.xphs.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Oladipo AO, Iku SII, Ntwasa M, Nkambule TTI, Mamba BB, Msagati TAM. Doxorubicin conjugated hydrophilic AuPt bimetallic nanoparticles fabricated from Phragmites australis: characterization and cytotoxic activity against human cancer cells. J Drug Deliv Sci Technol. 2020;57:101749. doi: 10.1016/j.jddst.2020.101749. [DOI] [Google Scholar]
- Olerile LD, Liu Y, Zhang B, Wang T, Mu S, Zhang J, Selotlegeng L, Zhang N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf B. 2017;150:121–130. doi: 10.1016/j.colsurfb.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Ou Y.-C, Webb JA, Faley S, Shae D, Talbert EM, Lin S, … Bardhan R (2016) Gold Nanoantenna-Mediated Photothermal Drug Delivery from Thermosensitive Liposomes in Breast Cancer. ACS Omega 1(2): 234–243 [DOI] [PMC free article] [PubMed]
- Pala R, Anju V, Dyavaiah M, Busi S, Nauli SM. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int J Nanomed. 2020;15:3741–3769. doi: 10.2147/IJN.S250872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi M, Pozzi D, Palmieri V, Caracciolo G. Principles for optimization and validation of mRNA lipid nanoparticle vaccines against COVID-19 using 3D bioprinting. Nano Today. 2022;43:101403. doi: 10.1016/j.nantod.2022.101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng LH, Huang YF, Zhang CZ, Niu J, Chen Y, Chu Y, Jiang ZH, Gao JQ, Mao ZW. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials. 2016;103:137–149. doi: 10.1016/j.biomaterials.2016.06.057. [DOI] [PubMed] [Google Scholar]
- Poonia N, Kaur Narang J, Lather V, Beg S, Sharma T, Singh B, Pandita D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B. 2019;181:756–766. doi: 10.1016/j.colsurfb.2019.06.004. [DOI] [PubMed] [Google Scholar]
- Prasher P, Sharma M, Mudila H, Gupta G, Sharma AK, Kumar D, Bakshi HA, Negi P, Kapoor DN, Chellappan DK, Tambuwala MM, Dua K. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci Commun. 2020;35:100244. doi: 10.1016/j.colcom.2020.100244. [DOI] [Google Scholar]
- Prusty K, Swain SK. Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater Sci Eng C. 2018;85:130–141. doi: 10.1016/j.msec.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Qiu L, Li J-W, Hong C-Y, Pan C-Y. Silver nanoparticles covered with pH-sensitive camptothecin-loaded polymer prodrugs: switchable fluorescence “off” or “on” and drug delivery dynamics in living cells. ACS Appl Mater Interfaces. 2017;9(46):40887–40897. doi: 10.1021/acsami.7b14070. [DOI] [PubMed] [Google Scholar]
- Radmansouri M, Bahmani E, Sarikhani E, Rahmani K, Sharifianjazi F, Irani M. Doxorubicin hydrochloride - Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int J Biol Macromol. 2018;116:378–384. doi: 10.1016/j.ijbiomac.2018.04.161. [DOI] [PubMed] [Google Scholar]
- Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers (basel) 2019;11(4):745. doi: 10.3390/polym11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Ingle AP, Paralikar P, Gupta I, Medici S, Santos CA. Recent advances in use of silver nanoparticles as antimalarial agents. Int J Pharm. 2017;526(1–2):254–270. doi: 10.1016/j.ijpharm.2017.04.042. [DOI] [PubMed] [Google Scholar]
- Ravichandran R, Sridhar R, Venugopal JR, et al. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol Biosci. 2014;14(4):515–525. doi: 10.1002/mabi.201300407. [DOI] [PubMed] [Google Scholar]
