Abstract
Abstract
The compounds bearing naphthalene moiety can be used as medical preparations because of their wide spectrum of biological activity and low toxicity. In this study, a new series of azoles or azines were synthesized from the reaction of the key intermediate 1-(1-hydroxynaphthalen-2-yl)-3-phenylpropane-1,3-dione 3 with a variety of electrophilic and nucleophilic reagents under a variety of mild conditions. The chemical structures of these compounds were confirmed by various spectroscopic methods such as (IR, 1H-NMR, 13C-NMR, mass spectra and elemental analyses). The prepared compounds were screened in vitro for their anti-microbial activity against some species of Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeuroginosa). Anti-fungal activities of the compounds were tested against yeast and mycelial fungi,Candida albicans and Aspergillus flavus. The antimicrobial activity of this series was showed either weak or moderate activities.
Graphic abstract
Keywords: Heterocycles, Microwave irradiation, Spectral characteristics, Antimicrobial activities
Introduction
According to the literature survey, heterocyclic compounds containing nitrogen, sulfur and oxygen atom are found to possess a varity of biological activities. Among them, pyrazoles, cyanopyridines, pyrimidinethiones. pyranes or their fused ring systems and pyridazine are found to exhibit a wide spectrum of biological activities. Various biological applications have been reported for pyrazoles such as anti-viral [1], anti-cancer [2], anti-microbial [3], anti-inflammatory [4], anti-depressant [5] anti-convulsant [6] analgesic and antiplatelet [7, 8]. Cyanopyridine derivatives have attracted considerable attention as they appeared of interest to possess anti-cancer [9], anti-convulsant [10] and anti-microbial [11]. Pyrimidinethiones have been found to possess anti-tubercular [12]. Pyrane and fused 4H-pyrane derivatives have attracted a great interest owing to their anti-microbial [13, 14], anti-oxidant [15], inhibitors of influenza virus sialidases [16], mutagenic activity [17], anti-cancer [18] and anti-viral [19]. Also, Pyridazine ring is a nucleus of a many of drugs available in the market like cadralazine (anti-hypertensive), minaprine (anti-depressant), hydralazine (smooth muscle relaxant), pipofezine (tricyclic antidepressant) [20, 21]. The aim of the present work, was to synthesize new pyrazole, pyridine, pyrimidine, pyrane and pyridazine derivatives by using 1-(1-hydroxynaphthalen-2-yl)-3-phenylpropane-1,3-dione 3 as the key starting material. Followed by anti-microbial evaluations of newly synthesized products were done. In general, the novel synthesized compounds showed a moderate antimicrobial activity against the previously mentioned microorganisms.
Results and discussion
Organic synthesis by using microwave irradiation (MW) is a new and interesting technique and is becoming popular now. The reactions under microwave irradiation take place in few minutes and no solvent is required [22–25]. In continution to our research for chemistry developments [26–29], we report here the use of microwave irradiation for the synthesis of 1-(1-hydroxynaphthalen-2-yl)-3-phenyl-propane-1,3-dione 3 in a quantitative yield (91%) from the reaction of α- naphthol and ethyl benzoylacetate in the presence of zinc chloride [30]. Rather than the expected coumarin 4 as the literature [31]. The key intermediate 3 was prepared previously in the literature [32, 33]. The structure of the latter product 3 was established on the basis of its elemental analyses and spectral data and chemical transformation. Thus, the infrared spectrum of compound 3 revealed an absorption bands at 3365, 3061, 2924, 1720, 1639 cm−1 for hydroxyl, aromatic, aliphatic and carbonyl function groups, respectively. The 1H-NMR spectrum of compound 3 showed the following signals at δ = 4.49 (s, 2H, CH2), 7.20–8.68 (m, 11H, aromatic H), 8.69 (s, 1H, OH). Also, the mass spectrum of compound 3 is in agreement with the proposed structure, its showed a molecular ion peak at m/z = 290 (M+) corresponding to a molecular formula C19H14O3 (Scheme 1).
Scheme 1.
The synthesis route of compound 3
The active methylene group in compound 3 was exploited to synthesize some novel heterocyclic compounds by its reaction with some electophilic and nucleophilic reagents. Thus, the reaction of compound 3 with dimethylformamide-dimethylacetal (DMF-DMA) in dioxane afforded the enaminone derivative 5 in a good yield as demonstrated in (Scheme 2). Establishing the structure 5 was based on its elemental analyses and spectral data. Thus, the 1H-NMR spectrum of compound 5 showed the following signals, a singlet signal at δ = 3.00 ppm assigned to N(CH3)2, a singlet signal at δ = 7.19 ppm assigned to oleffinic proton, a multiplet signals at δ = 7.64–8.67 ppm assigned to aromatic protons and a singlet signal at δ = 8.68 ppm assigned to hydroxyl group. Moreover, the mass spectrum revealed a molecular ion peak at m/z = 345 (M+) related to a molecular formula C22H19NO3. Also, when compound 3 was alkylated with triethylorthoformate in refluxing acetic anhydride afforded the ethoxymethylene derivative 6. Establishing the structure 6 was based on spectral data in addition of elemental analyses. So, its 1H-NMR spectrum in DMSO-d6 revealed the following signals at δ = 1.11 (t, 3H, CH3), 4.19 (q, 2H, CH2), 6.33 (s, 1H, CH-oleffinic), 7.29–8.23 (m, 11H, aromatic H), 8.68 (s, 1H, OH). The mass spectrum of the same product is in accordance with the proposed structure. Thus, it showed a molecular ion peak at m/z = 346 (M+).
Scheme 2.

Synthetic route to pyridinethione and pyrazoles
Compound 5 readily reacted with cyanothioacetamide 7 as example of active methylene reagents in refluxing ethanolic sodium ethoxide yield the expected pyridinethione derivative 10 which established on its spectral data (IR, 1H-NMR, mass spectra, 13C-NMR and elemental analyses). So, its mass spectrum revealed a molecular ion peak at m/z = 384 (M+ + 2) corresponding to a molecular formula C23H14N2O2S. Formation of pyridinethione 10 is believed to be proceed via initial addition of active methylene moiety in cyanothioacetamide on the π-bond system of the carbonyl group of 5 to afford the Michael adduct 8 that cyclizes by losing water molecule under the same reaction condition to give intermediate 9 which eliminate dimethylamine molecule to yield the final structure 10 as demonstrated in (Scheme 2) [34]. Furthermore, the behavior of enaminone 5 toward binucleophilic reagents was also investigated. Thus, when compound 5 was allowed to react with hydrazine hydrate a compound with a molecular formula C20H14N2O2 = 314 (M+) is formed which may be formulated as structure 14 based on spectroscopic data. Thus, the 1H-NMR spectrum of compound 14 showed a singlet signal at δ = 7.22 assigned to CH-pyrazole, a multiplet signals at δ = 7.31–8.71 assigned for CH aromatic and NH protons and a singlet signal at δ = 8.73 assigned to OH group. On the other hand, compound 6 was reacted with hydrazine hydrate to afford the product identical in all respects (mp, mixed mp, and spectral data) with those corresponding to the pyrazole derivative 14 as demonstrated in (Scheme 2).
Similarly, reactions of enaminone 5 with urea or thiourea to yield the expected pyrimidine derivatives 16a,b. Compounds 16a,b were established on its correct spectral data (IR,1H-NMR, mass spectra and elemental analyses). Also, enaminone 5 reacted with guanidine hydrochloride to yield the pyrimidine derivative 18 which established on spectral data. On the other hand, the reaction of enaminone 5 with hydroxylamine hydrochloride in ethanol containing anhydrous sodium acetate afforded the isoxazole derivative 20. The structure of compound 20 was established based on its elemental analyses and spectroscopic data. Thus, the 1H-NMR spectrum of compound 20 revealed the presence of a singlet signal at δ = 7.21 ppm corresponding to the CH-isoxazole and a multiplet signals at δ = 7.31–8.70 ppm corresponding to aromatic protons and a singlet signal at 8.71 ppm corresponding to OH group. The mass spectrum of compound 20 revealed a molecular ion peak at m/z = 315 (M+) corresponding to a molecular formula C20H13NO3 as demonstrated in (Scheme 3).
Scheme 3.

Synthetic route to pyrimidine and isoxazole
3-Phenylpropane-1,3-dione derivative 3 was examined as a key precursor toward a variety of nucleophlic and electrophilic reagents aiming at exploring its synthetic potentiality. Thus, when compound 3 was reacted with hydroxylamine hydrochloride to yield the isoxazole derivative 23 in a good yield. Compound 23 was established based on its spectral data (IR, 1H-NMR, 13C-NMR, mass spectra) and elemental analyses. The 1H-NMR spectrum of compound 23 showed a singlet signal at δ = 7.21 ppm assigned to CH-isoxazole, a singlet signal at δ = 8.69 ppm assigned to OH group beside a multiplet signals at δ = 7.32–8.54 ppm assigned to aromatic protons. The mass spectrum showed a molecular ion peak at m/z = 287 (M+) corresponding to a molecular formula C19H13NO2 as demonstrated in (Scheme 4).
