Abstract
The outcome of herpes simplex virus (HSV) infections manifesting as encephalitis in healthy or immunocompromised individuals is generally very poor with mortality rates of about 8 to 28% with treatment. The long-term prognosis of survivors is often problematic, posing the need for alternative treatments that may decrease the mortality and morbidity associated with herpes encephalitis. This study addresses one such approach that includes a temporary permeabilization of the blood-brain barrier during treatment with acyclovir (ACV). In these studies we utilized a synthetic bradykinin analog, Cereport (RMP-7), in conjunction with ACV to treat HSV infection of the brain in a rat model. Cereport, infused intravenously via the jugular vein, was shown to increase [14C]ACV uptake in both the HSV-1-infected and -uninfected rat brain by approximately two- to threefold, correlating with enhanced efficacy of ACV in various brain compartments. In another series of experiments to determine efficacy, various doses of unlabeled ACV were administered during infusion with RMP-7. The decrease in viral titers in the temporal regions of the brain after 5 days of treatment suggested that this approach enhanced the efficacy of ACV treatment. These data indicated that Cereport infused with ACV enhances both the penetration and efficacy of this drug in the treatment of an experimental HSV-1 infection of the rat brain.
Herpes simplex virus (HSV) infections in humans can manifest as a life-threatening disease with high mortality and significant morbidity in survivors. These infections can be caused by either HSV-1 or HSV-2 (2, 46). In neonates, primary infection that occurs during or shortly after birth is usually symptomatic within 1 month and is frequently lethal (42, 43, 46). Although treatment with either vidarabine or acyclovir (ACV) significantly reduces the mortality rate, up to 15% of those with encephalitis still do not survive (28, 42, 43, 45). In older individuals, encephalitis caused by primary or recurrent HSV infection results in a 70% mortality rate without treatment and an 8 to 28% mortality rate with treatment depending on when treatment is initiated (32, 45, 46). This disease is characterized by acute necrotizing focal encephalopathy, inflammation and swelling of the brain tissue, and petechiae or larger hemorrhages in the brain (9, 26, 39, 47). Although therapy with either vidarabine or ACV (42, 44) has proven to be effective in reducing mortality rates of HSV encephalitis, the long-term prognosis of the survivors is less than optimal. A few survivors suffer relapses, but many others have learning and memory abnormalities and impairment of general orientation and perceptive-motor skills (12, 14, 29). This current state of HSV encephalitis-associated mortality and morbidity suggests that alternative approaches to treatment need to be developed.
One possible reason for reduced efficacy in the central nervous system (CNS) may be failure of the drug to penetrate the blood-brain barrier (BBB) and enter the CNS tissues. Inefficient penetration of ACV across the BBB was demonstrated by de Miranda et al. (13) where the concentration of [14C]ACV in brain was 1/10 that of the plasma concentration 30 min after administration. One approach to solving this problem would be to use a drug that would enhance the penetration of ACV across the BBB and further increase efficacy in brain tissue.
The BBB is a complex vascular structure composed of a continuous layer of endothelial cells that maintain tight junctions between themselves (10, 41). The properties of the BBB suggest that a highly selective exchange system has evolved between the blood and brain to provide a homeostatic environment for the brain in the normal physiological state (41). This controlled environment may be altered by an increase in permeability under physiological conditions like hypertension (1, 24, 35, 36) or by physical damage of the endothelial membranes occurring with pathological conditions such as trauma, ischemia, tumors, and allergic or inflammatory diseases (3, 30). The inflammation and swelling of brain vasculature and tissue during infection with viruses such as HSV-1 suggest that viral infection of the CNS may also alter the permeability of the BBB (39, 45). Lastly, an increase in permeability of the BBB can be caused by a release of chemical mediators such as bradykinins, serotonin, histamines, arachidonic acid, leukotrienes, and free radicals (41).
The use of chemical mediators that increase the permeability of BBB can be advantageous when employed to increase drug delivery into the brain parenchyma. In experimental and clinical applications, the synthetic nonapeptide and bradykinin analog, Cereport, previously referred to as RMP-7, was found to selectively increase drug delivery into brain tumors (4, 5, 6, 7, 8, 16, 19, 21, 25, 31, 33, 34, 37) and to increase the permeability of the blood-ocular barrier to ganciclovir in guinea pigs (17, 18). When administered by either intravenous or intracarotid routes, Cereport selectively opens the BBB via stimulation of the β2 subclass of receptors on the brain endothelium. This stimulation leads to a rapid, transient increase in free intracellular Ca2+, which in turn causes an increase in endothelial pore size (15, 38). This effect is temporary (∼20 min) due to tachyphylaxis or desensitization of β2 receptor stimulation (6, 21).
In the present study, we describe the effect of intravenous administration of Cereport on the enhanced permeability and efficacy of ACV in a rat model of HSV-1 encephalitis.
MATERIALS AND METHODS
Virus preparation and assay.
Stock pools of HSV-1, strain E-377, were grown in primary cultures of rabbit kidney cells as described previously (27).
To determine the amount of HSV viral replication in the brain at various time periods, tissue was harvested, homogenized (10%, wt/vol), and assayed for HSV using a plaque assay on rabbit kidney cells as described previously (27).
Mortality of rats infected with HSV-1, strain E-377.
In order to obtain an optimal inoculum of HSV-1, we initially determined the virus concentration that would infect 100% of the animals. Groups of nine 2-month-old male Fisher rats (180 to 200 g; Charles River Laboratories, Raleigh, N.C.) were weighed and anesthetized by intraperitoneal injection of 0.1 to 0.15 ml of 100-mg/ml ketamine and 15-mg/ml xylazine. Rats were inoculated intranasally with various concentrations of HSV-1, strain E-377, using an Eppendorf pipettor and tip to administer 20 μl into each nostril. Animals were examined daily for 21 days, and mortality was recorded. Using these data, the mortality rate and the mean day of death (MDD) were calculated for each group.
