Abstract
Rice bran is the main by-product of rice processing and contains approximately 64% of the nutrients in rice. Its various nutrient elements include rice bran proteins, oil, oryzanol, vitamins, polysaccharides, etc. The use of fermented technology can increase the content of bioactive peptides, promote the absorption efficiency, and further improve the functionality and added value of rice bran. In recent years, the nutritional value and function of the extracts and fermented products of rice bran have been emphatically studied. Rice bran extracts and fermentation products serve a critical role in the anti-inflammatory reaction, reducing the plasma lipid effect and increasing anti-cancer activity. Moreover, few review studies have been reported on the anti-cancer activity and potential mechanism of action of rice bran extract and its fermentation products. In this review, we focused on the anti-cancer function, mechanisms, and potential clinical usage of rice bran extracts and fermentation products in the adjuvant therapy of cancer patients.
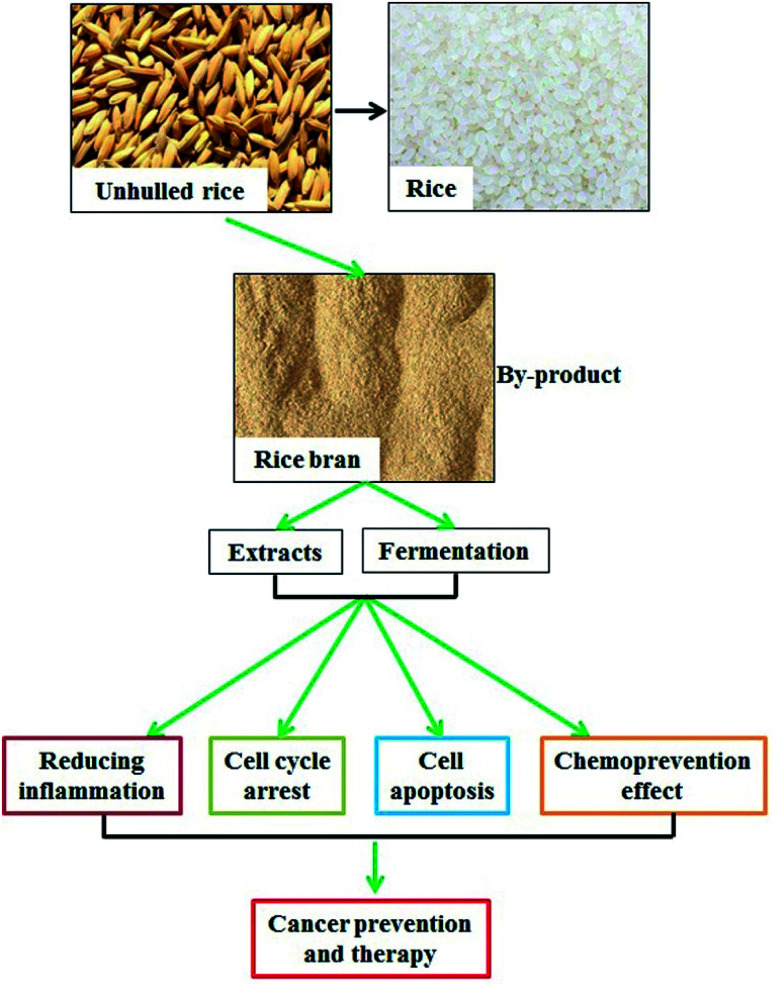
Extracts and fermentation products of rice bran serve important roles in mediating inflammation, cell cycel, cell apotosis, and cancer prevention.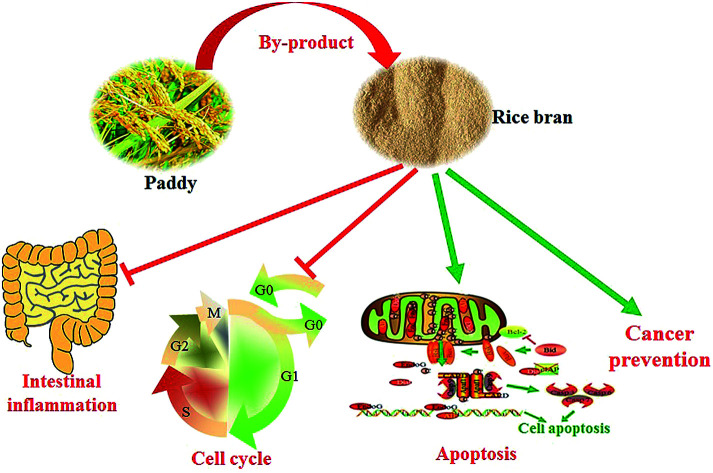
Introduction
Rice bran, which consists of a combination of aleurone and pericarp, is the hard outer layer that is obtained during rice milling. Rice bran is rich in essential fatty acids and dietary fiber and contains proteins, dietary minerals, and vitamins.1 Approximately 12–18.5% of rice bran is healthy oil, which contains 47% monounsaturated, 33% polyunsaturated, and 20% saturated fats as well as highly unsaponifiable ingredients including gamma-oryzanol, tocotrienols, and beta-sitosterol.2 However, the high oil content of rice bran leads to easy rancidity by endogenous lipase, and stabilization is an effective way to avoid rancidification.3 Heat (dry or damp), microwave application, refrigeration, and proteasome treatment to inactivate endogenous lipase are useful methods to stabilize rice bran and mitigate its rancidification.3,4 The protein content comprises approximately 10–15% of rice bran, and it is increased to 18% in defatted rice bran.5 The protein content includes albumin (water soluble), globulin (salt soluble), prolamin (alcohol soluble), and glutelin (alkali soluble).6 The rice bran protein contains all the essential amino acids. Compared with that of rice protein, the amino acid composition of rice bran is closer to the recommended model of the Food and Agriculture Organization (FAO)/World Health Organization (WHO), and its nutritional value is comparable to those of soybeans and egg proteins.7 Moreover, researchers are striving to isolate bioactive components from rice bran and reveal their functions.
Multiple isolation methods have been applied for the purification of rice bran fractions, and the method selection is based on the different physicochemical properties of the rice bran components. Rice bran oil (RBO) can be extracted using an aqueous method,8 an enzymatic method,9 and a screw press method.10 Subsequently, modified methods such as ultrasound and microwave-assisted aqueous extraction of RBO have been used.11,12 Rice bran protein (RBP) can be extracted using an alkali solution,13 enzymatic,14 or physical method.15 With the development of the extraction technology, the yield and purity of the rice bran components will be further improved.
