Abstract
Purpose:
Polypharmacy is common in the hemodialysis population and increases the likelihood that patients will be exposed to clinically significant drug-drug interactions. Concurrent use of proton pump inhibitors (PPIs) with citalopram or escitalopram may potentiate the QT-prolonging effects of these selective serotonin reuptake inhibitors through pharmacodynamic and/or pharmacokinetic interactions.
Methods:
We conducted a retrospective cohort study using data from the U.S. Renal Data System (2007–2017) and a new-user design to examine the differential risk of sudden cardiac death (SCD) associated with citalopram/escitalopram initiation vs. sertraline initiation in the presence and absence of PPI use among adults receiving hemodialysis. We studied 72,559 patients: 14,983 (21%) citalopram/escitalopram initiators using a PPI; 26,503 (36%) citalopram/escitalopram initiators not using a PPI; 10,779 (15%) sertraline initiators using a PPI; and 20,294 (28%) sertraline initiators not using a PPI (referent). The outcome of interest was 1-year SCD. We used inverse probability of treatment weighted survival models to estimate weighted hazard ratios (HRs) and 95% confidence intervals (CIs).
Results:
Compared with sertraline initiators not using a PPI, citalopram/escitalopram initiators using a PPI had the numerically highest risk of SCD (HR [95% CI] = 1.31 [1.11–1.54]), followed by citalopram/escitalopram initiators not using a PPI (HR [95% CI] = 1.22 [1.06–1.41]). Sertraline initiators using a PPI had a similar risk of SCD compared with those not using a PPI (HR [95% CI] = 1.03 [0.85–1.26]).
Conclusions:
Existing PPI use may elevate the risk of SCD associated with citalopram or escitalopram initiation among hemodialysis patients.
Keywords: Hemodialysis, citalopram, escitalopram, proton pump inhibitor, sudden cardiac death, USRDS
INTRODUCTION
Polypharmacy among individuals with dialysis-dependent kidney failure is common. Many hemodialysis patients have medication regimens involving more than 10 drugs,1, 2 placing them at high risk for drug-drug interactions and associated adverse events. To-date, investigations of drug-drug interactions in the hemodialysis population have focused on describing the prevalence of potentially dangerous medication combinations3–5 and less attention has been paid to evaluating their clinical consequences.
Drug-drug interactions are either pharmacokinetic or pharmacodynamic. A pharmacokinetic interaction occurs when one drug impacts the absorption, distribution, metabolism, or excretion of another drug, whereas a pharmacodynamic interaction occurs when the pharmacologic effect(s) of one drug is altered by another drug. Approximately 30% of clinically meaningful interactions are due to pharmacokinetic drug-drug interactions, which are primarily caused by alterations in cytochrome P450 (CYP)-mediated hepatic metabolism.6 Numerous drugs prescribed to individuals receiving hemodialysis are hepatically metabolized and may be subject to CYP-mediated interactions,4 including certain selective serotonin reuptake inhibitors (SSRIs). Drug-drug interactions involving citalopram and escitalopram are particularly worrisome because the use of interacting medications could augment their known QT-prolonging effects.7–9
Proton pump inhibitors (PPIs), are prescribed to over 35% of U.S. hemodialysis patients10 and have the potential to interact with citalopram and escitalopram pharmacokinetically and pharmacodynamically. PPIs are competitive inhibitors of CYP 2C19,11, 12 the isoenzyme primarily responsible for citalopram/escitalopram metabolism. In addition, PPIs can induce hypomagnesemia,13 an electrolyte abnormality known to cause QT-prolongation. Since both of these PPI-related effects can enhance the extent of citalopram/escitalopram-induced QT-prolongation, we undertook this study to examine the effect of concurrent PPI use on the risk of sudden cardiac death (SCD) associates with the initiation of citalopram or escitalopram vs. sertraline, an SSRI with both lower QT-prolonging and interaction potential, among individuals treated with hemodialysis.
