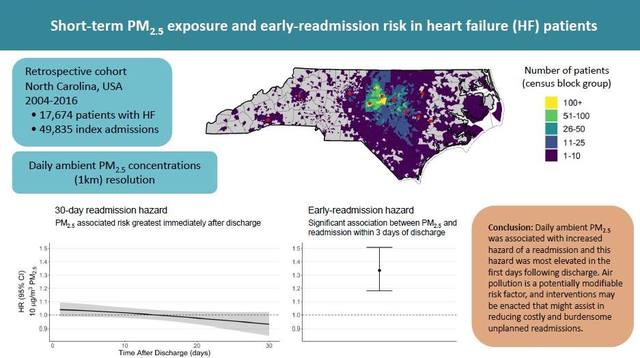
Abstract
Background:
Short-term changes in ambient fine particulate matter (PM2.5) increase the risk for unplanned hospital readmissions. However, this association has not been fully evaluated for high-risk patients or examined to determine if the readmission risk differs based on time since discharge. Here we investigate the relation between ambient PM2.5 and 30-day readmission risk in heart failure (HF) patients using daily time windows and examine how this risk varies with respect to time following discharge.
Methods:
We performed a retrospective cohort study of 17,674 patients with a recorded HF diagnosis between 2004 and 2016. The cohort was identified using the EPA CARES electronic health record resource. The association between ambient daily PM2.5 (μg/m3) concentration and 30-day readmissions was evaluated using time-dependent Cox proportional hazard models. PM2.5 associated readmission risk was examined throughout the 30-day readmission period and for early readmissions (1–3 days post-discharge). Models for 30-day readmissions included a parametric continuous function to estimate the daily PM2.5 associated readmission hazard. Fine-resolution ambient PM2.5 data were assigned to patient residential address and hazard ratios are expressed per 10 μg/m3 of PM2.5. Secondary analyses examined potential effect modification based on the time after a HF diagnosis, urbanicity, medication prescription, comorbidities, and type of HF.
Results:
The hazard of a PM2.5-related readmission within three days of discharge was 1.33 (95% CI 1.18–1.51). This PM2.5 readmission hazard was slightly elevated in patients residing in non-urban areas (1.43, 95%CI 1.22–1.67) and for HF patients without a beta-blocker prescription prior to the readmission (1.35; 95% CI 1.19–1.53).
Conclusion:
Our findings add to the evidence indicating substantial air quality-related health risks in individuals with underlying cardiovascular disease. Hospital readmissions are key metrics for patients and providers alike. As a potentially modifiable risk factor, air pollution-related interventions may be enacted that might assist in reducing costly and burdensome unplanned readmissions.
Keywords: Air pollution, particulate matter, PM2.5, heart failure, hospitalization, readmission
Graphical Abstract:
Introduction
Fine particulate air pollution (≤ 2.5 μm in diameter, PM2.5) is a global health risk factor, and in 2015 it was attributed to 7.6% of deaths and 4.2% of disability-adjusted life-years worldwide (1, 2). Notably, a greater proportion of this health burden occurs in sensitive populations including individuals with chronic health conditions (3, 4). Epidemiologic studies in populations with chronic conditions have associated short-term PM2.5 exposure with increased rates of hospitalization and readmission risk using windows of readmission, including multi-year, 1-yr, and 30-days (5–11). Though readmission windows provide a standardized way to report risk, they may obscure key risk periods as they do not evaluate the potential of time-varying risk. To better understand and communicate PM2.5-related risk following discharge it is necessary to examine how risk varies in the days following discharge, especially for individuals with chronic health conditions such as cardiovascular disease, who have high rates of readmission.
Unplanned inpatient readmissions are an indicator of patient morbidity and a statistic used by hospitals and healthcare systems as a quality-of-care metric (12). Minimizing readmissions is a priority for healthcare systems and primarily the focus has been on hospital-based standards of care; however, the role of the ambient environment on patient health should also be considered (13). Air pollution may contribute to exacerbating underlying cardiovascular and respiratory health conditions, resulting in increased readmissions (14). Indeed, elevated readmission risk in association with both long-term and short-term PM2.5 has been observed in populations with cardiovascular disease (5–7, 15) and kidney disease (9). These studies provide evidence of increased risk in individuals with comorbidities. Yet studies to date have not specifically focused on readmission risk and short-term air pollution among heart failure (HF) patients, who have some of the highest readmissions risks and for whom current national policies have had limited success at reducing readmissions. Additionally, studies have not examined beyond the standard readmission windows to characterize the daily risk. Such a study could identify potential changes in the air pollution related hazard following discharge (i.e. non-proportional hazard with respect to time), which is an important step for communicating exposure risks for improved patient care.
The aim of this study was to evaluate the association between daily PM2.5 exposure following hospital discharge and 30-day hospital readmissions among patients diagnosed with HF in North Carolina, USA between 2004 and 2016. A time-varying model with daily windows was used to capture changes in air quality-related readmission risk after discharge and potentially identify key post-discharge windows of susceptibility. We also examined changes in readmission risk based on the time after HF diagnosis, co-morbid conditions, type of HF, urbanicity, and medication prescription.
Materials and Methods
Study population and health outcome
The study cohort was identified using electronic health records (EHR) in the University of North Carolina Healthcare System and included individuals with a recorded HF diagnosis between July 1, 2004 and December 31, 2016. HF diagnoses were defined according to the International Classification of Diseases, Ninth Revision codes (ICD-9, 428.x) and Tenth Revision codes (ICD-10, I50.x). EHR were linked to patient demographics, address, and death records. Records removed included those with inconsistent birth, death, or visit dates or addresses that could not be geocoded. Full details about the data warehouse, cohort, and geocoding have been published (16). This study was approved by an Institutional Review Board at the University of North Carolina Chapel Hill (IRB #17–0150).
The primary outcome was hazard of a 30-day readmission for any cause following a hospital discharge. EHR were used to identify inpatient hospitalizations (index admission) and subsequent inpatient hospitalization (readmission). Hospitalizations could serve as both an index admission and a readmission. Patient hospitalizations that occurred on the same day as a discharge were assumed to represent facility transfers and were linked to a single index hospitalization. Consistent with other estimations of readmission risk, we only considered index admissions where patients were discharged alive (9, 17). As the analysis was focused on 30-day readmissions, censoring occurred at 30 days. Patients could be represented more than once if they were hospitalized multiple times during the study period.
