Abstract
Ochrobactrum anthropi, formerly known as CDC group Vd, is an oxidase-producing, gram-negative, obligately aerobic, non-lactose-fermenting bacillus of low virulence that occasionally causes human infections. It is highly resistant to all β-lactams except imipenem. A clinical isolate, SLO74, and six reference strains were tested. MICs of penicillins, aztreonam, and most cephalosporins tested, including cefotaxime and ceftazidime, were >128 μg/ml and of cefepime were 64 to >128 μg/ml. Clavulanic acid was ineffective and tazobactam had a weak effect in association with piperacillin. Two genes, ampR and ampC, were cloned by inserting restriction fragments of genomic DNA from the clinical strain O. anthropi SLO74 into pBK-CMV to give the recombinant plasmid pBK-OA1. The pattern of resistance to β-lactams of this clone was similar to that of the parental strain, except for its resistance to cefepime (MIC, 0.5 μg/ml). The deduced amino acid sequence of the AmpC β-lactamase (pI, 8.9) was only 41 to 52% identical to the sequence of other chromosomally encoded and plasmid-encoded class C β-lactamases. The kinetic properties of this β-lactamase were typical for this class of β-lactamases. Upstream from the ampC gene, the ampR gene encodes a protein with a sequence that is 46 to 62% identical to those of other AmpR proteins and with an amino-terminal DNA-binding domain typical of transcriptional activators of the Lys-R family. The deduced amino acid sequences of the ampC genes of the six reference strains were 96 to 99% identical to the sequence of the clinical strain. The β-lactamase characterized from strain SLO74 was named OCH-1 (gene, blaOCH-I).
The species of the genus Ochrobactrum form two groups: Ochrobactrum anthropi and O. intermedium (25). O. anthropi, formerly classified as CDC group Vd, is a nonfastidious, gram-negative bacillus that is strictly aerobic, oxidase positive, and motile (with peritrichous flagella), does not ferment lactose, and has strong urease activity (8, 16). O. anthropi is widespread and is distributed in water and hospital environments. In some cases, it has been isolated from water-based environments in hospitals (antiseptic solutions, dialysis fluids) (12). It has often been found on human clinical material: it often adheres to catheters, but pacemakers, intraocular lenses, and silicon tubing may also become infected (10, 18). Although only weakly virulent, O. anthropi causes hospital-acquired infections, often in immunocompromised hosts (7, 11, 14, 27). O. anthropi is usually resistant to β-lactams, such as broad-spectrum penicillins and oxyimino cephalosporins, except for cefepime in some cases and aztreonam (3). It is generally susceptible to carbapenems and aminoglycosides (19), trimethoprim-sulfamethoxazole (4), ciprofloxacin, and tetracyclines. The most effective antimicrobial agents for treating human infections are imipenem, trimethoprim-sulfamethoxazole, and ciprofloxacin (7), sometimes in conjunction with catheter removal (28). As this bacterium displays extensive resistance to β-lactams, we screened for and cloned a β-lactamase gene.
MATERIALS AND METHODS
Bacterial strains.
Table 1 shows the bacterial strains used in this study. O. anthropi SLO74 was isolated in November 1991 at Saint-Louis Hospital (Paris, France) from a blood culture from a leukemic patient with catheter-related sepsis. Six reference strains of O. anthropi were obtained from the collection of the Pasteur Institute (Paris, France). Escherichia coli XL1-Blue (Stratagene, Amsterdam, The Netherlands) and E. coli HB101 (Bio-Rad, Marnes-La-Coquette, France) were used for cloning and subcloning experiments, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR47 supE44 relA1 lacIq ZDM15Tn10 (Tetr) | Stratagene |
| HB101 | F−mcrB mrr hsdS20 (rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 (Smr) supE44 | Bio-Rad |
| O. anthropi | ||
| SLO 74 | Extended-spectrum cephalosporin resistance | Saint-Louis Hospital |
| CIP 82113 | Collection of the Pasteur Institute | |
| CIP 82115 T | ||
| CIP 82116 | ||
| CIP 102332 | ||
| CIP 103949 | ||
| CIP 103952 | ||
| Plasmids | ||
| pBK-CMV phagemid | Neor Kanr | Stratagene |
| pBC SK+ phagemid | Camr | Stratagene |
| pBK-OA1 | 4.7-kb DNA fragment from O. anthropi SLO74 that contained blaOCH-1 in the EcoRI site of pBK-CMV | This study |
| pSK+-OA2 | 3-kb DNA fragment from the recombinant plasmid pBK-OA1 that contained blaOCH-1 in pBC SK+ digested with SacII and EcoRI | This study |
Antimicrobial agents and MIC determination.
