Abstract
A baseline involved to uninvolved free light chain ratio (FLCr) ≥100 with involved FLC ≥10 mg/dL is a multiple myeloma (MM)-defining event (MDE). However, multimeric light chain aggregates may contribute to increased FLC levels and impair renal light chain clearance. Therefore, we conducted a retrospective study to assess the association between urine monoclonal protein (uMCP) excretion and the risk of progression. We included 822 asymptomatic MM patients without MDE besides elevated FLCr (n=120 with FLCr ≥100, n=702 with FLC <100). Patients with a FLC ≥100 were grouped based on 24-hour uMCP excretion (≥200 mg/24h [n=35], <200 mg/24h [n=85]). The 2-year risk of progression to symptomatic MM or AL amyloidosis was significantly higher in patients with uMCP excretion ≥200 versus <200 mg/24h (36.2% versus 13.5%, respectively; HR 2.79, 95%CI 1.57-4.96, p<0.001). However, the progression risk was similar in patients with a baseline FLCr <100 versus FLC≥100 with uMCP <200 mg/24h (log rank p=0.127). We showed that increased uMCP excretion in the setting of a FLCr ≥100 is an unfavorable prognostic marker. This underscores the importance of conducting a diagnostic 24-hour urine assessment and may help refine the subset of patients warranting therapy if the FLCr is the only MDE.
Introduction
In 2014 the multiple myeloma (MM) diagnostic criteria were revised to reclassify patients with “ultra-high risk” smoldering MM (SMM) as having MM (1). Based on retrospective data, a baseline involved to uninvolved free light chain ratio (FLCr) ≥100 with an involved FLC (iFLC) ≥10 mg/dL is considered a myeloma defining event (MDE) (2, 3). Additional “SLiM” diagnostic criteria include a baseline bone marrow plasma cell burden (BMPC) ≥60% or >1 focal lesion on MRI, and identify asymptomatic patients who would now be treated for MM (1). However, the risk of progression in patients with an elevated FLCr as the sole MDE has since been questioned by some experts (4). A prior case report demonstrated that serum light chain multimers can impede renal clearance, thereby resulting in a high serum FLC despite low urinary monoclonal protein (uMCP) excretion (5). Therefore, we conducted a retrospective study to compare the disease progression risk in patients with high versus low uMCP excretion in the setting of an elevated serum FLCr.
Methods
We evaluated asymptomatic SMM and “SLiM” MM patients diagnosed between January 1, 2000 and January 10, 2020. Included patients were untreated within 6 weeks of diagnosis, had a baseline BMPC burden of 10-59%, without end organ damage (hypercalcemia, anemia, renal failure, or lytic lesions; “CRAB” MDE). Patients with a baseline FLCr ≥100 and iFLC >10 mg/dL were included if they had a 24-hour urine collection for electrophoresis and no other MDE at diagnosis. Cross-sectional imaging (whole body CT, PET-CT, whole body or spine/pelvis MRI) was not a requirement for study inclusion due to practice pattern variability during the study period. The Freelite test (Binding Site, Birmingham, United Kingdom) was used to quantify FLC (references range for serum kappa and lambda light chains was 0.33-1.94 and 0.57-2.63 mg/dL, respectively) (6). The Kaplan-Meier method was used to assess the time to progression (TTP). Progression was defined as treatment for systemic AL amyloidosis or symptomatic MM (typical “CRAB” MDE), and SMM patients treated due to evolving biomarkers or “SLiM” MDE were censored. Cox proportional hazards models were used for uni- and multivariable analyses. Between group comparisons were conducted using non-parametric tests. A two-sided p-value <0.05 was considered statistically significant.