Environmental data
Daily PM2.5 (μg/m3) was estimated on a 1km × 1km grid using a previously described exposure prediction model that incorporates satellite aerosol optical depth measurements, chemical transport model simulations, meteorology, and land-use. Reported cross-validation and mean square error indicated good concurrence between monitor readings and predicted values (R2 of 0.84, mean square error of 1.3 μg/m3) (3, 18–20). Temperature and relative humidity data were obtained using the National Climatic Data Center API. Air pollution and meteorological data were linked to a patient’s geocoded home address, using the nearest 1km × 1km centroid for PM2.5 and monitoring station location for meteorological data.
Statistical analysis
Risk of a 30-day readmission
The 30-day readmission risk associated with same day ambient PM2.5 was estimated using time-dependent Cox proportional hazards models. Models incorporated both time-dependent and time-independent risk factors. Time-dependent variables included daily PM2.5, daily temperature, daily relative humidity, and discharge day-of-the-week. Non-linear relationships with temperature and relative humidity have been observed previously (9, 21), and were modeled using the average of lag days 0, 1, and 2 and natural splines (df = 4). Time-independent factors included patient-specific, hospitalization event-specific, and local aggregated variables. Patient-specific variables included indicator of sex, race, baseline smoking status at the time of HF diagnosis, whether the patient had three or more previous hospital visits in the year prior, and age at discharge. Event-specific variables included whether the discharge occurred on a holiday and length of stay. Models were additionally adjusted for local social characteristics, including the levels of public assistance, greater than high-school level education, urbanicity at the census block group level (2010 US Census), and the percentage of adults reporting fair or poor health at the county level (22). Models included a continuous logarithmic function to model risk and examine non-proportional hazards with respect to the time following discharge. To account for repeated measures from individuals contributing multiple events, models were adjusted for patient-specific clusters. We acknowledged death as a competing risk and calculated the cause-specific hazard to estimate the instantaneous all-cause readmission hazard (23). The calculated PM2.5 associated readmission risk reflects a short-term hazard and is expressed as the daily hazard (hazard ratio, HR) of readmission.
In secondary analyses, we assessed potential effect modification by sex, race, urbanicity, prescription history, time since HF diagnosis, HF subtype, and patient comorbidities. The median percent urbanicity (86% urban) was used as a cut off to examine readmission hazards in non-urban (below median) and urban (above median) strata. Prescription of a beta blocker was assessed as a binary factor. Once a patient was prescribed a medication listed in Supplemental Table 1 they were assumed to be on the medication for the remainder of the study period. We focused on beta-blockers for the class of medications examined because they have been observed to attenuate the effect of air pollution (24–27). The impact of time since HF diagnosis was examined by stratifying on tertiles of time since HF diagnosis. This was examined because the readmission risk associated with PM2.5 could also change based on potential time periods of increased sensitivity following hospitalization or time since HF diagnosis. HF subtype was examined for systolic and diastolic heart failure. Comorbidities included type 2 diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), emphysema, hypertension, hyperlipedemia, peripheral artery disease (PAD), and ischemic heart disease (IHD).
Risk of an early-readmission (1–3 days)
To evaluate PM2.5-readmission impacts immediately after discharge, the risk of a readmission occurring within 3 days of discharge (early-readmission) was measured using similar methods as the 30-day readmission models. Risk was examined using Cox proportional hazards models censored at 3 days. In secondary analyses we also assessed effect modification by sex, race, urbanicity, time since HF diagnosis, HF subtype, and patient comorbidities. The association with respect to time since HF diagnosis was explored further using models stratified on different windows of time (120 days) that were incremented every 10 days. Results are expressed as the hazard of an early-readmission.
As a post-hoc analysis we also estimated the number of annual excess readmissions in North Carolina. Annual excess readmissions were calculated using the difference in daily PM2.5 between the average PM2.5 concentration in NC and that observed in each zip code over a 5-year period (2012–2016). The number of annual hospitalizations was estimated using state statistics from the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP). In 2016, The HCUP State Inpatient Databases and State Emergency Department Databases reported 4,831,532 emergency department visits with 11.25% being admitted to a hospital, resulting 543,547 estimated hospitalization (28). Hospitalizations estimates were population weighted by US Census population in 2010. We assumed that temporal and spatial variability in these hospitalizations was small.
Sensitivity analyses
To assess potential bias in the estimation of PM2.5 associated readmission risk, changes in risk estimates were evaluated after adjustments for census tract socioeconomic variables and county level pollutant and health outcomes (ozone, health behaviors, clinical care, socioeconomic factors, and physical environment variables) (22). Changes were also compared after restricting the analysis to individuals with street-level geocoded addresses, imputing missing smoking status using the mice package in R, and restricting the analysis to individuals with <10 and ≥ 10 30-day readmissions during the study period.
All statistical analyses were performed with R software (version 3.6.0) (29). Results are expressed as the HR per 10 μg/m3 increase in PM2.5 and associated 95% confidence interval (CI). This work was supported by internal US Environmental Protection Agency grants and by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Characterization of clinical cohort
Among the 18,223 patients who had a heart failure diagnosis during the study period, 549 patients were excluded due to missing demographic values (3%). Of the remaining 17,674 patients, the average age was 68.6 years, 49.6% were male, and 64.1% were white (Table 1). Medications classified as a beta-blocker were prescribed to 4,075 patients (23%) at some point during the study period (Table 1, Supplemental Table 1). The spatial distribution of study participants is shown in Supplemental Figure 1.
Table 1.