The antimicrobial agents used were standard laboratory powders. The antimicrobial agents used were as follows: amoxicillin, clavulanic acid, and ticarcillin (SmithKline Beecham, Nanterre, France); piperacillin and tazobactam (Wyeth-Lederle, Oullins, France); cephalothin and cefamandole (Eli-Lilly, Saint-Cloud, France); cefepime (Bristol-Myers Squibb, Nanterre, France); cefotaxime (Aventis, Paris, France); cefoxitin and imipenem (Merck Sharp & Dohme-Chibret, Paris, France); aztreonam (Sanofi, Paris, France); and ceftazidime (GlaxoWellcome, Marly-le-Roy, France).
MICs were determined with the standard agar dilution technique on Mueller-Hinton agar (Bio-Rad) with a multiple inoculator and an inoculum of 105 CFU per spot. All plates were incubated at 37°C for 18 h. The MICs of some β-lactams (amoxicillin, ticarcillin, and piperacillin) were determined alone or in combination with 2 μg of clavulanic acid per ml or 4 μg of tazobactam per ml.
Cloning experiments and recombinant plasmids.
The chromosomal DNA of O. anthropi SLO74 was prepared as described by Grimont and Grimont (13). It was digested with EcoRI (Roche Biochemicals France S.A., Meylan, France) and ligated using T4 DNA ligase (Amersham Pharmacia Biotech, Saclay, France) into the EcoRI site of the pBK-CMV phagemid (Table 1) (Stratagene). Recombinant plasmids were introduced into E. coli XL1 by the standard CaCl2 technique. Antibiotic-resistant colonies were selected on Drigaski agar (Bio-Rad) containing ceftazidime (2 μg/ml) and kanamycin (25 μg/ml) (Sigma, Saint-Quentin Falavier, France). Recombinant plasmid DNA was recovered using Qiagen columns (Qiagen, Courtaboeuf, France), and the size of the inserts was estimated by restriction enzyme digestion and electrophoresis in 1 to 3% agarose gels.
The recombinant plasmid pBK-OA1 was double digested with SacII (Roche Biochemicals) and EcoRI, and the resulting fragment was ligated into the pBC SK+ phagemid (Table 1) (Stratagene) digested with the same enzymes. Transformants were selected on the basis of resistance to amoxicillin (40 μg/ml) and chloramphenicol (25 μg/ml) (Sigma) using Drigalski agar plates.
Preparation of crude extracts of β-lactamase.
The O. anthropi SLO74 strain and the six reference strains of O. anthropi (Table 1) were cultured overnight at 37°C in 100 ml of Trypticase soy broth. For cultures of the E. coli XL1(pBK-OA1) clone and the E. coli HB101(pSK+-OA2) subclone, amoxicillin (40 μg/ml) and more kanamycin (25 μg/ml) or more chloramphenicol (25 μg/ml), respectively, were added to the medium to maintain selection pressure. Bacterial suspensions were pelleted (30 min at 5,800 × g), resuspended in 2 ml of 20 mM Tris buffer (pH 7.5), and disrupted by sonication (two times for 30 s each at 20 Hz) (Vibra Cell; Bioblock Scientific, Illkirch, France). The crude extracts were cleared by centrifugation at 48,000 × g for 30 min at 4°C.
IEF.
All β-lactamase extracts were subjected to analytical isoelectric focusing (IEF) (1) on an ampholine polyacrylamide gel with a pH range of 3.5 to 10. The gel was put in a Multiphor apparatus (Amersham Pharmacia Biotech) for 18 h at 200 V, 15 mA, and 6 W. The focused β-lactamases were detected by overlaying the gel with 1 mM nitrocefin (Oxoid, Paris, France). The pI markers were those for the reference β-lactamases: for TEM-3 (pCFF04), pI is 6.3; for SHV-4 (pUD21), pI is 7.8; and for CMY-2 (pSenf), pI is 9.2.
β-Lactamase purification and kinetic measurements.