Results and discussion
We included 822 patients; 702 SMM patients (baseline FLCr <100 or baseline FLCr ≥100 and iFLC <10 mg/dL) and 120 “SLiM” (asymptomatic) MM patients (baseline FLCr ≥100 and iFLC ≥10 mg/dL). Patients with a baseline FLCr ≥100 had a median involved FLC of 79.9 (IQR 42.9-131.5) mg/dL and median FLC ratio of 207.1 (IQR 137.7-400.3). Patients with a baseline FLC≥100 were dichotomized into those with a “measurable” baseline 24-hour urine monoclonal protein (uMCP) of ≥200 (n=35, “high excretors”) versus <200 mg/24h (n=85, “low excretors”). At diagnosis, high versus low excretors had a median iFLC of 101 versus 66.5 mg/dL (p=0.001), median serum creatinine of 0.9 mg/dL (p=0.495), median estimated glomerular filtration rate (eGFR) 74 mL/min/1.73m2 (p=0.505), and median BMPC burden of 30% versus 21% (p=0.088). Pertinent baseline characteristics are summarized in table 1.
Table 1.
Baseline characteristics of patients meeting the FLCr SLiM criteria for MM, and SMM patients
| Baseline FLCr <100 |
Baseline FLCr≥100 |
||
|---|---|---|---|
| SMM (n=702) |
uMCP <200 mg/24h (n=85) |
uMCP ≥200 mg/24h (n=35) |
|
| Median age at diagnosis - years (IQR) | 65 (57–79) | 66 (56–72) | 67 (55–74) |
| Male - n (%) | 418 (60) | 37 (44) | 16 (46) |
| Median diagnostic disease paramaters (IQR) | |||
| serum MCP - mg/dL | 1.7 (1–2.5) | 1.9 (1–2.9) | 1.8 (0–2.6) |
| urine MCP - mg/24h | 0 (0–24) | 0 (0–83) | 465 (290–879) |
| BMPC - % | 15 (10–22) | 21 (15–32) | 30 (20–40) |
| iFLC - mg/dL | 7.2 (2.8–20.6) | 66.5 (33.2–116.5) | 101 (44.9–169) |
| FLCr - n | 8 (3–25) | 195 (133–360) | 259 (144–554) |
| light chain isotype (kappa/lambda) - n(%) | 456 (65) / 243 (35) | 55 (65) / 30 (35) | 15 (43) / 20 (57) |
| Calcium - mg/dL | 9.4 (8.9–10.1) | 9.5 (8.8–10.1) | 9.6 (8.7–10.5) |
| Hemoglobin - g/dL | 12.9 (10.6–13.9) | 12.5 (10.8–13.2) | 12.5 (10–13.5) |
| Creatinine - mg/dL | 1 (0.7–1.2) | 0.9 (0.6–1.1) | 0.9 (0.7–1.3) |
| eGFRa - mL/min/1.73m2 | 68.9 (41.1–82.9) | 74.6 (50.2–88) | 74.4 (53.2–88.3) |
| Cross-sectional imaging at diagnosis or prior to progression - n (%) | 489 (70) | 53 (62) | 14 (40) |
| Missing 24hUP at baseline - n (%) | 332 (47) | - | - |
| Mayo 2018 SMM risk score at baseline (Low/ Intermediate/ High) - n (%) | 217 (39) /227 (32) / 204 (29) | - | - |
Calculated using CKD-Epi formula, assuming that all patients were Caucasian
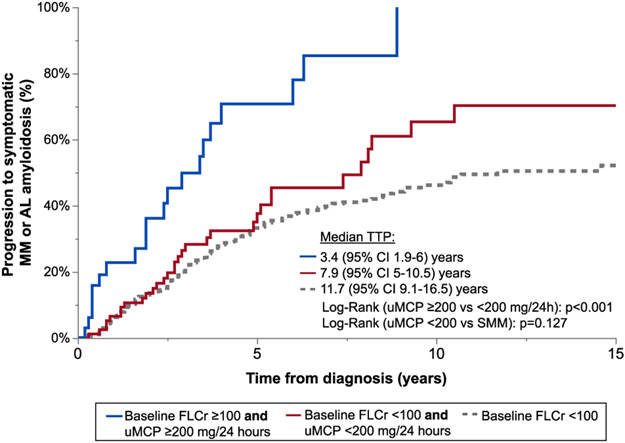
As shown in figure 1, the risk of progression to symptomatic MM or systemic AL amyloidosis was similar in patients with a baseline FLCr <100 versus those with a FLCr ≥100 and low uMCP excretion (2-year progression risk 13% versus 13.5% respectively; log rank p=0.127). Among patients with a baseline FLCr ≥100 the risk of progression to symptomatic MM or systemic AL amyloidosis was 2.8 times higher in patients with high versus low uMCP excretion (2-year progression risk 36.2% versus 13.5%, respectively, as shown in figure 1; HR 2.79, 95%CI 1.57-4.96, p<0.001). After adjusting for baseline eGFR, BMPC burden, iFLC, and light chain isotype, the progression risk was still 2.7 times higher in patients with high versus low uMCP excretion (HR 2.66, 95%CI 1.39-5.10, p=0.003).
