Abstract
Purpose
This study evaluated the efficacy of treating periodontitis using subgingival nano-hydroxyapatite powder with an air abrasion device (NHAPA) combined with scaling and root planing (SRP).
Methods
A total of 28 patients with stage III periodontitis (grade B) were included in this study, although 1 was lost during follow-up and 3 used antibiotics. The patients were divided into a test group and a control group. All patients first received whole-mouth SRP using hand instruments, and a split-mouth approach was used for the second treatment. In the test group, the teeth were treated with NHAPA for 15 seconds at 70% power per pocket. Subgingival plaque samples were obtained from the 2 deepest pockets at the test and control sites before treatment (baseline) and 3 months after treatment. The full-mouth plaque index (PI), gingival index (GI), papillary bleeding index (PBI), bleeding on probing (BOP), probing depth (PD) and clinical attachment level (CAL) were recorded at baseline and at 1- and 3-month post-treatment. Real-time polymerase chain reaction was used to determine the colonisation of Treponema denticola (Td), Porphyromonas gingivalis (Pg), and Aggregatibacter actinomycetemcomitans in the subgingival plaque.
Results
From baseline to the first month, the test group showed significantly larger changes in BOP and CAL (43.705%±27.495% and 1.160±0.747 mm, respectively) than the control group (36.311%±27.599% and 0.947±0.635 mm, respectively). Periodontal parameters had improved in both groups at 3 months. The reductions of PI, GI, BOP, PD, and CAL in the test group at 3 months were greater and statistically significant. The total bacterial count and Td and Pg species had decreased significantly by the third month in both groups (P<0.05).
Conclusions
Applying NHAPA in addition to SRP improves clinical periodontal parameters more than SRP alone. Subgingival NHAPA may encourage clot adhesion to tooth surfaces by increasing surface wettability.
Keywords: Anaerobic bacteria, Hydroxyapatites, Periodontal debridement, Periodontitis, Real-time polymerase chain reaction
Graphical Abstract
INTRODUCTION
Periodontitis is a chronic inflammatory condition caused by microbial dental plaque; in some cases it is complicated by attachment loss in alveolar supporting bone and connective tissue [1]. Microbial dental plaque and calculus must be removed at the initial stage of periodontal therapy, as well as during periodontal maintenance care. Scaling and root planing (SRP) procedures, conducted either manually and/or using ultrasonic devices, are currently the gold standard in non-surgical periodontal treatment [2]. However, SRP has certain challenges; specifically, it is difficult to access deep pockets, furcation areas, and root concavities and to remove periodontopathogens, which attack dentinal tubules [3] and enter gingival tissue [4]. Because it is not always possible to manage inflammation effectively using SRP alone, a variety of ancillary therapeutic strategies are used in combination with it. These involve systemic or local antibiotics, antiseptics [5], lasers [6], air abrasion devices [7,8] and photodynamic therapy [9]. These approaches may improve the efficacy of conservative periodontal treatment and reduce the need for periodontal surgery.
Air abrasion or air polishing devices are utilised to clean both teeth and their root surfaces by applying compressed air containing abrasive particles and water. Such devices are effective and suitable means of mechanically removing microbial dental plaque and calculus [10,11]. Flemmig et al. [12] suggested that using an air polishing device to treat moderate to deep periodontitis was more effective than SRP in eradicating subgingival biofilms. However, Wennström et al. [11] found no significant differences between SRP and glycine powder used with air abrasion devices in terms of clinical or microbiological outcomes throughout maintenance treatment. Various powders, such as calcium carbonate, erythritol, glycine, and trehalose, have been used subgingivally with air polishing devices. However, it has not been determined which powder is most effective for clinical and microbiological results [13]; therefore, new and improved procedures are needed.
In recent years, hydroxyapatite (HA) has been found to have many applications in the field of health care, including bone growth stimulation, promoting remineralisation, slow degradation, biocompatibility, and strength. Furthermore, the size of HA nanoparticles makes HA the most abundant mineral in bones and teeth [14,15].
An in vitro study found that HA, which can be used as a bioactive and natural surface cleaning powder, removed calculus and various stains on cement surfaces very effectively without damaging them. A qualitative analysis of cement images taken after air polishing with HA revealed that the cement surface was fully saturated with calcium and phosphorus [16].
The literature indicates that, to date, no study has yet investigated the effect of the use of nano-HA powder with an air abrasion device (NHAPA) as an adjunct to SRP. We aimed to assess the clinical and microbiological effects of nano-HA on wound healing after SRP. Using NHAPA in addition to SRP may increase the wettability of the cement surface, improve the efficacy of periodontitis treatment, and prepare a better environment for new attachment.