Demographic and clinical characteristics of the study population described for the entire cohort and by urbanization and beta blocker (BB) prescription. Characteristics by urbanization and BB prescription represent patient data at the first visit.
| Characteristic | Unstratified | Urban | Non-urban | No BB | Yes BB |
|---|---|---|---|---|---|
| Patients (#) | 17,674 | 8,868 | 8,798 | 13,599 | 4,075 |
| Age (mean, yrs) | 68.6 | 69.0 | 68.2 | 68.6 | 68.7 |
| Sex (% male) | 8,768 (49.6%) | 4,225 (47.6%) | 4,540 (51.6%) | 6,758 (49.7%) | 2,010 (49.3%) |
| White (%) | 11,331 (64.1%) | 5,368 (60.5%) | 5,958 (67.7%) | 8,743 (64.3%) | 2,588 (63.5%) |
| Black (%) | 5,263 (29.8%) | 2,930 (33%) | 2,331 (26.5%) | 3,996 (29.4%) | 1,267 (31.1%) |
| Other race (%) | 1,080 (6.1%) | 570 (6.4%) | 5,09 (5.8%) | 860 (6.3%) | 220 (5.4%) |
| Systolic HF (%) | 5,803 (32.8%) | 2,821 (31.8%) | 2,977 (33.8%) | 4,401 (32.4%) | 1,402 (34.4%) |
| Diastolic HF (%) | 5,893 (33.3%) | 3,018 (34%) | 2,873 (32.7%) | 4,178 (30.7%) | 1,715 (42.1%) |
| Type 2 diabetes (%) | 6,909 (39.1%) | 3,346 (37.7%) | 3,561 (40.5%) | 5,119 (37.6%) | 1,790 (43.9%) |
| IHD (%) | 11,799 (66.8%) | 5,781 (65.2%) | 6,014 (68.4%) | 8,768 (64.5%) | 3,031 (74.4%) |
| COPD (%) | 7,655 (43.3%) | 3,771 (42.5%) | 3,878 (44.1%) | 5,655 (41.6%) | 2,000 (49.1%) |
| CKD (%) | 11,591 (65.6%) | 5,846 (65.9%) | 5,742 (65.3%) | 8,480 (62.4%) | 3,111 (76.3%) |
| BP (%) | 14,037 (79.4%) | 7,012 (79.1%) | 7,020 (79.8%) | 10,486 (77.1%) | 3,551 (87.1%) |
| Lipid (%) | 14,292 (80.9%) | 7,140 (80.5%) | 7,146 (81.2%) | 10,713 (78.8%) | 3,579 (87.8%) |
| PAD (%) | 7,476 (42.3%) | 3,750 (42.3%) | 3,724 (42.3%) | 5,503 (40.5%) | 1,973 (48.4%) |
Description of inpatient admissions, readmissions, and daily ambient PM2.5
There were 49,835 inpatient hospitalizations considered as index admissions. Of these admissions, 25.0% resulted in a readmission within 30 days and 8.1% within a week. The median length of stay was 3 days (Table 2). Hospitalizations not preceded by a beta-blocker prescription had a median time since HF diagnosis of 183 d and median percent urbanicity of 87.3%. Hospitalizations preceded by a beta-blocker prescription had a greater median time since HF diagnosis (799 d) and a slightly lower median percent urbanicity (80.0%) (Table 2).
Table 2.
Hospital admission characteristics and average (25th, 75th percentiles) PM2.5, temperature, and relative humidity for event-days described for the entire cohort and by urbanicity and beta blocker (BB) prescription.
| Characteristic | Unstratified | Urban | Non-urban | No BB | Yes BB |
|---|---|---|---|---|---|
| Index hospitalizations (#) | 49,835 | 24,945 | 24,863 | 35,348 | 14,487 |
| 30-day readmissions (%) | 12,477 (25%) | 6,314 (25.3%) | 6,152 (24.7%) | 8,399 (23.8 %) | 4,078 (28.1 %) |
| Readmissions in 3 days (%) | 4,037 (8.1%) | 880 (3.5%) | 820 (3.3%) | 1143 (3.2%) | 555 (3.8%) |
| Time to readmission, median (d) | 122 | 117 | 126 | 137 | 91 |
| LOS, median (d) | 3 | 3 | 3 | 4 | 3 |
| Time since HF diagnosis, median (d) | 316 | 303 | 329 | 183 | 799 |
| Urbanicity, median (%) | 86.4 | 100 | 10.7 | 87.3 | 80 |
| PM2.5 (μg/m3) | 8.8 (6.3, 12.1) | 9.1 (6.5, 12.4) | 8.6 (6.1, 11.8) | 8.9 (6.4, 12.2) | 8.6 (6.2, 11.8) |
| Temp (°C) | 16.6 (9.1, 23.4) | 16.8 (9.3, 23.5) | 16.5 (9.0, 23.3) | 16.7 (9.2, 23.4) | 16.5 (9.0, 23.3) |
| RH (%) | 67.5 (57.7, 75.6) | 66.6 (57.1, 74.6) | 68.4 (58.3, 76.7) | 67.5 (57.8, 75.7) | 67.3 (57.6, 75.5) |
The average daily PM2.5 concentration for the time between discharge and readmission or censoring was 9.8 μg/m3 (IQR: 6.3–12.1 μg/m3, range: 0.00025–133.0 μg/m3) (Table 2). The annual average PM2.5 declined over the study period (Supplemental Figure 2).
Associations between daily PM2.5 and hospital readmission
Hazard of a readmission within 30 days (non-proportional hazard)
Examination of the Schoenfeld residuals suggested there was a non-proportional PM2.5 associated readmission hazard with respect to the time following discharge. The readmission hazard was most elevated in the first days following discharge and then decreased. One day after discharge, the hazard ratio for a 30-day readmission was elevated with an estimated HR of 1.04 (95%CI 0.99–1.09) (Figure 1, Supplemental Table 2). When considering the hazard for the full 30-day period no association was observed (HR 1.04, 95%CI 0.97–1.48).
Figure 1.
