Abstract
Acute spinal cord injury (SCI) is a devastating condition that causes enormous damage to a patient’s physical, mental, and economic situation and requires a multidisciplinary approach to treatment. Research on SCI has been performed for a long time, and the management of SCI has developed dramatically in recent decades as a mechanism of injury and the pathophysiology of SCI have been revealed from the primitive stage in the past. In the treatment of patients with acute SCI, there is a lot of debate regarding surgical treatment strategies and pharmacological management, such as steroid use. In particular, the efficacy of steroid use, such as methylprednisolone sodium succinate, has been increasing and decreasing and is still intensely debated. The practice guidelines reported so far for this are also at the “suggest” stage with weak recommendations. Therefore, this review aims to summarize the effects of steroid use on SCI. This review provides an overview of current practical guidelines and clinical studies on steroid use in patients with SCI.
Keywords: Spinal cord injuries, Pathophysiology, Steroid, Guideline
INTRODUCTION
Spinal cord injury (SCI) is a catastrophic problem that can cause severe dysfunction in neurologic condition and decrease in quality of life for affected individuals.11) In addition, the ongoing cost of treatment and rehabilitation for SCI has a huge impact on the socio-economic status of individuals and families, further placing a significant financial burden on the national health care system.1,3) The prevalence and incidence of SCI vary according to geopolitical and economic conditions, and about 1,000 new cord injury patients occur every year in South Korea.14) Also, until now, the pathophysiology of SCI has been studied very deeply in terms of cellular and molecular aspects, and accordingly, there have been many studies and discussions on the management of SCI, but there are still controversies especially regarding the use of steroid. Therefore, we aim to provide updated reviews and guidelines for the use of steroids in acute SCI. And later, it is considered necessary to updated review the contents surgical and cellular treatment strategies for SCI and future perspective.
PATHOPHYSIOLOGY OF SCI
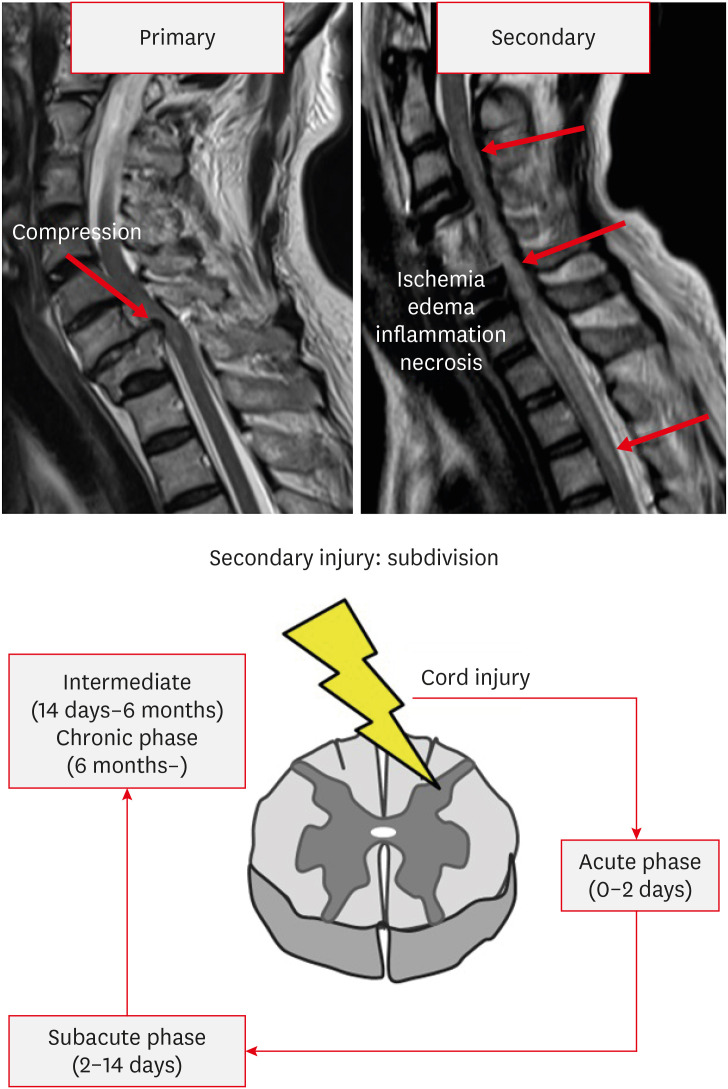
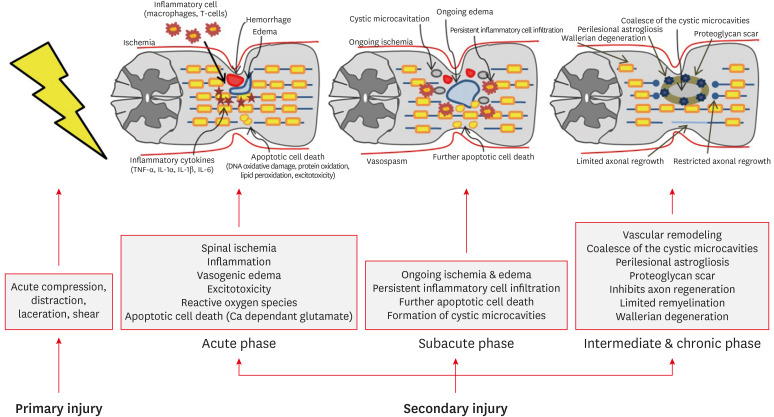
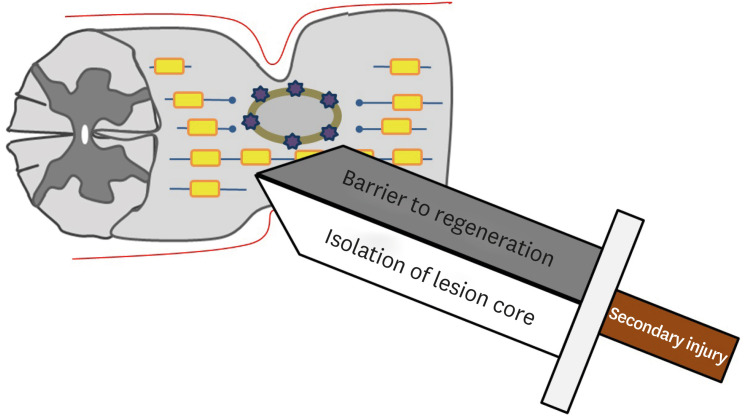
SCI can be classified into primary and secondary injuries.1) The primary injury is an initial mechanical injury that causes damage to the axon, blood vessel, and cell membrane by direct force applied to the spinal cord through persistent or transient compression, vertebral fracture, and cord laceration or transection.1,3,43) Primary injuries are the direct result of mechanical forces at the time of injury and lead to cell death and bleeding. However, anatomically, it is rare for the spinal cord to be fully transected or disrupted.44) So, the remaining extra axons are very important because they act as neural substrates for new treatment strategies. Previous studies have reported that only 5% of the original axons in animal experiments can maintain nerve function.20) After the primary injuries, a secondary injury begins immediately, resulting in the extension of the nerve injury site and exacerbating the neurologic deficit.52) The therapeutic focus of SCI is to avoid and inhibit secondary injuries. Secondary injury can be divided into acute (within 48 hours), subacute (2–14 days), intermediate (14 days–6 months), and chronic (more than 6 months) phases (FIGURE 1). During the acute phase, edema, hemorrhage, and ischemia occur at the injury site. Inflammatory cells (macrophages, microglia, T-cells, and neutrophils) also infiltrate the injury site and these cells trigger the secretion of inflammatory cytokines (tumor