METHODS
Data source
We used data from the U.S. Renal Data System (USRDS), a national surveillance system that collects, analyzes, and distributes information on individuals with end-stage kidney disease in the U.S.14 The USRDS database includes the Medical Evidence Report, the Death Notification form, and Medicare standard analytic files, including enrollment information and final action Part A, B, and D claims.14
Study design and population
We conducted a retrospective cohort study using a new-user design15 to evaluate the comparative risk of SCD among patients receiving maintenance hemodialysis treated with an SSRI (citalopram, escitalopram, or sertraline) who were and were not concomitantly using a PPI at the time of SSRI initiation (Figure 1). We selected citalopram and escitalopram (the S-enantiomer of the citalopram racemate) as the SSRIs of interest because these related compounds have greater QT-prolonging potential than other SSRIs (i.e., can prolong the QT-interval at therapeutic doses)16 and are primarily metabolized by CYP 2C19.17, 18 We selected sertraline as the comparator SSRI because it has lower QT-prolonging potential than citalopram and escitalopram (i.e., can prolong the QT-interval in certain scenarios such as supratherapeutic doses),16 and despite being hepatically metabolized, sertraline is unlikely to be the subject of clinically significant CYP-mediated drug-drug interactions.19 We selected PPIs (dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole) as the interacting drugs of interest, because they are frequently prescribed to hemodialysis patients10 and are competitive inhibitors of CYP 2C19.11, 12, 20
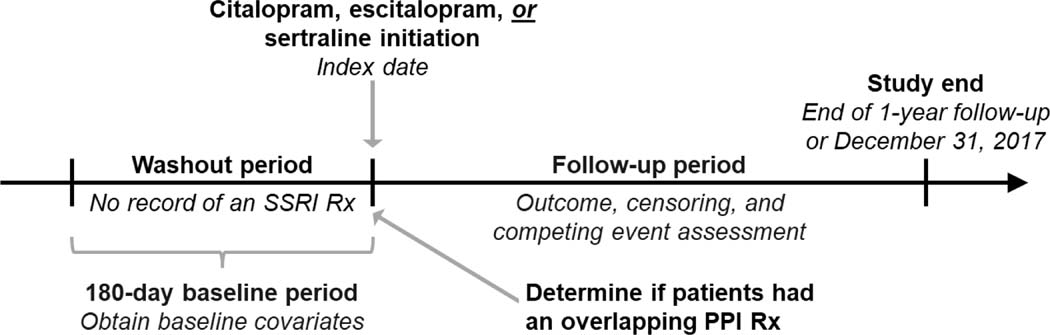
Figure 1. Study design.
We determined if citalopram, escitalopram, and sertraline new-users were or were not using a PPI at the time of SSRI initiation by determining if they filled a prescription for a PPI that overlapped the index date.
Abbreviations: PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor; Rx, prescription.
To construct the study cohort, we identified patients treated with in-center maintenance hemodialysis with continuous baseline Medicare Part A, B, and D coverage who newly initiated citalopram, escitalopram, or sertraline therapy from 1/1/2007 to 12/30/2017. The baseline period was the 180-days prior to SSRI initiation. We then applied the following exclusion criteria: 1) age <18 years at the beginning of baseline, 2) time on maintenance dialysis ≤90 days at the beginning of baseline, 3) receipt of hospice care during baseline, 4) presence of an implantable cardioverter defibrillator, and 5) missing demographic data.
Study exposure
We used Medicare Part D prescription drug claims to identify new-users of citalopram, escitalopram, or sertraline and defined the index date as the date of the first prescription for one of these medications after a 180-day washout period free of SSRI prescription fills. Next, we determined if citalopram, escitalopram, and sertraline new-users were or were not using a PPI at the time of SSRI initiation. To do so, we assessed if they had a prescription fill for a PPI that overlapped the SSRI index date. We then classified patients into four exposure groups: 1) citalopram/escitalopram initiators using a PPI, 2) citalopram/escitalopram initiators not using a PPI, 3) sertraline initiators using a PPI, and 4) sertraline initiators not using a PPI (referent group). We selected sertraline initiators not using a PPI as the referent because sertraline has lower QT-prolonging potential compared to citalopram/escitalopram, and this group was not using PPIs, the interacting drug of interest.16
Study outcomes
We obtained dates and causes of death from the End Stage Renal Disease Death Notification form. The primary outcome was SCD within 1 year of the index date. We defined SCD using the established USRDS definition, death due to cardiac arrhythmia or cardiac arrest listed as the primary cause of death on the Death Notification form.21 We also considered two broader cardiac outcomes in secondary analyses: a composite of SCD or hospitalized ventricular arrhythmia and, separately, cardiovascular mortality (Table S1).