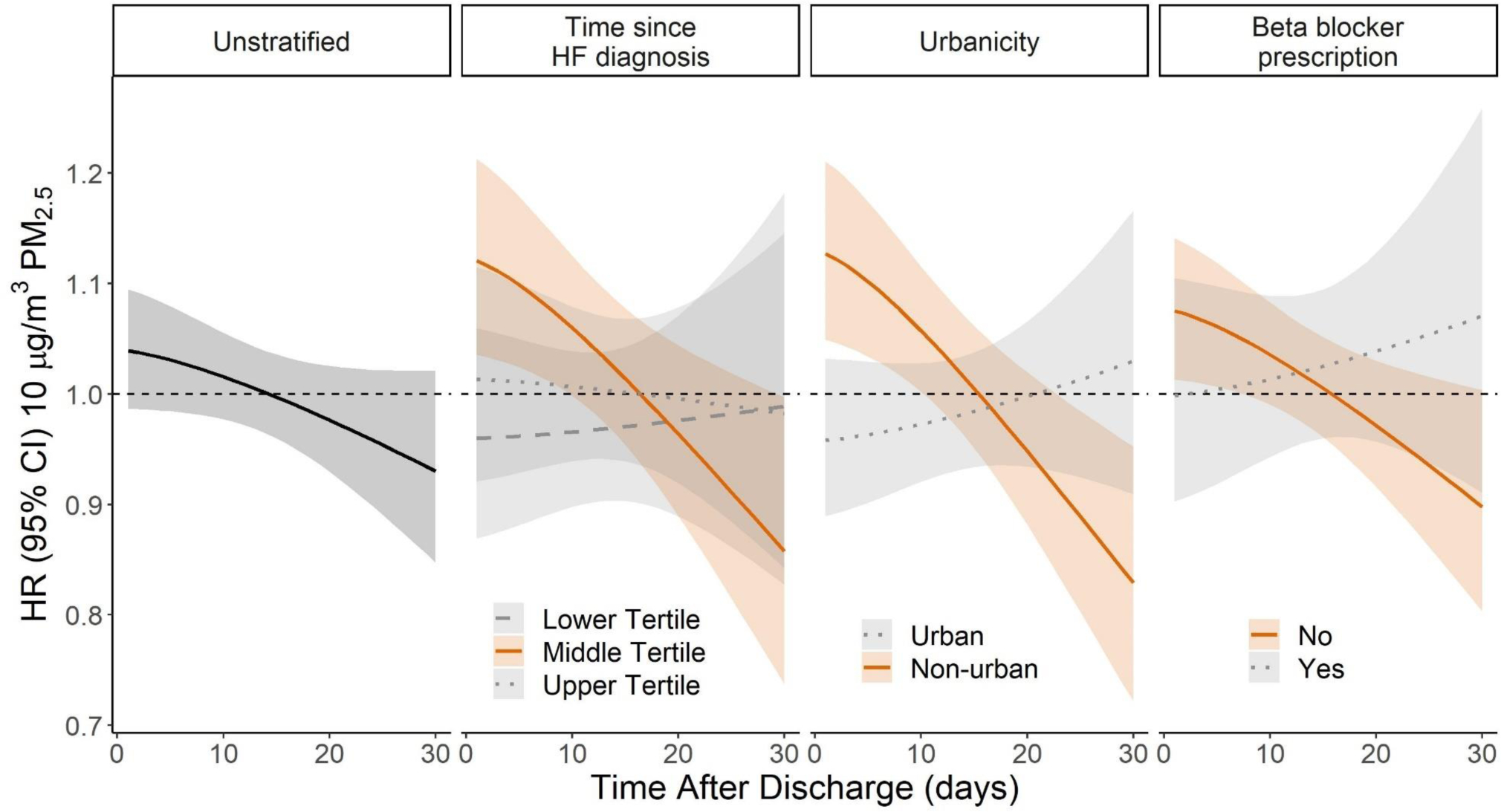
The hazard ratio (HR, 95% CI) of a 30-day readmission for any cause with respect to time after discharge for the main unstratified model and stratified by time since HF diagnosis, urbanicity, and beta blocker prescription. The median percent urbanicity (86% urban) was used as a cut off to examine readmission hazards in non-urban (below) and urban (above) strata. Time since HF diagnosis tertiles, lower tertile (< 68 days), middle tertile (68–682 days), upper tertile (> 683 days). HR is expressed per 10 μg/m3 increase in PM2.5.
The PM2.5-related readmission hazard was also observed to vary based on tertile of the time since initial HF diagnosis, recorded prescription of a beta-blocker, and urbanicity (Supplemental Table 2). On the day after discharge, the readmission hazard was elevated for middle tertile of time after HF diagnosis (68–683 days; HR 1.12; 95% CI 1.03–1.21), before the first recorded prescription of a beta-blocker (HR 1.07, 95% CI 1.01–1.14), and in non-urban areas (HR 1.13; 95% CI 1.05–1.21). The readmission hazard in these strata remained elevated for 7–10 days (Figure 1, Supplemental Table 2). No association was observed in the lower (<68 days after HF diagnosis) and upper tertiles (>683 days after HF diagnosis) for time since HF diagnosis, after a recorded beta-blocker prescription, or in urban areas (Supplemental Table 2). In stratified analyses, PM2.5 associations were slightly stronger in males, but similar between races (Supplemental Table 2).
Including census tract socioeconomic variables and county level ozone and health outcomes as further adjustments resulted in minimal change (< 5% change in the HR for the day following discharge). There was high geocoding precision in this study and street-level geocoding was available for over 15,489 patients (88% of patients). Obtaining patients’ smoking status is a challenge for many studies using electronic health records and smoking status was imputed for 10,571 patients (60% of patients). Greater HR estimates were observed when restricting the analysis to individuals with street-level geocoded addresses, imputing smoking status, and stratifying on an individual’s number of 30-day readmissions; however, these additional considerations had considerable overlap in confidence intervals with other models, indicating no meaningful deviation from the original model (Supplemental Figure 3). Observed HR estimates within the 30-day period were also similar in models stratified and adjusted by HF subtype and comorbidity (Supplemental Figure 4).
Hazard of an early-readmission (1–3 days)
The PM2.5-related hazard of an early-readmission (1–3 days following discharge) was elevated with an observed HR of 1.33 (95% CI 1.18–1.51). This association was observed for hospitalizations occuring prior to the first beta blocker prescription (HR 1.35; 95% CI 1.19–1.53), but not for hospitalizations occuring after. When index admissions were stratified based on their time since HF diagnosis, we observed that greater hazard occurred for the middle tertile of 68 to 683 days since HF diagnosis (1.60, 95% CI 1.39–1.84), with attenuated associations observed for the first and third tertiles (Supplemental Table 3). The hazard of early-readmission was slightly more elevated in non-urban strata (1.43, 95%CI 1.22–1.67) compared to the urban strata (1.25, 95% CI 1.05–1.49), but this interaction was not significant. No apparent differences were observed by sex or race (Figure 2, Supplemental Figure 5, Supplemental Table 3). When stratifying by patient comorbidity, patients indicated to have type 2 diabetes had a slightly higher HR (1.41, 95% CI 1.17–1.69); however, the confidence intervals overlapped with other comorbid conditions and the unstratified model. Overall, there were minimal differences between models stratified on or adjusted for HF subtype and comorbidity (Supplemental Figure 6). Based on results from the unstratified model for a 30-day readmission, we estimated the annual number of excess readmissions. Given that in NC, there are approximately 543,547 hospitalizations annually (30), we estimated that based on daily deviances in ambient PM2.5 above the NC mean, each year 71,020 of these hospitalizations could result in a 30-day readmission.