- Remaut K, Oorschot V, Braeckmans K, Klumperman J, De Smedt SC. Lysosomal capturing of cytoplasmic injected nanoparticles by autophagy: an additional barrier to non viral gene delivery. J Control Release. 2014;195:29–36. doi: 10.1016/j.jconrel.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Ren W, Zeng L, Shen Z, Xiang L, Gong A, Zhang J, Mao C, Li A, Paunesku T, Woloschak GE, Hosmane NS, Wu A. Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv. 2013;3:20855–20861. doi: 10.1039/c3ra42863j. [DOI] [Google Scholar]
- Riley MK, Vermerris W. Recent advances in nanomaterials for gene delivery-a review. Nanomaterials (basel) 2017;7(5):94. doi: 10.3390/nano7050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312(5776):1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- Ruzycka-Ayoush M, Kowalik P, Kowalczyk A, et al. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nano. 2021;12:8. doi: 10.1186/s12645-021-00077-9. [DOI] [Google Scholar]
- Sadhukhan P, Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, Sil PC. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater Sci Eng C. 2019;100:129–140. doi: 10.1016/j.msec.2019.02.096. [DOI] [PubMed] [Google Scholar]
- Sadrjavadi K, Shahbazi B, Fattahi A (2018) De-esterified tragacanth-chitosan nano-hydrogel for methotrexate delivery; optimization of the formulation by Taguchi design. Artif Cells Nanomed Biotechnol 1–11. 10.1080/21691401.2018.1471482 [DOI] [PubMed]
- Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, Moroni L, Dubruel P. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41(21):7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- Satalkar P, Elger BS, Hunziker P. Shaw D Challenges of clinical translation in nanomedicine: a qualitative study. Nanomedicine. 2016;12(4):893–900. doi: 10.1016/j.nano.2015.12.376. [DOI] [PubMed] [Google Scholar]
- Sawant VJ, Bamane SR. PEG-beta-cyclodextrin functionalized zinc oxide nanoparticles show cell imaging with high drug payload and sustained pH responsive delivery of curcumin in to MCF-7 cells. J Drug Deliv Sci Technol. 2018;43:397–408. doi: 10.1016/j.jddst.2017.11.010. [DOI] [Google Scholar]
- Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, Crommelin DJA. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz L, Novak B, Hoeh AK, et al. Epidermal penetration and protoporphyrin IX formation of two different 5 -aminolevulinic acid formulations in ex vivo human skin. Photodiagnosis Photodyn Ther. 2016;14:40–46. doi: 10.1016/j.pdpdt.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Severino P, Silveira EF, Loureiro K, Chaud MV, Antonini D, Lancellotti M, Sarmento VH, da Silva CF, Santana MHA, Souto EB. Antimicrobial activity of polymyxin-loaded solid lipid nanoparticles (PLX-SLN): Characterization of physicochemical properties and in vitro efficacy. Eur J Pharm Sci. 2017;106:177–184. doi: 10.1016/j.ejps.2017.05.063. [DOI] [PubMed] [Google Scholar]
- Shafiee S, Ahangar HA, Saffar A. Taguchi method optimization for synthesis of Fe3O4 @chitosan/Tragacanth Gum nanocomposite as a drug delivery system. Carbohyd Polym. 2019;222:114982. doi: 10.1016/j.carbpol.2019.114982. [DOI] [PubMed] [Google Scholar]
- Shakil MS, Hasan MA, Sarker SR. Iron oxide nanoparticles for breast cancer theranostics. Curr Drug Metab. 2019;20(6):446–456. doi: 10.2174/1389200220666181122105043. [DOI] [PubMed] [Google Scholar]
- Shao D, Zhang X, Liu W, Zhang F, Zheng X, Qiao P, Li J, Dong WF, Chen L. Janus silver-mesoporous silica nanocarriers for SERS traceable and pH-sensitive drug delivery in cancer therapy. ACS Appl Mater Interfaces. 2016;8(7):4303–4308. doi: 10.1021/acsami.5b11310. [DOI] [PubMed] [Google Scholar]