Study covariates
We identified baseline covariates in the 180-day baseline period using Medicare Part A, B, and D claims. Covariates included patient demographics, comorbid conditions, prescription medication use, and metrics of health care utilization (Tables S2 and S3).
Statistical analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Baseline characteristics of each SSRI-PPI exposure group are displayed as count (%) for categorical variables and as mean ± standard deviation or median (interquartile range) for continuous variables. We compared baseline covariate distributions across the groups using absolute standardized mean differences. A standardized mean difference >0.10 represents an imbalance between exposure groups.22
We used an on-treatment analytic approach to evaluate the association between SSRI-PPI exposure group and the 1-year risk of SCD. We followed individuals from the index date to the first occurrence of an outcome, censoring, or competing event. Censoring events included: 1) change of dialysis modality, 2) kidney transplantation, 3) recovery of kidney function, 4) loss of Medicare Part A, B, or D coverage, 5) loss to follow-up, 6) index SSRI discontinuation (using a 7-day grace period), 7) switch to a non-index SSRI, 8) PPI discontinuation (using a 7-day grace period) among those using a PPI on the index date, 9) PPI initiation among those not using a PPI on the index date, 10) completion of one year of follow-up, and 11) study end (12/31/2017).
In primary analyses, we assessed the SSRI-PPI exposure group—SCD associations using Fine and Gray proportional subdistribution hazards models,23 treating death due to a cause other than SCD (i.e., non-SCD) as a competing event. These models estimate hazard ratios (HRs) and 95% confidence intervals (CIs). We used inverse probability of treatment (IPT) weighting for confounding control. Because we considered four different exposure groups, we estimated propensity scores and generated IPT weights using standard methods for multicategory exposures.15, 24 Briefly, we calculated the predicted probability (i.e., propensity score) of receiving the treatment actually received as a function of baseline covariates, Pr (actual SSRI-PPI exposure category | baseline covariates), using multinomial logistic regression. We calculated stabilized inverse probability of treatment weights as the reciprocal of the propensity score multiplied by the marginal probability of treatment, and estimated weighted HRs by applying stabilized IPT weights in our regression models.
We used an analogous analytic approach to conduct secondary analyses considering alternative outcomes and exposure variations. We evaluated the association between SSRI-PPI exposure groups and two broader cardiac outcomes, specified above. In analyses evaluating cardiovascular mortality, we treated non-cardiovascular death as a competing event. We also evaluated if SCD associations were consistent for both citalopram and escitalopram by comparing each of these SSRIs, separately, to sertraline.
We conducted sensitivity analyses to evaluate the robustness of our primary findings, including: 1) using a more stringent 0-day grace period to define SSRI and PPI discontinuation, 2) excluding patients using another non-PPI CYP 2C19 inhibitor on the index date, 3) restricting the study cohort to patients with the Medicare Part D low income subsidy (i.e., individuals more likely to use prescription PPIs), and 4) conducting stratified analyses based on the duration of PPI use (>30 and ≤30 days) at the time of SSRI initiation to assess the potential influence of PPI-related prevalent user bias. In addition, we evaluated the associations among SSRI-PPI exposure groups and death due to a cause other than SCD (i.e., non-SCD), a negative control outcome. In these analyses, we treated SCD as a competing event.