MATERIALS AND METHODS
Study design, ethical approval, and patient population
This prospective randomised controlled clinical trial complied with the principles of the Helsinki Declaration, as revised in 2008. Approval from the Ethics Committee of Bolu Abant Izzet Baysal University was obtained before the study (date/number: 2018/268). All subjects signed an informed written consent form prior to being enrolled in the study.
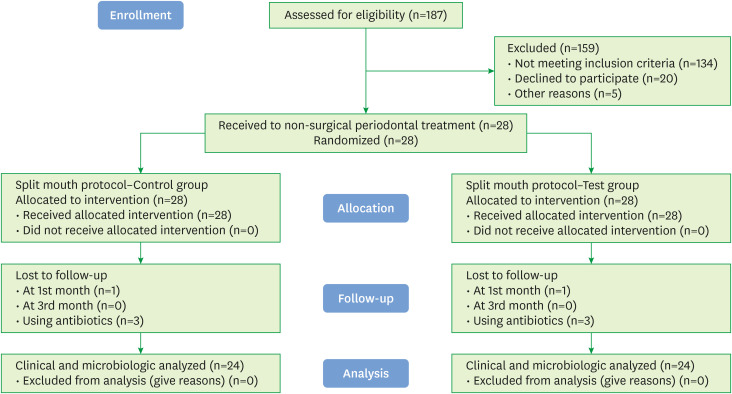
Patients who were referred to the Department of Periodontology of the Faculty of Dentistry of our university between January 2019 and September 2019 were recruited. All participants provided written informed consent. The sample size was estimated based on previous publications [17] with a power of 95%; α=0.05; and an effect size for probing depth (PD)=0.5±0.7 mm. The minimum number of participants required for comparison was 19 for every group between 2 periods (G*Power version 3.1.9.2; Heinrich Heine University, Dusseldorf, Germany). Nevertheless, after considering the possibility of losing participants prior to follow-up, the number of participants included was 28. Four participants were later excluded, so the study was completed with 24 participants (14 men and 10 women). The Consolidated Standards of Reporting Trials flowchart is shown in Figure 1.
Figure 1. Flow chart of the study.
Determination of periodontal status and eligibility criteria
According to the new classification, stage III periodontitis is indicated by a clinical attachment level ≥5 mm, a radiographical middle triad of the root and bone loss up to the apical, loss of teeth due to periodontitis ≤4 mm, PD ≥6 mm, vertical bone loss ≥3 mm and class II or III furcation. The inclusion criteria for this study were as follows: 1) stage III periodontitis (grade B), good general health, and being between the ages of 18 and 60; 2) ≥20 natural teeth in the whole mouth (except the third molar); and 3) having a minimum of 2 symmetrical teeth, PD ≥6 mm and loss of mandibular or maxillary bone (radiologically verified). The exclusion criteria were as follows: 1) smoking; 2) comorbidities that may influence the progression of periodontitis; 3) being pregnant or lactating; 4) use of systemic antibiotics in the preceding 6 months and/or use of anti-inflammatory drugs in the preceding 3 months; 5) having received periodontal treatment in the preceding year; and 6) extensive prosthetic involvement.
Subgingival plaque sampling
Subgingival plaque samples were obtained from test and control sites before periodontal treatment (baseline) and at the third month post-treatment. The 2 deepest pockets were chosen for microbiological sampling. After supragingival plaque was removed, subgingival plaque samples were obtained using sterile Gracey curettes (Hu-Friedy Group, Chicago, IL, USA) and transferred to 1.5-mL Eppendorf tubes. The samples were maintained at −80°C until analysis.
Clinical parameters
For each patient, the full-mouth plaque index (PI) [18], gingival index (GI) [19], papillary bleeding index (PBI) [20], bleeding on probing (BOP), PD, and clinical attachment level (CAL) were recorded at baseline (BL) and at the first and third months after treatment. PI, GI and BOP were measured at 4 sites for every tooth, while PD and CAL were measured at 6 sites for each tooth (mesiobuccal, mid-buccal, distobuccal, mesio-lingual, mid-lingual and disto-lingual). PD was measured as the distance between the bottom of a pocket and the gingival margin, while CAL was recorded as the distance between the bottom of the pocket to the cemento-enamel junction. All measurements were taken by a single calibrated examiner (Ö.U.) using a Williams probe (Hu-Friedy Group). Supragingival calculus was removed from all teeth using a Piezon Instrument A (EMS, Nyon, Switzerland). All clinical crowns were polished with a polishing brush and Prophy paste (Sultan Healthcare, York, PA, USA). Patients were educated about dental care and brushing techniques. For each patient, an appointment for SRP was set 7–10 days following cleaning.