Figure 1.
Time to progression (TTP) to symptomatic MM or systemic AL amyloidosis stratified by baseline FLC ratio (FLCr) and urinary monoclonal protein (uMCP) excretion at diagnosis.
We conducted multiple sensitivity analyses amongst the cohort of patients with a baseline FLCr ≥100 to ensure our findings were robust. Patients with cross-sectional imaging at diagnosis or prior to progression confirming the absence of osteolytic lesions had a significantly higher progression risk if they had high versus low uMCP excretion (n=14 versus n=21, respectively; HR 4.20, 95%CI 1.86-9.49, p<0.001). If progression was defined as the time to therapy initiation for symptomatic MM/amyloidosis or other “SLiM” MDE or evolving biomarkers, the 2-year progression risk was still significantly higher in patients with high versus low uMCP excretion (51% versus 26%, respectively; HR 2.11, 95%CI 1.33-3.37, p=0.002). Furthermore, if we defined progression as those with symptomatic MM only, the results remained similar (HR 2.90, 95%CI 1.58-5.34, p<0.001). Among the 85 patients with a uMCP <200 mg/24h and FLCr >100, 44 patients had no quantifiable uMCP excretion (uMCP =0 mg/24h), and 45 patients had a quantifiable uMCP less than 200 mg/24h (median 84 [IQR 35-132] mg/24h). Even among the low uMCP excretor patients, the risk of progression was significantly higher among patients with versus without a quantifiable uMCP (n=45 versus n=44, respectively; HR 2.37, 95% CI 1.14-4.93, p=0.021).
In patients with FLCr ≥100, 52 patients had end organ damage at progression (26 [50%] patients with anemia, 8 [15%] with renal failure, 21 [40%] with lytic lesions, 6 [12%] with systemic AL amyloidosis). The baseline median uMCP was 664 (IQR 309-898) versus 20 (IQR 0-96) mg/24h in patients with high versus low (n=32) uMCP excretion that met progression criteria. Among patients who progressed, 5 (25%) of 20 patients with high uMCP excretion and 3 (9.4%) of 32 patients with low uMCP excretion presented with renal failure as the MDE. In high versus low uMCP excretors with renal progression, at the time of progression the median serum creatinine was 2.7 (range 1.9-4.1) versus 2.5 (range 1.9-3.2) mg/dL (p=0.653), median uMCP 4964 (range 738-5910) versus 512 (range 196-734) mg/24h (p=0.052), and median iFLC 388 (range 317-1640) versus 437 (range 199-1030) mg/dL (p=1.00).