- Sharma H, Kumar K, Choudhary C, Mishra PK, Vaidya B. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif Cells Nanomed Biotechnol. 2014;44(2):672–679. doi: 10.3109/21691401.2014.978980. [DOI] [PubMed] [Google Scholar]
- Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle-based drug delivery systems. J Pharm Sci. 2018;107(7):1741–1753. doi: 10.1016/j.xphs.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Shen M-Y, Liu T-I, Yu T-W, Kv R, Chiang W-H, Tsai Y-C, Chen HH, Lin S-C, Chiu H-C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials. 2019;197:86–100. doi: 10.1016/j.biomaterials.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang L, Zhang J, Ma R, Gao J, Liu Y, Zhang C, Zhang Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@ Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials. 2014;35(22):5847–5861. doi: 10.1016/j.biomaterials.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Shillitoe EJ. Gene therapy: the end of the rainbow? Head Neck Oncol. 2009;1(1):1–5. doi: 10.1186/1758-3284-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. doi: 10.3390/ijms19071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanathan T, Krishna VM, Saravanan V, Kumar R, Kumar R. MgO nanoparticles for effective uptake and release of doxorubicin drug: pH sensitive controlled drug release. J Nanosci Nanotechnol. 2016;16(9):9421–9431. doi: 10.1166/jnn.2016.12164. [DOI] [Google Scholar]
- Sonali, Singh RP, Singh N, Sharma G, Vijayakumar MR, Koch B, Singh S, Singh U, Dash D, Pandey BL, Muthu MS. Transferrin liposomes of docetaxel for brain-targeted cancer applications: formulation and brain theranostics. Drug Deliv. 2016;23(4):1261–1271. doi: 10.3109/10717544.2016.1162878. [DOI] [PubMed] [Google Scholar]
- Soni N, Soni N, Pandey H, Maheshwari R, Kesharwani P, Tekade RK. Augmented delivery of gemcitabine in lung cancer cells exploring mannose anchored solid lipid nanoparticles. J Colloid Interface Sci. 2016;481:107–116. doi: 10.1016/j.jcis.2016.07.020. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Thapa U, Saha M, Jalees M. Aggregation behaviour of tetracaine hydrochloride with Gemini surfactants and the formation of silver nanoparticles using drug-Gemini surfactants mixture. J Mol Liq. 2019;276:399–408. doi: 10.1016/j.molliq.2018.12.006. [DOI] [Google Scholar]
- Su Z, Erdene-Ochir T, Ganbold T, Baigude H. Design of Curdlan-Based pH-Sensitive Polymers with Endosome Buffering Functionality for Sirna Delivery. Int J Biol Macromol. 2020;146:773–780. doi: 10.1016/j.ijbiomac.2019.10.129. [DOI] [PubMed] [Google Scholar]
- Su S, Zuo X, Pan D, Pei H, Wang L, Fan C, Huang W. Design and applications of gold nanoparticle conjugates by exploiting biomolecule-gold nanoparticle interactions. Nanoscale. 2013;5:2589–2599. doi: 10.1039/c3nr33870c. [DOI] [PubMed] [Google Scholar]
- Sulaiman GM, Waheeb HM, Jabir MS, Khazaal SH, Dewir YH, Naidoo Y (2020) Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep 10(1):9362. 10.1038/s41598-020-66419-6 [DOI] [PMC free article] [PubMed]
- Sundraraman G, Jayakumari LS. Meticulous taxifolin releasing performance by the zinc oxide nanoparticles: as a short road to drug delivery system for cancer therapeutics. J Cluster Sci. 2020;31:241–255. doi: 10.1007/s10876-019-01642-4. [DOI] [Google Scholar]