Five periodontitis patients were randomly selected for intra-examiner calibration. Full-mouth PI, GI, PBI, BOP, PD and CAL were measured twice, 24 hours apart, before the study. The intraclass correlation coefficients were as follows: PI, 0.934; GI, 0.997; PBI, 0.969; BOP, 0.863; PD, 0.867; and CAL, 0.882.
Periodontal treatment and intervention
The periodontal pockets and root surfaces in both groups were treated with SRP using Gracey curettes until the operator confirmed smoothness. Flattening of the root surface and isotonic saline irrigation (5 mL/tooth) were then performed on all periodontal pockets. When necessary, the treatment was conducted under local anaesthesia (Maxicaine Forte; Vem Ilac, Istanbul, Turkey).
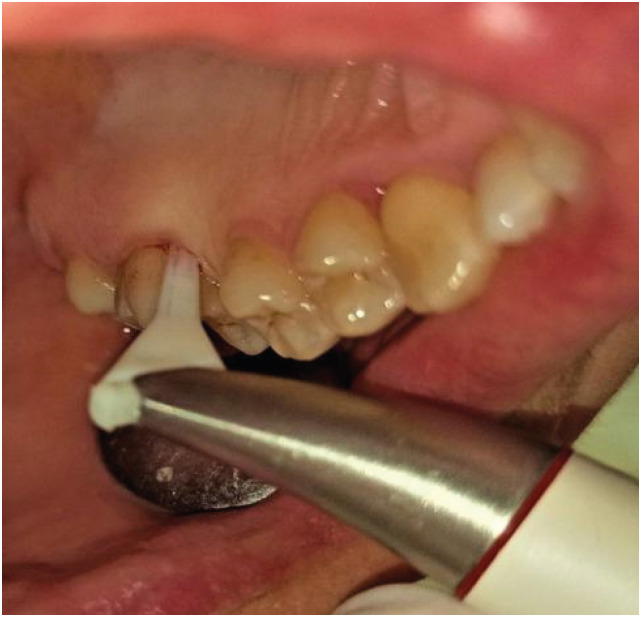
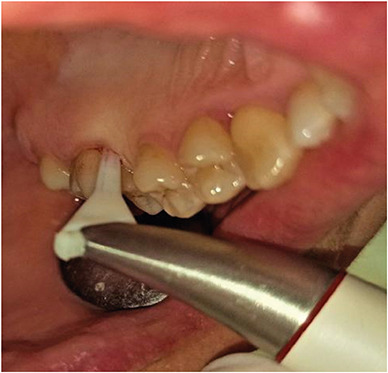
In the test group, nano-HA powder was administered to the root surface using a Perio-Flow System (EMS, Geneva, Switzerland) for 15 seconds, at 70% power per pocket. A nozzle tip specially designed for the gingival sulcus was embedded in the periodontal pocket, as instructed by the manufacturer (Figure 2). After this procedure, the nano-HA powder spray was applied to the root surface. The particle size of the powder was approximately 40 nm and its calcium content was 34%–40% (Alfa Aesar No. 36731). All therapeutic interventions were carried out by a periodontist experienced with the use of air abrasion devices. The SRP was completed by polishing the clinical crowns with a polishing brush and Prophy paste. Patients whose oral hygiene was found to be inadequate were again instructed in oral hygiene practices.
Figure 2. Application of nano-hydroxyapatite powder with a specially designed nozzle tip of the air polishing system into the periodontal pocket.
Microbiological analysis
Treponema denticola (Td), Porphyromonas gingivalis (Pg), and Aggregatibacter actinomycetemcomitans (Aa) are oral pathogens linked to dental caries and periodontitis. Our purpose was to determine the colonisation of these 3 microorganisms on the subgingival plaque using quantitative real-time polymerase chain reaction (PCR) assays. Until DNA extraction, each sample was maintained at −80°C. DNA was then extracted using a Kurabo Tissue kit (Quick Gene DNA; Kurabo, Osaka, Japan) and extraction machine (Quick Gene-mini 80; Kurabo). All PCR assays were performed using NZYtech (Lisbon, Portugal) Td (Cat No.: MD02971), Pg (Cat No.: MD02421), and Aa (Cat No.: MD00011) bacteria kits and a rotor-Gene Q (Qiagen, Hilden, Germany) device. The amount of fluorescent signal (threshold cycle) given by the bacteria was calculated for each cycle. In evaluating the quantitative results, positive controls prepared according to the manufacturer's instructions were used. The positive control values were entered in the relevant section in the Rotor-Gene Q Series software 2.0.2 and the copy quantity (copies/µL) in the microliter corresponding to the threshold cycle for each sample was calculated automatically.