Figure 2.
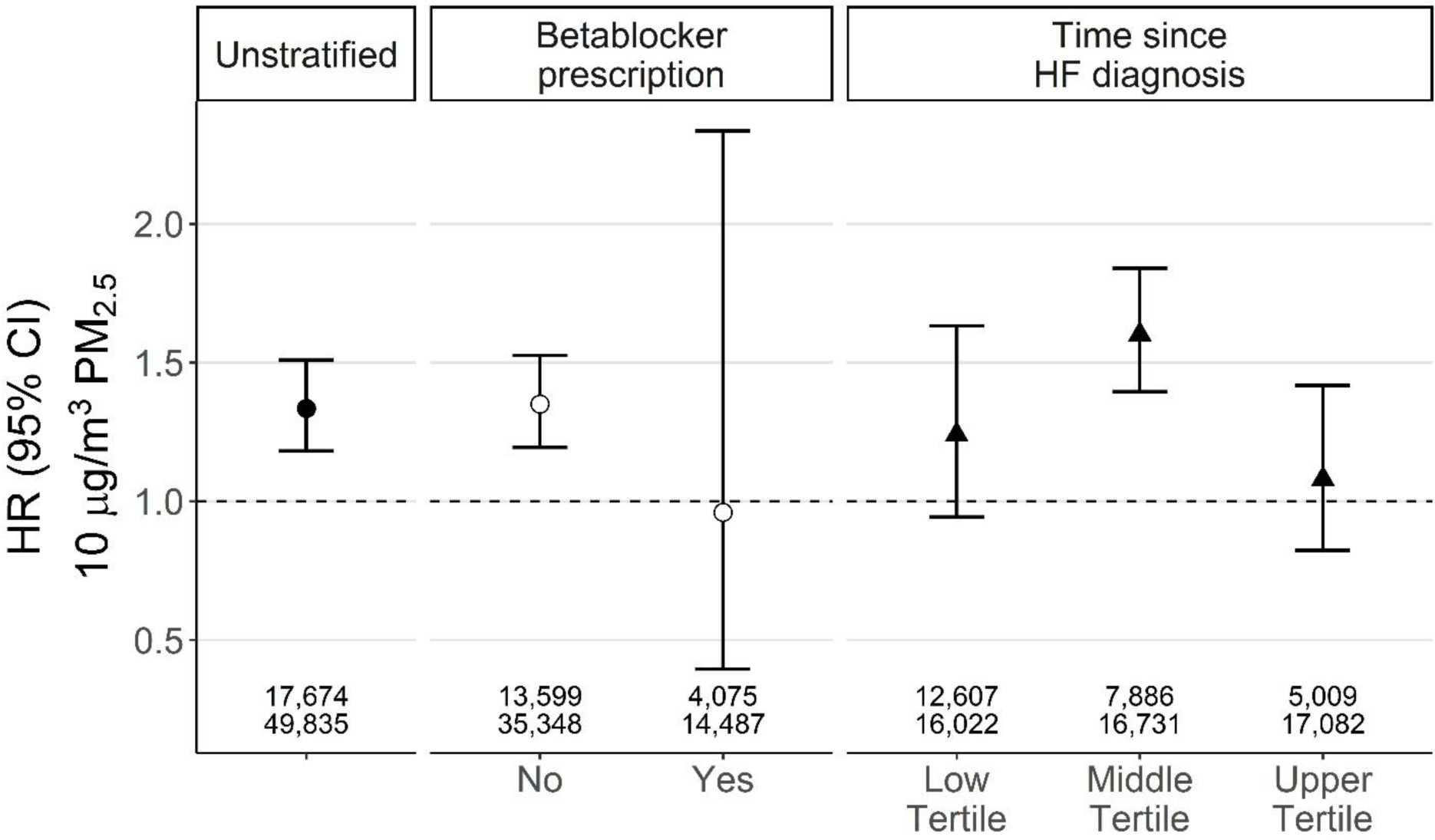
The hazard ratio (HR, 95%CI) for an early-readmission for any cause for the main unstratified model and stratified by betablocker prescription and time since HF diagnosis. Early readmission was defined as occuring within three days of discharge. Time since HF diagnosis tertiles, lower tertile (< 68 days), middle tertile (68–682 days), upper tertile (> 683 days). HR is expressed per 10 μg/m3 increase in PM2.5. Numbers on the bottom of each grouping represent the respective number of patients (top) and index hospitalizations (bottom) included in each model.
Early-readmission hazard (1–3 days) examined using rolling windows of the time since HF diagnosis
In examining the influence of time since HF diagnosis further using rolling windows of time (120 day), we observed that the period of greatest PM2.5-related hazard appeared to occur between 130 and 530 days since HF diagnosis (Figure 3). This period of increased PM2.5-related hazard differed based on urbanicity. In the non-urban strata, elevated hazard was observed 150–350 days after a HF diagnosis, while in the urban strata it was observed later (330–480 days) (Supplemental Figure 7, Supplemental Table 4). Differences between sex and race strata were not observed (Supplemental Table 4).
Figure 3.