To validate the rat model, the effect of ACV treatment on the mortality of rats infected with HSV-1 was determined. Five groups of 10 2-month-old male Fisher rats (180 to 200 g) were weighed and anesthetized as described previously. Each animal was then inoculated intranasally with 2.4 × 104 PFU of HSV-1. Twenty-four hours after viral inoculation, intraperitoneal treatment with phosphate-buffered saline (placebo) or 120, 100, 80, or 60 mg of ACV/kg of body weight was initiated and continued twice daily for 5 days. Animals were monitored for mortality for 21 days after viral inoculation. Toxicity of ACV was determined in uninfected animals using the same treatment regime as described above. The mortality and MDD were determined for all groups. Efficacy was determined using the Fisher exact test for mortality and Mann-Whitney U rank sum test for MDD. A P value of 0.05 was considered significant.
Pathogenesis of HSV-1 infection in rat brains.
Groups of male Fisher rats were inoculated intranasally with 2.4 × 104 PFU of HSV-1, as described above. On each of days 1, 2, 3, 4, 5, 6, 7, and 10 three animals were sacrificed and the olfactory lobes, cerebral cortex, cerebellum, diencephalon, and pons-medulla sections of the brain were removed. The brain sections were then weighed and 10% (wt/vol) homogenates were prepared in minimal essential medium containing 10% fetal bovine serum. Samples were centrifuged at 4°C in a Beckman refrigerated tabletop centrifuge at 1,500 rpm for 15 min. The supernatant was removed, aliquoted, and frozen at −70°C until assayed for HSV.
Administration of Cereport and [14C]ACV.
The uptake of [14C]ACV (Glaxo SmithKline, Inc., Research Triangle Park, N.C.) into rat brains, either uninfected or 6 days after infection with HSV-1, was determined after treatment with either Cereport or saline by using catheters placed in the jugular and femoral veins. To insert the catheters, rats were weighed and anesthetized as previously described. The neck area and the lower left abdominal quadrant including the medial face of the left thigh were shaved and sterilized with a betadine solution followed by ethanol. For the jugular vein catheter, a midline incision was made from the lower mandible to the suprasternal notch. The jugular vein was then exposed, and the caudal portion was clamped. A nick was made in the vein with fine scissors, and a PE-50 catheter was fed into the vein. The clamp was removed, and the catheter was pushed further into the cranial vena cava. The vein was then checked for patency and secured with Vet-Bond. For the femoral vein catheter, an incision was made at the junction of the left hind leg and the trunk. The internal femoral vein was exposed and nicked with fine scissors. A PE-50 catheter was inserted and tied off with a proximal suture. Once the vein was assessed for patency, the catheter was further secured with Vet-Bond.
After catheterization, animals were attached to a perfusion pump (Razel Scientific Instruments, Inc., Stamford, Conn.) and infused with either saline or 6 μg of Cereport/kg in saline for 20 min at a rate of 50 μl/min. Five minutes into the infusion 50 μCi of [14C]ACV (specific activity, 12,472 dpm/pmol) was added as a 150-μl bolus. The [14C]ACV bolus was followed with 300 μl of saline to flush the infusion line. At 10 and 20 min into the infusion, heparinized blood was taken from the femoral vein. The samples were centrifuged to separate plasma from the red blood cells. After collecting the 20-min blood sample, 100 μl of cerebral spinal fluid (CSF) was obtained via cisternal puncture caudal to the occipital protuberance. Following the infusion, the animal was sacrificed by decapitation and the brain was carefully removed and blotted for determination of [14C]ACV content.
Analysis of [14C]ACV in plasma, blood cells, CSF, and brain tissue.
The amount of [14C]ACV in plasma and CSF was determined by adding 100 μl of plasma or CSF to 5 ml of liquid scintillation fluid and counting in a liquid scintillation counter. To determine the amount of [14C]ACV in red blood cells, 50 μl of blood was added to 600 μl of NCSII solubilizer for liquid scintillation counting (Amersham Corp., Arlington Heights, Ill.) and vortexed. This solution was then heated at 50°C in a water bath for 20 to 30 min. After heating, 600 μl of 20% benzoyl peroxide (wt/vol) in acetone and 18 μl of acetic acid were added to each tube. The tubes were then heated at 50°C for an additional 20 to 30 min. After cooling, the solution was added to 5 ml of liquid scintillation fluid for counting.
The amount of [14C]ACV in brain tissue was determined by mincing the whole brain in 2 to 3 ml of water so that the process of digestion would proceed more rapidly. The minced sample was then separated into vials containing approximately 150 mg of tissue and reweighed. NCSII was added to each vial at the ratio of 1 ml/100 mg of tissue. Vials were capped, vortexed, and heated at 50°C until a homogeneous solution was obtained (1 to 16 h). Thirty microliters of glacial acetic acid was added per ml of NCSII to maximize the efficiency of the reaction and to minimize background. The solution was then cooled and added to 5 ml of liquid scintillation fluid for counting.
To determine if [14C]ACV was preferentially taken up by certain tissues in the infected rat brain, the distribution of [14C]ACV was determined in both uninfected and HSV-1-infected animals infused with either Cereport or saline. To prepare the rat brain tissue for radioactivity determinations, brains were first minced into pieces weighing no more than 150 mg each. For each brain, the weight of each piece versus the radioactivity level obtained from each piece was plotted and a linear regression analysis was performed.
Efficacy of ACV during infusion with Cereport.