Randomisation
After the whole-mouth SRP procedure, the patients' jaws were treated as separate parts, according to the split-mouth protocol. The closed envelope technique was used for randomisation. To determine which side of the jaw to apply NHAPA to, each patient selected a closed envelope. Fourteen of the envelopes enclosed a note saying ‘left’ and 14 a note saying ‘right’; each patient received treatment on the side of the jaw indicated by the envelope they chose. To avoid the Hawthorne effect, the same operation was applied to the control area without operation of the device.
The following treatments were performed on the 4 groups of 7 patients each: group 1 received SRP + NHAPA + saline on the right side of the jaw and SRP + saline on the left side of the jaw; group 2 received SRP + NHAPA + saline on the left side of the jaw and SRP + saline on the right side of the jaw; group 3 received SRP + NHAPA + saline on the right side of the mandible and SRP + saline on the left side of the mandible; and group 4 received SRP + NHAPA + saline on the left side of the mandible and SRP + saline on the right side of the mandible.
Statistical analysis
The data were analysed using SPSS version 23.0 (IBM, Armonk, NY, USA). The Shapiro–Wilk test was used to evaluate whether the measured data showed a normal distribution. The paired t-test was used for intergroup comparisons and repeated measures. Analysis of variance was utilised for intra-group comparisons of variables with a normal distribution. The Dunn-Šidák correction was performed to identify time points with significant differences. Microbiological analyses were evaluated using non-parametric tests. The Wilcoxon signed-rank test was used to analyse the microbiological data. The relationships between bacterial colonisation and alterations in clinical parameters were determined using Spearman rank correlation analysis. A P value <0.05 was considered to indicate statistical significance.
RESULTS
Although 28 patients were initially included, 1 patient was lost prior to the first-month follow-up and 3 patients had to be excluded because they required antibiotics. Therefore, the study was completed with a total of 24 patients (14 men, 10 women) with a mean age of 39.79±7.988 years (range: 23–53 years). The number of teeth in this series was 26.46±1.50 (range: 23–28).
Clinical results
The number of teeth with a PD ≥5 mm was 738, and 238 were included in the test group. In the whole mouth, there were significant decreases in PI, GI, PBI, BOP, PD, and CAL values between BL and the first month, as well as between the first and third months (data not shown). The intragroup comparison revealed significant decreases in the PI, GI, PBI, BOP, PD, and CAL values at the third month compared to BL in both groups (Table 1).
Table 1. Clinical parameters at baseline, 1 month, and 3 months after treatment.
| Parameter | SRP+NHAPA (n=24) | SRP (n=24) | ||||
|---|---|---|---|---|---|---|
| BL | 1M | 3M | BL | 1M | 3M | |
| PI (index) | 2.072±0.642 | 1.113±0.576a) | 0.761±0.479a)b) | 2.06±0.667 | 1.119±0.479a) | 0.791±0.48a)b) |
| Δ‡ | 0.959±0.680 | 1.311±0.570c) | 0.940±0.702 | 1.269±0.611c) | ||
| GI (index) | 2.018±0.498 | 1.191±0.48a) | 0.91±0.423a)c) | 2.01±0.457 | 1.214±0.479a) | 1.002±0.407a)c) |
| Δ‡ | 0.827±0.687 | 1.109±0.583c) | 0.796±0.660 | 1.008±0.595c) | ||
| PBI (index) | 1.612±0.901 | 0.504±0.573a)c) | 0.23±0.402a)b) | 1.648±0.862 | 0.64±0.694a)c) | 0.247±0.425a)b) |
| Δ‡ | 1.107±0.819 | 1.382±0.797 | 1.008±0.859 | 1.401±0.794 | ||
| BOP (%) | 81.409±20.562 | 37.704±22.081a)c) | 28.22±18.052a)c) | 81.136±20.33 | 44.825±21.29a)c) | 33.931±19.504a)b)c) |
| Δ‡ | 43.705±27.495c) | 53.189±23.577c) | 36.311±27.599c) | 47.205±25.432c) | ||
| PD (mm) | 3.458±1.052 | 2.262±0.686a) | 1.932±0.671a)b)c) | 3.361±0.96 | 2.348±0.726a) | 2.048±0.642a)b)c) |
| Δ‡ | 1.196±0.709 | 1.527±0.605c) | 1.013±0.600 | 1.313±0.602c) | ||
| CAL (mm) | 3.673±1.126 | 2.513±0.845a)c) | 2.176±0.868a)b)c) | 3.561±1.073 | 2.614±0.89a)c) | 2.303±0.778a)b)c) |
| Δ‡ | 1.160±0.747c) | 1.497±0.710c) | 0.947±0.635c) | 1.258±0.769c) | ||
Values are given as mean±standard deviation.