RESULTS
Study cohort characteristics
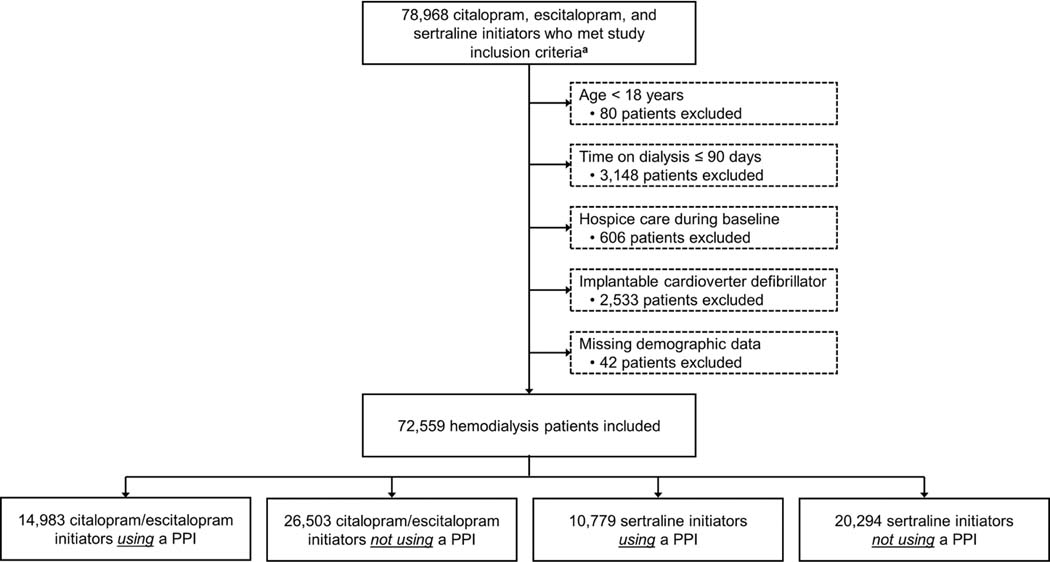
Figure 2 displays study cohort selection. Overall, 72,559 patients receiving maintenance hemodialysis were included: 14,983 (21%) citalopram/escitalopram initiators using a PPI, 26,503 (36%) citalopram/escitalopram initiators not using a PPI, 10,779 (15%) sertraline initiators using a PPI, and 20,294 (28%) sertraline initiators not using a PPI. The average patient age was 60 ± 15 years, 53% were women, 36% were Black, and 17% were Hispanic. Of the 25,762 patients using a PPI at the time of SSRI initiation, the median (interquartile range) time on PPI therapy was 49 (6–145) days, and the most frequently used PPIs were omeprazole (45%), pantoprazole (30%), and esomeprazole (17%). Propensity score distributions are displayed in Figure S1, and baseline characteristics of the study cohort stratified by SSRI-PPI exposure group are presented in Table 1, Table S4, and Table S5.
Figure 2. Flow diagram depicting study cohort assembly.
a To be included in the study, patients had to receive in-center hemodialysis during the 180-days prior to study medication initiation (i.e., the baseline period) and also have continuous Medicare Part A, B, and D coverage during this period.
Abbreviations: PPI, proton pump inhibitor.
Table 1.
Select baseline characteristics of the SSRI–PPI exposure groups after inverse probability of treatment weighting
| Citalopram/escitalopram | Sertraline | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | PPI n = 14,860a | No PPI n = 26,581a | PPI n = 10,726a | No PPI n = 20,341a |
| Age (years) | 61 ±15 | 61 ± 15 | 61 ± 15 | 61 ± 15 |
| Female | 7,872 (53%) | 13,989 (53%) | 5,646 (53%) | 10,709 (53%) |
| Race | ||||
| Black | 5,303 (36%) | 9,528 (36%) | 3,872 (36%) | 7,304 (36%) |
| White | 8,884 (60%) | 15,867 (60%) | 6,375 (59%) | 12,119 (60%) |
| Other | 673 (5%) | 1,185 (4%) | 479 (4%) | 917 (5%) |
| Hispanic | 2,609 (18%) | 4,648 (17%) | 1,861 (17%) | 3,537 (17%) |
| Medicare Part D low income subsidy | 10,659 (72%) | 19,025 (72%) | 7,693 (72%) | 14,556 (72%) |
| Cause of dialysis-dependent kidney failure | ||||
| Diabetes | 7,530 (51%) | 13,453 (51%) | 5,457 (51%) | 10,296 (51%) |
| Hypertension | 3,809 (26%) | 6,794 (26%) | 2,713 (25%) | 5,204 (26%) |
| Glomerular disease | 1,523 (10%) | 2,721 (10%) | 1,097 (10%) | 2,089 (10%) |
| Other | 1,998 (13%) | 3,613 (14%) | 1,458 (14%) | 2,752 (14%) |
| Time on maintenance dialysis | ||||
| < 1.0 year | 2,461 (17%) | 4,405 (17%) | 1,788 (17%) | 3,374 (17%) |
| 1.0 – 1.9 years | 2,769 (19%) | 4,974 (19%) | 1,976 (18%) | 3,796 (19%) |