Scheme 4.

The synthesis route of compound 23
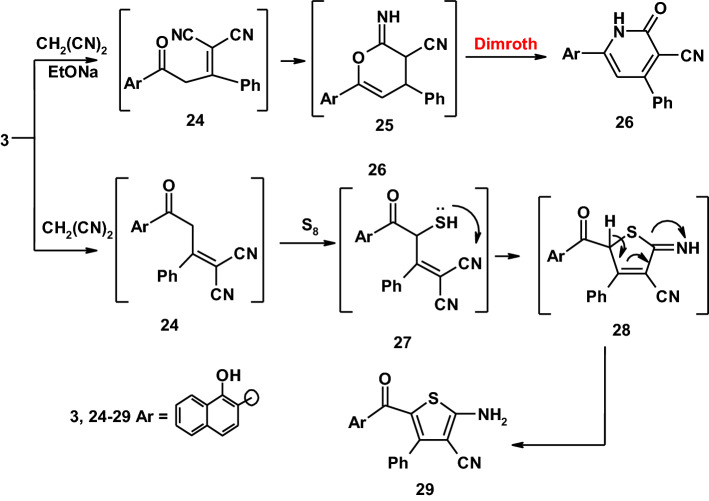
The reactivity of compound 3 toward active methylene reagents was also investigated and found to afford new pyridinone derivative. Thus, when compound 3 was reacted with malononitrile in ethanolic sodium ethoxide, a product 26 with molecular formula C22H14N2O2 was obtained as the sole isolable product through the intermediates 24 and 25 [35]. On the other hand, the reaction of compound 3 with a mixture of malononitrile and elemental sulfur in ethanolic piperidine afforded the expected thiophene derivative 29 as demonstrated in (Scheme 5) [34]. Assignment of structure 29 for the reaction product was based on its correct elemental analyses and compatible spectroscopic data, the 1H-NMR of the structure revealed a singlet signal at δ = 8.69 ppm corresponding to OH group beside a multiplet signals corresponding to the aromatic protons and NH2 at δ = 7.20–8.68 ppm. Also, the mass spectrum showed a molecular ion peak at m/z = 370 (M+) corresponding to a molecular formula C22H14N2O2S.
Scheme 5.
Synthetic route to pyridine and thiophene
One-pot reaction of a mixture of compound 31 and dimedone in the presence of ammonium acetate afforded the tetrahydroquinoline derivatives 35a–c. Assignment of structure 35b as example for the reaction product was based on its compatible spectroscopic data. Thus, its IR spectrum showed an absorption bands at 3371, 3255 cm−1 for (OH/NH), 3059 cm−1 for (CH-arom), 2954–2835 cm−1 for (CH-aliph) and 1639, 1620 cm−1 for (2C=O) group. However, the 1H-NMR of compound 35b for example showed a singlet signals at δ = 0.82, 1.19 ppm corresponding to 2CH3, two singlet signals at δ = 2.70 ppm and at δ = 2.86 ppm corresponding to two methylene groups of dimedone, a singlet signal at δ = 3.70 ppm assigned to OCH3 protons, a singlet signal at δ = 4.49 ppm assigned to CH-pyridine, a singlet signal at δ = 8.45 ppm assigned to OH group beside a multiplet signals at δ = 7.21–8.28 ppm assigned to aromatic protons and NH. The mass spectrum of the same compound revealed a molecular ion peak at 529 (M+) and a number of fragments which agree with the proposed structure. The formation of 35a–c can be understood in terms of the Michael type addition of the active methylene group in the dimedone molecule to the activated double bond in the compound 31 to yield the Michael adduct 33 and cyclized to 34, the intermediate 34 was oxidized by loss of water molecule to yield the final product 35 [36–38] as demonstrated in (Scheme 6).
Scheme 6.
Synthetic route to fused pyridine
Reactions of compound 3 with some electrophilic reagents under alkaline conditions were also investigated. Thus, 4H-pyran-3-carbonitrile derivatives 40a,b were synthesized in an excellent yield upon treatment of 3 with arylidinemalononitrile 36a–b in the presence of a catalytic amount of piperidine. The structures of compounds 40a,b were established based on analytical and spectral data.The products 40a,b are apparently formed via a Michael type addition of the active methylene group in compound 3 to the activated double bond in arylidinemalononitrile 36a,b to form the non-isolable intermediate 39 through an intramolecular cyclization and subsequent tautomerization to give 40a,b. Also, compound 3 was reacted with arylidinecyanothioacetamide to yield the acceptable pyridinethione derivatives 45a–c. We first assumed that the reaction product is formed via addition of active methylene to α, β-unsaturated linkage and subsequent water elimination from carbonyl benzoyl group to yield a cyclic intermediate 44 which aromatized by loss of H2 to yield the pyridinethione 45 as demonstrated in (Scheme 7). Confirmation of pyridinethiones 45 based on its compatible spectroscopic data. Thus, the 1H-NMR spectrum of compound 45b for example showed a singlet signal at δ = 3.87 ppm assigned for OCH3, a singlet signal at δ = 8.74 ppm assigned to OH, a singlet signal at δ = 9.87 ppm assigned for NH group in addition to a multiplet signals at δ = 7.12–8.73 ppm assigned to aromatic protons [34, 39, 40].
Scheme 7.

Reaction of 1,3-diketone with some electrophilic reagents
Similarly, The reaction of compound 3 with cyclopentylidenecynothiocetamide and cyclohexyliden-cynothioacetamide 46a,b yield the azaspiro derivatives 49a,b through the intermediates 47and 48 [41] as demonstrated in (Scheme 8). Establishing the spiro derivatives was based on its compatible spectroscopic data and elemental analyses.
Scheme 8.
The synthetic route of compound 49
Coupling of propane-1,3-dione derivative 3 with diazotized aromatic amines in ethanol buffered with sodium acetate at 0–5 °C afforded the aryl hydrazones 51a–c. The aryl hydrazones were established based on its compatible spectroscopic data and elemental analyses. Condensation of 51 with malononitrile proceeded very readily by fusion in the presence of ammonium acetate to yield the pyridazine derivatives 54 a–c. Compounds 54a–c were established based on spectral data (IR, 1H-NMR and mass spectra) and elemental analyses. Thus, the 1H-NMR spectrum of 54a for example revealed a singlet signal at δ = 3.99 ppm assigned to OCH3, a singlet signal at δ = 8.71 ppm assigned to OH group in addition to a multiplet signals at δ = 6.68–8.25 ppm corresponding to aromatic protons and NH. Also, the mass spectrum of the same compound revealed a molecular ion peak at 472 (M+) corresponding to the molecular formula C29H20N4O3 and a number of fragments which agree with the proposed structure [42] (Scheme 9).
Scheme 9.
Synthetic route to pyridaznes
In vitro antimicrobial activity
The newly synthesized compounds have been screened for antibacterial activity against some species of Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeuroginosa). Anti-fungal activities of the compounds were tested against yeast and mycelial fungi; Candida albicans and Aspergillus flavus, respectively. Each tested compound was dissolved in DMSO making a solution concentration of 1.00 mg/mL and loaded separately in paper disks of Whatman filter paper with equal diameter size (10 mm), Paper disks were sterilized in an autoclave. The paper disks loaded with the desired concentration of the complex solution, were placed aseptically in the petri dishes containing nutrient agar medium (agar 20 g + beef extract 3 g + peptone 5 g) inoculated with Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeuroginosa, Candida albicans and Aspergillus flavus. The petri dishes were incubated at 36 °C. The inhibition zones were recorded after 24 h of incubation in case of bacteria and yeast and after 5–6 days in case of mycelial fungi. Each treatment was replicated three times [43, 44]. Ampicillin and clotrimazole, were used as a common standard antibiotic and antifungal agents, respectively. They prepared using the same procedure as above at the same concentration and solvents. The % activity index was calculated for the tested compounds by using the given formula in Eq. (1).
| 1 |
Minimum inhibitory concentration (MIC) measurement
The minimum inhibitory concentration (MIC) was determined using the disk diffusion technique by preparing disks containing 1.9–1000 µg/mL of each compound against Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeuroginosa). The anti-fungal activities of the compounds were tested against two fungi Candida albicans and Aspergillus flavus. The twofold dilutions of the solution were prepared. The microorganism suspensions at 10 CFU/mL (colony forming unit/mL) concentration were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24 h for the bacteria. The standard antibiotic ampicillin and antifungal clotrimazole was also recorded using the same procedure as above at the same concentration and solvents. At the end of the incubation period, the minimum inhibitory concentrations (MIC) values were recorded as the lowest concentration of the substance that had no visible turbidity. Control experiments with DMSO and uninoculated media were run parallel to the test compounds under the same condition. Tables 1 and 2 illustrated the results of antimicrobial and antifungal activity and it's MIC. The results which are illustrated in Table 1 showed that most of tasted compounds were active against most of micro-organisms used. Both of compounds 45c and 5 showed no antibacterial or antifungal activity. On the other side each of compound 29 and 54a showed maximum antibacterial and antifungal activity. Compound 51a has no antibacterial activity against Gram-negative bacteria only, although it have broad spectrum antibacterial activity against Gram-positive bacteria and antifungal activity against C. albicans and A. flavus. On the other hands, compound 23 showed narrow spectrum antibacterial activity against P. aeuroginosa (a Gram-negative bacteria) and S. aureus (a Gram-positive bacteria) and revealed no antibacterial activity against E. coli (a Gram-negative bacteria) and B. subtilis (a Gram-positive bacteria), but in case of compound 40b it has no antibacterial activity against B. Subtilis only and has narrow range spectrum as antibacterial agent against S. aureus, E. Coli and P. aeuroginosa with also small rang spectrum antifungal activity. All the other compounds (3, 35b, 26, 35c, 35a, 40a, 10, 16a, 14,and 49a) indicated wide range spectrum antibacterial and antifungal activity.