PI: plaque index, GI: gingival index, PBI: papillary bleeding index, BOP: bleeding on probing, PD: probing depth, CAL: clinical attachment level, SRP: scaling and root planning, NHAPA: nano-hydroxyapatite powder with an air abrasion device, 1M: 1-month check, 3M: 3-month check; Δ‡: changes from baseline.
a)Significant difference from baseline, P<0.05; b)Significant difference from 1 month, P<0.05; c)Significant difference between the test and control groups, P<0.05.
Comparisons of the PI, GI, PBI, BOP, PD, and CAL mean scores between the test and control groups at BL and at the first and third months are shown in Table 1. No statistically significant differences were detected between the test and control groups for all clinical variables at BL.
When we compared the time-dependent changes (hereafter denoted as Δ) in clinical parameters between groups, the mean ΔBOP and ΔCAL values in the test group between BL and the first month were statistically significantly higher than those of the control group (P<0.005). The mean ΔPI, ΔGI, ΔBOP, ΔPD, and ΔCAL values in the test group between BL and the third month showed statistically significant differences compared to those of the control group (P<0.005). There were no statistically significant differences in the mean ΔPBI values between the 2 groups (Table 1).
The mean ΔPD and ΔCAL scores of the subgingival plaque samples taken from the teeth with PD ≥6 mm between BL and the third month were significantly higher in the test group. There were no noteworthy differences in the ΔPI, ΔGI, and ΔBOP values between the 2 groups between BL and the third month (Table 2).
Table 2. ΔPI, ΔGI, ΔBOP, ΔPD, and ΔCAL of teeth from which subgingival plaque samples were obtained in the study groups.
| Changes (BL-3M) | SRP+NHAPA (n=48) | SRP (n=48) | P values |
|---|---|---|---|
| ∆PI (index) | 1.240±0.649 | 1.198±0.630 | 0.336 |
| ∆GI (index) | 1.170±0.639 | 1.097±0.812 | 0.478 |
| ∆BOP (%) | 54.167±44.027 | 52.083±40.322 | 0.818 |
| ∆PD (mm) | 2.568±0.656 | 2.106±0.838 | 0.028a) |
| ∆CAL (mm) | 2.558±0.825 | 2.107±0.822 | 0.047a) |
Values are given as mean±standard deviation.
Δ: changes from baseline, PI: plaque index, GI: gingival index, PBI: papillary bleeding index, BOP: bleeding on probing, PD: probing depth, CAL: clinical attachment level, SRP: scaling and root planning, NHAPA: nano-hydroxyapatite powder with an air abrasion device, 1M: 1-month check, 3M: 3-month check.
a)Significant difference from baseline, P<0.05.
Microbiological results
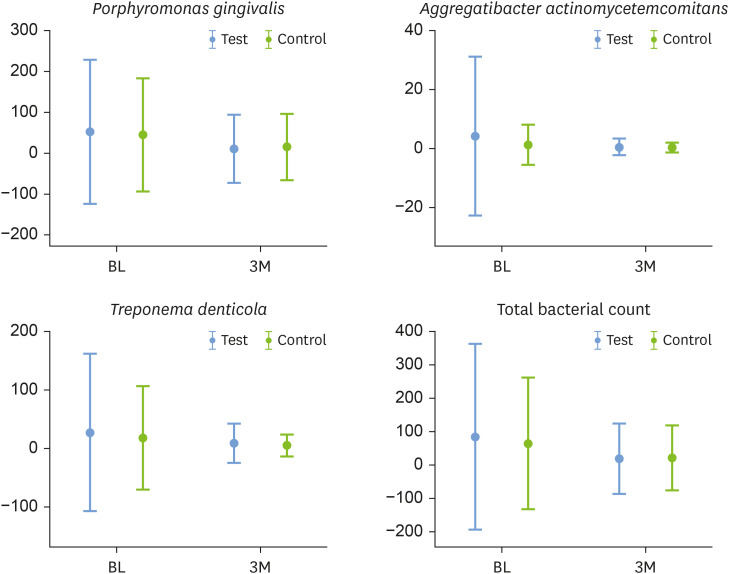
The total bacterial count (TBC) and counts of the Td and Pg species decreased significantly between BL and the third month in both the test and control groups (P<0.05). However, the quantity of the Aa species was not significantly different at the third month compared to BL in either group. There were no significant differences in TBC, Td, Pg, or Aa species between the test and control groups at BL and the third month (Figure 3).