necrosis factor-α, interleukin [IL]-1α, IL-1β, and IL-6) and causes disruption of the blood-spinal cord barrier. The level of release of these cytokines peaks at 6 to 12 hours after injury and is maintained until 4 days after injury.42,48) In addition, loss of ionic homeostasis of nerve cells by injury increases intracellular calcium ion, which activates calcium-dependent proteases, causing mitochondrial dysfunction, which ultimately leads to apoptotic cell death (Ca2+-dependent glutamate-associated cell death).24,45) During process of apoptotic cell death, ATP, potassium ions, and DNA are released, which induce the microglia to release additional proinflammatory cytokines and attract more inflammatory cells to the injury site. In addition, debris from process of apoptotic cell death is cleared by phagocytes. In this process, phagocytes release reactive oxygen species (oxygen and nitrogen free radicals), which causes delayed necrosis and apoptosis through DNA oxidative damage, protein oxidation, and lipid peroxidation.4,15,24) Oligodendrocytes are particularly sensitive to apoptosis. Therefore, the sensitive response of oligodendrocytes to apoptosis extends far from the epicenter of SCI, eventually leading to demyelination of preserved axons.16) Also, a prominent feature in the acute phase is excitotoxicity caused by excitatory neurotransmitters such as glutamate and aspartate. Excitatory neurotransmitters are overproduced by damaged nerve cells, and the excessive activation of excitatory neurotransmitter receptors causes excitotoxicity. This process also eventually leads to apoptosis of glial cells and neurons.30,31,41,50) During the subacute phase, edema progresses, leading to vascular compromise and further exacerbation of ischemia. Persistent inflammatory cell infiltration is maintained. Ongoing ischemia and persistent inflammatory cell infiltration induce a more cytotoxic microenvironment, resulting in further apoptotic cell death and formation of cystic microcavities.29,30,31,50) In the intermediate and chronic phases, vascular remodeling, alterations in the extracellular matrix composition, and reorganization of neural circuits around the injury site occur. The continuous loss of neurons causes coalesce of the cystic microcavities, which acts as an important barrier for axon regrowth, regeneration, and cell migration (FIGURE 2).3,37,40) Astrocytes within the perilesional area proliferate and strongly interweave their extended processes together. This prevents further expansion of the lesion by isolating the lesion core. However, these tightly interwoven astrocytes form a physical barrier called a glial scar to inhibit axonal regeneration (FIGURE 3). Astrocytes also form a chemical barrier by secreting a lot of chondroitin sulfate proteoglycan (CSPGs) that inhibits axon regeneration. CSPGs bind to leukocyte common antigen-related receptors such as protein tyrosine phosphatase and activate the GTPase RhoA and Rho-associated protein kinase to cause regeneration failure.3,12,13,22,34) The infiltrated fibroblast also acts as a chemical barrier for axonal regeneration by depositing inhibitory extracellular matrix molecules.1,10) Prolonged apoptosis of oligodendrocytes progresses to chronic demyelination, eventually leading to fiber with disrupted myelin sheath called Wallerian degeneration.24,49)
FIGURE 1. Phases and subdivisions of spinal cord injury.
FIGURE 2. Pathophysiology of spinal cord injury.
FIGURE 3. A double-edged sword for secondary injury.
UPDATE OF STEROID MANAGEMENT FOR ACUTE SCI