| 2.0 – 2.9 years | 2,193 (15%) | 3,965 (15%) | 1,603 (15%) | 3,005 (15%) |
| ≥ 3.0 years | 7,437 (50%) | 13,237 (50%) | 5,358 (50%) | 10,165 (50%) |
| Anxiety | 3,359 (23%) | 6,003 (23%) | 2,411 (22%) | 4,547 (22%) |
| Depression | 5,310 (36%) | 9,484 (36%) | 3,818 (36%) | 7,237 (36%) |
| GERD | 4,797 (32%) | 8,413 (32%) | 3,400 (32%) | 6,488 (32%) |
| Arrhythmia | 4,799 (32%) | 8,509 (32%) | 3,492 (33%) | 6,538 (32%) |
| Conduction disorder | 1,456 (10%) | 2,580 (10%) | 1,038 (10%) | 1,979 (10%) |
| Heart failure | 7,278 (49%) | 12,968 (49%) | 5,314 (50%) | 9,930 (49%) |
| Ischemic heart disease | 7,422 (50%) | 13,249 (50%) | 5,392 (50%) | 10,151 (50%) |
| ECG during the last 30 days of baseline | 4,450 (30%) | 7,984 (30%) | 3,258 (30%) | 6,096 (30%) |
| Diabetes | 10,509 (71%) | 18,815 (71%) | 7,626 (71%) | 14,361 (71%) |
| ≥ 1 CYP 3A4 inhibitor | 1,068 (7%) | 1,919 (7%) | 786 (7%) | 1,487 (7%) |
| ≥ 1 other CYP 2C19 inhibitor | 1,846 (12%) | 3,279 (12%) | 1,319 (12%) | 2,505 (12%) |
| ≥ 1 med with any TdP risk c | 5,417 (36%) | 9,620 (36%) | 3,916 (37%) | 7,345 (36%) |
| ≥ 1 med with known TdP risk c | 1,513 (10%) | 2,694 (10%) | 1,095 (10%) | 2,048 (10%) |
| ≥ 1 med with possible TdP risk b | 1,495 (10%) | 2,671 (10%) | 1,081 (10%) | 2,016 (10%) |
| ≥ 1 med with conditional TdP risk b | 3,664 (25%) | 6,452 (24%) | 2,630 (25%) | 4,970 (24%) |
| Hospitalized in the last 30 days of baseline | 4,565 (31%) | 8,102 (30%) | 3,287 (31%) | 6,167 (30%) |
| ED visit in the last 30 days of baseline | 5,131 (35%) | 9,143 (34%) | 3,711 (35%) | 7,004 (34%) |
Values are given as % for categorical variables and as mean ± standard deviation for continuous variables. All covariates were measured during the 180-day baseline period. Tables S4 and S5 display the full list of baseline covariates considered in our analyses stratified by SSRI-PPI exposure group for both the unweighted and inverse probability of treatment weighted cohorts.
Effective sample size after inverse probability of treatment weighting. Absolute standardized mean differences comparing covariate distributions across all exposure group pairs were ≤ 0.10 after inverse probability of treatment weighting.
The CredibleMeds website (https://crediblemeds.org) is a reliable online clinical resource with up-to-date information about medications that can cause QT-prolongation and/or torsade de pointes. CredibleMeds classifies QT-prolonging medications as having a known, possible, or conditional TdP risk. Lists of medications falling into each category are provided in Table S3. Medications classified as having any level of TdP risk are those falling into any of the three CredibleMeds categories.