Figure 3. Total bacterial count and relative levels of Treponema denticola, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans at baseline and 3 months after treatment, compared between the test and control groups.
No meaningful correlations were found between the changes in clinical parameters and the counts of Td, Pg, or Aa of the teeth from which subgingival plaque samples were gathered at BL and at the third month (Table 3).
Table 3. Correlation between changes of clinical periodontal parameters and counts of the periodontopathogens.
| Variables | SRP+NHAPA | SRP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ∆TBC | ∆Td | ∆Pg | ∆Aa | ∆TBC | ∆Td | ∆Pg | ∆Aa | ||
| ∆PI (index) | |||||||||
| r | 0.139 | 0.210 | −0.122 | −0.121 | −0.167 | −0.209 | −0.154 | −0.054 | |
| P | 0.518 | 0.326 | 0.571 | 0.573 | 0.437 | 0.326 | 0.472 | 0.803 | |
| ∆GI (index) | |||||||||
| r | 0.332 | 0.365 | 0.039 | 0.125 | 0.251 | 0.174 | 0.053 | −0.021 | |
| P | 0.113 | 0.079 | 0.858 | 0.560 | 0.237 | 0.417 | 0.805 | 0.923 | |
| ∆BOP (%) | |||||||||
| r | 0.181 | 0.287 | 0.034 | 0.277 | 0.301 | 0.329 | 0.129 | 0.205 | |
| P | 0.396 | 0.174 | 0.873 | 0.190 | 0.154 | 0.116 | 0.547 | 0.335 | |
| ∆PD (mm) | |||||||||
| r | −0.262 | 0.075 | −0.201 | −0.067 | 0.061 | 0.101 | −0.186 | 0.023 | |
| P | 0.216 | 0.726 | 0.346 | 0.757 | 0.776 | 0.640 | 0.385 | 0.914 | |
| ∆CAL (mm) | |||||||||
| r | −0.185 | 0.050 | 0.004 | −0.005 | 0.030 | 0.150 | −0.290 | −0.154 | |
| P | 0.388 | 0.818 | 0.985 | 0.980 | 0.889 | 0.485 | 0.169 | 0.471 | |
PI: plaque index, GI: gingival index, PBI: papillary bleeding index, BOP: bleeding on probing, PD: probing depth, CAL: clinical attachment level, SRP: scaling and root planning, NHAPA: nano-hydroxyapatite powder with an air abrasion device, Δ: Change between baseline and third month, r: correlation coefficient, TBC: total bacterial count, Aa: Aggregatibacter actinomycetemcomitans, Pg: Porphyromonas gingivalis, Td: Treponema denticola.
DISCUSSION
Several studies have shown the technical limitations of conventional mechanical debridement for controlling subgingival dental biofilms. New methods are needed to increase the efficiency of non-surgical periodontal treatment. To our knowledge, this is the first trial designed to assess the clinical and microbiological effects of NHAPA application and SRP in periodontitis patients [21,22].
We found significant decreases in clinical parameters in both groups at the first and third months compared to BL. Similarly, studies have shown that both using glycine powder in addition to SRP and SRP alone significantly reduced clinical parameters compared to BL [23,24]. One study found that the use of erythritol powder in addition to SRP improved clinical parameters at the first month compared to BL; however, the results at 3 months after treatment were found to be worse than those at the first month after treatment [17]. These results may be associated with patients losing the motivation to maintain oral hygiene. In our study, the changes in GI and PBI between BL and at the third month, as well as the changes in BOP, PD and CAL between BL and at the first month, were significantly greater in the group treated with air abrasion devices, including nano-HA and SRP, than in the group that received SRP alone. In contrast, Caygur et al. [23] and Tsang et al. [24] found no noteworthy differences in the clinical parameters between their test and control groups after the use of glycine powder in addition to SRP treatment. These variations may be attributed to the type of powder used. The timing of treatment and the power setting used are also important factors. In the current study, we utilised a powder composed of ingredients similar to those of cement and bone to provide a suitable surface for wound healing and clot adhesion on the root surface. In previous studies, nano-HA has been used as a clot-blended graft material and as a powder with air abrasion device in the peri-implantitis decontamination method, and was found to improve clot adhesion to titanium implant surfaces and increase surface wettability [25,26].