Table 1.
Antibacterial and antifungal activities of synthesized compounds
| Compound | Gram-negative bacteria (−ve) | Gram-positive bacteria (+ve) | Fungal Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeuroginosa | S. aureus | B. subtilis | C. albicans | A. flavus | |||||||
| DIZ (mm) | % Activity index | DIZ (mm) | % Activity index | DIZ (mm) | % Activity index | DIZ (mm) | % Activity index | DIZ (mm) | % Activity index | DIZ (mm) | % Activity index | |
| 35c | 4.1 | 13.7 | 9 | 33.5 | 13.4 | 52.1 | 12 | 45.6 | 13 | 44.6 | 15 | 57.0 |
| 40a | 5.2 | 18.8 | 13 | 44.5 | 14.7 | 56.8 | 14.3 | 54.4 | 16.3 | 55.2 | 17.4 | 66.6 |
| 51a | NA | – | NA | – | 10 | 39.6 | 8 | 32.4 | 13 | 45.7 | 14 | 49.2 |
| 29 | 18.4 | 79.9 | 23 | 94.5 | 25 | 93.5 | 24.8 | 98.9 | 19.1 | 77.3 | 24 | 88.1 |
| 10 | 15 | 64.6 | 17.5 | 70.9 | 22.5 | 86.3 | 19 | 87.9 | 19.3 | 73.8 | 24.8 | 83.7 |
| 40b | 3 | 10.5 | 7 | 23.8 | 5 | 14.7 | NA | – | 4 | 13.5 | 6 | 24.0 |
| 14 | 8 | 29.6 | 10 | 48.7 | 9 | 26.7 | 6 | 18.9 | 8 | 25.2 | 13 | 43.4 |
| 16a | 14.3 | 54.5 | 15 | 66.7 | 16.7 | 80.0 | 19.6 | 85.6 | 19 | 59.7 | 17.8 | 69.1 |
| 3 | 9 | 37.8 | 19 | 61.5 | 14 | 46.3 | 11 | 36.8 | 11 | 22.8 | 10 | 33.0 |
| 35a | 15 | 70.0 | 20 | 83.9 | 19.7 | 73.8 | 19.6 | 77.3 | 17.6 | 63.5 | 19.4 | 73.6 |
| 35b | 11 | 40.8 | 17.3 | 63.9 | 16.9 | 71.7 | 17.8 | 69.4 | 12.9 | 56.0 | 18 | 62.0 |
| 23 | NA | – | 4 | 13.8 | 3 | 8.9 | NA | – | 5 | 12.3 | 7 | 17.0 |
| 26 | 13 | 49.5 | 16 | 69.2 | 14 | 65.5 | 14 | 58.5 | 10 | 25.6 | 14 | 42.0 |
| 45c | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – |
| 49a | 5 | 24.8 | 12 | 54.2 | 8 | 35.3 | 6 | 21.7 | 8 | 33.3 | 11 | 44.0 |
| 5 | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – |
| 54a | 11 | 77.2 | 27 | 83.9 | 24 | 84.5 | 31 | 97.6 | 24.7 | 85.3 | 28.5 | 97.0 |
| Ampicillin | 25 | 100 | 25 | 100 | 24 | 100 | 25 | 100 | NA | – | NA | – |
| Clotrimazole | NA | – | NA | – | NA | – | NA | – | 26 | 100 | 26 | 100 |
NA No activity, DIZ Diameter of inhibition zone
Table 2.
Minimum inhibitory concentrations (MIC) for selected compounds
| Compounds | Minimum inhibitory concentration (MIC) of the synthesized compounds (µg/mL) | |||||
|---|---|---|---|---|---|---|
| E. coli | P. aeuroginosa | S. aureus | B. subtilis | C. albicans | A. flavus | |
| 35c | 760 | 530 | 270 | 500 | 65.5 | 50.9 |
| 40a | 780 | 385 | 260 | 500 | 48.9 | 29.4 |
| 51a | NA | NA | 530 | 740 | 95.7 | 69.5 |
| 29 | 98.7 | 68.5 | 66.5 | 135 | 14.6 | 8.8 |
| 10 | 197.5 | 135 | 90.7 | 189.4 | 22.4 | 9.8 |
| 40b | NA | 750 | NA | NA | 510 | 365 |
| 14 | 540 | 260 | 740 | NA | 188.5 | 93.7 |
| 16a | 188.4 | 125 | 125 | 177.5 | 37.2 | 17.6 |
| 3 | 385 | 187.5 | 375 | 740 | 260 | 187.5 |
| 35a | 145 | 92.7 | 135.7 | 240 | 24.4 | 14.7 |
| 35b | 365 | 197.5 | 177.9 | 240 | 63.5 | 39.2 |
| 23 | NA | NA | NA | NA | 760 | 600 |
| 26 | 240 | 136 | 187.8 | 385 | 260 | 145 |
| 45c | NA | NA | NA | NA | NA | NA |
| 49a | 520 | 260 | 500 | NA | 125 | 97.7 |
| 5 | NA | NA | NA | NA | NA | NA |
| 54a | 125 | 93.7 | 93.7 | 125 | 11.7 | 3.9 |
| Ampicillin | 125 | 187.5 | 93.7 | 187.5 | NA | NA |
| Clotrimazole | NA | NA | NA | NA | 7.8 | 5.8 |
NA No activity
From Table 2, we observed that compounds 29, 35a, 10, 54a and 16a showed the lowest minimum inhibitory concentrations (MIC) for most tested bacteria and fungi, while compounds 40a, 40b, 35c and 49a exhibited high concentrations of MIC as compared with standard antimicrobial agents used.
Structure–activity relationship: By analyzing the previous results, it is noted that the substitutes do not play a clear role in the biological activity. However, in most of the results it was observed that the compounds that contain electron-withdrawing groups have a higher biological activity than the compounds that contain electron-donating groups and that the biological activity depends on the formation of the new fused rings and the type of strains chosen from bacteria and fungi.
Conclusion
In conclusion, compounds 3 and 5 were used as efficient precursors for the synthesis of new heterocycles including α-naphthol moiety with expected biological activities.
Experimental
The melting points, the elemental analyses and the spectral data were recorded as reported in reference [29].
General procedure of the synthesis of 1-(1-hydroxynaphthalen-2-yl)-3-phenyl-propane-1,3-dione (3)
A mixture of α-naphthol 1 (0.01 mol, 1.44 g), ethyl benzoylacetate 2 (0.01 mol, 1.92 g) and zinc chloride (0.5 gm) was exposed to microwave irradiation (Microwave assisted synthesis was performed on a CEM Microwave synthesizer, the irradiation power was 200 W as the maximum level of irradiation and a maximum level of internal vessel pressure at 250 Psi for about 5 min), the reaction mixture was allowed to reach room temperature, then diluted with ethanol with stirring and the solid product that formed, was filtrated off and crystallized from ethanol to give (3) as brown crystals: M.P.: 147–149 °C, yield: 2.65 g (91%). IR (KBr) (vmax/cm−1): 3365 (OH), 3061 (Ar–H), 2924 (Aliph-H), 1720, 1639 (2C=O). MS (EI, 70 eV): m/z (%) = 290 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.49 (s, 2H, CH2), 7.20–8.68 (m, 11H, aromatic H), 8.69 (s, 1H, OH). Anal. Calcd. for C19H14O3 (290): C, 78.61; H, 4.86. Found: C, 78.63; H, 4.88.