The efficacy of ACV administered during infusion with Cereport or saline was determined for HSV-1-infected rats. Initially, rats were weighed, anesthetized, and inoculated intranasally with HSV-1 as described previously. The next day rats were again anesthetized and a jugular vein catheter was inserted, also as described above. To prevent catheter removal by the animal, the catheter was threaded under the skin and out the dorsal surface behind the neck of the rat. The incision was sutured, and the area was wrapped with Vet Wrap both to protect the incision from infection and to keep the catheter in place. The catheter was flushed with heparin and capped at all times except during treatments. On day 2 following viral inoculation, rats with patent catheters were divided into two treatment groups. The first group of rats was infused with Cereport (6 μg/kg) via the jugular vein catheter for 20 min at a rate of 50 μl/min. Five minutes into the infusion a bolus of ACV (20, 40, or 80 mg/kg) was administered through the catheter followed by 300 μl of saline. In the second group of rats, saline instead of Cereport was infused prior to the ACV bolus. After 5 days of treatments every 12 h, surviving rats were sacrificed and brain sections were removed, homogenized, and assayed for HSV-1 as described previously.
RESULTS
Mortality of rats infected with HSV-1, strain E-377.
Intranasal inoculation of rats with HSV-1, strain E-377, results in the development of HSV encephalitis in a majority of the animals and, depending on the quantity of viral inoculum, can be lethal. To determine the virus inoculum needed to result in an infection rate of 90 to 100%, rats were inoculated with concentrations of HSV-1 ranging from 4.8 × 103 PFU to 6.0 × 105 PFU. Animals were monitored for 21 days following viral inoculation, and mortality was recorded. The results (Table 1) indicated that the highest titers resulted in 100% mortality whereas a viral input of 2.4 × 104 PFU resulted in 88% mortality and an inoculum of 4.8 × 103 PFU resulted in 67% mortality. Based on these data, we chose an input of 2.4 × 104 PFU for all future experiments. This inoculum would insure significant infection rates without an overwhelming rate of virus-induced mortality.
TABLE 1.
Mortality of rats inoculated intranasally with HSV-1
| Virus inoculum (PFU)a | Mortality
|
MDD | |
|---|---|---|---|
| No. | % | ||
| 6.0 × 105 | 9/9 | 100 | 6.2 |
| 1.2 × 105 | 9/9 | 100 | 6.0 |
| 2.4 × 104 | 7/8 | 88 | 8.0 |
| 4.8 × 103 | 6/9 | 67 | 7.3 |
Animals were inoculated intranasally with 0.04 ml of the indicated concentrations of HSV-1, strain E-377.
To determine the optimal ACV concentration for treatment, we assessed the effect of ACV treatment on the mortality of rats infected with 2.4 × 104 PFU of HSV-1. Beginning 24 h after infection, animals were treated with phosphate-buffered saline (placebo) or 60, 80, 100, or 120 mg of ACV/kg intraperitoneally twice daily for 5 days. Animals were monitored for 21 days, and mortality was recorded. The results (Table 2) indicated that only the 120- or 100-mg/kg dose of ACV significantly reduced mortality. While the 120-mg/kg dose appeared to be slightly toxic to the animals, the 100-mg/kg dose of ACV resulted in no mortality in either infected or control animals. ACV at 80 and 60 mg/kg did not significantly reduce mortality rates but did increase the MDD.
TABLE 2.
Effect of treatment with acyclovir on the mortality of rats inoculated with HSV-1
| Treatmenta | Mortality
|
MDD | P | ||
|---|---|---|---|---|---|
| No. | % | P | |||
| Placebo (PBS) | 9/10 | 90 | 7.3 | ||
| ACV (mg/kg) | |||||
| 120 | 3/10 | 30 | <0.05 | 7.7 | NS |
| 120b | 1/6 | 17 | 5.0 | ||
| 100 | 0/10 | 0 | <0.0001 | ||
| 100b | 0/6 | 0 | |||
| 80 | 4/10 | 40 | NSc | 14.8 | <0.0001 |
| 80b | 0/6 | 0 | |||
| 60 | 4/10 | 40 | NS | 11.3 | <0.01 |
| 60b | 1/6 | 17 | 4.0 | ||
Treatment was initiated 24 h after infection and continued twice daily for 5 days.
Uninfected animals; toxicity.
NS, not significant.
Pathogenesis of HSV-1 infection in rat brain.
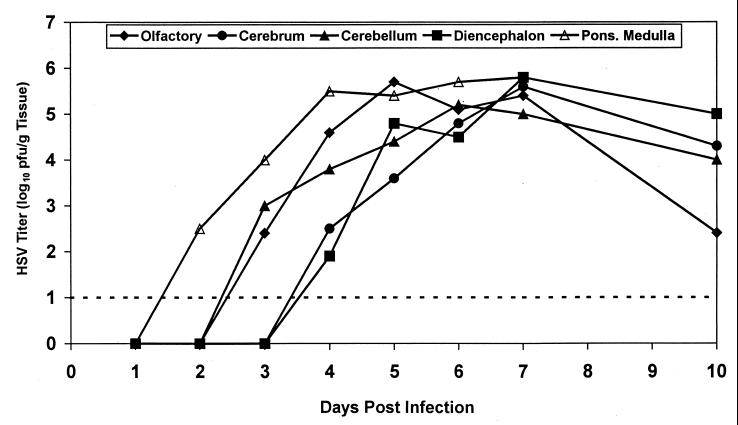
To determine the extent of viral replication in the brain, we investigated the pathogenesis of HSV-1 infection after intranasal inoculation with 2.4 × 104 PFU of HSV-1. On each of days 1, 2, 3, 4, 5, 6, 7, and 10, three animals were sacrificed and the olfactory lobes, cerebral cortex, cerebellum, diencephalon, and pons-medulla were removed and assayed for HSV. The results (Fig. 1) indicated that viral replication increased over time in all regions of the brain, reaching peak levels by day 7. In animals that survived greater than 7 days, viral titers declined. HSV-1 was first detectable in the pons-medulla 2 days after infection. Three days after infection, virus was detected in the olfactory lobes and the cerebellum. Lastly, virus was detected in the cerebral cortex and diencephalon 4 days after HSV-1 inoculation.