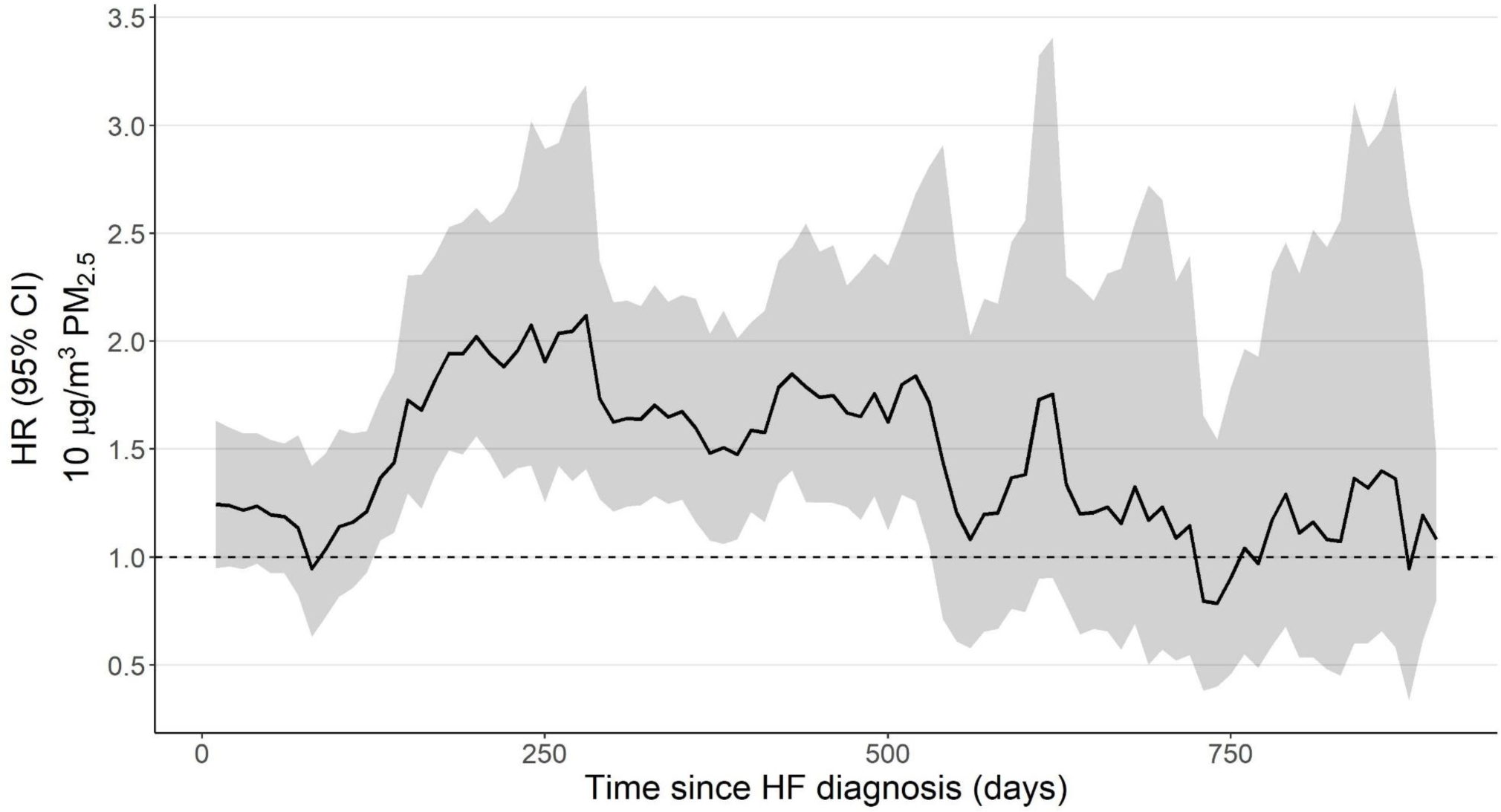
The hazard ratio (HR, 95%CI) for an early-readmission for any cause with respect to time since HF diagnosis for the main unstratified model. HRs were evaluated at different times since HF diagnosis by using 120-day rolling windows that were incremented in 10-day intervals. Early readmission was defined as occurring within three days of discharge. HR is expressed per 10 μg/m3 increase in PM2.5.
Discussion
In this cohort study of 17,674 patients with a recorded HF diagnosis in North Carolina over a 13-year period, we evaluated the association between 30-day readmissions resulting from 49,835 inpatient admissions and the variation in daily ambient PM2.5. Associations between readmission and daily variation in PM2.5 varied based on time since discharge and were greatest immediately after discharge. In North Carolina, there are approximately 543,000 hospitalizations annually with comorbid HF (28). We estimate that annually 71,020 could result in a readmission within 30 days of discharge, due to elevated PM2.5. Stronger associations were observed for individuals who resided in non-urban areas, hospitalizations that occurred 2–9 months after a patient’s initial HF diagnosis, and hospitalizations with no prior indication of prescription of beta-blocker medication(s). These results were observed in areas where annual PM2.5 concentrations were estimated to be below the annual PM2.5 National Ambient Air Quality Standard (12 μg/m3).
Readmissions are a substantial burden for both patients, their caregivers, healthcare system, and insurers. Hospital systems are encouraged to improve processes and communication to reduce avoidable readmissions and may be penalized for excess 30-day readmissions for outcomes such as HF. Primary targets for readmission reduction have been facility and patient centered and do not consider air quality when addressing excess readmissions. However, short-term PM2.5 is associated with considerable healthcare cost and social burden. For cardiovascular and respiratory related diseases, a 1 μg/m3 increase in daily PM2.5 increases annual inpatient and post-acute care costs by $3–7 million (31). Our results suggest that poorer air quality may increase readmission risks for HF patients, which is relevant for individual patients and hospital systems seeking to understand and monitor health risks in their communities.
Our findings are comparable to the growing number of studies that measure the impact of air pollution on hospital readmissions in vulnerable populations. Elevated risk has been observed in other studies focusing on short-term PM2.5-related readmissions, with most reporting increased risk following discharges from a cardiovascular related health events in older adults (5–7, 15) and patients with chronic kidney disease (9). In these patient groups, increases in cardiovascular related readmission hazard ranges 1–14% per 10 μg/m3 PM2.5 (5, 6, 9, 15). Increased cardiovascular related readmission risk has also been observed with other air pollutants including PM10, CO, NO2, and O3 (7). Greater hazard in vulnerable populations may relate to increased sensitivity to air pollution in individuals with chronic health conditions. Effect modification related to certain health conditions has also been theorized to potentially increase susceptibility to air pollution but is an area that requires further study (16, 32). In this study, our examination of potential effect modification by heart failure subtype and certain cardiovascular and respiratory conditions did not reveal a significant increase in PM2.5-realated readmission hazard associated with certain chronic health conditions.