Abbreviations: CYP, cytochrome P450; ECG, electrocardiogram; ED, emergency department; GERD, gastroesophageal reflux disease; med, medication; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor; TdP, Torsades de Pointes
Primary analyses
The primary study cohort was followed for a total of 15,489 person-years (3,064 person-years for citalopram/escitalopram initiators using a PPI; 5,703 person-years for citalopram/escitalopram initiators not using a PPI; 2,269 person-years for sertraline initiators using a PPI; and 4,454 person-years for sertraline initiators not using a PPI). A total of 1,197 SCDs occurred during the 1-year follow-up period at a rate of 77.3/1,000 person-years (292 events at a rate of 95.3/1,000 person-years among citalopram/escitalopram initiators using a PPI; 448 events at a rate of 78.6/1,000 person-years among citalopram/escitalopram initiators not using a PPI; 177 events at a rate of 78.0/1,000 person-years among sertraline initiators using a PPI; and 280 events at a rate of 62.9/1,000 person-years among sertraline initiators not using a PPI).
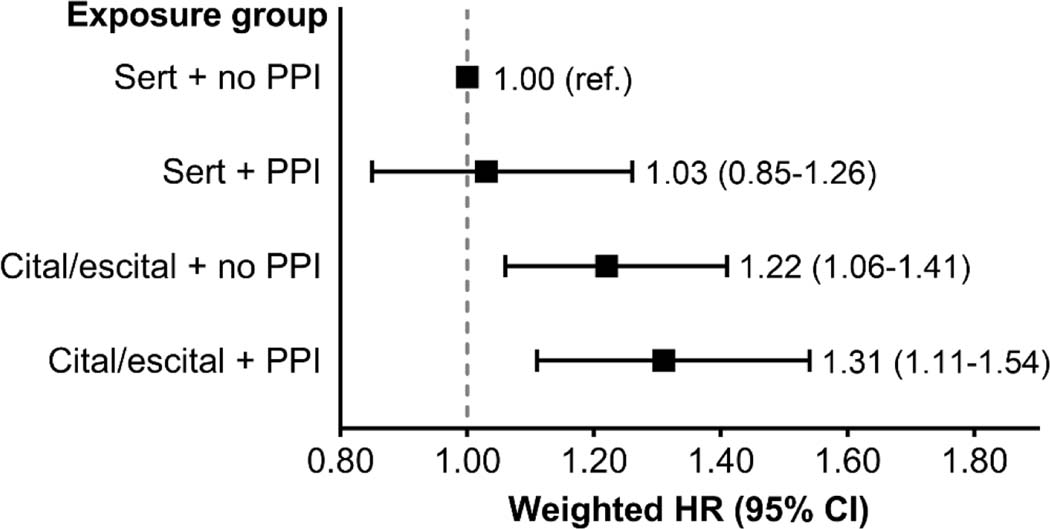
Figure 3 and Table S6 display the associations among SSRI-PPI exposure groups and SCD. Compared with sertraline initiators not using a PPI, citalopram/escitalopram initiators using a PPI had the numerically highest risk of SCD (weighted HR [95% CI] = 1.31 [1.11–1.54]), followed by citalopram/escitalopram initiators not using a PPI (weighted HR [95% CI] = 1.22 [1.06–1.41]. Sertraline initiators using vs. not using a PPI had similar risks of SCD, weighted HR [95% CI] = 1.03 [0.85–1.26].
Figure 3. Association between SSRI–PPI exposure status and the 1-year risk of sudden cardiac death.
An on-treatment analytic approach was used in all analyses. Fine and Gray proportional subdistribution hazards models were used to estimate hazard ratios, and inverse probability of treatment weighting was used for confounding control.
Abbreviations: CI, confidence interval; cital/escital, citalopram or escitalopram; HR hazard ratio; PPI, proton pump inhibitor; ref., referent; sert, sertraline.