Our study findings demonstrate the added importance of a 24-hour uMCP assessment to the serum FLC assay at diagnosis of SMM. However, the 2-year risk of progression in patients with a FLC ≥100 was substantially lower than previously reported (2). In the study by Larsen et al. 12% of patients with a FLC ≥100 had a BMPC burden >60% and would therefore have been excluded from our study, as this would now be considered a MDE. Furthermore, the Larsen et al. study was conducted prior to the revision of the MM diagnostic criteria, and therefore the proportion of patients that received cross-sectional imaging at diagnosis to exclude lytic lesions was not reported (2). Therefore, we suspect that the cohort of patients with a FLC ≥100 in the Larsen et al. study may have had other concomitant MDE at diagnosis that increased the progression risk.
While prior case reports have demonstrated the presence of light chain multimers in the serum (5, 7-10), the nephrotoxic potential of light chain multimers is unclear (7, 11). We hypothesize that large light chain aggregates may result in falsely high serum light chain estimation due to both impaired renal clearance and increased light scatter which may falsely increase nephelometric quantification. To our knowledge, this is the first study to assess the association between uMCP excretion and progression risk in patients presenting with a high FLCr. We showed the risk of disease progression is significantly higher among patients with ≥200 versus <200 mg/24h uMCP excretion in the setting of a serum FLC ratio >100. Importantly, patients with a low uMCP excretion had a similar risk of progression to symptomatic MM/AL amyloidosis compared to SMM patients with a baseline FLCr <100. These findings underscore the importance of conducting a 24-hour urine assessment at diagnosis and may help refine the subset of patients in whom therapy is warranted if an elevated FLCr is the only MDE. The progression risk in our study was lower than previously reported among patients with an elevated baseline FLCr ≥100. Therefore, in patients with low urine monoclonal protein excretion where the elevated FLCr is the only myeloma defining event (MDE), it is reasonable to observe without treatment and monitor closely for CRAB MDE.
Footnotes
Disclosure of Conflicts of Interest
The authors of this manuscript have no relevant conflicts of interest to disclose.
Competing Interests
None
References:
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. [DOI] [PubMed] [Google Scholar]
- 2.Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia. 2013;27(4):941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastritis E, Terpos E, Moulopoulos L, Spyropoulou-Vlachou M, Kanellias N, Eleftherakis-Papaiakovou E, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia. 2013;27(4):947–53. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca R, Gonzalez-Velez M. Treatment of Smoldering Multiple Myeloma: Expectant Observation Should Still Be the Standard. American Society of Clinical Oncology Educational Book. 2020(40):364–70. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RS, Charlesworth MC, Owen BA, Benson LM, Katzmann JA, Reeder CB, et al. Trimolecular complexes of lambda light chain dimers in serum of a patient with multiple myeloma. Clin Chem. 2002;48(10):1805–11. [PubMed] [Google Scholar]
- 6.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–44. [PubMed] [Google Scholar]
- 7.Solling K, Solling J, Lanng Nielsen J. Polymeric Bence Jones proteins in serum in myeloma patients with renal insufficiency. Acta Med Scand. 1984;216(5):495–502. [DOI] [PubMed] [Google Scholar]
- 8.Kozuru M, Benoki H, Sugimoto H, Sakai K, Ibayashi H. A case of lambda type tetramer Bence-Jones proteinemia. Acta Haematol. 1977;57(6):359–65. [DOI] [PubMed] [Google Scholar]
- 9.Berggard I, Peterson PA. Polymeric forms of free normal kappa and lambda chains of human immunoglobulin. J Biol Chem. 1969;244(16):4299–307. [PubMed] [Google Scholar]
- 10.Grey HM, Kohler PF. A case of tetramer Bence Jones proteinaemia. Clinical and experimental immunology. 1968;3(3):277–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Myatt EA, Westholm FA, Weiss DT, Solomon A, Schiffer M, Stevens FJ. Pathogenic potential of human monoclonal immunoglobulin light chains: relationship of in vitro aggregation to in vivo organ deposition. Proc Natl Acad Sci U S A. 1994;91(8):3034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]