FIG. 1.
Replication of HSV-1, E-377, in brains of rats inoculated intranasally. The pathogenesis of HSV-1 infection in rat brain was determined after intranasal inoculation of 2.4 × 104 PFU of HSV-1. Following inoculation, animals were sacrificed on days 1, 2, 3, 4, 5, 6, 7, and 10. Olfactory lobes, cerebral cortex, cerebellum, diencephalon, and pons-medulla were removed, homogenized, and assayed for HSV-1. HSV-1 titers are expressed as log10 PFU/gram of tissue. A value of 1 log10 PFU/g of tissue is the lowest detectable limit.
Uptake of [14C]ACV into rat brains.
The pharmacokinetic profile of ACV in rat brain administered during infusion with and without Cereport was examined using 14C-labeled ACV. Rats were catheterized, infused with Cereport or saline, given a [14C]ACV bolus, and sampled as previously described in Materials and Methods. The results are presented in Table 3. In uninfected animals, Cereport increased the uptake of [14C]ACV from 1.4 ± 0.2 to 4.1 ± 0.7 nmol/g of rat brain. These values indicate an increase in [14C]ACV uptake in animals infused with Cereport of approximately threefold over animals infused with saline. In HSV-1-infected animals, the increase in uptake was approximately twofold, from 1.7 ± 0.3 to 3.5 ± 0.7 nmol of ACV/g of brain tissue. In both infected and uninfected animals, the increase in [14C]ACV uptake in rat brain was shown to be statistically significant in the analysis of variance test (P = 0.0001). The levels of radioactivity in plasma, blood cells, and CSF were shown to be higher in uninfected animals infused with Cereport than observed in saline-infused animals. In HSV-1-infected animals, this situation was reversed. That is, radioactivities in plasma, blood, and CSF were shown to be higher in the saline-infused group than in the group infused with Cereport. Although this variability cannot be easily explained, the expression of similar ratios of the radioactivity found in plasma at 20 min to that found in the CSF at 20 min suggests that [14C]ACV was distributed similarly throughout the tissues in both Cereport- and saline-infused animals.
TABLE 3.
Uptake of [14C] ACV in uninfected and HSV-1-infected rats infused with Cereport
| Organ or fluid (min) | Uptakea of [14C]ACV in rats
|
|||
|---|---|---|---|---|
| Uninfected
|
HSV-1 infected
|
|||
| Saline | Cereport | Saline | Cereport | |
| Brain | 1.4 ± 0.2 | 4.1 ± 0.7 | 1.7 ± 0.3 | 3.5 ± 0.7 |
| Plasma (10) | 43.0 ± 4.6 | 107.7 ± 23.1 | 103.7 ± 24.5 | 73.6 ± 17.3 |
| Plasma (20) | 20.7 ± 3.5 | 84.1 ± 19.0 | 74.2 ± 14.3 | 29.5 ± 5.0 |
| Blood cells (10) | 6.8 ± 3.4 | 8.4 ± 5.4 | 6.3 ± 1.3 | 7.1 ± 4.1 |
| Blood cells (20) | 3.3 ± 1.2 | 6.2 ± 3.7 | 5.7 ± 2.3 | 2.9 ± 1.4 |
| CSF | 1.2 ± 0.2 | 4.9 ± 3.8 | 7.9 ± 3.0 | 4.6 ± 2.9 |
| Plasma/CSF ratio (20) | 17.3 ± 4.2 | 22.5 ± 10.2 | 10.0 ± 2.8 | 9.4 ± 7.3 |
Values are micromolar, except for those of brain, which are expressed in nanomoles per gram.
One of the pathogenic manifestations of HSV encephalitis in humans is acute necrosis of certain brain structures such as the temporal lobe. As this may cause damage to the BBB and alter the absorption of [14C]ACV, the distribution of labeled ACV in both uninfected and HSV-1-infected brain and in Cereport- and saline-infused brain was determined. An analysis of variance test on the coefficients of determination from each linear regression of brain piece weight versus radioactive content indicated that there was no statistical difference in the distribution of [14C]ACV in HSV-1-infected versus -uninfected rat brains (P = 0.211) or in that of brains from Cereport- versus saline-infused animals (P = 0.880).
Efficacy of ACV in Cereport-infused, HSV-1-infected rats.