Synthesis of 2-((dimethylamino)methylene)-1-(1-hydroxynaphthalen-2-yl)-3-phenyl-propane-1,3-dione (5)
A mixture of 3 (0.01 mol, 2.9 g) and DMF-DMA (0.01 mol, 1.19 g) in dioxane (30 ml) was heated under reflux for 6 h. The reaction mixture was allowed to cool. The separated solid was filtered off, washed with ethanol and crystallized from ethanol to give (5) as pale brown crystals: M.P.: 173–175 °C, yield: 2.65 g (77%). IR (KBr) (vmax/cm−1): 3422 (NH), 3062 (Ar–H), 2932–2854 (Aliph- H), 1723, 1636 (2C=O). MS (EI, 70 eV): m/z (%) = 345 (M+). 1H NMR (400 MHz, DMSO-d6): δ 3.00 (s, 6H, 2CH3), 7.19 (s, 1H, CH-oleffinic), 7.64–8.67 (m, 11H, aromatic H), 8.68 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 44.5, 44.5, 116.2, 122.4, 124.3, 126.1, 126.7, 127.6, 127.6, 128.1, 128.1, 128.2, 128.6, 129.1, 130.1, 132.4, 132.5, 138.1, 163.2, 166.5, 194.7, 198.0. Anal. calcd for C22H19NO3 (345): C, 76.50; H, 5.54; N, 4.06. Found: C, 76.52; H, 5.56; N, 4.08.
Synthesis of 2-(ethoxymethylene)-1-(1-hydroxynaphthalen-2-yl)-3-phenylpropane-1,3-dione (6)
A mixture of 3 (0.01 mol, 2.9 g) and triethoxy methane (3 ml) in acetic anhydride (10 ml) was heated under reflux for 12 h. The reaction mixture was allowed to cool. The separated solid was filtered off, washed with ethanol and crystallized from ethanol to give (6) as pale brown crystals: M.P.: 120–122 °C, yield: 3.46 g (83%). IR (KBr) (vmax/cm−1): 3426 (OH), 3062 (Ar–H), 2930 (Aliph-H), 1767, 1636 (2C=O). MS (EI, 70 eV): m/z (%) = 346 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.11 (t, 3H, CH3), 4.19 (q, 2H, CH2), 6.33 (s, 1H, CH-oleffinic), 7.29–8.23 (m, 11H, aromatic H), 8.68 (s, 1H, OH). Anal. calcd for C22H18O4 (346): C, 76.29; H, 5.24. Found: C, 76.31; H, 5.26.
Synthesis of 5-(1-hydroxy-2-naphthoyl)-4-phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (10)
A mixture of 5 (0.01 mol, 3.45 g) and cyanothioacetamide 7 (0.01 mol, 1 g), in presence of sodium ethoxide (30 ml) was heated under reflux for 24 h. The solution was allowed to cool and poured into crushed ice then acidified with HCl. The separated solid was filtered off, washed with water and crystallized from dioxane to give (10) as brown crystals: M.P.: 238–240 °C, yield: 3.35 g (87%). IR (KBr) (vmax/cm−1): 3447, 3423 (NH), 3062 (AR–H), 2209 (CN), 1636 (C=O).). MS (EI, 70 eV): m/z (%) = 382 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.20–8.24 (m, 13H, aromatic H and NH), 8.70 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 107.1, 117.0, 118.2, 122.3, 123.2, 126.3, 126.4, 126.8, 127.3, 127.3, 127.5, 127.5, 128.3, 128.4, 129.1, 130.0, 130.1, 132.2, 144.6, 166.1, 168.0, 168.6, 196.0. Anal. calcd for C23H14N2O2S (382): C, 72.23; H, 3.69; N, 7.33. Found: C, 72.25; H, 3.71; N, 7.35.
Synthesis of (1-hydroxynaphthalen-2-yl)(3-phenyl-1H-pyrazol-4-yl)methanone (14)
Method (A): A mixture of 5 (0.01 mol, 3.45 g) and hydrazine hydrate (10 ml) was heated under reflux for 12 h. The reaction mixture was allowed to cool and poured into crushed ice. The separated solid was filtered off, washed with water and crystallized from DMF to give (14).
Method (B): A mixture of 6 (0.01 mol) and hydrazine hydrate (10 ml) was heated under reflux for 12 h. The reaction mixture was allowed to cool and poured into crushed ice. The separated solid was filtered off, washed with water and crystallized from DMF to give (14).
as white crystals: M.P.: > 300 °C, yield: 2.50 g (79%). IR (KBr) (vmax/cm−1): 3300, 3228 (NH), 3062 (Ar–H), 1670 (C=O).). MS (EI, 70 eV): m/z (%) = 314 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.22 (s, 1H, CH-pyrazole), 7.31–8.71 (m, 12H, aromatic and NH), 8.73 (s, 1H, OH). Anal. calcd for C20H14N2O2 (314): C, 76.42; H, 4.49; N, 8.91. Found: C, 76.44; H, 4.51; N, 8.93.
General procedure for preparation of compounds (16a,b and 18)
A mixture of 5 (0.01 mol, 3.45 g) with urea (0.01 mol, 0.6 g), thiourea (0.01 mol, 0.76 g) and guanidine hydrochloride (0.01 mol, 0.95 g), in presence of sodium ethoxide (30 ml) was heated under reflux for 24 h. The solutions were allowed to cool and poured into crushed ice then acidified with HCl. The separated solids were filtered off, washed with water and crystallized from the proper solvent to give (16a,b and 18).
5-(1-hydroxy-2-naphthoyl)-4-phenylpyrimidin-2(1H)-one (16a)
It was obtained as beige crystals from DMF: M.P.: > 300 °C, yield: 2.7 g (78%). IR (KBr) (vmax/cm−1): 3447 (OH), 3423 (NH), 3061 (Ar–H), 1740, 1641 (2C=O). MS (EI, 70 eV): m/z (%) = 342 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.22 (s, 1H, CH-pyrimidine), 7.65–8.70 (m, 12H, aromatic H and NH), 8.71 (s, 1H, OH). Anal. calcd for C21H14N2O3 (342): C, 73.68; H, 4.12; N, 8.18. Found: C,73.70; H, 4.14; N,8.20.
(1-hydroxynaphthalen-2-yl)(4-phenyl-2-thioxo-1,2-dihydropyrimidin-5-yl)methanone (16b)
It was obtained as brown crystals from DMF: M.P.: > 300 °C, yield: 2.8 g (78%). IR (KBr) (vmax/cm−1): 3449 (OH), 3400 (NH), 1645 (C=O). MS (EI, 70 eV): m/z (%) = 358 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.19 (s, 1H, CH-pyrimidine), 7.59–8.67 (m, 12H, aromatic H and NH), 8.68 (s, 1H, OH). Anal. calcd for C21H14N2O2S (358): C, 70.37; H, 3.94; N, 7.82. Found: C, 70.39; H, 3.96; N, 7.84.
(2-amino-4-phenylpyrimidin-5-yl)(1-hydroxynaphthalen-2-yl)methanone (18)
It was obtained as brown crystals from dioxane: M.P.: 250–252 °C, yield: 2.45 g (71%). IR (KBr) (vmax/cm−1): 3449–3400 (OH, NH2), 1642 (C=O). MS (EI, 70 eV): m/z (%) = 341 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 6.20 (s, 2H, NH2), 7.31–8.39 (m, 12H, aromatic H), 8.67 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 118.0, 119.3, 123.2, 124.3, 124.8, 126.4, 126.4, 126.4, 126.6, 127.6, 128.2, 128.7, 128.9, 128.9, 130.7, 132.7, 155.3, 162.7, 165.8, 166.3, 194.0. Anal. calcd for C21H15N3O2 (341). Anal. Calcd. for C21H15N3O2: C, 73.89; H, 4.43; N, 12.31. Found: C, 73.91; H, 4.45; N, 12.33.
Synthesis of (1-hydroxynaphthalen-2-yl)(3-phenylisoxazol-4-yl)methanone (20)
A mixture of 5 (0.01 mol, 3.45 g), hydroxylamine hydrochloride in ethanol (30 ml) containing anhydrous sodium acetate (1 g) was heated under reflux for 24 h. The reaction mixture was allowed to cool and poured into cold water (60 ml). The separated solid was filtered off and crystallized from ethanol to give (20) as brown crystals: M.P.: 150–152 °C, yield: 2.25 g (71%). IR (KBr) (vmax/cm−1): 3425 (OH), 3060 (Ar–H), 1636 (C=O). MS (EI, 70 eV): m/z (%) = 315 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.21 (s, 1H, CH-isoxazole), 7.31–8.70 (m, 11H, aromatic H), 8.71 (s, 1H, OH). Anal. calcd for C20H13NO3 (315): C, 76.18; H, 4.16; N, 4.44%. Found:C, 76.20; H, 4.18; N, 4.46%.