Fermentation is an important method for the deep processing of rice bran; thus, the fermentation products can be used as high-quality feed16 and raw materials for food and beverage production.17 The traditional zymogenous microorganisms include yeast, Aspergillus oryzae, lactic acid bacteria, and Lentinus edodes.18–21 Fermentation contributes to an increased amino acid content and a decreased fatty acid content.22 Moreover, the bioactive components obtained from fermented rice bran (FRB) exert various biological activities (Table 1).
Rice bran fermentation and function.
| Raw material | Type of fermentation microorganism | Model | Function | Reference |
|---|---|---|---|---|
| Brown rice and rice bran | Aspergillus oryzae | Mice bearing tumors derived from QR-32 cells, ApcMin/+ mice, female A/J mice, male F344 rats, or female LEC rats | Fermented brown rice and rice bran administration reduced the expression of the inflammation-related genes TNF-α, Mac-1, CCL3 and CXCL2 in tumor-bearing mice; suppressed the multiplicity of colon tumors in ApcMin/+ mice exposed to dextran sodium sulfate; inhibited NNK-induced lung tumorigenesis and NMBA-induced esophageal tumorigenesis; protected against the development of hepatitis; and suppressed diethylnitrosoamine (DEN)- and phenobarbital (PB)-induced hepatocarcinogenesis | 22,34–38 |
| Rice bran | Fungi and lactic acid bacteria | Colitis in mice | Dietary supplementation with fermented rice bran attenuated dextran sodium sulfate-induced intestinal inflammation via elevating short-chain fatty acids and tryptamine production | 19 |
| Rice bran along with Angelicae gigantis, Cnidium officinale, Artemisia princeps, and Camellia sinensis | Lactobacillus rhamnosus and Pichia deserticola | Atopic dermatitis in mice | Fermented rice bran combined with Angelicae gigantis, Cnidium officinale, Artemisia princeps, and Camellia sinensis mitigated 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis through inhibiting the expression of cytokines and Cox-2 | 1 |
| Rice bran | Lentinus edodes | Healthy participants | Supplementation with fermented rice bran significantly increased IFN-γ secretion without causing obvious adverse effects and with no significant effects on the production of IL-2, IL-4, IL-10, IL-12, or TNF-α | 21 |
| Rice bran | Issatchenkia orientalis MFST1 | 3T3-L1 adipocytes | Fermented rice bran treatment reduced reactive oxygen species generation and oxidative stress-induced insulin resistance | 39 |
| Neptune rice bran | Saccharomyces boulardii | Raji B lymphomas | Treatment with fermented Neptune rice bran extracts significantly inhibited the viability of Raji B lymphomas | 23 |
| Rice bran | Lactobacillus rhamnosus and Saccharomyces cerevisiae | B16F1 melanoma | Fermented rice bran extracts reduced the expression of microphthalmia-associated transcription factor and inhibited the melanogenesis of B16F1 melanoma cells | 20 |
| Rice bran | Saccharomyces cerevisiae | Rats or mice | Oral administration of fermented rice bran induced an anti-stress and anti-fatigue response | 40 |
With the development of nutraceuticals, researchers and consumers are currently paying increasing interest to the possible preventive/therapeutic effects of rice bran on pathological conditions;24–27 nutraceuticals have attracted significant attention due to their abilities to regulate the immune response, glucose and lipid metabolism. Black rice bran, which is rich in gamma-oryzanol, could promote the expression of CD14 and toll-like receptor 4 and enhance the phagocytic activity of the macrophages RAW264.7.28 The extracts, such as phenolic, flavonoid, anthocyanin, and proanthocyanidin extracts, obtained from rice bran inhibit alpha-glucosidase activity and promote glucose uptake in 3T3-L1 adipocytes, which are critical factors for glucose homeostasis.29 Monacolins (present in large amounts in red yeast rice, which is produced by the fermentation of Monascus purpureus mold) have been shown to be bioactive for cholesterol reduction.30
Studies have demonstrated that rice bran extracts and FRB serve critical roles in the regulation of oxidative response, inflammatory reaction, immune function, cholesterol metabolism, and tumor suppression.31–33 In this study, we emphasized the potential function of rice bran extracts and FRB in cancer prevention and therapy.
Anti-cancer activity: mechanism of action and chemopreventive effects
Reducing inflammatory reaction
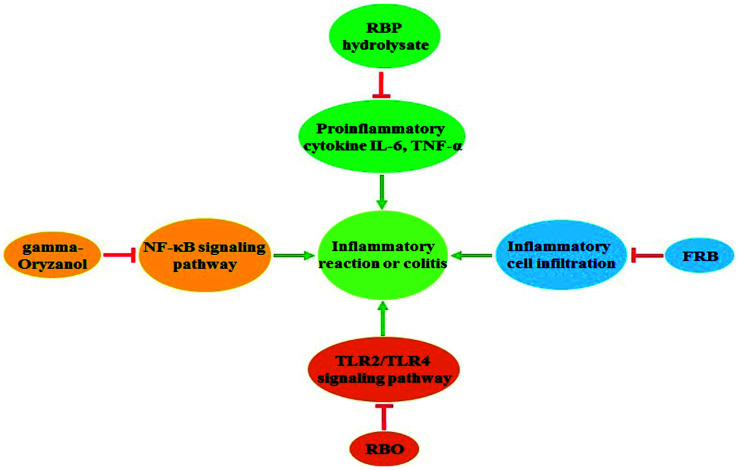
Inflammation is an important inducer of tumor progression, and many cancers arise from infection and inflammation.41 Inflammation also aids in cancer cell proliferation and promotes angiogenesis and cell mobility.42 Thus, reducing inflammation is a promising therapeutic target for treating cancer.43 Recent data demonstrate that rice bran extracts and FRB are helpful in attenuating inflammation (Fig. 1). A high-fat diet results in chronic intestinal inflammation,44 and rice bran enzymatic extract supplements mitigate high-fat diet-induced inflammatory factor secretion.45 The rice bran protein hydrolysate also contributes to the suppression of proinflammatory cytokine expression in rats fed a high-carbohydrate, high-fat diet.46 RBO also exerts an anti-inflammatory response and can reduce the inflammatory response of rats fed partially hydrogenated vegetable fat.47 A subsequent study has shown that the gamma-oryzanol component of RBO serves an essential role in the anti-inflammatory activity.48 Gamma-tocotrienol is another important component of rice bran extracts that represses pancreatic tumor growth by reducing the NF-κB-mediated inflammatory microenvironment.49 The colitis effect is a critical inducer of colon cancer,50 and a dietary supplement of FRB effectively inhibits dextran sodium sulfate-induced colitis via reducing inflammatory cell infiltration and inflammatory cytokine production.19 Overall, the rice bran extracts and FRB might serve essential roles in preventing cancer development via downregulation of the inflammatory reaction.