This study is the first to examine readmissions associated with short-term exposures in a population with a history of HF and observe that the PM2.5 associated hazard of readmissions did not follow the proportional hazards assumption after discharge. The association between PM2.5 exposure and rehospitalization was highest immediately after discharge and declined thereafter. This is one of the few studies to report on this effect as much prior work has censored readmission occurring in the first 7–29 days from analysis (6, 7, 15). Elevated associations between PM2.5 exposure and early-readmissions, within a week of discharge, have also been observed for end stage renal disease patients (9). Immediate readmissions are more likely to be influenced by acute conditions present at the time of discharge, thus our results may indicate an acute worsening of post-discharge symptoms resulting in a rapid rehospitalization for some individuals. Future studies that examine clinical conditions observed during discharge may be able to determine if a specific set of post-discharge conditions drive associations between PM2.5 exposure and early-readmission.
While all-cause readmissions are a substantial source of public health interest, studies examining cause-specific readmissions expand our understanding of pathophysiologic mechanisms. There is evidence that PM2.5 is more strongly associated with readmissions from some causes than others, including ischemic heart disease and myocardial infarction (5, 6, 9). Elevated PM2.5-related risk for readmissions caused by rhythm and conduction disorders has also been observed, though not consistently. Increased PM risk for readmissions attributed to rhythm and conduction disorders was observed in the Medicare population with chronic kidney disease population (9), but not in a more general older adult population in Utah (5). Additionally, there is evidence of increased risk for respiratory related readmissions for general respiratory causes and pneumonia (9).
Medication usage is another source of potential variability in air quality-related readmission risk. In our study, we did not observe associations with readmissions occurring after the initial prescription of beta-blockers, according to the available medical records. The observation that beta-blockers may attenuate the effects of air pollution has been previously observed both in human cohort studies and animal models (15, 33–35). There is evidence that medication usage may modify subclinical measures such as heart rate variability (HRV) (34, 36) and inflammation (33, 35), but this has not be observed consistently across studies (37, 38). The possibility that beta-blocker usage may attenuate air pollution-related readmissions risks opens a new dimension in understanding of medication-related attenuation of environmental health risks where overt clinical outcomes may be related to medication usage. Given the high prevalence and tolerability of beta-blockers, this effect should be observable in other populations and is worth future exploration.
Our study has several strengths that add validity to prior observations regarding air pollution associated readmissions. First, this study defines risk in a large patient cohort that included 17,674 individuals diagnosed with HF between 2004 and 2016, a 13-year period. Second, we were able to estimate the risk associated daily fluctuations in ambient PM2.5 by adjusting for time-dependent risk factors and confounders. Lastly, patient exposure was defined using PM2.5 modeled at a fine resolution that was linked to patient residence. Many previous studies assigned exposure based on larger geographic areas that could contribute to exposure misclassification.
This study also has some limitations. First, interpretation of the results should be taken in the context of patients in the UNC healthcare system, who are more likely to reside in central NC. Results may have limited generalization to patients with HF in other states or nationally; however, previous analyses of this HF patient population have been concordant with those from the southeastern United States (8), suggesting our results may have regional generalizability, if not national relevance. Our results are also only focused on associations with total PM2.5, other exposures and potential interactions were not examined. Second, though a strength of this study includes capturing patients from more rural areas, patients residing in rural areas may have differing co-morbidities and healthcare needs as compared to urban populations at initial diagnosis. We acknowledge that hazard differences between urban and non-urban areas may reflect these health differences. Our observations by strata of urbanicity could also reflect differences in PM2.5 composition, in particular traffic-related air pollution. Third, there is a possibility of exposure misclassification resulting from using residential address to assign exposure, a challenge common to many other epidemiological studies. However, the residential address is likely relevant for recently discharged, older HF patients who are less likely to be working or traveling far from home. Fourth, the use of EHR data, specifically ICD codes, to define the diagnosis of HF may have low sensitivity to identifying patients with this condition. Similarly, as an observational study using EHR data, we were also limited in what we could say about time-varying conditions with sparse or incomplete data, such as smoking status. However, our models that modified the smoking classification had minimal impact on our PM2.5 estimates. Likewise, knowledge of beta-blocker medication was limited to hospital orders for inpatient or outpatient prescriptions and lacked information on prescription filling and adherence. We based our definition of beta-blockers on the earliest prescription recorded and assumed use of this medication until the end of the study period, a reasonable assumption for most patients given the common usage of beta-blockers in HF patients, but nevertheless subject to some treatment misclassification.
Conclusions
Our findings add to the knowledge about increased health risks that are related to air pollution. We observed increased PM2.5-related risk of hospital readmission in patients that have been diagnosed with heart failure and that this risk is potentially dependent on time since HF diagnosis and medication usage. The excess readmissions associated with air pollution have great economic and social costs. Though knowledge regarding air pollution related hazard following readmission is limited, our identification of greater PM2.5-related risk in the first days after discharge could assist health care providers in targeting specific patient care instructions during this period. Air pollution is a modifiable risk factor, and thus the risks presented here might be mitigated by the collective actions of public health officials, health care providers, and individual patients.
Supplementary Material
Highlights.
Short-term PM2.5 exposure and early-readmission risk: a retrospective cohort study in North Carolina heart failure patients”
Evaluated association between daily PM2.5 and hospital readmissions in heart failure patients
Association was measured using time-dependent Cox proportional hazard models
Continuous hazard for 30-day readmissions was modeled using a parametric function
PM2.5-associated readmission hazard was elevated in first days following discharge
Hazard elevated in non-urban areas and patients without a beta-blocker prescription
Acknowledgments
This work was supported by internal US Environmental Protection Agency grants. The source of funding had no role in study design, data collection, analyses, interpretation, and decision to submit the article for publication. This project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
Dr. Cavin Ward-Caviness is a paid advisor for the Clock Foundation. The Clock Foundation had no role in any aspect of this work.