Synthesis of (2-(3-Phenylisoxazol-5-yl)naphthalen-1-ol (23)
A mixture of 3 (0.01 mol), hydroxylamine hydrochloride (0.01 mol) in ethanol (30 ml) containing anhydrous sodium acetate (1 g) was heated under reflux for 24 h. The reaction mixture was allowed to cool and poured into cold water (60 ml). The separated solid was filtered off and crystallized from ethanol to give (23) as beige crystals: M.P.: 132–134 °C, yield: 2.25 g (80%). IR (KBr) (vmax/cm−1): 3382 (NH), 3061 (Ar–H). MS (EI, 70 eV): m/z (%) = 287 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.21 (s, 1H, CH- isoxazole), 7.32–8.54 (m, 11H, aromatic H), 8.69 (s, 1H, OH); 13C NMR (100 MHz, DMSO-d6) δ 99.6, 118.7, 120.6, 123.5, 124.1, 126.1, 126.3, 126.5, 126.5, 127.3, 127.5, 127.6, 127.9, 128.8, 128.8, 132.4, 161.0, 163.2, 170.1. Anal. calcd for C19H13NO2 (287): C, 79.43; H, 4.56; N, 4.88. Found: C, 79.45; H, 4.58; N, 4.90.
Synthesis of 6-(1-hydroxynaphthalen-2-yl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (26)
A mixture of 3 (0.01 mol, 2.90 g), malononitrile (0.01 mol, 0.66 g) in sodium ethoxide (30 ml) was heated under reflux for 12 h. The reaction mixture was allowed to cool and poured into crushed ice then acidified with HCl. The separated solid was filtered off, washed with water and crystallized from ethanol to give (26) as brown crystals: M.P.: 163–165 °C, yield: 2.35 g (69%). IR (KBr) (vmax/cm−1): 3311, 3294, 3062, 2193, 1639 cm−1. MS (EI, 70 eV): m/z (%) = 338 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.16 (s, 1H, CH-Pyridine), 7.51–8.63 (m, 12H, aromatic H and NH), 8.64 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 108.3, 118.3, 118.6, 120.1, 123.4, 124.1, 125.8, 126.9, 127.1, 127.2, 127.6, 127.6, 127.9, 127.9, 128.3, 133.9, 135.2, 153.6, 155.2, 155.3, 157.8, 162.3. Anal. calcd for C22H14N2O2 (338): C, 78.09; H, 4.17; N, 8.28. Found: C, 78.11; H, 4.19; N, 8.30.
Synthesis of 2-amino-5-(1-hydroxy-2-naphthoyl)-4-phenylthiophene-3-carbonitrile (29)
Equimolar amounts of 3 (0.01 mol, 2.90 g), malononitrile and elemental sulfur (0.01 mol, 0.66 and 0.32 g) in ethanol (30 ml) containing piperidine were refluxed for 15 h, poured onto cold water (60 ml) and acidified with HCl. The solid product thus formed was filtered off and crystallized from ethanol to give (29) as yellow crtstals: M.P.: 130–132 °C, yield: 2.95 g (80%). IR (KBr) (vmax/cm−1): 3421–3400 (NH2), 3061 (Ar–H), 2192 (CN), 1639 (C=O). MS (EI, 70 eV): m/z (%) = 370 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.20–8.68 (m, 13H, aromatic H and NH2), 8.69 (s, 1H, OH). Anal. calcd for C22H14N2O2S (370): C, 71.33; H, 3.81; N, 7.56. Found:C, 71.35; H, 3.84; N, 7.58.
General procedure for preparation of compounds (35a-c)
A mixture of 31a–c (which prepared by a mixture of 3 and 30a–c in ethanol/piperidine/ refluxing) (0.01 mol, 2.90 g), dimedone (0.01 mol, 1.40 g) and ammonium acetate (2 gm) was fused for 30 min. The reaction mixture was allowed to cool, then triturated with ethanol. The separated solid was filtered off, washed with water and crystallized from the proper solvent to give (35a–c).
3-(1-hydroxy-2-naphthoyl)-7,7-dimethyl-2,4-diphenyl-4,6,7,8-tetrahydroquinolin-5(1H)-one (35a)
It was obtained as yellow crystals from dioxane: M.P.: 280–282 °C, yield: 4.30 g (86%). IR (KBr) (vmax/cm−1): 3402, 3400 (NH), 3059 (Ar–H), 2954–2870 (Aliph-H), 1642, 1620 (2C=O). MS (EI, 70 eV): m/z (%) = 499 (M+). 1H NMR(400 MHz, DMSO-d6): δ (ppm) 0.86 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.73 (s, 2H, CH2), 2.89 (s, 2H, CH2), 4.80 (s, 1H, CH-pyridine), 7.14–8.70 (m, 17H, aromatic H and NH), 8.70 (s, 1H, OH). Anal. calcd for C34H29NO3 (499): C, 81.74; H, 5.85; N, 2.80. Found: C, 81.76; H, 5.87; N, 2.82.
3-(1-hydroxy-2-naphthoyl)-4-(4-methoxyphenyl)-7,7-dimethyl-2-phenyl-4,6,7,8-tetrahydro-quinolin-5(1H)-one (35b)
It was obtained as yellow crystals from dioxane: M.P.: 277–279 °C, yield: 4.25 g (80%). IR (KBr) (vmax/cm−1): 3371 (OH), 3255 (NH), 3059 (Ar–H), 2954–2835 (Aliph-H), 1639, 1620 (2C=O). MS (EI, 70 eV): m/z (%) = 529 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.82(s, 3H, CH3), 1.19 (s, 3H, CH3), 2.70 (s, 2H, CH2), 2.86 (s, 2H, CH2), 3.70 (s, 3H, OCH3), 4.49 (s, 1H, CH-pyridine), 7.21–8.28 (m, 16H, aromatic H and NH), 8.45 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 29.2, 29.8, 33.8, 41.0, 41.4, 52.3, 57.8, 107.0, 113.2, 116.3, 116.3, 122.0, 123.1, 124.3, 126.4, 126.8, 127.2, 127.6, 127.6, 127.6, 127.8, 128.0, 128.2, 128.9, 132.1, 132.1, 132.7, 137.2, 137.2, 143.6, 150.2, 159.5, 166.0, 193.4, 195.1. Anal. calcd for C35H31NO4 (529): C, 79.37; H, 5.90; N, 2.64. Found: C, 79.39; H, 5.92; N, 2.66.
4-(4-chlorophenyl)-3-(1-hydroxy-2-naphthoyl)-7,7-dimethyl-2-phenyl-4,6,7,8-tetra-hydroquinolin-5(1H)-one (35c)
It was obtained as pale yellow crystals from dioxane: M.P.: 290–292 °C, yield: 4.30 g (83%). IR (KBr) (vmax/cm−1): 3356 (OH), 3271 (NH), 3059 (Ar–H), 2954–2870 (Aliph-H), 1643, 1620 (2C=O). MS (EI, 70 eV): m/z (%) = 533 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.83 (s, 3H, CH3), 1.20 (s, 3H, CH3), 2.70 (s, 2H, CH2), 2.89 (s, 2H, CH2), 4.49 (s, 1H, CH-pyridine), 7.28–8.11 (m, 16H, aromatic H and NH), 8.50 (s, 1H, OH). Anal. calcd for C34H28ClNO3 (533): C, 76.47; H, 5.28; N, 2.62. Found:C, 76.49; H, 5.30; N, 2.64.
General procedure for preparation of compounds (40a,b)
A mixture of 3 (0.01 mol, 2.90 g) and arylidenemalononitriles (36a,b) (0.01 mol, 1.88 and 1.70 g) in ethanol (30 ml) containing a catalytic amount of piperidine was heated under reflux for 12 h. The reaction mixture was allowed to cool and poured into crushed ice then acidified with HCl. The separated solid was filtered off, washed with water and crystallized from the proper solvent to give (40a,b).
2-amino-4-(4-chlorophenyl)-5-(1-hydroxy-2-naphthoyl)-6-phenyl-4H-pyran-3-carbonitrile (40a)
It was obtained as brown crystals from ethanol: M.P.: 162–164 °C, yield: 4.30 g (92%). IR (KBr) (vmax/cm−1): 3448- 3421 (OH, NH2), 3063 (Ar–H), 2929 (Aliph-H), 2191 (CN), 1630 (C=O). MS (EI, 70 eV): m/z (%) = 480 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.40 (s, 1H, 4H-pyrane), 7.23–8.72 (m, 17H, aromatic H and NH2), 8.74 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 43.7, 60.3, 118.9, 122.1, 123.4, 124.2, 125.2, 125.2, 126.2, 126.8, 126.8, 126.8, 126.9, 126.9, 127.5, 127.5, 127.6, 127.6, 128.1, 128.2, 131.3, 131.3, 132.6, 133.2, 135.0, 140.8, 163.3, 165.2, 193.5. Anal. calcd for C29H19ClN2O3 (478): C, 72.73; H, 4.00; N, 5.85. Found: C, 72.75; H, 4.02; N, 5.87.