Fig. 1. The role of rice bran extracts and FRB in reducing inflammation. Supplementation of RBP hydrolysate and RBO inhibited the production of proinflammatory factors. Gamma-oryzanol inactivated the NF-κB signaling pathway and reduced inflammatory reactions. FRB effectively mitigated dextran sodium sulfate-induced colitis by suppressing the infiltration of inflammatory cells and the secretion of inflammatory factors.
Cell cycle arrest
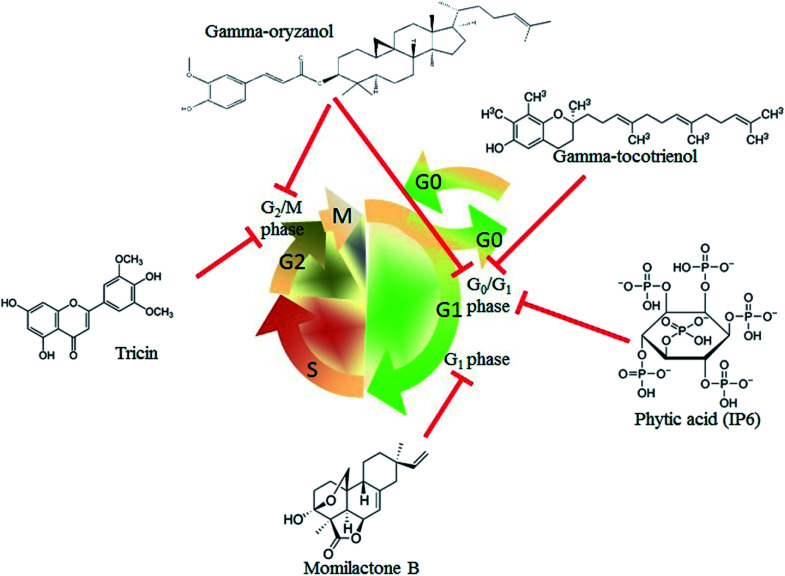
Aberrant cell cycle protein expression leads to uncontrolled cell proliferation, which is one of the critical factors for cancer occurrence.51 Cell cycle blockers are ideal targets for cancer therapy.52 As shown in Fig. 2, tricin is an important flavone component of rice bran. A study has indicated that tricin arrests the human malignant breast cancer cell line MDA-MB-468 at the G2/M phase. Furthermore, the volume of tumors is significantly decreased in nude mice implanted with tricin-pretreated MDA-MB-468 cells as compared to that in the control group.53 Thus, tricin treatment results in arrest of the cancer cell cycle and inhibition of the tumor growth. Gamma-tocotrienol also inhibits human gastric adenocarcinoma SGC-7901 cell proliferation via arresting the cell cycle at the G0/G1 phase.54 Momilactone B, a terpenoid phytoalexin derived from rice bran, exerts its anti-cancer effect by arresting the human leukemia U937 cells at the G1 phase.55 Gamma-oryzanol can arrest the cell cycle of the prostate cell lines PC3 and LNCaP (at the G2/M phase) and DU145 (at the G0/G1 phase).56 Phytic acid, which is also known as inositol hexakisphosphate (IP6), is a bioactive compound existing in rice bran, and it induces cell cycle arrest at the G0/G1 phase of the human colon cancer cell line HT-29 and breast cancer cell line MCF-7.57,58 In summary, rice bran extracts play an important role in suppressing cancer cell proliferation by blocking cell cycle progression.
Fig. 2. Rice bran extracts arrest the cancer cell cycle. Gamma-tocotrienol, gamma-oryzanol, and phytic acid (IP6) arrested the cell cycle at the G0/G1 phase of gastric adenocarcinoma SGC-7901 cells; prostate cells DU145, colon cancer cells HT-29, and breast cancer cells MCF-7. Momilactone B treatment resulted in human leukemia cell U937 arrest at the G1 phase. Tricin and gamma-oryzanol could also arrest the breast cancer cells MDA-MB-468 and prostate cell lines (PC3 and LNCaP) at the G2/M phase.
Promoting cell apoptosis
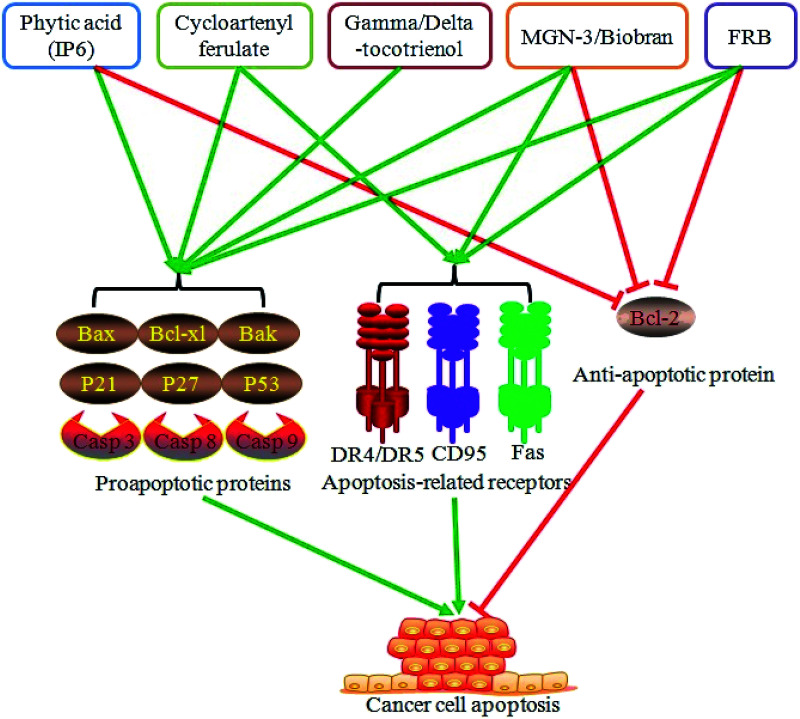
Dysregulation of cell apoptosis is one of the vital aspects of cancer development. Promotion of cancer cell apoptosis serves a critical role in cancer prevention and therapy. Moreover, rice bran extracts and FRB exhibit a role in regulating cancer cell apoptosis. Water-based extracts of rice bran dramatically induce colon cancer cell apoptosis.59 Phytic acid from rice bran elicits a proapoptotic effect on the colon cancer cells HT-29 through the regulation of Bax and Bcl-xl protein expression.60 Moreover, phytic acid induces the apoptosis of the liver cancer cells HepG2 by upregulating the expression of the proapoptotic genes p53 and Bax and downregulating the expression of the antiapoptotic gene Bcl-2.61 Cycloartenyl ferulate, one of the phenolic compounds from rice bran, promotes the activity of the death receptors DR4 and DR5, suppresses the antiapoptotic Bcl-2 expression, elevates the proapoptotic Bak expression, and leads to the apoptosis of the colon cancer cells SW480.62 Tocotrienol is also involved in mediating cancer cell apoptosis. Gamma-tocotrienol induces caspase 3 activation and contributes to the apoptosis of the gastric adenocarcinoma SGC-7901 cells,54 whereas delta-tocotrienol elevates the expression of p21, p27, and caspases 3 and 9, represses Akt phosphorylation, and results in human colorectal adenocarcinoma DLD-1 cell apoptosis.63 Administration of MGN-3/Biobran, a modified arabinoxylan rice bran, can promote tumor cell apoptosis and reduce tumor volume and weight in mice bearing a solid Ehrlich carcinoma.64 MGN-3/Biobran also enhances yeast-induced breast cancer cell apoptosis via activating the caspases 8 and 9 in the MCF-7 cells and caspases 3, 8, and 9 in the HCC70 cells.65 Other studies indicate that MGN-3/Biobran exhibits a proapoptotic effect on the human leukemic HUT78 cells in a death receptor CD95-dependent manner66 and induces the human multiple myeloma cell line U266 cell apoptosis by decreasing the Bcl-2 expression and increasing the Bax expression.67 A recent study has also showed that FRB with Aspergillus oryzae activates the expression of the death receptor DR5 and Fas protein, promotes the protein levels of caspases 3, 8, and 9, reduces the Bcl-2 expression, and leads to the human acute lymphoblastic leukemia Jurkat cell apoptosis.68 In general, rice bran extracts and FRB might promote cancer cell apoptosis by enhancing the proapoptotic protein expression and repressing the antiapoptotic protein expression (Fig. 3).