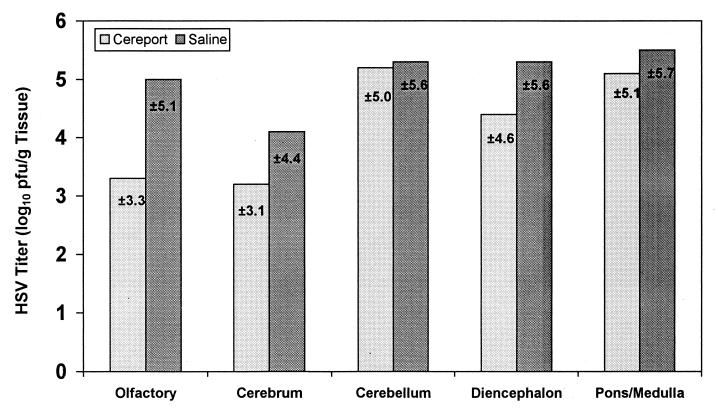
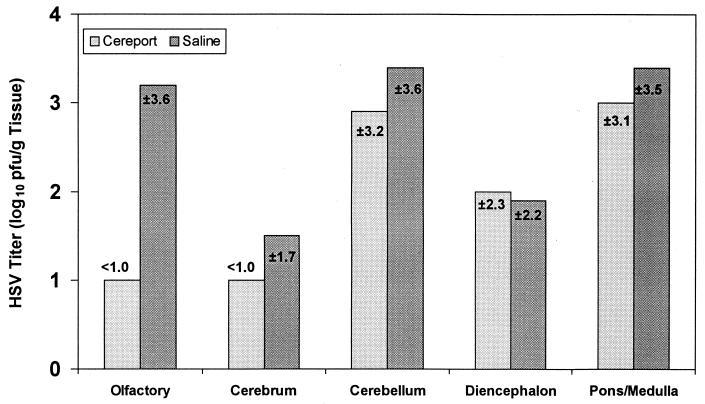
HSV-1-infected rats catheterized in the jugular vein were used to determine the efficacy of ACV administered during infusion with Cereport. Since HSV titers in the various brain structures were shown to peak at day 7, viral determinations of HSV-1 titers in various sections of the brain were determined 7 days after intranasal inoculation with HSV-1. The results in Fig. 2 to 4 suggested that ACV administered during Cereport infusion was more effective in the olfactory lobes and cerebral cortex. Viral replication was unaffected in the pons-medulla, diencephalon, and cerebellum at all doses administered with or without Cereport infusion. The most dramatic effect was seen in the olfactory lobes, where ACV was shown to reduce the amount of virus in animals given Cereport in a dose-dependent manner. This reduction ranged from 1.7 log10 PFU/g of tissue in animals treated with 20 mg of ACV/kg to >3.6 log10 PFU/g in animals treated with 40 mg of ACV/kg. At the highest dose of ACV used in these experiments (80 mg/kg), there appeared to be an increase in efficacy of the drug in various brain tissues in animals infused only with saline. Thus, in Cereport-infused animals, ACV was effective at much lower doses than was required in saline-infused rats. As an antiviral effect was apparent in the saline-infused group treated only with 80 mg of ACV/kg, higher doses of ACV would not be expected to further improve efficacy in animals infused with Cereport.
FIG. 2.
Effect of 20 mg of ACV/kg on the replication of HSV-1 in rat brain. The efficacy of 20 mg of ACV/kg administered during infusion with 6 μg of Cereport/ml was determined 7 days after infection in various portions of the rat brain. HSV-1 titers are expressed as the mean log10 PFU/gram of tissue ± the standard deviation. Means reflect values obtained from four animals that survived out of five in each group. A value of 1 log10 PFU/g of tissue is the lowest detectable limit.
FIG. 4.
Effect of 80 mg of ACV/kg on the replication of HSV-1 in rat brain. The efficacy of 80 mg of ACV/kg administered during infusion with 6 μg of Cereport/ml was determined 7 days after infection in various portions of the rat brain. HSV-1 titers are expressed as the mean log10 PFU/gram of tissue ± the standard deviation. Means reflect values obtained from six animals infused with Cereport and seven animals infused with saline (100% survival). A value of 1 log10 PFU/g of tissue is the lowest detectable limit.
DISCUSSION
In the present study, intravenous infusion of Cereport significantly increased the uptake of [14C]ACV in both uninfected and HSV-1-infected rat brain by two- to threefold. In addition, these studies have indicated that the increase in ACV uptake results in an improvement in drug efficacy in specific parts of the brain such as the temporal and olfactory lobes, as shown by decreased HSV titers after 5 days of treatment. It has been previously reported that Cereport increases the uptake of various-sized hydrophilic compounds, including α-aminoisobutyric acid (Mr, 103), boronophenylalanine (Mr, 208), ganciclovir (Mr, 255), sucrose (Mr, 342), carboplatin (Mr, 373), methotrexate (Mr, 455), inulin (Mr, 5,000), cytokines (Mr, 10,000 to 20,000), and dextran (Mr, 70,000), into brain tumors of both rat and humans (5, 7, 11, 16, 21, 23, 25, 31, 33, 34, 37). In these studies, Cereport infusion selectively increased the permeability of these compounds ranging in molecular weight from 100 to 70,000 from 2- to 3-fold for the smallest compounds to 6-fold for the intermediate-sized cytokines and 12-fold for dextran. Thus, a 2- to 3-fold increase in ACV (Mr, 225) uptake in rat brain observed in our studies is in agreement with previously reported data. In addition, the infusion of Cereport with carboplatin (8, 11, 33), loperamide (20), boronophenylalanine (5), and ganciclovir (31) significantly improved the efficacy of the drug in tumoricidal or analgesic therapy.
Although Cereport was primarily designed to increase permeability across the BBB in tumors, it has also been shown to enhance the permeability of the blood-ocular barrier to compounds such as sucrose and ganciclovir (17, 18), as well as the BBB of normal brain to loperamide (20). In those studies there was a 2-fold increase in the permeability to ganciclovir, a 4.5-fold increase in the uptake of sucrose, and a 2-fold increase in the response time to the analgesic loperamide (Mr, 514) after infusion with Cereport. In comparison, our studies showed a 3-fold increase in [14C]ACV uptake in uninfected rat brain. These effects of increased permeability in the normal BBB, the blood-brain tumor barrier, and the blood-ocular barrier can be explained by the highly preferential binding of the bradykinin analog, Cereport, to constitutively expressed β2 receptors on vascular endothelial cells in the CNS (6). Upon binding to these receptors, the β2 receptor agonist causes an increase in intracellular calcium (15) that is mediated by the nitric oxide-cyclic GMP second messenger system (40), leading to an increase in the intercellular spaces of tight junctions between endothelial cells (38).