2-amino-5-(1-hydroxy-2-naphthoyl)-4-(4-hydroxyphenyl)-6-phenyl-4H-pyran-3-carbonitrile (40b)
It was obtained as brown crystals from ethanol: M.P.: 200–202 °C, yield: 4.30 g (90%). IR (KBr) (vmax/cm−1): 3447–3390 (OH, NH2), 3062 (Ar–H), 2928 (Aliph-H), 2196 (CN), 1630 (C=O). MS (EI, 70 eV): m/z (%) = 460 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.20 (s, 1H, 4H-pyrane), 6.87 (s, 2H, NH2), 6.90–8.71 (m, 15H, aromatic H), 8.72 (s, 1H, OH), 10.00 (s, 1H, OH). Anal. calcd for C29H20N2O4 (460): C, 75.64; H, 4.38; N, 6.08. Found: C, 75.66; H, 4.40; N, 6.10.
General procedure for preparation of compounds (45a–c)
A mixture of 3 (0.01 mol, 2.90 g) and arylidenecyanothioacetamide (41a–c) (0.01 mol, 1.88, 2.18 and 2.22 g) in ethanol (30 ml) containing a catalytic amount of TEA was heated under reflux for 12 h. The reaction mixture was allowed to cool and poured into crushed ice then acidified with HCl. The separated solid was filtered off, washed with water and crystallized from the proper solvent to give (45a–c).
5-(1-hydroxy-2-naphthoyl)-4,6-diphenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (45a)
It was obtained as pale yellow crystals from ethanol: M.P.: 146–148 °C, yield: 3.60 g (78%). IR (KBr) (vmax/cm−1): 3444 (OH), 3390 (NH), 3059 (Ar–H), 2191 (CN), 1620 (C=O). MS (EI, 70 eV): m/z (%) = 458 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.23–8.73 (m, 17H, aromatic H and NH), 8.74 (s, 1H, OH). Anal. calcd for C29H18N2O2S (458): C, 75.96; H, 3.96; N, 6.11. Found: C, 75.98; H, 3.98; N, 6.13.
5-(1-hydroxy-2-naphthoyl)-4-(4-methoxyphenyl)-6-phenyl-2-thioxo-1,2-dihydro-pyridine-3-carbonitrile (45b)
It was obtained as pale yellow crystals from ethanol: M.P.: 150–152 °C, yield: 4.00 g (82%). IR (KBr) (vmax/cm−1): 3448, 3387, 3059, 2931, 2194, 1630 cm−1. MS (EI, 70 eV): m/z (%) = 488 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.87 (s, 3H, OCH3), 7.12–8.73 (m, 15H, aromatic H), 8.74 (s, 1H, OH), 9.87 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.6, 108.2, 111.9, 118.2, 118.2, 118.8, 122.3, 124.6, 124.8, 125.2, 126.4, 126.8, 126.8, 127.3, 127.3, 127.5, 127.6, 128.0, 128.1, 128.7, 132.1, 132.1, 132.7, 135.2, 155.8, 160.8, 163.7, 165.2, 172.6, 193.3. Anal. calcd for C30H20N2O3S (488): C, 73.75; H, 4.13; N, 5.73. Found: C, 73.77; H, 4.15; N, 5.75.
4-(4-chlorophenyl)-5-(1-hydroxy-2-naphthoyl)-6-phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (45c)
It was obtained as yellow crystals from ethanol: M.P.: 136–138 °C, yield: 3.60 g (88%). IR (KBr) (vmax/cm−1): 3390 (OH), 3255 (NH), 3059 (Ar–H), 2191 (CN), 1635 (C=O). MS (EI, 70 eV): m/z (%) = 494 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.23–8.73 (m, 16H, aromatic H and NH), 8.74 (s, 1H, OH). Anal. calcd for C29H17ClN2O2S (492): C, 70.65; H, 3.48; N, 5.68. Found: C, 70.67; H, 3.50; N, 5.71.
General procedure for preparation of compounds (49a,b)
A mixture of 3 (0.01 mol, 2.90 g) and 2-cyano-2-cyclopentylidene-ethaneethioamide 46a (0.01 mol, 1.66 g) or 2-cyano-2-cyclohexylidene-ethaneethioamide 46b (0.01 mol, 1.88 g) in ethanol (30 ml) containing a catalytic amount of piperidine was heated under reflux for 24 h. The reaction mixture was allowed to cool and poured into crushed ice then acidified with HCl. The separated solid was filtered off, washed with water and crystallized from the proper solvent to give (49a,b).
10-(1-hydroxy-2-naphthoyl)-9-phenyl-7-thioxo-8-azaspiro[4.5]dec-9-ene-6-carbonitrile (49a)
It was obtained as pale yellow crystals from ethanol: M.P.: 180–182 °C, yield: 3.25 g (74%). IR (KBr) (vmax/cm−1): 3387 (OH), 3248 (NH), 3059 (Ar–H), 2935–2854 (Aliph-H), 2183 (CN), 1627 (C=O). MS (EI, 70 eV): m/z (%) = 440 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.85–1.91 (m, 4H, 2CH2), 1.95 (s, 1H, CH-pyridine), 2.00–2.94 (m, 4H, 2CH2), 7.14–8.69 (m, 11H, aromatic H), 8.70 (s, 1H, OH)), 9.40 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 27.8, 27.8, 38.3, 38.3, 39.0, 57.8, 116.2, 119.2, 122.3, 123.3, 123.9, 126.1, 126.4, 126.9, 127.3, 127.3, 127.5, 127.8, 128.3, 128.3, 128.8, 132.5, 135.2, 155.3, 166.7, 193.2, 198.9. Anal. calcd for C27H22N2O2S (438): C, 73.95; H, 5.06; N, 6.39%. Found: C, 73.97; H, 5.08; N, 6.41%.
5-(1-hydroxy-2-naphthoyl)-4-phenyl-2-thioxo-3-azaspiro[5.5]undec-4-ene-1-carbonitrile (49b)
It was obtained as pale yellow crystals from dioxane: M.P.: 278–280 °C, yield: 3.95 g (87%). IR (KBr) (vmax/cm−1): 3394 (OH), 3325 (NH), 3062 (Ar–H), 2931–2854 (Aliph-H), 2191 (CN), 1643 (C=O). MS (EI, 70 eV): m/z (%) = 454 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.86–1.02 (m, 4H, 2CH2), 1.76 (s, 2H, CH2), 1.96 (s, 1H, CH-pyridine), 2.00–2.94 (m, 4H, 2CH2), 6.70–8.71 (m, 11H, aromatic H), 9.23 (s, 1H, OH), 9.87 (s, 1H, NH). Anal. calcd for C28H24N2O2S (452): C, 74.31; H, 5.35; N,6.19%. Found: C, 74.33; H, 5.37; N,6.21%.
General procedure for preparation of compounds (51a–c)
A cold suspension of aryl diazonium salts 50a–c (0.02 mol, 0.85, 0.86 and 0.77 g) (prepared from 0.02 mol of aromatic amines with the appropriate quantities of sodium nitrite and hydrochloric acid) was gradually added to a cold solution (0–5 °C) of 3 (0.002 mol, 1.45 g) in ethanol (50 ml) containing anhydrous sodium acetate (2 gm) with continuous stirring for 1 h. The resulting reaction product was filtered off, washed with water and crystallized from the proper solvent to give compounds (51a–c).
1-(1-hydroxynaphthalen-2-yl)-2-(2-(4-methoxyphenyl)hydrazono)-3-phenyl propane-1,3-dione (51a)
It was obtained as brown crystals from ethanol: M.P.: 170–172 °C, yield: 3.45 g (81%). IR (KBr) (vmax/cm−1): 3420 (OH), 3400 (NH), 3062 (Ar–H), 2930 (Aliph-H), 1724, 1659 (2C=O). MS (EI, 70 eV): m/z (%) = 424 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.87 (s, 3H, OCH3), 6.96–8.73 (m, 15H, aromatic H), 8.74 (s, 1H, OH), 11.00 (s, 1H, NH). Anal. calcd for C26H20N2O4 (424): C, 73.57; H, 4.75; N, 6.60. Found: C, 73.59; H, 4.77; N, 6.62.
2-(2-(4-chlorophenyl)hydrazono)-1-(1-hydroxynaphthalen-2-yl)-3-phenylpropane-1,3-dione (51b)
It was obtained as brown crystals from ethanol: M.P.: 161–162 °C, yield: 3.30 g (77%). IR (KBr) (vmax/cm−1): 3449 (OH), 3419 (NH), 3063 (Ar–H), 1723, 1650 (2C=O). MS (EI, 70 eV): m/z (%) = 430 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.23–8.71 (m, 15H, aromatic H), 8.72(s, 1H, OH), 12.00 (s, 1H, NH). Anal. calcd for C25H17ClN2O3 (428): C, 70.01; H, 4.00; N, 6.53. Found:C, 70.02; H, 4.03; N, 6.55.