Fig. 3. Rice bran extracts and FRB promote cancer cell apoptosis. Rice bran extracts (phytic acid, cycloartenyl ferulate, gamma/delta-tocotrienol, and MGN-3/Biobran) and FRB administration dramatically induced cancer cell apoptosis via promoting the expression of proapoptosis proteins (Bax, Bcl-xl, Bak, P21, P27, P53, and caspase 3/8/9), activating apoptosis-related receptors (DR4/5, CD95, and Fas) or inhibiting the expression of the anti-apoptotic protein Bcl-2.
Enhancing the chemopreventive effects
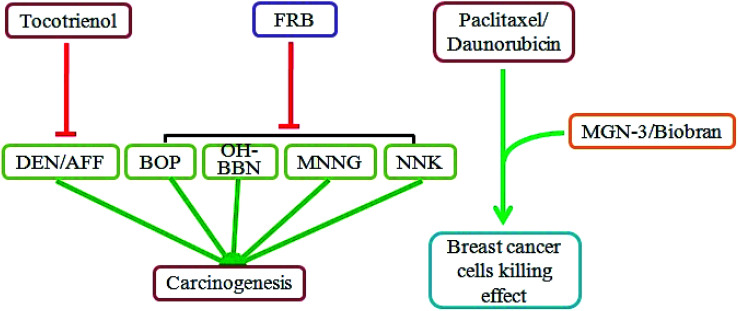
Chemoprevention is the use of natural or synthetic agents to inhibit, delay, or reverse carcinogenesis.69 As shown in Fig. 4, studies have demonstrated that rice bran extracts and FRB can be effective natural agents for chemoprevention. Diethylnitrosamine (DEN) and 2-acetylaminofluorene (AAF) are carcinogens that can induce the development of liver cancer. An in vivo study has indicated that DEN/AFF exposure elevates hepatic lipid peroxidation and low-density lipoprotein oxidation and leads to liver carcinogenesis in Sprague-Dawley rats.70 However, treatment with tocotrienol-rich fraction reduced the DEN/AFF-induced activation of the abovementioned parameters and hepatocarcinogenesis.70 The total phenolics and gamma-tocotrienol in rice bran have a positive correlation with reduced colorectal cancer cell growth.71N-Nitrosobis(2-oxopropyl)amine (BOP), N-butyl-N-(4-hydroxybutyl)-nitrosamine (OH-BBN), N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) are well-known carcinogens. Diets with 5% or 10% fermented brown rice and rice bran have significantly inhibited BOP-induced pancreatic cancer,72 OH-BBN-induced bladder carcinogenesis,73 MNNG-induced gastric cancer,74 NNK-induced lung cancer,35 and prostate carcinogenesis in the transgenic rat adenocarcinoma of the prostate (TRAP) strain.75
Fig. 4. Rice bran extracts and FRB have chemopreventive effects. Tocotrienol treatment reduced DEN/AFF-induced liver carcinogenesis. A diet of FRB effectively inhibited BOP-induced pancreatic cancer, OH-BBN-induced bladder cancer, MNNG-induced gastric cancer, and NNK-induced lung cancer. MGN-3/Biobran supplementation enhanced the susceptibility of breast cancer cells to paclitaxel and daunorubicin.
In addition to directly reducing chemical-induced carcinogenesis, the rice bran extracts can enhance the sensitivity of cancer cells to chemotherapy drugs. MGN-3/Biobran treatment significantly increases the susceptibility of the human nonmetastatic breast cancer cells MCF-7 and metastatic murine breast cancer cells 4T1 to paclitaxel.76 Another study showed that MGN-3/Biobran contributes to enhanced sensitivity of the breast cancer cells MCF-7 and HCC-70 to daunorubicin.77 Overall, rice bran extracts and FRB are potential chemopreventive agents that act by reducing carcinogen-related cancer induction and enhancing the susceptibility of cancer cells to chemotherapy drugs (Fig. 4).