- Taghavi S, HashemNia A, Mosaffa F, Askarian S, Abnous K, Ramezani M. Preparation and evaluation of polyethylenimine-functionalized carbon nanotubes tagged with 5TR1 aptamer for targeted delivery of Bcl-xL shRNA into breast cancer cells. Colloids Surf B. 2016;140:28–39. doi: 10.1016/j.colsurfb.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Taghavi S, Nia AH, Abnous K, Ramezani M. Polyethylenimine-functionalized carbon nanotubes tagged with AS1411 aptamer for combination gene and drug delivery into human gastric cancer cells. Int J Pharm. 2017;516(1–2):301–312. doi: 10.1016/j.ijpharm.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Tahover E, Patil YP, Gabizon AA. Emerging delivery systems to reduce doxorubicin cardiotoxicity and improve therapeutic index. Anticancer Drugs. 2015;26(3):241–258. doi: 10.1097/CAD.0000000000000182. [DOI] [PubMed] [Google Scholar]
- Tapeinos C, Battaglini M, Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release. 2017;264:306–332. doi: 10.1016/j.jconrel.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NTT, Wang T-H, Lin C-Y, Tai Y. Synthesis of methotrexate-conjugated gold nanoparticles with enhanced cancer therapeutic effect. Biochem Eng J. 2013;78:175–180. doi: 10.1016/j.bej.2013.04.017. [DOI] [Google Scholar]
- Vakilinezhad MA, Amini A, Akbari Javar H, Baha’addini Beigi Zarandi BF, Montaseri H, Dinarvand R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. DARU J Pharm Sci. 2018;26:165–177. doi: 10.1007/s40199-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke R, Lentacker I, De Smedt SC, Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J Control Release. 2021 doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinhas R, Mendes R, Fernandes AR, Baptista PV. Nanoparticles-emerging potential for managing leukemia and lymphoma. Front Bioeng Biotechnol. 2017;5:79. doi: 10.3389/fbioe.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang Y-C, Dou S, Xiong M-H, Sun T-M, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011;5(5):3679–3692. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R, Liu H, Wang Y, Yan L, Chen S, Yang Q, Chen C. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules. 2018;23(7):1578. doi: 10.3390/molecules23071578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang L, Chen T, Guo W, Bao X, Wang D, Chen S, Tang B, Yang Q, Chen C. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017;22(11):1814. doi: 10.3390/molecules22111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wavikar P, Pai R, Vavia P. Nose to brain delivery of rivastigmine by in situ gelling cationic nanostructured lipid carriers: enhanced brain distribution and pharmacodynamics. J Pharm Sci. 2017;106(12):3613–3622. doi: 10.1016/j.xphs.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Wen S, Zhao Q, An X, Zhu J, Hou W, Li K, Huang Y, Shen M, Zhu W, Shi X. Multifunctional PEGylated multiwalled carbon nanotubes for enhanced blood pool and tumor MR imaging. Adv Healthcare Mater. 2014;3(10):1568–1577. doi: 10.1002/adhm.201300631. [DOI] [PubMed] [Google Scholar]
- WHO: Accessed on 27 March 2022. https://www.who.int/news-room/fact-sheets/detail/cancer
- Wu D, Wang H, Hou X, Chen H, Ma Y, Hou Y, Hong J, Ding Y. Effects of gold core size on regulating the performance of doxorubicin-conjugated gold nanoparticles. Nano Res. 2018;11(6):3396–3410. doi: 10.1007/s12274-017-1963-y. [DOI] [Google Scholar]
- Wu Y, Li YH, Gao XH, Chen HD. The application of nanoemulsion in dermatology: an overview. J Drug Target. 2013;21:321–327. doi: 10.3109/1061186X.2013.765442. [DOI] [PubMed] [Google Scholar]
- Xia B, Zhang W, Tong H, Li J, Chen Z, Shi J. Multifunctional chitosan/porous silicon@Au nanocomposite hydrogels for long-term and repeatedly localized combinatorial therapy of cancer via a single injection. ACS Biomater Sci Eng. 2019 doi: 10.1021/acsbiomaterials.8b01533. [DOI] [PubMed] [Google Scholar]