Secondary analyses
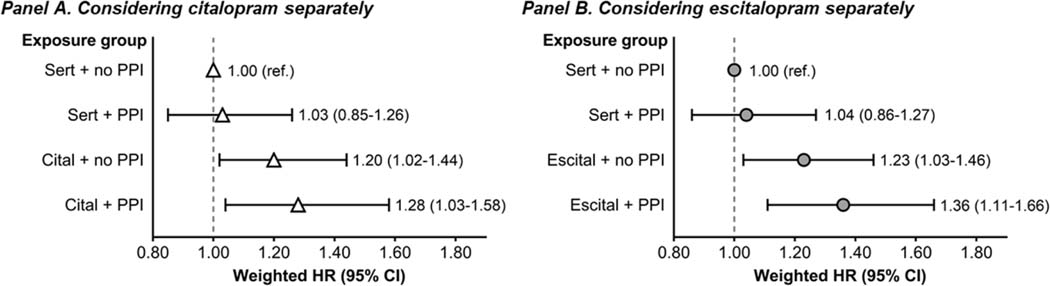
Secondary analyses evaluating alterative cardiac outcomes produced consistent results (Table 2). Relative to sertraline initiation without PPI use, citalopram/escitalopram initiation with concurrent PPI use was associated with the numerically highest risk of the composite outcome, SCD or hospitalized ventricular arrhythmia (weighted HR [95% CI] = 1.29 [1.10–1.51]), followed by citalopram/escitalopram initiation without PPI use (weighted HR [95% CI] = 1.20 [1.04–1.37]). Initiation of sertraline with vs. without concurrent PPI use was not associated with the composite outcome, weighted HR [95% CI] = 0.98 [0.81–1.19]. Analogous patterns were seen for cardiovascular mortality. In addition, analyses evaluating citalopram and escitalopram separately generated similar results (Figure 4 and Table S7).
Table 2.
Association between SSRI–PPI exposure group and the 1-year risk of other cardiac outcomes
| Sudden cardiac death or hospitalized ventricular arrhythmia – secondary outcome | |||||
|---|---|---|---|---|---|
|
| |||||
| Exposure group | n | No. of events | Rate per 1,000 p-y | Crude HR (95% CI) | Weighted HR (95% CI) |
| Sert + no PPI | 20,294 | 303 | 68.1 | 1.00 (ref.) | 1.00 (ref.) |
| Sert + PPI | 10,779 | 182 | 80.2 | 1.18 (0.98–1.42) | 0.98 (0.81–1.19) |
| Cital/escit + no PPI | 26,503 | 474 | 83.2 | 1.22 (1.06–1.41) | 1.20 (1.04–1.37) |
| Cital/escit + PPI | 14,983 | 309 | 100.9 | 1.47 (1.25–1.72) | 1.29 (1.10–1.51) |
|
| |||||
| Cardiovascular death – secondary outcome | |||||
|
| |||||
| Exposure group | n | No. of events | Rate per 1,000 p-y | Crude HR (95% CI) | Weighted HR (95% CI) |
| Sert + no PPI | 20,294 | 416 | 93.4 | 1.00 (ref.) | 1.00 (ref.) |
| Sert + PPI | 10,779 | 256 | 112.9 | 1.21 (1.04–1.42) | 1.06 (0.91–1.25) |
| Cital/escit + no PPI | 26,503 | 641 | 112.4 | 1.20 (1.06–1.36) | 1.16 (1.03–1.31) |
| Cital/escit + PPI | 14,983 | 417 | 136.1 | 1.45 (1.26–1.66) | 1.26 (1.10–1.45) |
An on-treatment analytic approach was used in all analyses. Fine and Gray proportional subdistribution hazards models were used to estimate hazard ratios and inverse probability of treatment weighting was used for confounding control.
Abbreviations: CI, confidence interval; cital/escital, citalopram or escitalopram; HR hazard ratio; No., number PPI, proton pump inhibitor; p-y, person-years; ref., referent; sert, sertraline.
Figure 4. Association between SSRI–PPI exposure status and the 1-year risk of sudden cardiac death considering citalopram and escitalopram separately.
An on-treatment analytic approach was used in all analyses. Fine and Gray proportional subdistribution hazards models were used to estimate hazard ratios, and inverse probability of treatment weighting was used for confounding control.
Abbreviations: CI, confidence interval; cital, citalopram; escital, escitalopram; HR hazard ratio; PPI, proton pump inhibitor; ref., referent; sert, sertraline.
Sensitivity analyses
Sensitivity analyses using a shorter grace period to define SSRI and PPI discontinuation, excluding patients using another non-PPI CYP 2C19 inhibitor, restricting the study cohort to individuals with the Medicare Part D low-income subsidy, and stratifying by duration of PPI use produced results that were consistent with our primary analyses (Table S8). Negative control outcome analyses evaluating the association between SSRI-PPI exposure group and death due to a cause other than SCD (i.e., non-SCD) produced null results (Table S9).