Clinical trials
In addition to studies that have focused on the function of rice bran extracts and FRB in cell or animal models, increasing research is being concentrated on their clinical effects. Dietary supplementation of rice bran dramatically downregulates the level of the inflammatory factor IL-6 in overweight and obese adults.78 In patients with type II diabetes, the dietary consumption of rice bran oil significantly reduces the blood levels of total cholesterol, triglyceride, and low-density lipoprotein cholesterol.79,80 In addition, RBO containing gamma-oryzanol81 or rice bran extract containing an acylated steryl glucoside fraction82 effectively decreases low-density lipoprotein cholesterol levels. Thus, rice bran administration could mitigate the inflammatory reaction and reduce the level of blood lipids in obese and hyperlipidemic subjects. Rice bran extracts still play a crucial role in regulating innate immune activity. MGN-3/Biobran consumption for 30 days dramatically increased the NK cell activity in geriatric subjects (over 65 years old).83 A study that enrolled patients with multiple myeloma has also indicated that dietary supplementation with MGN-3/Biobran contributes to elevated NK cell activity.84 The function of activated NK cells is to help recognize and kill cancer cells, which is critical for cancer prevention and immunotherapy. Another clinical effect of rice bran extracts is the regulation of gastrointestinal function. Administration of MGN-3/Biobran remarkably attenuates the clinical symptoms of patients with diarrhea-predominant or mixed-type irritable bowel syndrome.85 A heat-stabilized rice bran diet offers the benefits of bacterial richness and diversity in the gut, helps with microbial metabolism, modulates stool metabolite profiles, and contributes to chemoprevention in colorectal cancer survivors.86–88 The function of rice bran in altering gut microbiota might contribute to the relief of adverse gastrointestinal effects in cancer patients during chemotherapy. A study that enrolled patients with cervical cancer indicated that the oral intake of hydrolyzed rice bran effectively attenuated the diarrheal side effects of chemotherapy.89 Rice bran extracts also exert an adjuvant therapeutic effect on cancer patients. A study of hepatocellular carcinoma patients receiving interventional therapy demonstrated that dietary supplementation of MGN-3/Biobran significantly decreased the recurrence and increased the two-year survival rate.90 Therefore, rice bran extracts play a large role in regulating inflammation, lipid metabolism, and clinical cancer therapy via the modulation of gastrointestinal function, chemotherapy effects, and interventional therapy effects (Table 2).
Detailed information of rice bran extracts in clinical trials.
| Types of extract | Types of trial | Registration number | Target enroll number | Disease type | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Rice husk powder or rice bran | Randomized trial | IRCT2015040721652N1 | 105 | Overweight and obese adults | Rice husk powder and rice bran consumption combined with an energy-restricted diet significantly reduced the levels of inflammatory factor IL-6 in overweight and obese subjects | 78 |
| Rice bran oil (RBO) or canola oil (CO) | Randomized, controlled, parallel-group trial | IRCT2014050417568N1 | 75 | Postmenopausal women with type 2 mellitus | RBO or CO treatment attenuated lipid disorders in type 2 diabetic women. RBO improved the lipid profile more efficiently than CO | 79 |
| Rice bran and sesame blend oil | Open-label randomized trial | 300 | Patients with type 2 mellitus | A novel blend of 20% cold-pressed unrefined sesame oil and 80% physically refined rice bran oil, when used as cooking oil, lowered hyperglycemia and improved the lipid profile in type 2 diabetes mellitus patients | 80 | |
| Rice bran oil (RBO) containing gamma-oryzanol | Randomized, double-blind, controlled trial | 59 | Hyperlipidemic subjects | RBO with gamma-oryzanol decreased LDL-C levels and increased the antioxidant capacity in hyperlipidemic subjects. Thus, RBO consumption may reduce cardiovascular disease risk factors | 81 | |
| Rice bran extract containing acylated steryl glucoside fraction (RB-ASG) | Randomized, double-blinded trial | 51 | Obese men | RB-ASG fraction might reduce the blood LDL cholesterol levels and the risk of arteriosclerosis in obese Japanese men | 82 | |
| MGN-3/Biobran | Randomized, double-blind, placebo-controlled trial | 12 | Healthy geriatric subjects | Biobran/MGN-3 induced a significant increase in NK activity, which may increase resistance to viral infections and cancers in the geriatric population | 83 | |
| MGN-3/Biobran | Randomized, placebo-controlled trial | 48 | Multiple myeloma patients | MGN-3 might represent an immunologically relevant product for activating innate immunity in multiple myeloma patients and warrants further testing to demonstrate clinical efficacy | 84 | |
| MGN-3/Biobran | Pilot, randomized, controlled trial | 40 | Diarrhea-predominant or mixed-type irritable bowel syndrome | The administration of Biobran/MGN-3 improved IBS symptoms. The anti-inflammatory and/or immune modulatory effects of Biobran might be useful in IBS patients | 85 | |
| Heat-stabilized rice bran or cooked navy bean powder | Randomized-controlled pilot trial | NCT01929122 | 29 | Colorectal cancer survivors | Dietary supplementation of heat-stabilized rice bran or cooked navy bean powder had benefits for gut microbiotic richness and microbial metabolism | 86 |
| Heat-stabilized rice bran | Randomized controlled trial | NCT01929122 | 29 | Colorectal cancer survivors | Heat-stabilized rice bran consumption favorably modulated the stool metabolome of colorectal cancer survivors | 87 |
| Heat-stabilized rice bran or cooked navy bean powder | Randomized controlled trial | NCT01929122 | 29 | Colorectal cancer survivors | Increased dietary heat-stabilized rice bran or cooked navy bean powder consumption contributed to colorectal cancer chemoprevention | 88 |
| Hydrolyzed rice bran | Randomized, double-blind pilot trial | UMIN000004350 | 20 | Cervical cancer patients | Hydrolyzed rice bran administration may relieve diarrhea, an acute-phase gastrointestinal side effect of chemoradiotherapy | 89 |
| MGN-3/Biobran | Three-year randomized trial | NCT01018381 | 68 | Patients with hepatocellular carcinoma | Biobran/MGN-3, in conjunction with intervention therapy, may be useful for the treatment of hepatocellular carcinoma; this treatment showed lower recurrence and an increased the two-year survival rate | 90 |
Conclusion
Rice bran extracts and FRB are promising adjuvant therapeutic agents for cancer prevention and therapy. They might play a critical role in suppressing local inflammation, arresting the cancer cell cycle, promoting cancer cell apoptosis, and enhancing the chemopreventive effects (Fig. 5). Furthermore, the benefits of dietary supplementation of rice bran include modulation of the microbiota richness of the gut, maintenance of the gastrointestinal function, attenuation of the side effects and enhancement of the therapeutic effects in cancer patients. In general, as potential nutraceuticals, rice bran extracts and fermentation products are greatly expected to contribute health benefits to those suffering from pathological conditions. Furthermore, nutraceutical supplementation is a new clinically auxiliary strategy for the prevention or therapeutic treatment of acute and chronic diseases.
Fig. 5. The potential mechanism of treatment with rice bran extracts and fermented products for cancer prevention and therapy. Rice bran is a by-product during rice processing, and the bioactive components of rice bran extracts and fermented products exert important roles in cancer prevention and therapy. Administration of these bioactive components contributes to reducing inflammatory reactions, arresting the cell cycle, promoting cell apoptosis, and preventing cancer occurrence.
Conflicts of interest
The authors declare no conflict of interest.