Cells of the E. coli HB101 subclone containing the recombinant plasmid pSK+-OA2 (Table 1) were obtained from 4-liter cultures in brain heart infusion broth (Difco) at 37°C. Cells were harvested by centrifugation at 5,800 × g for 30 min. The pellets (about 12 g [wet weight]) were washed by resuspending them in 24 ml of 0.15 M NaCl and centrifuging at 5,800 × g for 20 min. The supernatants were discarded and the pellets were resuspended under the same conditions, lysed by sonication (Branson Sonifier), and centrifuged at 5,800 × g for 1 h. The pellets were discarded. The crude extracts were cleared by centrifugation at 48,000 × g for 30 min at 4°C. Nucleic acids were precipitated by adding spermine (0.2 M) (Sigma) and collected by centrifugation (30,000 × g for 30 min at 4°C). The supernatant was dialyzed three times against 5 liters of distilled water and lyophilized. The enzyme was purified by chromatography on Bio-Rex 70 resin (weakly acidic cation exchanger) equilibrated with 10 mM Tris hydrochloride buffer, pH 7.0. The β-lactamase was eluted with a linear gradient of 0 to 0.6 M NaCl, and active fractions were pooled, desalted by three centrifugations and dilutions on an Ultrafree-20 centrifuge filter unit with a nominal molecular weight limit of 10,000 (Sigma), and used rapidly for kinetic studies. The kinetic constants kcat and Km for substrates were determined by a computerized microacidimetric assay at pH 7.0 and 37°C in 0.1 M NaCl as described by Labia et al. (20). One β-lactamase unit is defined as the amount of enzyme hydrolyzing 1 μmol of benzylpenicillin in 1 min at pH 7.0 and 37°C. In this test, the initial benzylpenicillin concentration is 500 μM.
DNA sequencing, PCR amplification, and sequence analysis.
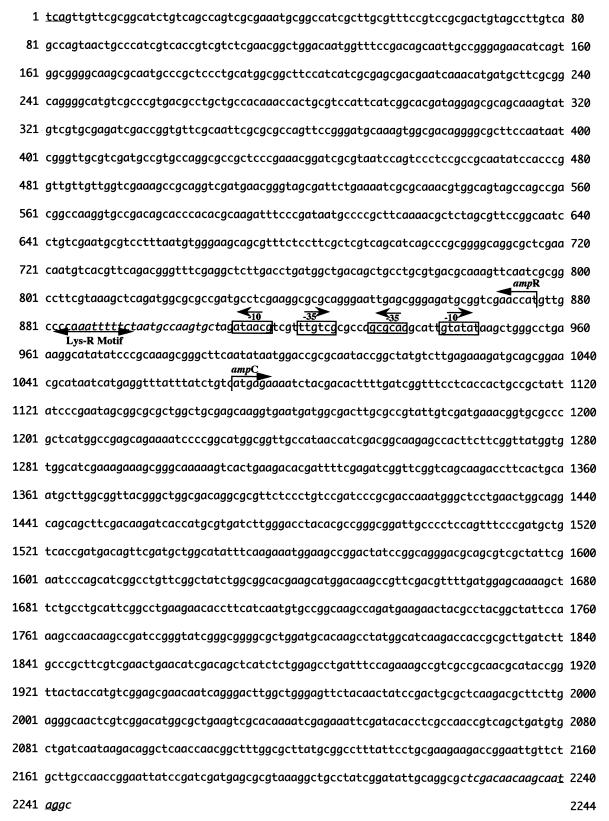
The insert of the recombinant plasmid pBK-OA1 was sequenced using the method of Sanger et al. (30), with fluorescent dye-labeled dideoxynucleotides, thermal cycling with Taq polymerase (Amersham), and an ABI 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). We studied the variability of the ampC genes of O. anthropi using two primers designed to amplify the entire coding region in the six reference strains: upper ochro (5′-AATTTTTCTAATGCCAAGTGCT-3′) and lower ochro (5′-GCCTATTGCTTGTTGTCGAG-3′) (see Fig. 2). The PCR products were sequenced and analyzed. The BLASTN program of NCBI (http://www.ncbi.nlm.nih.gov) was used for database searches, and Clustal W (http://www.2.eib.ac.uk./clustalw) was used to align multiple protein sequences.
FIG. 2.
Nucleotide sequence of the 2,244-bp fragment of pBK-OA1 containing the ampC and ampR coding regions. The putative promoter sequences are indicated as −35 and −10 regions (boxed). The start codons and the Lys-R motif are indicated by arrows, and the stop codons are underlined. The PCR primers upper ochro and lower ochro are in italics.
Nucleotide sequence accession numbers.
The blaOCH gene nucleotide sequence data appear in the EMBL nucleotide sequence database under accession no. AJ401618 for blaOCH-1, AJ295340 for blaOCH-2, AJ295341 for blaOCH-3, AJ295342 for blaOCH-4, AJ295343 for blaOCH-5, AJ295344 for blaOCH-6, and AJ294345 for blaOCH-7.
RESULTS
Antimicrobial agent susceptibility.