Many studies have been conducted to prevent or reduce the effects of secondary injury, and among them, research on steroids with neuroprotective effects have been discussed for a long time. Steroids are the only drugs that have been evaluated in Phase III trials. The use of corticosteroids in patients with acute SCI began in the 1960s with the idea that corticosteroids with anti-inflammatory properties would also reduce the spinal cord edema based on the experience of using steroids for brain swelling.18) Although animal studies cannot absolutely explain the beneficial effects of steroid use, the results of many animal studies support the positive effects of steroid use in acute SCI.18,19) Corticosteroids are known to have neuroprotective effects, including improvement of vascular perfusion, prevention of calcium influx and accumulation, modulation of the inflammatory cells, prevention of the loss of spinal cord neurofilament proteins facilitate neuronal excitability and impulse conduction, and inhibition of lipid peroxidation and inflammatory cytokines. Among them, inhibition of lipid peroxidation has the strongest neuroprotective feature. Among these glucocorticoids, methylprednisolone sodium succinate (MPSS) is particularly effective for neuroprotection than other glucocorticoids, so MPSS is currently mainly used.9) The best-known large prospective randomized multicenter clinical trials for evaluating the effects of corticosteroids in SCI are the National Acute Spinal Cord Injury Studies (NASCIS). This was implemented up to NASCIS I, II, and II. A summary of the NASCIS trial is as follows. NASCIS I, published in 1984, compared the effects of low-dose (100-mg bolus and 100 mg/daily) and high-dose (1,000-mg bolus and 1,000 mg/daily) MPSS in 330 patients within 48 hours after SCI, and there was no difference in neurological improvement. Rather, complications such as wound infection and sepsis occurred more in patients using high-dose MPSS.5) However, since not using corticosteroids for SCI patients in actual clinical practice could be ethically problematic, a placebo that can compare the MPSS and natural history of spinal cord recovery was not used in this study. In animal experiments, the 1,000-mg dose was a low dose to achieve effective neuroprotection, and it was suggested that an initial dose of 30 to 40 mg/kg followed by intravenous maintenance was more appropriate.8,23) Therefore, in NASCIS II, MPSS (initial bolus of 30 mg/kg followed by a 23-hour infusion of 5.4 mg/kg per hour), naloxone (opioid receptor antagonist), or placebo were randomly used to 487 patients within 12 hours after SCI and compared with each other. There was no difference in neurological effect between groups, but motor and sensory recovery were significantly improved in both complete and incomplete patients treated with MPSS within 8 hours after SCI. In the group using MPSS, the risk of wound infection and pulmonary embolism increased, so there were concerns about the risk of complications after using steroid.6) NASCIS II was the first clinical study to show the effects of pharmacological agents after SCI, and it was a study that became the basis for the use of steroids after SCI worldwide, and contributed to promoting the activation of research on other neuroprotective agents after SCI. In NASCIS III, the using duration of MPSS in 499 patients within 8 hours after SCI was compared, and the effect of tirilazad mesylate was additionally evaluated. Tirilazad mesylate, a member of the 21-aminosteroid family of antioxidant molecules, inhibits lipid peroxidation without activation of glucocorticoid receptors, and was expected to have a positive effect on SCI without complications of steroid.2,27) Patients in the study received 30-mg/kg bolus of methylprednisolone (MP) during the first hour and randomly maintained either 5.4 mg/kg/hour of 23 hour MPSS or 5.4 mg/kg/hour of 47 hour MPSS infusion or maintained for 48 hours at 2.5-mg/kg every 6 hours of Tirilazad mesylate from initial time. Motor and sensory recovery showed similar effects in all 3 group arms