Abbreviations
- RBO
Rice bran oil
- RBP
Rice bran protein
- FRB
Fermented rice bran
- DR
Death receptor
- Bcl-2
B-cell lymphoma-2
- Bax
BCL2-associated X protein
- Bak
Bcl-2 homologous antagonist/killer
- Bcl-xl
B-cell lymphoma-extra large
- MGN-3/Biobran
Arabinoxylan rice bran
- DEN
Diethylnitrosamine
- AAF
2-Acetylaminofluorene
- BOP
N-Nitrosobis(2-oxopropyl) amine
- OH-BBN
N-Butyl-N-(4-hydroxybutyl)-nitrosamine
- MNNG
N-Methyl-N′-nitro-N-nitrosoguanidine
- NNK
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone
- CD95
Cluster of differentiation 95
- IL
Interleukin
- NK
Natural killer
- TNF
Tumor necrosis factor
- Mac-1
Macrophage-1
- CCL
Chemokine (C–C motif) ligand
- CXCL
Chemokine (C–X–C motif) ligand
- LDL
Low-density lipoprotein
Supplementary Material
Acknowledgments
This work was supported by the Beijing Natural Science Foundation (7172210), the Beijing Nova Program (Z181100006218043), the National Natural Science foundation of China (NSFC 81471873), the Beijing Excellent Talents Funding for Youth Scientist Innovation Team (No. 2016000026833TD01), the High-level Teachers in Beijing Municipal Universities (IDHT20180506), the Shenzhen Municipal Commission Science and Technology Innovation JSGG20170413151359491.
Notes and references
- Shin H. Y. Kim S. M. Lee J. H. Lim S. T. Food Chem. 2019;272:235–241. doi: 10.1016/j.foodchem.2018.07.174. [DOI] [PubMed] [Google Scholar]
- Bhosale S. Curr. Res. Nutr. Food Sci. 2015;3:74–80. doi: 10.12944/CRNFSJ.3.1.08. [DOI] [Google Scholar]
- Saikia D. Deka S. C. Int. Food Res. J. 2011;18:21–30. [Google Scholar]
- Weerathilake W. A. D. V., Ranawana S. S. E., Abeynayake N. R., Perera A. N. F. and Wijeratne W. M. M. P., 2012
- Laokuldilok T. Rattanathanan Y. Cereal Chem. 2014;91:560–565. doi: 10.1094/CCHEM-02-14-0022-R. [DOI] [Google Scholar]
- Fabian C. Ju Y. H. Crit. Rev. Food Sci. Nutr. 2011;51:816–827. doi: 10.1080/10408398.2010.482678. [DOI] [PubMed] [Google Scholar]
- Hamada J. S. J. Food Sci. 2000;65:305–310. doi: 10.1111/j.1365-2621.2000.tb15998.x. [DOI] [Google Scholar]
- Han S. W. Chee K. M. Cho S. J. Food Chem. 2015;172:766–769. doi: 10.1016/j.foodchem.2014.09.127. [DOI] [PubMed] [Google Scholar]
- Amarasinghe B. Kumarasiri M. P. M. Gangodavilage N. C. Food Bioprod. Process. 2009;87:108–114. doi: 10.1016/j.fbp.2008.08.002. [DOI] [Google Scholar]
- Hanmoungjai P. Pyle D. L. Niranjan K. J. Am. Oil Chem. Soc. 2001;78:817–821. doi: 10.1007/s11746-001-0348-2. [DOI] [Google Scholar]
- Sayasoonthorn S. Kaewrueng S. Ul P. P. A. T. T. A. Rice Science. 2012;1:75–78. doi: 10.1016/S1672-6308(12)60024-9. [DOI] [Google Scholar]
- Khoei M. Chekin F. Food Chem. 2016;194:503–507. doi: 10.1016/j.foodchem.2015.08.068. [DOI] [PubMed] [Google Scholar]
- Chao M. A. Liao Y. J. Yang T. Zou H. R. Yao W. U. Wan D. J. Food Sci. Technol. 2014;39:132–135. [Google Scholar]
- Gnanasambandam R. Hettiarachchy N. S. J. Food Sci. 1995;60:1066–1069. doi: 10.1111/j.1365-2621.1995.tb06293.x. [DOI] [Google Scholar]
- Grossman M. V. Rao C. S. Da silva R. S. F. Journal of Food Biochemistry. 1980;4:181–188. doi: 10.1111/j.1745-4514.1980.tb00656.x. [DOI] [Google Scholar]
- Feng X. U. Wang C. Y. Food Sci. 2014;35:11–16. [Google Scholar]
- K San Mu A. K. Ideris A. Saad C. R. J. Anim. Vet. Adv. 2011;10:2990–2995. [Google Scholar]
- Liu L. Zhang R. Deng Y. Zhang Y. Xiao J. Huang F. Wen W. Zhang M. Food Chem. 2017;221:636–643. doi: 10.1016/j.foodchem.2016.11.126. [DOI] [PubMed] [Google Scholar]
- Islam J. Koseki T. Watanabe K. Budijanto S. Oikawa A. Alauddin M. Goto T. Aso H. Komai M. Shirakawa H. Nutrients. 2017;9:E747. doi: 10.3390/nu9070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. Y. Seo Y. K. Park J. M. Seo M. J. Park J. K. Kim J. W. Park C. S. Biosci., Biotechnol., Biochem. 2009;73:1704–1710. doi: 10.1271/bbb.80766. [DOI] [PubMed] [Google Scholar]
- Choi J. Y. Paik D. J. Kwon D. Y. Park Y. Nutr. J. 2014;13:35. doi: 10.1186/1475-2891-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma K. Kanda Y. Suzuki Ikeda S. Sakaki R. Nonomura T. Kobayashi M. Osaki M. Shikanai M. Kobayashi H. Okada F. Nutrients. 2015;7:10237–10250. doi: 10.3390/nu7125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. P. Heuberger A. L. Weir T. L. Barnett B. Broeckling C. D. Prenni J. E. J. Agric. Food Chem. 2011;59:1862–1870. doi: 10.1021/jf1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliu P. Santini A. Novellino E. Expert Opin. Ther. Pat. 2018;28:875–882. doi: 10.1080/13543776.2018.1552260. [DOI] [PubMed] [Google Scholar]
- Santini A. Novellino E. Armini V. Ritieni A. Food Chem. 2013;140:843–849. doi: 10.1016/j.foodchem.2012.10.098. [DOI] [PubMed] [Google Scholar]
- Santini A. Novellino E. Expert Rev. Clin. Pharmacol. 2018;11:545–547. doi: 10.1080/17512433.2018.1464911. [DOI] [PubMed] [Google Scholar]
- Andrew R. Izzo A. A. Br. J. Pharmacol. 2017;174:1177–1194. doi: 10.1111/bph.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. Y. Kim H. W. Jang H. H. Hwang Y. J. Choe J. S. Lim Y. Kim J. B. Lee Y. H. J. Med. Food. 2017;20:855–863. doi: 10.1089/jmf.2017.3966. [DOI] [PubMed] [Google Scholar]