The MICs of β-lactams for all strains of O. anthropi showed that these strains were resistant to all β-lactams tested except imipenem (Table 2). Clavulanic acid was ineffective and tazobactam slightly reduced resistance to piperacillin. The E. coli XL1(pBK-OA1) clone and the E. coli HB101(pSK+-OA2) subclone had similar resistance phenotypes: resistance to all penicillins (intermediate resistance to piperacillin in the clone harboring pBK-OA1), resistance to all cephalosporins except cefepime, intermediate susceptibility to aztreonam, and susceptibility to imipenem.
TABLE 2.
MICs of β-lactams for O. anthropi SLO74, six reference strains of O. anthropi, the E. coli XL1(pBK-OA1) clone, the E. coli HB101(pSK+-OA2) subclone, and the strains E. coli XL1 and E. coli HB101
| Strain | MIC (μg/ml) for the given strainc
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMX | AMC | TIC | TCC | PIP | TZP | CEF | FAM | CFM | FOX | CXM | CTX | FEP | CAZ | ATM | IPM | |
| O. anthropi | ||||||||||||||||
| SLO74 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 0.5 |
| CIP 82113 | ≥128 | 128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 128 | ≥128 | ≥128 | 64 | ≥128 | ≥128 | 1 |
| CIP 82115 T | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 0.5 |
| CIP 82116 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 1 |
| CIP 102332 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 0.25 |
| CIP 103949 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 1 |
| CIP 103952 | ≥128 | 128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | ≥128 | 64 | ≥128 | ≥128 | 64 | ≥128 | ≥128 | 0.25 |
| E. coli | ||||||||||||||||
| XL1(pBK-OA1)a | ≥128 | ≥128 | ≥128 | ≥128 | 16 | 8 | ≥128 | ≥128 | ≥128 | 128 | ≥128 | 32 | 0.5 | 128 | 8 | 0.12 |
| HB101(pSK+-OA2)b | ≥128 | 128 | ≥128 | ≥128 | 64 | 16 | ≥128 | ≥128 | ≥128 | 64 | ≥128 | 64 | 0.5 | ≥128 | 16 | 0.25 |
| XL1 | 4 | 1 | 0.5 | 0.12 | 1 | 0.25 | 2 | 0.5 | 0.25 | 2 | 0.5 | 0.06 | 0.06 | 0.12 | 0.06 | 0.5 |
| HB101 | 4 | 1 | 0.5 | 0.12 | 0.5 | 0.25 | 4 | 0.5 | 0.25 | 2 | 0.5 | 0.06 | 0.06 | 0.12 | 0.06 | 0.5 |
This strain, harboring the recombinant multicopy plasmid pBK-OA1, produced OCH-1 β-lactamase (in the presence of the regulator gene).
This strain, harboring the recombinant multicopy plasmid pSK+-OA2, produced OCH-1 β-lactamase (in the absence of the regulator gene).
AMX, amoxicillin; AMC, AMX + clavulanic acid (2 μg/ml), TIC, ticarcillin; TCC, TIC + clavulanic acid; PIP, piperacillin; TZP, PIP + tazobactam (4 μg/ml); CEF, cephalothin; FAM, cefamandole; CFM, cefixime; FOX, cefoxitin; CXM, cefuroxime; CTX, cefotaxime; FEP, cefepime; CAZ, ceftazidime; ATM, aztreonam; IPM, imipenem.
IEF.
A band of β-lactamase activity was detected in each strain of O. anthropi in analytical IEF experiments. The corresponding pI was 8.9 for the parental strain O. anthropi SLO74. The pI values for the six reference strains of O. anthropi were from 8 to 9.2 (CIP 82113, pI = 9; CIP 83115T, pI = 8; CIP 83116, pI ≥ 9.2; CIP 102332, pI ≥ 9.2; CIP 103949, pI = 9.2; CIP 103952, pI = 8.5) (data not shown).
Cloning and sequence analysis of blaOCH-1 and subcloning.