1-(1-hydroxynaphthalen-2-yl)-3-phenyl-2-(2-p-tolylhydrazono) propane-1,3-dione (51c)
It was obtained as brown crystals from ethanol: M.P.: 166–168 °C, yield: 2.90 g (71%). IR (KBr) (vmax/cm−1): 3422, 3400 (OH), 3063 (NH), 2926 (Aliph-H), 1723, 1650 (2C=O). MS (EI, 70 eV): m/z (%) = 408 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.91 (s, 3H, CH3), 7.14–8.73 (m, 15H, aromatic H), 8.74 (s, 1H, OH)), 12.00 (s, 1H, NH). Anal. calcd for C26H20N2O3 (408): C, 76.45; H, 4.94; N, 6.86. Found: C, 76.46; H, 4.96; N, 6.88.
General procedure for preparation of compounds (54a–c)
A mixture of compounds 51a–c (0.001 mol, 0.424, 0.428 and 0.408 g), ammonium acetate (3 gm) and malononitrile (0.001 mol, 0.066 g) were fused for 10 min. The solid precipitate so formed was treated with ethanol and filtered out and crystallized from the proper solvent to give (54a–c).
6-(1-hydroxy-2-naphthoyl)-3-imino-2-(4-methoxyphenyl)-5-phenyl-2,3-dihydropyridazine-4-carbonitrile (54a)
It was obtained as brown crystals from dioxane: M.P.: 230–232 °C, yield: 3.75 g (79%). IR (KBr) (vmax/cm−1): 3421 (OH), 3400 (NH), 3065 (Ar–H), 2928 (Aliph-H), 2200 (CN), 1650 (C=O). MS (EI, 70 eV): m/z (%) = 472 (M+). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.99 (s, 3H, OCH3), 6.68–8.25 (m, 16H, aromatic H and NH), 8.71 (s, 1H, OH). Anal. calcd for C29H20N4O3 (472): C, 73.72; H, 4.27; N, 11.86. Found: C, 73.75; H, 4.29; N, 11.88.
2-(4-chlorophenyl)-6-(1-hydroxy-2-naphthoyl)-3-imino-5-phenyl-2,3-dihydropyridazine-4-carbonitrile (54b)
It was obtained as brown crystals from dioxane: M.P.: 258–260 °C, yield: 3.60 g (75%). IR (KBr) (vmax/cm−1): 3406 (OH), 3400 (NH), 3063 (Ar–H), 2200 (CN), 1650 (C=O). MS (EI, 70 eV): m/z (%) = 478 (M+ + 2). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 6.67–8.25 (m, 16H, aromatic H and NH), 8.71 (s, 1H, OH). Anal. calcd for C28H17ClN4O2 (476): C, 70.52; H, 3.59; N, 11.75%. Found: C, 70.55; H, 3.63; N, 11.78%.
6-(1-hydroxy-2-naphthoyl)-3-imino-5-phenyl-2-p-tolyl-2,3-dihydropyridazine-4-carbonitrile (54c)
It was obtained as brown crystals from dioxane: M.P.: 272–274 °C, yield: 3.85 g (84%). IR (KBr) (vmax/cm−1): 3400, 3385 (OH), 3062 (NH), 2963 (Aliph-H), 2202 (CN), 1650 (CO). MS (EI, 70 eV): m/z (%) = 457 (M+ + 1). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.76 (s, 3H, CH3), 6.67–8.27 (m, 16H, aromatic H and NH), 8.72 (s, 1H, OH). Anal. calcd for C29H20N4O2 (456): C, 76.30; H, 4.42; N, 12.27. Found: C, 76.33; H, 4.45; N, 12.30.
Acknowledgements
The authors are very grateful to Prof. Dr. A. E. Khodair, Department of Chemistry, Faculty of Science, Suez Canal University, Ismailia, Egypt, for valuable support and reviewing this manuscript, and the authors are very grateful to Department of Microbiology, Faculty of Science, Arish University, Arish, Egypt for performing the antimicrobial evaluation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Helw EAE, Gado MM, El-Ziaty AK. Synthesis and anti-rotavirus activity of some nitrogen heterocycles integrated with pyrazole scaffold. J Iran Chem Soc. 2020;17:1479–1492. doi: 10.1007/s13738-020-01873-7. [DOI] [Google Scholar]
- 2.Wahyuningsih TD, Suma AAT, Astuti E. Synthesis, anticancer activity, and docking study of N-acetyl pyrazolines from veratraldehyde. J Appl Pharm Sci. 2019;9(3):14–20. doi: 10.7324/JAPS.2019.90303. [DOI] [Google Scholar]
- 3.Kumar RS, Arif IA, Ahmad A, Idhayadhulla A. Anti-inflammatory and antimicrobial activities of novel pyrazole analogues. Saudi J Biol Sci. 2013;23:614–620. doi: 10.1016/j.sjbs.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherian B, Kumar RA, Vinod B. Pyrazole carbaldehydes as novel anti-inflammatory agents: synthesis and in vitro membrane stabilization method. J Pharm Sci Res. 2020;12(2):252–257. [Google Scholar]
- 5.Kaplancikli ZA, Ozdemir A, Turan-Zitouni G, Altintop MD, Can OD. New pyrazoline derivatives and their antidepressant activity. Eur J Med Chem. 2010;45:4383–4387. doi: 10.1016/j.ejmech.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 6.El-Sawy ER, Ebaid MS, Abo-Salem HM, Al-Sehemi AG, Mandour AH. Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of some new 4,6-dimethoxy-5-(heterocycles)benzofuran starting from naturally occurring visnagin. Arabian J Chem. 2014;7:914–923. doi: 10.1016/j.arabjc.2012.12.041. [DOI] [Google Scholar]
- 7.Abd El Razik HA, Badr MH, Atta AH, Mouneir SM, Abu-Serie MM. Benzodioxole-pyrazole hybrids as anti-inflammatory and analgesic agents with COX-1,2/5-LOX inhibition and antioxidant potential. Arch Pharm Chem Life Sci. 2017;7(350):e1700026. doi: 10.1002/ardp.201700026. [DOI] [PubMed] [Google Scholar]
- 8.Kucukguzel SG, Senkardes S. Recent advances in bioactive pyrazoles. Eur J Med Chem. 2015;97:786–815. doi: 10.1016/j.ejmech.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Shi L, Qiu S, Chen S, Lin M, Xiang Y, Zhao CH. Design, synthesis, and evalution of cyanopyridines as anti-colorectal cancer agents via inhbiting STAT3 pathway. Drug Des Dev Ther. 2019;13:3369–3381. doi: 10.2147/DDDT.S217800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulloora SH, Shabaraya R, Aamir S, Adhikari AV. New imidazol[1,2-a]pyridines carrying active pharmacophores: synthesis and anticonvulsant studies. Bioorg Med Chem Lett. 2013;23:1502–1506. doi: 10.1016/j.bmcl.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Malladi V, Isloor AM, Peethambar SK, Ganesh BM, Goud PS. Synthesis and antimicrobial activity of some new pyrazole containing cyanopyridone derivatives. Der Pharma Chemica. 2012;4(1):43–52. [Google Scholar]
- 12.Paghdar DJ, Akabari JD, Tala SD, Dhaduk MF, Joshi HS. Synthesis of some new thiopyrimidine and oxopyrimidine heterocycles bearing 4-(methylsulfonyl)phenyl nucleus as potent antitubercular and antimicrobial agents. Indian J Heterocycl Chem. 2007;17:113–116. [Google Scholar]
- 13.Faidallah HM, Khan KhA, Asiri AM. Synthesis and characterization of a novel series of benzenesulfonylurea and thiourea derivatives of 2H-pyran and 2h-pyridine-2-ones as antibacterial, antimycobacterial and antifungal agents. Eur J Chem. 2011;2(2):243–250. doi: 10.5155/eurjchem.2.2.243-250.257. [DOI] [Google Scholar]
- 14.Sangani CHB, Mungra DC, Patel MP, Patel RG. Synthesis and in vitro antimicrobial screening of new pyrano[4,3-b]pyrane derivatives of 1H-pyrazole. Chin Chem Lett. 2012;23:57–60. doi: 10.1016/j.cclet.2011.09.012. [DOI] [Google Scholar]
- 15.Aghajani M, Asghari S, Pasha GHF, Mohseni M. Study of three-componenet reaction of α-ketoesters and active methylenes with OH-acids to synthesize new 2-amino-4H-pyran derivatives and evaluation of their antibacterial and antioxidant activities. Res Chem Intermed. 2020;46:1841–1855. doi: 10.1007/s11164-019-04066-x. [DOI] [Google Scholar]
- 16.Taylor NR, Cleasby A, Singh O, Skarzynski T, Wonacott AJ, Smith PW, Sollis SL, Howes PD, Cherry PC, Bethell R, Colman P, Varghese J. Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 2. Crystallographic and molecular modeling study of complexes of 4-amino-4H-pyran-6-carboxamides and sialidase from infuenza virus types A and B. J Med Chem. 1998;41:798–807. doi: 10.1021/jm9703754. [DOI] [PubMed] [Google Scholar]