when treatment was started within 3 hours after SCI. However, patients who started MPSS within 3 hours after SCI and received 24-hour infusion showed improvement in neurologic function after 1 year and who received MPSS within 3–8 hour followed by a 48-hour infusion also improved.7) In actual clinical practice, the method of maintaining the MPSS for 24 hours after the initial bolus was mainly used because of the low complications of steroid. Many criticisms have been raised about measurement of primary outcome, poor randomization, failure to demonstrate functional improvement, manipulation of data, and interpretation and conclusion of data in the NASCIS series. Although the increase in the incidence of complications after steroid use in this series was not statistically significant, it was found that the wound infection rate, pulmonary embolism, sepsis, and even secondary death due to respiratory complications after MPSS use were higher. A question arose as to whether there was an overall benefit to using steroid for SCI. Nevertheless, NASCIS II and III have established the administration of MPSS as the standard clinical practice for acute SCI worldwide. In the early 2000s, papers on the occurrence of complications of high-dose steroids after SCI were continuously reported.26,28,33,38) As the evidence for serious side effects has been accumulating, a gradual change in the practice of steroid for SCI in actual clinical practice has become inevitable. In a survey conducted by spine surgeons in Canada, British, Switzerland, and Germany, it was reported that the rate of using of steroid for SCI decreased from 70%–80% to 20%–30%.17,25,51) Also, in a survey conducted on Cervical Spine Research Society members in 2014, the usage rate of MP for SCI decreased significantly compared to the past, reaching about 50%.46) Therefore, the need for recommendation of professional organization for the use of MP in SCI for clinical practice has emerged. In 2013, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guideline on the use of MP for SCI was published as a level 1 recommendation, and unlike the guidelines recommended by the same joint committee in 2002, the use of MP for SCI was not recommended. Afterwards, the European spine society, which includes the British National Institute for Health and Care Excellence guidelines and the guidelines of The Polish Society of Spinal Surgery, also did not recommend the use of steroids for SCI.35,36,39) Despite the recommendations of professional organizations that do not use steroid for SCI, in 2017, AOSpine practice guideline suggested that 24-hour infusion of high-dose MPSS should be provided in patients with SCI within 8 hours as a treatment option without presenting any new evidence.21) However, a meta-analysis including 3 randomized controlled trials and 13 observation studies did not show the effectiveness of high-dose MPSS in patients with SCI within 8 hours of injury.32) Also, the latest meta-analysis published in 2020 showed the same results.47) Globally, the prescription rate of MPSS has decreased from 76% to 24% in Canada, from 68% to 19% in the UK, and from 73% to 27% in Poland.25,36,51) In South Korea, the prescription rate of MPSS in SCI patients for the past 11 years was 59%, and it was the highest at 76% in 2012, and then gradually decreased to 41% in 2017.14) Most prevalent rationale for using steroids in SCI patients is because of the belief that it will work and medical litigation.36) Compared to the North America or Europe, South Korea still has a high proportion of steroid prescriptions for SCI. Therefore, it is necessary to educate and inform the spine surgeon that steroid use is not effective in SCI patients, and that there is no objective rationale for steroid use even for patients within 8 hours after injury.