Disclaimer
The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 2017;389(10082):1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopalan S, Al-Kindi Sadeer G, Brook Robert D. Air Pollution and Cardiovascular Disease. Journal of the American College of Cardiology. 2018;72(17):2054–70. [DOI] [PubMed] [Google Scholar]
- 3.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of Short-term Exposure to Air Pollution With Mortality in Older AdultsAssociation of Short-term Exposure to Air Pollution With Mortality in Older AdultsAssociation of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA. 2017;318(24):2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabass A, Talbott EO, Venkat A, Rager J, Marsh GM, Sharma RK, et al. Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants (2001–2008). International Journal of Hygiene and Environmental Health. 2016;219(3):301–10. [DOI] [PubMed] [Google Scholar]
- 5.Leiser CL, Smith KR, VanDerslice JA, Glotzbach JP, Farrell TW, Hanson HA. Evaluation of the Sex-and-Age-Specific Effects of PM(2.5) on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009. J Clin Med. 2019;8(12):2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Tian Y, Cao Y, Song J, Huang C, Xiang X, et al. Fine particulate air pollution and hospital admissions and readmissions for acute myocardial infarction in 26 Chinese cities. Chemosphere. 2018;192:282–8. [DOI] [PubMed] [Google Scholar]
- 7.von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D’Ippoliti D, et al. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation. 2005;112(20):3073–9. [DOI] [PubMed] [Google Scholar]
- 8.Ward-Caviness CK, Danesh Yazdi M, Moyer J, Weaver AM, Cascio WE, Di Q, et al. Long-Term Exposure to Particulate Air Pollution Is Associated With 30-Day Readmissions and Hospital Visits Among Patients With Heart Failure. Journal of the American Heart Association. 2021:e019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt LH, Xi Y, Kshirsagar A, Di Q, Ward-Caviness C, Wade TJ, et al. Association of short-term exposure to ambient PM2.5 with hospital admissions and 30-day readmissions in end-stage renal disease patients: population-based retrospective cohort study. BMJ Open. 2020;10(12):e041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. Long-term exposure to PM(2.5) and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFlorio-Barker S, Lobdell DT, Stone SL, Boehmer T, Rappazzo KM. Acute effects of short-term exposure to air pollution while being physically active, the potential for modification: A review of the literature. Preventive Medicine. 2020;139:106195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieden TR. A framework for public health action: the health impact pyramid. American journal of public health. 2010;100(4):590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abohashem S, Osborne MT, Dar T, Naddaf N, Abbasi T, Ghoneem A, et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. European heart journal. 2021;42(7):761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh QL, Blizzard CL, Marwick TH, Negishi K. Association of ambient particulate matter with heart failure incidence and all-cause readmissions in Tasmania: an observational study. BMJ Open. 2018;8(5):e021798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward-Caviness CK, Weaver AM, Buranosky M, Pfaff ER, Neas LM, Devlin RB, et al. Associations Between Long-Term Fine Particulate Matter Exposure and Mortality in Heart Failure Patients. Journal of the American Heart Association. 2020;9(6):e012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett M RS, Andrews R. Overview of Key Readmission Measures and Methods. 2012. HCUP Methods Series Report #2012–04. U.S. Agency for Healthcare Research and Quality; 2012. [Google Scholar]
- 18.Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing PM2.5 Exposures with High Spatiotemporal Resolution across the Continental United States. Environ Sci Technol. 2016;50(9):4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int. 2019;130:104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFlorio-Barker S, Crooks J, Reyes J, Rappold AG. Cardiopulmonary Effects of Fine Particulate Matter Exposure among Older Adults, during Wildfire and Non-Wildfire Periods, in the United States 2008–2010. Environ Health Perspect. 2019;127(3):37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute UoWPH. County Health Rankings 2010. 2010. [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken, N.J.: Hoboken, N.J. : J. Wiley, ©2002.; 2002. [Google Scholar]
- 24.Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Schwartz JD, Gold DR, et al. Particulate air pollution and nonfatal cardiac events. Part II. Association of air pollution with confirmed arrhythmias recorded by implanted defibrillators. Research report (Health Effects Institute). 2005(124):83–126; discussion 7–48. [PubMed] [Google Scholar]
- 25.Barclay JL, Miller BG, Dick S, Dennekamp M, Ford I, Hillis GS, et al. A panel study of air pollution in subjects with heart failure: negative results in treated patients. Occupational and environmental medicine. 2009;66(5):325. [DOI] [PubMed] [Google Scholar]
- 26.Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, et al. Short-Term Effects of Air Pollution on Heart Rate Variability in Senior Adults in Steubenville, Ohio. Journal of occupational and environmental medicine. 2006;48(8):780–8. [DOI] [PubMed] [Google Scholar]
- 27.Warburton DER, Bredin SSD, Shellington EM, Cole C, de Faye A, Harris J, et al. A Systematic Review of the Short-Term Health Effects of Air Pollution in Persons Living with Coronary Heart Disease. J Clin Med. 2019;8(2):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quality AfHRa. HCUPnet, Healthcare Cost and Utilization Project. Rockville, MD2016.
- 29.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2019. [Google Scholar]
- 30.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circulation Heart failure. 2018;11(12):e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei YG, Wang Y, Di Q, Choirat C, Wang Y, Koutrakis P, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. BMJ-British Medical Journal. 2019;367:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring). 2014;22(7):1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiarella SE, Soberanes S, Urich D, Morales-Nebreda L, Nigdelioglu R, Green D, et al. β₂-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. The Journal of clinical investigation. 2014;124(7):2935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Hartog JJ, Lanki T, Timonen KL, Hoek G, Janssen NA, Ibald-Mulli A, et al. Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease. Environ Health Perspect. 2009;117(1):105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Xu M, Zhang Y, Guo Y, Zhang Y, He B. Effects of long-term application of metoprolol and propranolol in a rat model of smoking. Clinical and experimental pharmacology & physiology. 2014;41(9):708–15. [DOI] [PubMed] [Google Scholar]
- 36.Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J, et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation. 2002;106(8):933–8. [DOI] [PubMed] [Google Scholar]
- 37.Hampel R, Schneider A, Brüske I, Zareba W, Cyrys J, Rückerl R, et al. Altered cardiac repolarization in association with air pollution and air temperature among myocardial infarction survivors. Environ Health Perspect. 2010;118(12):1755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang KJ, Coull BA, Zanobetti A, Suh H, Schwartz J, Stone PH, et al. Particulate air pollution as a risk factor for ST-segment depression in patients with coronary artery disease. Circulation. 2008;118(13):1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