Although many pharmacokinetic and physiological characteristics of Cereport have been elucidated, it is unclear why Cereport can be administered to selectively affect tumor tissue without affecting distant nontumor brain tissue. In one report, Elliott et al. (19) suggested that this selectivity in tumor tissue was due to a “leaky” tumor barrier permitting Cereport to diffuse to the β2 receptors on the abluminal side of the endothelial cells, thus eliciting a larger response. In another study, Cereport selectively increased permeability in vasculature of focal irradiated brain lesions induced in canine brains (22). These experiments suggest that Cereport can selectively enhance the permeability of damaged vasculature of the brain without affecting distant undamaged blood vessels. In contrast, our results indicated that the effect of Cereport on the uptake of acyclovir in HSV-1-infected rat brain did not significantly differ from the effect observed in uninfected brain. Although there is damage to blood vasculature within focal encephalopathies and large hemorrhages in HSV encephalitis, there was no statistical difference between the distribution of [14C]ACV in HSV-1-infected rat brain and that in uninfected brain.
One concern in the usage of Cereport is the route of administration and the optimal timing of target drug addition. Intracarotid administration of Cereport is advantageous for a direct and rapid response in the CNS whereas the intravenous route is the preferred one for many therapeutic protocols. In some reports, intracarotid infusion of Cereport was used to allow for better control over timing of the drug to target sites as well as to maintain constant drug levels over specific time intervals (6). Comparisons between intracarotid and intravenous administration of Cereport showed that both routes resulted in an equally significant and selective increase in uptake of compounds like carboplatin (19) and ganciclovir (17, 18). Other studies suggested that a greater concentration of Cereport was necessary to elicit the optimal response with intravenous infusions (21). In those experiments, which were done to optimize the clinical utility of Cereport by refining intravenous administration protocols, the maximum permeation of carboplatin into rat tumors was noted when carboplatin was given as a bolus 5 min into the Cereport infusion. Comparably, in our studies, the optimum protocol was to add ACV as a bolus 5 min into a 20-min Cereport infusion. These studies indicate that by refining each of the administration protocols, both intracarotid and intravenous infusions of Cereport can result in a physiological and clinically effective response in modulating the BBBs to increase delivery of hydrophilic drugs to the brain.
In summary, the results presented in this report demonstrate that intravenous infusion of Cereport significantly increased uptake of ACV into HSV-1-infected as well as -uninfected rat brains. The increase in ACV uptake was in agreement with previously reported studies on the effect of Cereport and correlated with an inhibition of HSV-1 replication in specific regions of the HSV-1-infected rat brain. Although the increase in efficacy was limited to the frontal and temporal portions of the brain, a dramatic decrease in HSV-1 replication was demonstrated in these areas. Although additional experimental studies are needed, these data indicate that infusing Cereport with ACV can enhance the penetration of the drug into the brain parenchyma and improve efficacy in the treatment of experimental HSV infections. The results of these studies further suggest that approaches of this type to better facilitate the penetration of an active drug into CNS tissue may have potential for treatment of these infections in humans.
FIG. 3.
Effect of 40 mg of ACV/kg on the replication of HSV-1 in rat brain. The efficacy of 40 mg of ACV/kg administered during infusion with 6 μg of Cereport/ml was determined 7 days after infection in various portions of the rat brain. HSV-1 titers are expressed as the mean log10 PFU/gram of tissue ± the standard deviation. Means reflect values obtained from three animals that survived out of five in each group. A value of 1 log10 PFU/g of tissue is the lowest detectable limit.
ACKNOWLEDGMENTS
We thank Raymond Bartus and Peter Elliot at Alkermes, Inc., for providing Cereport and for their input in the experimental design. We also thank Karen Biron, GlaxoSmithKline, Inc., Research Triangle Park, N.C., for providing us with the 14C-labeled acyclovir and Richard J. Whitley for his critical review of the manuscript.
This work was supported by Public Health Service contract NO1-AI-65290 from the NIAID, National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Auer L, Johansson B, MacKenzie E T. Cerebral venous pressure during actively induced hypertension and hypercapnia in cats. Stroke. 1980;11:180–183. doi: 10.1161/01.str.11.2.180. [DOI] [PubMed] [Google Scholar]
- 2.Aurelius E, Johansson B, Sköldenberg B, Forsgren M. Encephalitis in immunocompetent patients due to herpes simplex virus 1 or 2 as determined by PCR or type-specific antibody assays of cerebrospinal fluid. J Med Virol. 1993;39:179–186. doi: 10.1002/jmv.1890390302. [DOI] [PubMed] [Google Scholar]