In both groups, TBC and the numbers of Td and Pg decreased significantly between BL and the third month after treatment. Hägi et al. [27] compared the microbiological outcomes of SRP and EPAP treatment modalities for up to 6 months: In both the treatment and control groups, they noted only mild alterations in microbiological composition after treatment and no remarkable alterations in Td, Pg, or Aa. The microbiological results of these tests, which used the same treatment methods with different powders, indicate that powders do not strengthen the effect of SRP on bacteria.
The microbiological results of our study indicated that the number of Aa did not diminish significantly in either group between BL and the third month after treatment. These results confirm the findings of Darby et al. [28], who noted that the reduction in Aa levels after SRP was less than that of other possible periodontopathogens. The lack of any noteworthy differences in the number of Aa may be due to a failure to eliminate Aa from deep pockets due to its deep invasion into the gums and migration from other regions of the mouth.
In addition to SRP, nano-HA application with an air abrasion device may produce greater improvement in clinical periodontal parameters. In our study, the mean ΔPD and ΔCAL scores of subgingival plaque samples taken from teeth with deep pockets between BL and 3 months were significantly higher in the test group. We think that the positive effect of our treatment on clinical parameters may have resulted from modification of the cement surface, as shown in in vitro studies [16]; it may also have contributed to periodontal wound healing because it increases the hydrophilicity of the root surface by covering it with nano-HA powder in alveolar bone and cement. However, the effects of applying nano-HA to the dentin surface with an air abrasion device and the extent to which nano-HA treatment influences the healing of periodontal pockets have not yet been clinically studied. Our findings need to be supported by additional in vivo and clinical studies on this subject.
The present study is subject to certain limitations. Only 3 predominant periodontal pathogens were evaluated, and the follow-up period was short. Therefore, further trials with larger groups of patients and longer follow-up periods are warranted to determine the advantages and disadvantages of NHAPA treatment for the clinical, inflammatory and microbiological outcomes of periodontal disease.
To conclude, our results indicate that the treatment modality analysed herein can be a safe and effective means of increasing the effectiveness of SRP in stage III periodontitis. Subgingival nano-HA powder applied with an air abrasion device can improve clot adhesion to the tooth surface and increase surface wettability. These features of nano-HA application may increase clinical attachment, thus reducing the need for surgical periodontal treatment in selected cases.
Footnotes
Funding: This study was supported by Bolu Abant Izzet Baysal University Scientific Research Center (Project No. 2020.06.05.1443).
- Conceptualization: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet, Mustafa Tunalı.
- Formal analysis: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet.
- Investigation: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet, Mustafa Tunalı, Önder Albayrak.
- Methodology: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet, Mustafa Tunalı, Önder Albayrak.
- Project administration: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet.
- Writing - original draft: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet, Mustafa Tunalı, Önder Albayrak.
- Writing - review & editing: Özge Uysal, Gülbahar Ustaoğlu, Mustafa Behçet, Mustafa Tunalı, Önder Albayrak.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan ME. Nonsurgical approaches for the treatment of periodontal diseases. Dent Clin North Am. 2005;49:611–636. doi: 10.1016/j.cden.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Giuliana G, Ammatuna P, Pizzo G, Capone F, D'Angelo M. Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: microbiological findings. J Clin Periodontol. 1997;24:478–485. doi: 10.1111/j.1600-051x.1997.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 4.Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52:68–83. doi: 10.1111/j.1600-0757.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71:82–112. doi: 10.1111/prd.12121. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson MR, Diogo Löfgren CI, Jansson HM. The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol. 2008;79:2021–2028. doi: 10.1902/jop.2008.080197. [DOI] [PubMed] [Google Scholar]