CONCLUSION
In 2017, AOSpine practice guideline suggested that 24-hour infusion of high-dose MPSS should be provided in patients with SCI within 8 hours as a treatment option without presenting any new evidence. However, subsequent meta-analysis studies consistently show that high dose MPSS use in SCI patients has no effect on neurological improvement. However, South Korea still has high prescription rate of MPSS for SCI compare to other countries. Therefore, it is necessary to fully understand the effects and side effects of steroid use and to apply them appropriately in clinical practice.
Footnotes
Conflict of Interest: Je Hoon Jeong serves as an Editor-in-Chief of the Korean Journal of Neurotrauma, but has no role in the decision to publish this article.
Except for that, no potential conflict of interest relevant to this article was reported.
References
- 1.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–SS22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DK, Braughler JM, Hall ED, Waters TR, McCall JM, Means ED. Effects of treatment with U-74006F on neurological outcome following experimental spinal cord injury. J Neurosurg. 1988;69:562–567. doi: 10.3171/jns.1988.69.4.0562. [DOI] [PubMed] [Google Scholar]
- 3.Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine. 2018;30:1–18. doi: 10.3171/2018.9.SPINE18682. [DOI] [PubMed] [Google Scholar]
- 4.Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience. 2002;115:839–849. doi: 10.1016/s0306-4522(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 5.Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985;63:704–713. doi: 10.3171/jns.1985.63.5.0704. [DOI] [PubMed] [Google Scholar]
- 6.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 7.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 8.Braughler JM, Hall ED. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+ + K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J Neurosurg. 1982;56:838–844. doi: 10.3171/jns.1982.56.6.0838. [DOI] [PubMed] [Google Scholar]
- 9.Braughler JM. Lipid peroxidation-induced inhibition of gamma-aminobutyric acid uptake in rat brain synaptosomes: protection by glucocorticoids. J Neurochem. 1985;44:1282–1288. doi: 10.1111/j.1471-4159.1985.tb08755.x. [DOI] [PubMed] [Google Scholar]
- 10.Brazda N, Müller HW. Pharmacological modification of the extracellular matrix to promote regeneration of the injured brain and spinal cord. Prog Brain Res. 2009;175:269–281. doi: 10.1016/S0079-6123(09)17518-0. [DOI] [PubMed] [Google Scholar]
- 11.Budh CN, Osteråker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007;21:89–96. doi: 10.1177/0269215506070313. [DOI] [PubMed] [Google Scholar]
- 12.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Sung CH, Heo DR, Jeong SY, Kang CN. Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: a national population-based study in South Korea. Spinal Cord. 2020;58:232–237. doi: 10.1038/s41393-019-0357-2. [DOI] [PubMed] [Google Scholar]
- 15.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druschel C, Schaser KD, Schwab JM. Current practice of methylprednisolone administration for acute spinal cord injury in Germany: a national survey. Spine (Phila Pa 1976) 2013;38:E669–E677. doi: 10.1097/BRS.0b013e31828e4dce. [DOI] [PubMed] [Google Scholar]
- 18.Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30:693–697. doi: 10.3171/jns.1969.30.6.0693. [DOI] [PubMed] [Google Scholar]
- 19.Faden AI. Therapeutic approaches to spinal cord injury. Adv Neurol. 1997;72:377–386. [PubMed] [Google Scholar]
- 20.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Fehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: Recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7:203S–211S. doi: 10.1177/2192568217703085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forgione N, Fehlings MG. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82:e535–e539. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol. 1982;18:320–327. doi: 10.1016/0090-3019(82)90140-9. [DOI] [PubMed] [Google Scholar]