- 17.Hiramoto K, Nasuhara A, Michikoshi K, Kato T, Kikugawa K. DNA strnd-breaking activity and mutagenicity of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyrn-4-one (DDMP), a maillard reaction product of glucose and glycine. Mutat Res. 1997;395:47–56. doi: 10.1016/S1383-5718(97)00141-1. [DOI] [PubMed] [Google Scholar]
- 18.Hadiyal SD, Parmar ND, Kalavadiya PL, Lalpara JN, Joshi HS. Microwave-assisted three-component domino synthesis of polysubstituted 4H-pyran derivaitves and their anticancer activity. Russ J Org Chem. 2020;56(4):671–678. doi: 10.1134/S1070428020040168. [DOI] [Google Scholar]
- 19.Hegab MI, Rashad AE, Shamroukh AH, Hamza IA. Synthesis and derivatization of angular 3-chloro-3-chlorosulfenyl naphtho[1,2-b]pyran(4H)-4-ones with evalution of antiviral activity. J Sulfur Chem. 2006;27(3):1–12. doi: 10.1080/17415990600622620. [DOI] [Google Scholar]
- 20.Thakur AS, Verma P, Chandy A. a review on biological profile of pyridazinone containing drugs. Asian J Res Chem. 2010;3(2):265–271. [Google Scholar]
- 21.Alam M, Zaman MS, Alam MM, Arora K, Ahmad A, Husain A. Synthesis and antimicrobial activities of new pyrazolo-pyridazine derivatives. Int J Pharma Sci Res. 2015;6(3):495–501. [Google Scholar]
- 22.El Ashry ESH, Kassem AA, Ramadan E. Microwave irradiation for accelerating organic reactions-part II: six-, seven-membered, spiro, and fused heterocycles. Adv Heterocycl Chem. 2006;90:1–123. doi: 10.1016/S0065-2725(05)90001-9. [DOI] [Google Scholar]
- 23.El Ashry ESH, Awad LF, Ibrahim EI, Bdeewy OKH. Microwave irradiation for accelerating the synthesis of acridine and xanthene derivatives from dimedone. ARKIVOC. 2006;2:178–186. doi: 10.3998/ark.5550190.0007.220. [DOI] [Google Scholar]
- 24.Chauhan SS, Joshi YC. Solid phase synthesis of isoxazole derivatives from diaryl 1,3-diketones under microwave irradiation. Rasayan J Chem. 2008;1(3):475–480. [Google Scholar]
- 25.Cirka V, Relich S. Microeave photochrmistry. Application in organic synthesis. Mini Rev Org Chem. 2011;8:282–293. doi: 10.2174/157019311796197472. [DOI] [Google Scholar]
- 26.Ramiz MMM, Abdel Hafiz IS, Abdel Reheim MAM, Gaber HM. Ptrazolones as building blocks in heterocyclic synthesis: synthesis of new pyrazolopyran, pyrazolopyridazine and pyrazole derivatives of expected antifungicidal activity. J Chin Chem Soc. 2012;59:72–80. doi: 10.1002/jccs.201100194. [DOI] [Google Scholar]
- 27.Abdel Hafiz IS, Abdel Reheim MAM, Ramiz MMM. Hydantion in heterocyclic synthesis: synthesis of new imidazpyridine, imidazotriazole, pyrazolopurinone, pyranoimidazole, imidazopyridazine and imidazopyrazole derivatives. J Chem Soc Pak. 2014;36(5):884–889. [Google Scholar]
- 28.Abdel Reheim MAM, Abdel Hafiz IS, Elian MA. A simple and convenient synthesis of isolated fused heterocycles based on: 6-phenyl-2-thioxo-2,3-dihydropyrimidin-4(5H)-one and 5-acetyl-6-phenyl-2-thioxo-2,3-dihydropyrimidn-4(5H)-one. Heterocycles. 2016;92(8):1397–1414. doi: 10.3987/COM-16-13467. [DOI] [Google Scholar]
- 29.Abdel Reheim MAM, Abdel Hafiz IS, Abdel Rady HSE. Utility of diketone in heterocyclic synthesis: synthesis of new substituted pyrimidines and fused pyrimidine of potential biosignificant interest. Curr Org Synth. 2018;15(8):1171–1181. doi: 10.2174/1570179415666180918161101. [DOI] [Google Scholar]
- 30.Abdel Reheim MAM, Abdel Hafiz IS, Mohamed S. Utility of β-diketones in heterocyclic synthesis: synthesis of new tetrahydro-pyrimidinethione, pyrazole, thiophene, dihydropyridine, dihydropyrane, pyridazine derivatives and investigation of their antimicrobial activity. Eur J Chem. 2016;7(3):298–308. doi: 10.5155/eurjchem.7.3.298-308.1447. [DOI] [Google Scholar]
- 31.Kini SG, Choudhary S, Mubeen M. Synthesis docking study and anticancer activity of coumarin substituted derivatives of benzothiazole. J Comput Methods Mol Des. 2012;2(1):51–60. [Google Scholar]
- 32.Garazd MM, Garazd YAL, Khilya VP. Neoflavones. 2. Methods for synthesizing and modifying 4-arylcoumarins. Chem Nat Compd. 2005;41(3):245–271. doi: 10.1007/s10600-005-0126-7. [DOI] [Google Scholar]
- 33.Pandya AB, Prajapati DG, Pandya SS. Synthesis of novel naphthalene COX inhibitors for anti-inflammatory activity. J Appl Pharm Sci. 2016;2(8):226–232. [Google Scholar]
- 34.Reheim MAM. β-ketoesters in heterocylic synthesis: synthesis of new dihydropyridine, tetrahydropyrimidine, pyrazole, aminothiophene, pyrazolopyrimidine derivatives, and investigation of their antimicrobial activity. Int J Pharma Sci. 2016;6(3):1468–1479. [Google Scholar]
- 35.Mohareb RM, Mohamed MH. Reaction of 2-amino-3-cyano-4,5,6,7-tetrahydrobenzo-[b]thiophene with ethyl acetoacetate: novel syntheses of pyridines, pyrazoles, and their fused derivatives. Heteroat Chem. 2001;12(6):518–526. doi: 10.1002/hc.1079. [DOI] [Google Scholar]
- 36.Gein VL, Kazantseva MI, Kurbatova AA, Vahrin MI. Synthesis of 4-aryl-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamides. Chem Heterocycl Compd. 2010;46(5):629–630. doi: 10.1007/s10593-010-0560-8. [DOI] [Google Scholar]
- 37.Gein VL, Kazantseva MI, Kurbatova AA. Synthesis of 4, N-diaryl-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-3-quinolinecarboxamides. Chem Heterocycl Compd. 2011;47(6):728–730. doi: 10.1007/s10593-011-0826-9. [DOI] [Google Scholar]
- 38.Gein VL, Kazantseva MI, Kurbatova AA. synthesis of 4, N-diaryl-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamides. Russ J Org Chem. 2011;47(6):886–888. doi: 10.1134/S1070428011060091. [DOI] [Google Scholar]
- 39.Hussein AM, Gad-Elkareem MAM, El-Adasy AAM, Othman IM. N-1-naphthyl-3-oxobutanamide in heterocyclic synthesis: a facile synthesis of nicotinamide, thieno[2,3-b]pyridine, and Bi- or tricyclic annulated pyridine derivatives containing naphthyl moiety. Phosphorus Sulfur Silicon. 2009;184:2263–2280. doi: 10.1080/10426500802453658. [DOI] [Google Scholar]
- 40.Aly HM, Kamal MM. efficient one-pot preparation of novel fused chromeno[2,3-d]pyrimidine and pyrano[2,3-d]pyrimidine derivatives. Eur J Med Chem. 2012;47:18–23. doi: 10.1016/j.ejmech.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 41.Dyachenko AD, Desenko SM, Dyachenko VD, Rusanov EB. Synthesis and crystal structure of 3-carbamoyl-6-methyl-5-phenyl-carbamoyl-2-thioxo-1,2,3,4-tetrahydro-pyridine-4-spirocyclohexane. Chem Heterocycl Compd. 2003;39(6):744–748. doi: 10.1023/A:1025690927825. [DOI] [Google Scholar]
- 42.Othman IMM, Nasr HM, Hassan MI. synthesis of some novel pyridazine, thienopyridazine, pyrazolopyridine, pyridopyrazolopyrimidine and pyridopyrazolotriazine derivatives with their antimicrobial activity. Can Chem Trans. 2014;2:504–517. [Google Scholar]
- 43.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (1995) In: Wood GL, Washington JA (eds) Manual of clinical microbiology. The American Society for Microbiology, Washington, DC
- 44.Jones RN, Barry AL, Gavan TL, Washington IIA (1985) In: Lennette EH, Ballows A, HauslerJr WJ, Shadomy HJ (eds) Manual of clinical microbiology, 4th edn. The American Society for Microbiology, Washington DC