- 7.Petersilka GJ. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol 2000. 2011;55:124–142. doi: 10.1111/j.1600-0757.2010.00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Petersilka GJ, Steinmann D, Häberlein I, Heinecke A, Flemmig TF. Subgingival plaque removal in buccal and lingual sites using a novel low abrasive air-polishing powder. J Clin Periodontol. 2003;30:328–333. doi: 10.1034/j.1600-051x.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 9.Sgolastra F, Petrucci A, Severino M, Graziani F, Gatto R, Monaco A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2013;40:514–526. doi: 10.1111/jcpe.12094. [DOI] [PubMed] [Google Scholar]
- 10.Moëne R, Décaillet F, Andersen E, Mombelli A. Subgingival plaque removal using a new air-polishing device. J Periodontol. 2010;81:79–88. doi: 10.1902/jop.2009.090394. [DOI] [PubMed] [Google Scholar]
- 11.Wennström JL, Dahlén G, Ramberg P. Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J Clin Periodontol. 2011;38:820–827. doi: 10.1111/j.1600-051X.2011.01751.x. [DOI] [PubMed] [Google Scholar]
- 12.Flemmig TF, Arushanov D, Daubert D, Rothen M, Mueller G, Leroux BG. Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J Periodontol. 2012;83:444–452. doi: 10.1902/jop.2011.110367. [DOI] [PubMed] [Google Scholar]
- 13.John MT, Michalowicz BS, Kotsakis GA, Chu H. Network meta-analysis of studies included in the clinical practice guideline on the nonsurgical treatment of chronic periodontitis. J Clin Periodontol. 2017;44:603–611. doi: 10.1111/jcpe.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksakal B, Demirel M. Synthesis and fabrication of novel cuttlefish (Sepia officinalis) backbone biografts for biomedical applications. Ceram Int. 2015;41:4531–4537. [Google Scholar]
- 15.Kalita SJ, Bhardwaj A, Bhatt HA. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater Sci Eng C. 2007;27:441–449. [Google Scholar]
- 16.Janicki T, Sobczak-Kupiec A, Skomro P, Wzorek Z. Surface of root cementum following air-polishing with bioactive hydroxyapatite (Ca and P mapping). A pilot study. Acta Bioeng Biomech. 2012;14:31–38. [PubMed] [Google Scholar]
- 17.Park EJ, Kwon EY, Kim HJ, Lee JY,, Choi J, Joo JY. Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: a randomized clinical trial. J Periodontal Implant Sci. 2018;48:295–304. doi: 10.5051/jpis.2018.48.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silness J, Löe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 19.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 20.Saxer UP, Mühlemann HR. Motivation and education. SSO Schweiz Monatsschr Zahnheilkd. 1975;85:905–919. [PubMed] [Google Scholar]
- 21.Breininger DR, O'Leary TJ, Blumenshine RV. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J Periodontol. 1987;58:9–18. doi: 10.1902/jop.1987.58.1.9. [DOI] [PubMed] [Google Scholar]
- 22.Wylam JM, Mealey BL, Mills MP, Waldrop TC, Moskowicz DC. The clinical effectiveness of open versus closed scaling and root planing on multi-rooted teeth. J Periodontol. 1993;64:1023–1028. doi: 10.1902/jop.1993.64.11.1023. [DOI] [PubMed] [Google Scholar]
- 23.Caygur A, Albaba MR, Berberoglu A, Yilmaz HG. Efficacy of glycine powder air-polishing combined with scaling and root planing in the treatment of periodontitis and halitosis: a randomised clinical study. J Int Med Res. 2017;45:1168–1174. doi: 10.1177/0300060517705540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang YC, Corbet EF, Jin LJ. Subgingival glycine powder air-polishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J Periodontal Res. 2018;53:440–445. doi: 10.1111/jre.12532. [DOI] [PubMed] [Google Scholar]
- 25.Gamal AY, Abdel-Ghaffar KA, Iacono VJ. A novel approach for enhanced nanoparticle-sized bone substitute adhesion to chemically treated peri-implantitis-affected implant surfaces: an in vitro proof-of-principle study. J Periodontol. 2013;84:239–247. doi: 10.1902/jop.2012.120023. [DOI] [PubMed] [Google Scholar]
- 26.Gümüş KÇ, Ustaoğlu G, Kara L, Ercan E, Albayrak Ö, Tunali M. Nano-hydroxyapatite airborne-particle abrasion system as an alternative surface treatment method on intraorally contaminated titanium discs. Int J Periodontics Restorative Dent. 2020;40:e179–e187. doi: 10.11607/prd.4852. [DOI] [PubMed] [Google Scholar]
- 27.Hägi TT, Hofmänner P, Eick S, Donnet M, Salvi GE, Sculean A, et al. The effects of erythritol air-polishing powder on microbiologic and clinical outcomes during supportive periodontal therapy: six-month results of a randomized controlled clinical trial. Quintessence Int. 2015;46:31–41. doi: 10.3290/j.qi.a32817. [DOI] [PubMed] [Google Scholar]
- 28.Darby IB, Mooney J, Kinane DF. Changes in subgingival microflora and humoral immune response following periodontal therapy. J Clin Periodontol. 2001;28:796–805. doi: 10.1034/j.1600-051x.2001.280812.x. [DOI] [PubMed] [Google Scholar]