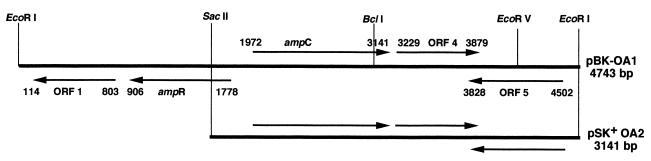
Total genomic DNA from O. anthropi SLO74 was digested with EcoRI and inserted into the EcoRI site of pBK-CMV. Four identical recombinant E. coli XL1 clones were obtained. One harboring pBK-OA1 (insert, 4.7 kb) was selected for further study. It produced a β-lactamase with a pI of 8.9. The entire 4,743-bp DNA insert was sequenced on both strands. We found that this insert contained five open reading frames (ORFs) (Fig. 1). Two ORFs (ORF4 and ORF5) showed no sequence identity with DNA sequences in databases in BLASTN searches. ORF1 was 98% identical to a gene encoding a 25-kDa outer membrane protein in Brucella abortus (9). ORF3 was 1,169 bp long and encoded a 390-amino-acid sequence. This ORF was preceded by putative −35 (TTGTCG) and −10 (GTATAT) promoter regions and a putative ATG initiation codon at position 1070 (Fig. 2). The consensus sites, SVSK and KTG, characteristic of serine β-lactamases were detected in the deduced amino acid sequence of the protein (17). The structural element characteristic of class C β-lactamases, YSN (24), was also detected (Fig. 3). The deduced amino acid sequence (OCH-1) was 42 to 52% identical to those of other chromosomally encoded and plasmid-encoded class C β-lactamases (Fig. 4). Immediately upstream from the blaOCH-1 gene was ORF2, an 867-bp ORF containing an ampR gene. This ampR gene had an overlapping and divergently oriented promoter (2): the sequences of boxes −35 and −10 were AACGCG and GCAATA, and a Lys-R motif (CTTTTTAAACC) (15, 21) was found (Fig. 2). The deduced amino acid sequence of the AmpR protein showed this protein to have a helix-turn-helix domain at the N terminus (15, 21). The AmpR protein of O. anthropi was 46 to 62% identical to other AmpR proteins (Fig. 5).
FIG. 1.
Schematic restriction endonuclease map of the recombinant plasmids pBK-OA1 and pSK+-OA2. The genes ampR and ampC and the other ORFs are indicated.
FIG. 3.
Multiple alignment of AmpC amino acid sequences deduced from the sequence of the ampC gene present in O. anthropi SLO74 and in the six reference strains of O. anthropi. The specific SVSK and KTG boxes of the serine-active β-lactamases and the KTG box specific for class C β-lactamases are boxed. Identical amino acids are indicated by dashes.
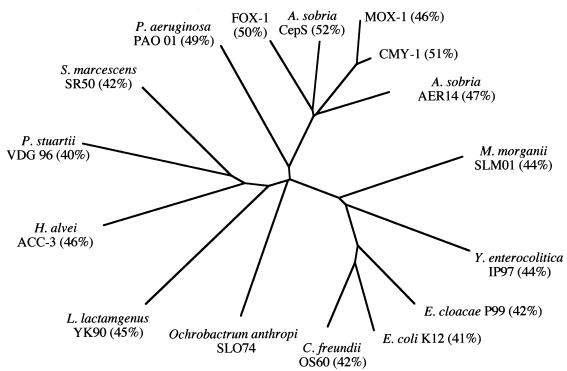
FIG. 4.
Schematic dendrogram obtained for 16 representative chromosomally encoded and plasmid-encoded class C β-lactamases. Percentages in brackets are percent identities between the indicated amino acid sequence and that of OCH-1.
FIG. 5.
Multiple alignment of deduced amino acid sequences of AmpR, which regulates cephalosporinase genes. The origins of the AmpR sequences are as follows: 1, E. cloacae MHN-1; 2, Citrobacter freundii OS 60; 3, Yersinia enterocolitica IP97; 4, Morganella morganii GUI-1; 5, O. anthropi SLO74; 6, P. aeruginosa PAO01; 7, Providencia stuartii VDG 96. Identical amino acids are indicated by an asterisk. The predicted helix-turn-helix motif (HTH) of the Lys-R family is indicated by arrows.
pBK-OA1 was double digested with SacII and EcoRI, and the resulting fragment was inserted into pBC SK+. The E. coli HB101 subclone harbored a recombinant plasmid, pSK+-OA2. DNA sequencing showed this plasmid to contain an insert of 3,141 bp. The ampC gene (ORF3) and a truncated ampR gene (ORF2) were identified in this insert (Fig. 1). This subclone was used for kinetic measurements for the OCH-1 β-lactamase.
Biochemical properties of OCH-1.
The β-lactamase of O. anthropi produced by the E. coli HB101(pSK+-OA2) subclone was overproduced, and crude extracts were found to have a specific activity of about 1,000 mU per mg. The enzyme was purified and the final preparation of the enzyme was >90% pure, as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, which was similar to results published for other class C β-lactamases (5). The isoelectric point of the enzyme, determined by analytical IEF (1), was 8.9. This enzyme had a low kcat for penicillins, a high kcat for cephalothin and cefaloridine, and similar kcat values for oxyimino cephalosporins (Table 3), but the Km measured for cefotaxime was low (9 μM), whereas those measured for cefepime, cefpirome, and ceftazidime were high. Aztreonam was a poor substrate, with no detectable hydrolysis and a high Ki (data not shown). For cephamycins (cefoxitin and cefotetan), no hydrolysis was detected, but the Kis were very low (excellent affinity) (Table 3).