- 3.Baethmann A, Kempski O, Unterberg A, Maier-Hauff K, Lange M, Schürer L. Mechanismen und Therapeutische Aspekte beim Zerebralen Sekundärschaden. Münch Med Wochenschr. 1982;124:941–944. [PubMed] [Google Scholar]
- 4.Barnett F H, Rainov N G, Ikeda K, Schuback D E, Elliott P, Kramm C M, Chase M, Qureshi N H, Harsh G, Chiocca E A, Breakefield X O. Selective delivery of herpes virus vectors to experimental brain tumors using RMP-7. Cancer Gene Ther. 1999;6:14–20. doi: 10.1038/sj.cgt.7700003. [DOI] [PubMed] [Google Scholar]
- 5.Barth R F, Yang W, Bartus R T, Moeschberger M L, Goodman J H. Enhanced delivery of boronophenylalanine for neutron capture therapy of brain tumors using the bradykinin analog Cereport (Receptor-Mediated Permeabilizer-7) Neurosurgery. 1999;44:351–360. doi: 10.1097/00006123-199902000-00062. [DOI] [PubMed] [Google Scholar]
- 6.Bartus R, Elliott P, Hayward N, Dean R, McEwen E, Fisher S. Permeability of the BBB by the bradykinin agonist, RMP-7: evidence for a sensitive, auto-regulated, receptor mediated system. Immunopharmacology. 1996;33:270–278. doi: 10.1016/0162-3109(96)00070-7. [DOI] [PubMed] [Google Scholar]
- 7.Bartus R T, Elliott P J, Dean R L, Hayward N J, Nagle T L, Huff M R, Snodgrass P A, Blunt D G. Controlled modulation of BBB permeability using the bradykinin agonist, RMP-7. Exp Neurol. 1996;142:14–28. doi: 10.1006/exnr.1996.0175. [DOI] [PubMed] [Google Scholar]
- 8.Black K L, Cloughesy T, Huang S-C, Gobin Y P, Zhou Y, Grous J, Nelson G, Farahani K, Hoh C K, Phelps M. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg. 1997;86:603–609. doi: 10.3171/jns.1997.86.4.0603. [DOI] [PubMed] [Google Scholar]
- 9.Booss J, Esiri M M. Viral encephalitis pathology, diagnosis, and management. London, England: Blackwell Scientific Publications; 1986. pp. 55–93. [Google Scholar]
- 10.Broadwell R D, Salcman M. Expanding the definition of the blood-brain barrier to protein. Proc Natl Acad Sci USA. 1981;78:7820–7824. doi: 10.1073/pnas.78.12.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloughesy T F, Black K L, Gobin Y P, Farahani K, Nelson G, Villablanca P, Kabbinavar F, Vinuela F, Wortel C H. Intra-arterial Cereport (RMP-7) and carboplatin: a dose escalation study for recurrent malignant gliomas. Neurosurgery. 1999;44:270–279. doi: 10.1097/00006123-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Davis L E, McLaren L C. Relapsing herpes simplex encephalitis following antiviral therapy. Ann Neurol. 1983;13:192–195. doi: 10.1002/ana.410130215. [DOI] [PubMed] [Google Scholar]
- 13.de Miranda P, Krasny H C, Page D A, Elion G B. Species differences in the disposition of acyclovir. In: King D H, Galasso G, editors. Proceedings of a symposium on acyclovir. New York, N.Y: Technical Publishing; 1982. pp. 31–35. [DOI] [PubMed] [Google Scholar]
- 14.Dix R D, Barringer J R, Panitch H S, Rosenberg S H, Hagedorn J, Whaley J. Recurrent herpes simplex encephalitis: recovery of virus after Ara-A treatment. Ann Neurol. 1983;13:196–200. doi: 10.1002/ana.410130216. [DOI] [PubMed] [Google Scholar]
- 15.Doctrow S R, Abelleira S M, Curry L A, Heller-Harrison R, Kozarich J W, Malfroy B, McCarroll L A, Morgan K G, Morrow A R, Musso G F, Smart J L, Straub J A, Turnbull B, Gloff C. The bradykinin analog RMP-7 increases intracellular free calcium levels in rat brain microvascular endothelial cells. J Pharmacol Exp Ther. 1994;271:229–237. [PubMed] [Google Scholar]
- 16.Elliott P, Hayward N, Dean R, Blunt D G, Bartus R. Intravenous RMP-7 selectively increases uptake of carboplatin into rat brain tumors. Cancer Res. 1996;56:3998–4005. [PubMed] [Google Scholar]
- 17.Elliott P, Mackic J, Graney W, Bartus R T, Zlokovic B. RMP-7, a bradykinin agonist, increases permeability of blood-ocular barriers in the guinea pig. J Investig Ophthalmol Vis Sci. 1995;36:2542–2547. [PubMed] [Google Scholar]
- 18.Elliott P J, Bartus R T, Makvic J B, Zlokovic B V. Intravenous infusion of RMP-7 increases ocular uptake of ganciclovir. Pharm Res. 1997;14:80–85. doi: 10.1023/a:1012011618785. [DOI] [PubMed] [Google Scholar]
- 19.Elliott P J, Hayward N J, Huff M R, Nagle T L, Black K L, Bartus R T. Unlocking the blood-brain barrier: a role for RMP-7 in brain tumor therapy. Exp Neurol. 1996;141:214–224. doi: 10.1006/exnr.1996.0156. [DOI] [PubMed] [Google Scholar]
- 20.Emerich D F, Snodgrass P, Pink M, Bloom F, Bartus R T. Central analgesic actions of loperamide following transient permeation of the blood brain barrier with Cereport (RMP-7) Brain Res. 1998;801:259–266. doi: 10.1016/s0006-8993(98)00571-x. [DOI] [PubMed] [Google Scholar]
- 21.Emerich D F, Snodgrass P, Dean R, Agostino M, Hasler B, Pink M, Xiong H, Kim B S, Bartus R T. Enhanced delivery of carboplatin in brain tumors with intravenous Cereport (RMP-7): dramatic differences and insight gained from dosing parameters. Br J Cancer. 1999;80:964–970. doi: 10.1038/sj.bjc.6690450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fike J R, Gobbel G T, Mesiwala A H, Shin H J, Nakagawa M, Lamborn K R, Seilhan T M, Elliot P J. Cerebrovascular effects of the bradykinin analog RMP-7 in normal and irradiated dog brain. J Neurooncol. 1998;37:199–215. doi: 10.1023/a:1005874206814. [DOI] [PubMed] [Google Scholar]
- 23.Ford J, Osborn C, Barton T, Bleehen N M. A phase I study of intravenous RMP-7 with carboplatin in patients with progression of malignant glioma. Eur J Cancer. 1998;34:1807–1811. doi: 10.1016/s0959-8049(98)00155-5. [DOI] [PubMed] [Google Scholar]