- Boue S. M. Daigle K. W. Chen M. H. Cao H. Heiman M. L. J. Agric. Food Chem. 2016;64:5345–5353. doi: 10.1021/acs.jafc.6b01909. [DOI] [PubMed] [Google Scholar]
- Nguyen T. Karl M. Santini A. Foods. 2017;6:E19. doi: 10.3390/foods6030019. doi: 10.3390/foods6030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Wang J. Liu Y. Gong L. Sun B. Food Funct. 2016;7:2747–2753. doi: 10.1039/C6FO00044D. [DOI] [PubMed] [Google Scholar]
- Gunasekaran S. Venkatachalam K. Namasivayam N. Mol. Cell. Biochem. 2019;451:117–129. doi: 10.1007/s11010-018-3398-5. [DOI] [PubMed] [Google Scholar]
- Noaman E. Badr El-Din N. K. Bibars M. A. Abou Mossallam A. A. Ghoneum M. Cancer Lett. 2008;268:348–359. doi: 10.1016/j.canlet.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Phutthaphadoong S. Yamada Y. Hirata A. Tomita H. Hara A. Limtrakul P. Iwasaki T. Kobayashi H. Mori H. Oncol. Rep. 2010;23:53–59. [PubMed] [Google Scholar]
- Phutthaphadoong S. Yamada Y. Hirata A. Tomita H. Taguchi A. Hara A. Limtrakul P. N. Iwasaki T. Kobayashi H. Mori H. Oncol. Rep. 2009;21:321–327. [PubMed] [Google Scholar]
- Shibata T. Nagayasu H. Kitajo H. Arisue M. Yamashita T. Hatakeyama D. Iwasaki T. Kobayashi H. Oncol. Rep. 2006;15:869–874. [PubMed] [Google Scholar]
- Kuno T. Hirose Y. Hata K. Kato K. Qiang S. H. Kitaori N. Hara A. Iwasaki T. Yoshimura T. Wada K. Kobayashi H. Mori H. Int. J. Oncol. 2004;25:1809–1815. [PubMed] [Google Scholar]
- Katayama M. Sugie S. Yoshimi N. Yamada Y. Sakata K. Qiao Z. Iwasaki T. Kobayashi H. Mori H. Oncol. Rep. 2003;10:875–880. [PubMed] [Google Scholar]
- Kim D. Han G. D. Plant Foods Hum. Nutr. 2011;66:285–290. doi: 10.1007/s11130-011-0243-3. [DOI] [PubMed] [Google Scholar]
- Kim K. M. Yu K. W. Kang D. H. Suh H. J. Phytother. Res. 2002;16:700–702. doi: 10.1002/ptr.1019. [DOI] [PubMed] [Google Scholar]
- Coussens L. M. Werb Z. Nature. 2002;420:860. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Allavena P. Sica A. Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Oncology. 2011;25:418–420. [PubMed] [Google Scholar]
- Gulhane M. Murray L. Lourie R. Tong H. Sheng Y. H. Wang R. Kang A. Schreiber V. Wong K. Y. Magor G. Denman S. Begun J. Florin T. H. Perkins A. Cuiv P. O. McGuckin M. A. Hasnain S. Z. Sci. Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justo M. L. Claro C. Zeyda M. Stulnig T. M. Herrera M. D. Rodriguez-Rodriguez R. Eur. J. Nutr. 2016;55:2011–2019. doi: 10.1007/s00394-015-1015-x. [DOI] [PubMed] [Google Scholar]
- Boonloh K. Kukongviriyapan V. Kongyingyoes B. Kukongviriyapan U. Thawornchinsombut S. Pannangpetch P. Nutrients. 2015;7:6313–6329. doi: 10.3390/nu7085292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y. P. Kumar P. P. Lokesh B. R. Lipids. 2016;51:451–467. doi: 10.1007/s11745-016-4132-2. [DOI] [PubMed] [Google Scholar]
- Rao Y. P. C. Sugasini D. Lokesh B. R. Biochem. Biophys. Res. Commun. 2016;479:747–752. doi: 10.1016/j.bbrc.2016.09.140. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B. Sung B. Ravindran J. Diagaradjane P. Deorukhkar A. Dey S. Koca C. Yadav V. R. Tong Z. Gelovani J. G. Guha S. Krishnan S. Aggarwal B. B. Cancer Res. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastille E. Frede A. McSorley H. J. Grab J. Adamczyk A. Kollenda S. Hansen W. Epple M. Buer J. Maizels R. M. Klopfleisch R. Westendorf A. M. PLoS Pathog. 2017;13:e1006649. doi: 10.1371/journal.ppat.1006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T. Sicinski P. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydbring P. Wang Y. Fassl A. Li X. Matia V. Otto T. Choi Y. J. Sweeney K. E. Suski J. M. Yin H. Bogorad R. L. Goel S. Yuzugullu H. Kauffman K. J. Yang J. Jin C. Li Y. Floris D. Swanson R. Ng K. Sicinska E. Anders L. Zhao J. J. Polyak K. Anderson D. G. Li C. Sicinski P. Cancer Cell. 2017;31:576–590. doi: 10.1016/j.ccell.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. Hudson E. A. Mann P. Verschoyle R. D. Greaves P. Manson M. M. Steward W. P. Gescher A. J. Br. J. Cancer. 2004;91:1364–1371. doi: 10.1038/sj.bjc.6602124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W. Xu W. Liu H. Liu J. Wang Q. Zhou J. Dong F. Chen B. J. Nutr. Biochem. 2009;20:276–284. doi: 10.1016/j.jnutbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Park C. Jeong N. Y. Kim G. Y. Han M. H. Chung I. M. Kim W. J. Yoo Y. H. Choi Y. H. Oncol. Rep. 2014;31:1653–1660. doi: 10.3892/or.2014.3008. [DOI] [PubMed] [Google Scholar]
- Hirsch G. E. Parisi M. M. Martins L. A. Andrade C. M. Barbe-Tuana F. M. Guma F. T. Prostate. 2015;75:783–797. doi: 10.1002/pros.22960. [DOI] [PubMed] [Google Scholar]
- Nurulhusna S. Norhaizan M. E. Hairuszah I. Abdah M. A. Norazalina S. Norsharina I. J. Med. Plants Res. 2010;4:2283–2289. [Google Scholar]
- El-Sherbiny Y. M. Cox M. C. Ismail Z. A. Shamsuddin A. M. Vucenik I. Anticancer Res. 2001;21:2393–2403. [PubMed] [Google Scholar]
- Takashima A. Ohtomo M. Kikuchi T. Iwashita J. Abe T. Hata K. J. Food Sci. Technol. 2013;50:595–599. doi: 10.1007/s13197-011-0368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafie N. H. Esa N. M. Ithnin H. Saad N. Pandurangan A. K. Int. J. Mol. Sci. 2013;14:23545–23558. doi: 10.3390/ijms141223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Fatlawi A. A. Irshad M. Zafaryab M. Rizvi M. M. Ahmad A. Asian Pac. J. Cancer Prev. 2014;15:3731–3736. doi: 10.7314/APJCP.2014.15.8.3731. [DOI] [PubMed] [Google Scholar]