TABLE 3.
Kinetic parameters of β-lactamase OCH-1 for various substrates
| β-Lactam | kcat (s−1)a | Km (μM) | kcatKm (s−1 μM−1) |
|---|---|---|---|
| Benzylpenicillin | 20b | 2.0 | 10.0 |
| Amoxicillin | 2.0 | 1.0 | 2.0 |
| Cephalothin | 480 | 260 | 1.84 |
| Cephaloridine | 400 | 415 | 0.96 |
| Cefepime | 2.8 | 760 | 0.004 |
| Cefpirome | 3.2 | 800 | 0.004 |
| Cefotaxime | 3.6 | 9.0 | 0.4 |
| Ceftazidime | 4.0 | 124 | 0.032 |
| Cefoxitin | —c | 0.1 | NAd |
| Cefotetan | —c | 0.1 | NAd |
For compounds with a kcat lower than 10 s−1, Ki values were determined instead of Km values, using cephalothin as the substrate.
Standard deviations for kcat values were 15%, and standard deviations were about 20% for Km and Ki values. Each determination was made at least in triplicate.
kcat of <0.05 s−1.
NA, not applicable.
Diversity of the O. anthropi AmpC β-lactamase.
An amplification product of about 1.3 kb was obtained for the six reference strains of O. anthropi. Sequencing of these products showed the ampC gene to be present in all six. Amino acid sequence analysis showed few differences (Fig. 3). The percentage of identity was between 96 and 99%.
DISCUSSION
O. anthropi is a gram-negative, mobile, aerobic, oxidase-positive, rod-shaped bacterium that often infects immunocompromised hosts (11, 14). The most frequent infection due to O. anthropi is central venous catheter-related bacteremia (10, 18). O. anthropi is generally resistant to all β-lactams except imipenem (4). The clinical strain SLO74 has this restoring resistance phenotype. The antimicrobial agent susceptibility of this strain and of the six reference strains confirmed this resistance to all β-lactams except imipenem. β-Lactamase inhibitors were inactive (clavulanic acid) or had only weak activity (tazobactam) in restoring susceptibility to penicillins. Among the gram-negative bacteria, O. anthropi is the most resistant to β-lactams (e.g., Stenotrophomonas maltophilia, which in addition is resistant to carbapenems) (31).
We investigated the cause of this high level of resistance to β-lactams by searching for β-lactamase production. All strains studied had a β-lactamase with a pI in the alkaline range (8 to >9.2). The pattern of susceptibility, inactivity of inhibitors, and alkaline pI suggested the presence of a class C β-lactamase (6). Using total DNA digested with EcoRI, we obtained four identical recombinant clones. The E. coli XL1 clone harboring the recombinant plasmid pBK-OA1 was resistant to all β-lactams (intermediate resistance to piperacillin and aztreonam) except cefepime and imipenem. This profile differed from that of the parental strain, O. anthropi SLO74, essentially in the level of resistance to cefepime. Cefepime is known to be stable and to be unaffected by class C β-lactamases (29). Therefore, the resistance to cefepime observed in the parental strain and in the six reference strains may be due to an impermeability mechanism (26). Analysis of the DNA sequence of the 4,743-bp insert of pBK-OA1 showed the presence of a gene with less than 50% identity to genes encoding class C β-lactamases. The deduced amino acid sequence contained two sites characteristic of active-site serine β-lactamases (SXXK and KXG) and a site characteristic of class C β-lactamases (YXN). The protein, OCH-1, was 42 to 52% identical to various different chromosomally encoded and plasmid-encoded class C β-lactamases. The highest level of sequence identity to OCH-1 was recorded with a class C β-lactamase called CepS (Aeromonas sobria) (32), with 52% identity. It is a new class C β-lactamase, very distantly related to other known class C enzymes.
The kinetic parameters of the purified OCH-1 β-lactamase from O. anthropi are typical of class C β-lactamases such as that produced by Enterobacter cloacae P99 (23). OCH-1 conferred resistance to all β-lactams except cefepime and imipenem. The level of resistance to extended-spectrum cephalosporins is high and comparable to that observed in Enterobacteriaceae overproducing the chromosomal class C β-lactamase, and it was therefore not possible to observe the inducible effect of cefoxitin or imipenem by standard diffusion (2).