- 24.Hansson H A, Johansson B B. Induction of pinocytosis in cerebral vessels by acute hypertension and hyperosmolar solution. J Neurosci Res. 1980;5:183–190. doi: 10.1002/jnr.490050303. [DOI] [PubMed] [Google Scholar]
- 25.Inamura T, Nomura T, Bartus R, Black K. Intracarotid infusion of RMP-7, a bradykinin analog: a method for selective drug delivery to brain tumors. J Neurosurg. 1994;81:752–758. doi: 10.3171/jns.1994.81.5.0752. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy P G E, Adams J H, Graham D I, Clements G B. A clinico-pathological study of herpes simplex encephalitis. Neuropathol Appl Neurobiol. 1988;14:395–415. doi: 10.1111/j.1365-2990.1988.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 27.Kern E R, Overall J C, Jr, Glascow L A. Herpesvirus hominus infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J Infect Dis. 1973;128:290–299. doi: 10.1093/infdis/128.3.290. [DOI] [PubMed] [Google Scholar]
- 28.Kimberlin D, Powell D, Gruber W, Diaz P, Arvin A, Kumar M, Jacobs R, Van Dyke R, Burchett S, Soong S-J, Lakeman A, Whitley R the NIAID Collaborative Antiviral Study Group. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to skin, eyes and mouth: results of a phase I/II trial. Pediatr Infect Dis J. 1996;15:247–254. doi: 10.1097/00006454-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Kosaburo A, Aso K, Kuzushima K, Hanada N, Shibata M, Morishima T. Relapse of herpes simplex encephalitis in children. Pediatrics. 1992;89:891–894. [PubMed] [Google Scholar]
- 30.Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;72:236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- 31.LeMay D R, Kittaka M, Gordon E M, Gray B, Stins M F, McComb K G, Jovanovic S, Tabrizi P, Weiss M H, Bartus R, Anderson W F, Zlokovic B V. Intravenous RMP-7 increases delivery of ganciclovir into rat brain tumors and enhances the effects of herpes simplex thymidine kinase gene therapy. Hum Gene Ther. 1998;9:989–995. doi: 10.1089/hum.1998.9.7-989. [DOI] [PubMed] [Google Scholar]
- 32.Lipton J D, Schafermeyer R W. Central nervous system infections: the usual and the unusual. Pediatr Emerg. 1995;13:417–443. [PubMed] [Google Scholar]
- 33.Matsukado K, Inamura T, Nakano S, Fukui M, Bartus R T, Black K L. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 1996;39:125–134. doi: 10.1097/00006123-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Matsukado K, Nakano S, Bartus R T, Black K L. Steroids decrease uptake of carboplatin in rat gliomas—uptake improved by infusion of bradykinin analog, RMP-7. J Neurooncol. 1997;34:131–138. doi: 10.1023/a:1005706329630. [DOI] [PubMed] [Google Scholar]
- 35.Mayhan W G, Faraci F M, Heistad D. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am J Physiol. 1986;251:H1171–H1175. doi: 10.1152/ajpheart.1986.251.6.H1171. [DOI] [PubMed] [Google Scholar]
- 36.Nag S, Robertson D M, Dinsdale H B. Quantitative estimate of pinocytosis in experimental acute hypertension. Acta Neuropathol. 1979;46:107–116. doi: 10.1007/BF00684811. [DOI] [PubMed] [Google Scholar]
- 37.Nakano S, Matsukado K, Black K L. Enhanced cytokines delivery and intercellular adhesion molecule 1 (ICAM-1) expression in glioma by intracarotid infusion of bradykinin analog, RMP-7. Neurolog Res. 1997;19:501–508. doi: 10.1080/01616412.1997.11740848. [DOI] [PubMed] [Google Scholar]
- 38.Sanovich E, Bartus R T, Friden P M, Dean R L, Le R Q, Brightman M W. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7. Brain Res. 1995;705:125–135. doi: 10.1016/0006-8993(95)01143-9. [DOI] [PubMed] [Google Scholar]
- 39.Sköldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl. 1996;100:8–13. [PubMed] [Google Scholar]
- 40.Sugita M, Hunt G E, Liu Y, Black K L. Nitric oxide and cyclic GMP attenuate sensitivity of the blood-tumor barrier permeability to bradykinin. Neurol Res. 1998;20:559–563. doi: 10.1080/01616412.1998.11740564. [DOI] [PubMed] [Google Scholar]
- 41.Wahl M, Unterberg A, Boethmann A, Schilling L. Mediators of blood-brain barrier dysfunction and formation of vasogenic brain edema. J Cereb Blood Flow Metab. 1988;8:621–634. doi: 10.1038/jcbfm.1988.109. [DOI] [PubMed] [Google Scholar]
- 42.Whitley R J. Neonatal herpes simplex infections. J Med Virol Suppl. 1993;1:13–21. doi: 10.1002/jmv.1890410505. [DOI] [PubMed] [Google Scholar]
- 43.Whitley R J. Neonatal herpes simplex virus infections: is there a role for immunoglobulin in disease prevention and therapy? Pediatr Infect Dis J. 1994;13:432–439. [PubMed] [Google Scholar]
- 44.Whitley R J, Alford C A, Hirsch M S, Schooley R T, Luby J P, Aoki F Y, Hanley D, Nahmias A J, Soong S J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. New Engl J Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 45.Whitley R J, Kimberlin D W. Viral encephalitis. Pediatr Rev. 1999;20:192–198. doi: 10.1542/pir.20-6-192. [DOI] [PubMed] [Google Scholar]
- 46.Whitley R J, Kimberlin D W, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–555. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 47.Whitley R J, Soong S J, Linneman C, Liu C, Pazin G, Alford C A. The NIAID Collaborative Study Group. Herpes simplex encephalitis: clinical assessment. JAMA. 1982;247:317–320. [PubMed] [Google Scholar]