- Kong C. K. Lam W. S. Chiu L. C. Ooi V. E. Sun S. S. Wong Y. S. Biochem. Pharmacol. 2009;77:1487–1496. doi: 10.1016/j.bcp.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Shibata A. Nakagawa K. Tsuduki T. Miyazawa T. J. Nutr. Biochem. 2015;26:832–840. doi: 10.1016/j.jnutbio.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Badr El-Din N. K. Noaman E. Ghoneum M. Nutr. Cancer. 2008;60:235–244. doi: 10.1080/01635580701627285. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. Gollapudi S. Anticancer Res. 2005;25:859–870. [PubMed] [Google Scholar]
- Ghoneum M. Gollapudi S. Cancer Lett. 2003;201:41–49. doi: 10.1016/S0304-3835(03)00458-0. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. Gollapudi S. Neoplasma. 2011;58:118–123. doi: 10.4149/neo_2011_02_118. [DOI] [PubMed] [Google Scholar]
- Horie Y. Nemoto H. Itoh M. Kosaka H. Morita K. Appl. Biochem. Biotechnol. 2016;178:1599–1611. doi: 10.1007/s12010-015-1970-y. [DOI] [PubMed] [Google Scholar]
- Kraus S. Arber N. Eur. J. Cancer. 2009;45(suppl. 1):360–366. doi: 10.1016/S0959-8049(09)70051-6. [DOI] [PubMed] [Google Scholar]
- Iqbal J. Minhajuddin M. Beg Z. H. Eur. J. Cancer Prev. 2004;13:515–520. doi: 10.1097/00008469-200412000-00009. [DOI] [PubMed] [Google Scholar]
- Forster G. M. Raina K. Kumar A. Kumar S. Agarwal R. Chen M. H. Bauer J. E. McClung A. M. Ryan E. P. Food Chem. 2013;141:1545–1552. doi: 10.1016/j.foodchem.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T. Takahashi S. Tomita H. Hisamatsu K. Hara A. Hirata A. Kobayashi H. Mori H. Oncol. Lett. 2015;10:3377–3384. doi: 10.3892/ol.2015.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T. Hirose Y. Yamada Y. Hata K. Qiang S. H. Asano N. Oyama T. Zhi H. Iwasaki T. Kobayashi H. Mori H. Oncol. Rep. 2006;15:533–538. [PubMed] [Google Scholar]
- Tomita H. Kuno T. Yamada Y. Oyama T. Asano N. Miyazaki Y. Baba S. Taguchi A. Hara A. Iwasaki T. Kobayashi H. Mori H. Oncol. Rep. 2008;19:11–15. [PubMed] [Google Scholar]
- Kuno T. Nagano A. Mori Y. Kato H. Nagayasu Y. Naiki-Ito A. Suzuki S. Mori H. Takahashi S. Nutrients. 2016;8:E421. doi: 10.3390/nu8070421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneum M. Badr El-Din N. K. Ali D. A. El-Dein M. A. Anticancer Res. 2014;34:81–87. [PubMed] [Google Scholar]
- Gollapudi S. Ghoneum M. Cancer Detect. Prev. 2008;32:1–6. doi: 10.1016/j.cdp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Edrisi F. Salehi M. Ahmadi A. Fararoei M. Rusta F. Mahmoodianfard S. Eur. J. Nutr. 2018;57:833–843. doi: 10.1007/s00394-017-1555-3. [DOI] [PubMed] [Google Scholar]
- Salar A. Faghih S. Pishdad G. R. J. Clin. Lipidol. 2016;10:299–305. doi: 10.1016/j.jacl.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Devarajan S. Chatterjee B. Urata H. Zhang B. Ali A. Singh R. Ganapathy S. Am. J. Med. 2016;129:731–739. doi: 10.1016/j.amjmed.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Bumrungpert A. Chongsuwat R. Phosat C. Butacnum A. J. Altern. Complement Med. 2019;25:353–358. doi: 10.1089/acm.2018.0212. [DOI] [PubMed] [Google Scholar]
- Ito Y. Nakashima Y. Matsuoka S. Int. J. Med. Invest. 2015;62:80–84. doi: 10.2152/jmi.62.80. [DOI] [PubMed] [Google Scholar]
- Elsaid A. F. Shaheen M. Ghoneum M. Exp. Ther. Med. 2018;15:2313–2320. doi: 10.3892/etm.2018.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholujova D. Jakubikova J. Czako B. Martisova M. Hunakova L. Duraj J. Mistrik M. Sedlak J. Cancer Immunol. Immunother. 2013;62:437–445. doi: 10.1007/s00262-012-1344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T. Shikano M. Tanaka M. Ozeki K. Ebi M. Katano T. Hamano S. Nishiwaki H. Tsukamoto H. Mizoshita T. Mori Y. Kubota E. Tanida S. Kataoka H. Okuda N. Joh T. J. Evidence-Based Complementary Altern. Med. 2014;2014:828137. doi: 10.1155/2014/828137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheflin A. M. Borresen E. C. Kirkwood J. S. Boot C. M. Whitney A. K. Lu S. Brown R. J. Broeckling C. D. Ryan E. P. Weir T. L. Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. G. Borresen E. C. Brown R. J. Ryan E. P. Br. J. Nutr. 2017;117:1244–1256. doi: 10.1017/S0007114517001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borresen E. C. Brown D. G. Harbison G. Taylor L. Fairbanks A. O'Malia J. Bazan M. Rao S. Bailey S. M. Wdowik M. Weir T. L. Brown R. J. Ryan E. P. Nutr. Cancer. 2016;68:1269–1280. doi: 10.1080/01635581.2016.1224370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y. Mizuno M. Ikeda M. Nakahara R. Kubota S. Ito J. Okada T. Kawamura M. Kikkawa F. Naganawa S. Evidence-based complementary and alternative medicine: eCAM. 2015;2015:974390. doi: 10.1155/2015/974390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M. H. Van Riep T. Thinh N. T. Song le H. Dung T. T. Van Truong L. Van Don L. Ky T. D. Pan D. Shaheen M. Ghoneum M. Anticancer Res. 2010;30:5145–5151. [PubMed] [Google Scholar]