Immediately upstream from the ampC gene was a gene in the opposite orientation that was 45 to 60% identical to known ampR genes. A helix-turn-helix motif was detected at the N-terminal end of the deduced amino acid sequence, as observed in other AmpR proteins and typical of transcriptional activators of the the Lys-R family (15, 21).
Analysis of the intercistronic region showed the presence of a Lys-R motif. The DNA sequence of this motif displayed a higher level of identity to the corresponding region in Pseudomonas aeruginosa PAO01 (22) than in Enterobacteriaceae (Fig. 6). This region is very long (193 bp) and is longer than the intercistronic region present in P. aeruginosa PAO01 (22). The putative promoters of the ampC and ampR genes overlapped and were divergently oriented, as previously described for the ampC-ampR regulatory system (23).
FIG. 6.
Alignment of the ampC-ampR intercistronic region from the β-lactamase of the following strains: 1, O. anthropi SLO74; 2, P. aeruginosa PAO01; 3, P. stuartii VDG 96; 4, M. morganii SLM 01; 5, Y. enterocolitica IP 97; 6, C. freundii OS 60. The Lys-R motif is boxed.
Comparison of the seven deduced AmpC amino acid sequences from the parental strain, SLO74, and the six reference strains of O. anthropi showed that there were seven different β-lactamases, with amino acid sequences that were 96 to 99% identical. We obtained seven different pIs, confirming this observation. These seven β-lactamases are very distantly related to the other group of class C β-lactamases. The β-lactamase present in strain SLO74 was named OCH-1, and the others present in the six reference strains of O. anthropi were named OCH-2 (CIP 82113), OCH-3 (CIP 82115T), OCH-4 (CIP 82116), OCH-5 (CIP 102332), OCH-6 (CIP 103949), and OCH-7 (CIP 103952).
ACKNOWLEDGMENTS
This work was financed by grants from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (Réseau β-lactamase), Paris, from the UFR Saint-Antoine, Paris, and from the Institut Beecham, La Défense, France.
REFERENCES
- 1.Barthélémy M, Guionie M, Labia R. β-Lactamases: determination of their isoelectric points. Antimicrob Agents Chemother. 1978;13:695–698. doi: 10.1128/aac.13.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett P M, Chopra I. Molecular basis of β-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizet C, Bizet J. Sensibilité comparée de Ochrobactrum anthropi, Agrobacterium tumefaciens, Alcaligenes faecalis, Alcaligenes denitrificans subsp. denitrificans, Alcaligenes denitrificans subsp. xylosoxydans et Bordetella bronchiseptica vis-à-vis de 35 antibiotiques dont 17 β-lactamamines. Path Biol. 1995;43:258–263. [PubMed] [Google Scholar]
- 4.Brivet F, Guibert M, Kiredjian M, Dormont J. Necrotizing fasciitis, bacteremia, and multiorgan failure caused by Ochrobactrum anthropi. Clin Infect Dis. 1993;17:516–518. doi: 10.1093/clinids/17.3.516. [DOI] [PubMed] [Google Scholar]
- 5.Bulychev A, Mobashery S. Class C β-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin substrates. Antimicrob Agents Chemother. 1999;43:1743–1746. doi: 10.1128/aac.43.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cieslak T J, Robb M L, Drabick C J, Fischer G W. Catheter-associated sepsis caused by Ochrobactrum anthropi: report of a case and review of related nonfermentative bacteria. Clin Infect Dis. 1992;14:902–907. doi: 10.1093/clinids/14.4.902. [DOI] [PubMed] [Google Scholar]
- 8.Déliere E, Vu-Thien H, Levy V, Barquins S, Schlegel L, Bouvet A. Epidemiological investigation of Ochrobactrum anthropi strains isolated from a haematology unit. J Hosp Infect. 2000;44:173–178. doi: 10.1053/jhin.1999.0690. [DOI] [PubMed] [Google Scholar]
- 9.de Wergifosse P. Cloning and nucleotide sequence of the gene coding for the 25-kilodalton outer membrane protein of Brucella abortus. J Bacteriol. 1995;177:1911–1914. doi: 10.1128/jb.177.7.1911-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earhart K C, Boyce K, Bone W D, Wallace M R. Ochrobactrum anthropi infection of retained pacemaker leads. Clin Infect Dis. 1997;24:281–282. doi: 10.1093/clinids/24.2.281. [DOI] [PubMed] [Google Scholar]
- 11.Gill M V, Ly H, Mueenuddin M, Schoch P E, Cunha B A. Intravenous line infection due to Ochrobactrum anthropi (CDC group Vd) in a normal host. Heart Lung. 1997;26:335–336. doi: 10.1016/s0147-9563(97)90092-3. [DOI] [PubMed] [Google Scholar]