- Xie C, Guo B, You H, Wang Z, Leng Q, Ding L, Wang Q. Synthesis and surface modification of mesoporous metal-organic framework (UiO-66) for efficient pH-responsive drug delivery and lung cancer treatment. Nanotechnology. 2021;32:295704. doi: 10.1088/1361-6528/abf7ea. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zhou D, Qi Y, Zhang Z, Xie Z, Chen X, Jin X, Meng F, Huang Y. Doxorubicin-loaded carborane-conjugated polymeric nanoparticles as delivery system for combination cancer therapy. Biomacromol. 2015;16(12):3980–3988. doi: 10.1021/acs.biomac.5b01311. [DOI] [PubMed] [Google Scholar]
- Yang T, Du G, Cui Y, Yu R, Hua C, Tian W, Zhang Y. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int J Nanomed. 2019;14:1997–2010. doi: 10.2147/IJN.S193170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye E, Loh XJ. Polymeric hydrogels and nanoparticles: a merging and emerging field. Aust J Chem. 2013;66(9):997–1007. doi: 10.1071/CH13168. [DOI] [Google Scholar]
- Ye E, Regulacio MD, Zhang S-Y, Loh XJ, Han M-Y. Anisotropically branched metal nanostructures. Chem Soc Rev. 2015;44(17):6001–6017. doi: 10.1039/C5CS00213C. [DOI] [PubMed] [Google Scholar]
- Yeo CI, Ooi KK, Tiekink E (2018) Gold-based medicine: a paradigm shift in anti-cancer therapy? Molecules (Basel, Switzerland), 23(6) [DOI] [PMC free article] [PubMed]
- Zabielska-Koczywąs K, Dolka I, Król M, Żbikowski A, Lewandowski W, Mieczkowski J, Wójcik M, Lechowski R. Doxorubicin conjugated to glutathione stabilized gold nanoparticles (Au-GSH-Dox) as an effective therapeutic agent for feline injection-site sarcomas—chick embryo chorioallantoic membrane study. Molecules. 2017;22(2):253. doi: 10.3390/molecules22020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani M, Rostami M, Aghajanzadeh M, Kheiri Manjili H, Rostamizadeh K, Danafar H. Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J Mater Sci. 2017;53(3):1634–1645. doi: 10.1007/s10853-017-1673-6. [DOI] [Google Scholar]
- Zeng F, Xu D, Zhan C, Liang C, Zhao W, Zhang J, Feng H, Ma X. Surfactant-free synthesis of graphene oxide coated silver nanoparticles for SERS biosensing and intracellular drug delivery. ACS Appl Nano Mater. 2018;1(6):2748–2753. doi: 10.1021/acsanm.8b00444. [DOI] [Google Scholar]
- Zhang W, Xu W, Lan Y, He X, Liu K, Liang Y. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int J Nanomed. 2019;14:5287–5301. doi: 10.2147/IJN.S203113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li J, Shi Z, Yang Y, Xie X, Lee SM, Wang Y, Leong KM, Chen M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–364. doi: 10.1016/j.actbio.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhu J, Zheng Y, Guo R, Wang S, Mignani S, Caminade AM, Majoral JP, Shi X. Doxorubicin-conjugated PAMAM dendrimers for pH-responsive drug release and folic acid-targeted cancer therapy. Pharmaceutics. 2018;10(3):162. doi: 10.3390/pharmaceutics10030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Li N, Astruc D. State of the art in gold nanoparticle synthesis. Coord Chem Rev. 2013;257:638–665. doi: 10.1016/j.ccr.2012.09.002. [DOI] [Google Scholar]
- Zhu J, Zheng L, Wen S, Tang Y, Shen M, Zhang G, Shi X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2014;35(26):7635–7646. doi: 10.1016/j.biomaterials.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Zhupanyn P, Ewe A, Büch T, Malek A, Rademacher P, Müller C, Reinert A, Jaimes Y, Aigner A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63–76. doi: 10.1016/j.jconrel.2019.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during this study.