- 24.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 25.Hurlbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41–45. doi: 10.1017/s031716710000754x. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine (Phila Pa 1976) 2009;34:2121–2124. doi: 10.1097/BRS.0b013e3181b613c7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen EJ, McCall JM, Ayer DE, Van Doornik FJ, Palmer JR, Belonga KL, et al. Novel 21-aminosteroids that inhibit iron-dependent lipid peroxidation and protect against central nervous system trauma. J Med Chem. 1990;33:1145–1151. doi: 10.1021/jm00166a010. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC, Cho DY, Lee WY, Chuang HC. Pitfalls in treatment of acute cervical spinal cord injury using high-dose methylprednisolone: a retrospect audit of 111 patients. Surg Neurol. 2007;68(Suppl 1):S37–S41. doi: 10.1016/j.surneu.2007.06.085. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Wu W, Li H, Li S, Huang LT, Yang YQ, et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J Spinal Cord Med. 2015;38:745–753. doi: 10.1179/2045772314Y.0000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Yang Y, He L, Pang M, Luo C, Liu B, et al. High-dose methylprednisolone for acute traumatic spinal cord injury: a meta-analysis. Neurology. 2019;93:e841–e850. doi: 10.1212/WNL.0000000000007998. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976) 2001;26:426–430. doi: 10.1097/00007632-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 34.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miękisiak G, Kloc W, Janusz W, Kaczmarczyk J, Łątka D, Zarzycki D. The use of methylprednisolone in the acute phase of spinal cord injury. The official position of the Polish Society of Spinal Surgery. J Spine Surg. 2013;1:11–24. [Google Scholar]
- 36.Miękisiak G, Łątka D, Jarmużek P, Załuski R, Urbański W, Janusz W. Steroids in acute spinal cord injury: all but gone within 5 years. World Neurosurg. 2019;122:e467–e471. doi: 10.1016/j.wneu.2018.09.239. [DOI] [PubMed] [Google Scholar]
- 37.Milhorat TH, Capocelli AL, Jr, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 38.Molano MR, Broton JG, Bean JA, Calancie B. Complications associated with the prophylactic use of methylprednisolone during surgical stabilization after spinal cord injury. J Neurosurg. 2002;96:267–272. doi: 10.3171/spi.2002.96.3.0267. [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence. Spinal injury: assessment and initial management. London: National Institute for Health and Care Excellence; 2016. [PubMed] [Google Scholar]
- 40.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 41.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 42.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 43.Rabinstein AA. Traumatic spinal cord injury. Continuum (Minneap Minn) 2018;24:551–566. doi: 10.1212/CON.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 44.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 45.Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder GD, Kwon BK, Eck JC, Savage JW, Hsu WK, Patel AA. Survey of Cervical Spine Research Society members on the use of high-dose steroids for acute spinal cord injuries. Spine (Phila Pa 1976) 2014;39:971–977. doi: 10.1097/BRS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 47.Sultan I, Lamba N, Liew A, Doung P, Tewarie I, Amamoo JJ, et al. The safety and efficacy of steroid treatment for acute spinal cord injury: a systematic review and meta-analysis. Heliyon. 2020;6:e03414. doi: 10.1016/j.heliyon.2020.e03414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16:1127–1129. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- 49.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377:125–151. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Werndle MC, Zoumprouli A, Sedgwick P, Papadopoulos MC. Variability in the treatment of acute spinal cord injury in the United Kingdom: results of a national survey. J Neurotrauma. 2012;29:880–888. doi: 10.1089/neu.2011.2038. [DOI] [PubMed] [Google Scholar]
- 52.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]