- 12.Grandsden W R, Eykyn S J. Seven cases of bacteremia due to Ochrobactrum anthropi. Clin Infect Dis. 1992;15:1068–1069. doi: 10.1093/clind/15.6.1068. [DOI] [PubMed] [Google Scholar]
- 13.Grimont F, Grimont P A D. Ribosomal nucleic acid gene restriction as a potential taxonomic tool. Ann Microbiol. 1986;137B:165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 14.Haditsch M, Binger L, Tschurtschenthaler G, Watschinger R, Zauner G, Mittermayer H. Bacteremia caused by Ochrobactrum anthropi in an immunocompromised child. Infection. 1994;22:291–292. doi: 10.1007/BF01739922. [DOI] [PubMed] [Google Scholar]
- 15.Henikoff S, Haughn G W, Wulff D L, Wallace J C. A large family of bacterial activator proteins. Proc Natl Acad Sci USA. 1988;85:6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1988;38:406–416. [Google Scholar]
- 17.Joris B, Ghuysen J M, Dive G, Renard A, Dideberg O, Charlier P, Frere J M, Kelly J A, Boyington J C, Moews P C, Knox J R. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Eur J Biochem. 1988;250:313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern W V, Oethinger M, Kaufhold A, Rozdzinski E, Marre R. Ochrobactrum anthropi bacteremia: report of four cases and short review. Infection. 1993;21:306–310. doi: 10.1007/BF01712451. [DOI] [PubMed] [Google Scholar]
- 19.Klein J D, Eppes S C. Ochrobactrum anthropi bacteremia in a child. Del Med J. 1993;65:493–495. [PubMed] [Google Scholar]
- 20.Labia R, Andrillon J, Le Goffic F. Computerized microacidimetric determination of β-lactamase Michaelis Menten constants. FEBS Lett. 1973;33:42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg F, Westman L, Normark S. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc Natl Acad Sci USA. 1987;82:4620–4624. doi: 10.1073/pnas.82.14.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge J M, Minchin S D, Piddock L J, Busby J W. Cloning and sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal β-lactamase. Biochem J. 1990;272:627–631. doi: 10.1042/bj2720627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matagne A, Dubus A, Galleni M, Frere J M. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat Prod Rep. 1999;16:1–19. doi: 10.1039/a705983c. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura N, Minami S, Mitsuhashi S. Sequences of homologous β-lactamases from clinical isolates of Serratia marcescens with different substrate specificities. Antimicrob Agents Chemother. 1998;42:176–179. doi: 10.1128/aac.42.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltroche-Llacsahuanga H, Brandenburg V, Riehl J, Hasse G. Ochrobactrum anthropi peritonitis in a CAPD patient. J Infect. 2000;40:299–301. doi: 10.1053/jinf.2000.0648. [DOI] [PubMed] [Google Scholar]
- 26.Piddock L J, Traynor E A. β-Lactamase expression and outer membrane protein changes in cefpirome-resistant and ceftazidime-resistant gram-negative bacteria. J Antimicrob Chemother. 1991;28:209–219. doi: 10.1093/jac/28.2.209. [DOI] [PubMed] [Google Scholar]
- 27.Roberto M, Anna N, Morena F, Leonardo C, Marina T, Francesco C. Ochrobactrum anthropi as an agent of nosocomial septicemia in the setting of AIDS. Clin Infect Dis. 1999;28:692–694. doi: 10.1086/517224. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra J, Garrido C, Folgueira D, Torres M J, Ramos J T. Ochrobactrum anthropi bacteremia associated with a catheter in an immunocompromised child and review of the pediatric literature. Pediatr Infect Dis J. 1999;18:586–660. doi: 10.1097/00006454-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Sanders C C. Cefepime: the next generation? Clin Infect Dis. 1993;17:369–379. [PubMed] [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer R C. The emergence of epidemic, multiple-antibiotic-resistant Stenotrophomonas (Xanthomonas) maltophilia and Burkholderia (Pseudomonas) cepacia. J Hosp Infect. 1995;30(Suppl.):453–464. doi: 10.1016/0195-6701(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T R, Hall J, MacGowan A P, Bennett P M. Sequence analysis of two chromosomally mediated inducible β-lactamases from Aeromonas sobria, strain 163a, one a class D penicillinase, the other an AmpC cephalosporinase. J Antimicrob Chemother. 1995;36:41–52. doi: 10.1093/jac/36.1.41. [DOI] [PubMed] [Google Scholar]