DISCUSSION
We evaluated the association of drug-drug interactions involving citalopram/escitalopram and PPIs with outcomes in the hemodialysis population. Relative to sertraline initiation without PPI use, citalopram/escitalopram initiation with concurrent PPI use was associated with the numerically highest risk of SCD, followed by citalopram/escitalopram initiation without PPI use. Relative to sertraline initiation without PPI use, sertraline initiation with concurrent PPI use was not associated with SCD. Results were consistent when we: assessed broader cardiac outcomes, evaluated citalopram and escitalopram separately, and performed several sensitivity analyses.
Observational studies have linked citalopram and escitalopram, SSRIs with the greatest QT-prolonging potential, to higher risks of adverse cardiac events in both the general25 and hemodialysis populations.26 However, few prior studies have considered if concurrent exposure to interacting medications has the potential to enhance the pro-arrhythmic risk associated with citalopram and escitalopram treatment. A nationwide cohort study in Taiwan showed that concomitantly using citalopram and omeprazole was associated with a higher risk of ventricular arrhythmias, cardiac arrest, and sudden death compared with using citalopram alone.27 Evidence in dialysis-dependent kidney failure is scant, and to our knowledge is limited to a case report of torsades de pointes in a peritoneal dialysis patient treated with escitalopram and omeprazole.28
Our finding that concurrent PPI use (vs. non-use) may enhance the risk of citalopram/escitalopram-associated SCD suggests the occurrence of a drug-drug interaction, which could be pharmacokinetic and/or pharmacodynamic. Citalopram and escitalopram are hepatically metabolized, primary by CYP 2C19.12, 29 Inhibition of this isoenzyme by other drugs, such as PPIs, will diminish citalopram/escitalopram clearance. Pharmacokinetic studies have demonstrated that PPI co-administration increases serum citalopram/escitalopram concentrations by 50 to 120%.30, 31 Such extensive accumulation can result in more profound citalopram/escitalopram-induced QT-prolongation. Moreover, PPIs themselves can prolong the QT-interval via induction of hypomagnesemia,13 and thus, have the potential to also interact with citalopram/escitalopram pharmacodynamically (i.e., the individual QT-prolonging effects citalopram/escitalopram and PPIs may be additive). Both of these interaction mechanisms could contribute to the development of clinically significant QT-prolongation. Notably, general population electrocardiogram studies have demonstrated that concurrent use of citalopram/escitalopram and a PPI can prolong the QT-interval by as much as 30 msec.32
While the interaction potential of medications is optimally studied experimentally via in vitro and/or clinical drug interaction studies,33, 34 epidemiologic investigations, such as ours can highlight adverse events associated with the concomitant use of drugs that have the potential to interact with one another. We hypothesize that the PPI-mediated competitive inhibition of CYP 2C19 might be driving the observed associations for two reasons. First, PPI-induced hypomagnesemia occurs in the setting of long-term PPI use. The majority of severe hypomagnesemia cases reported to the U.S. Food and Drug Administration (FDA) occurred among people on PPI therapy for longer than a year.35 In our study, the median length of PPI use at the time of SSRI initiation was 49 days, a duration likely too short to induce hypomagnesemia. In addition, because hypomagnesemia can cause QT-prolongation and is associated with fatal arrhythmias, we would expect that PPI-induced hypomagnesemia would elevate the risk of SCD even among patients using sertraline. In our study, the risk of SCD did not differ among sertraline initiators using and not using a PPI. Second, citalopram and escitalopram are primarily metabolized by CYP 2C19,17, 18 but sertraline is not.19 Relative to sertraline initiators not using a PPI, the risk of SCD was incremental across the exposure groups comprised of citalopram/escitalopram initiators. Citalopram/escitalopram initiators using a PPI had the numerically highest risk of SCD followed by citalopram/escitalopram initiators not using a PPI. SCD risk did not differ among sertraline initiators using and not using a PPI. Taken together these findings suggest that a pharmacokinetic CYP 2C19-mediated drug-drug interaction between citalopram/escitalopram and PPIs may explain our findings.
Irrespective of the exact mechanism(s) at play, our data suggest that clinicians should be cautious about prescribing citalopram/escitalopram in the setting of concurrent PPI therapy. This is consistent with guidance from the FDA and other pharmaceutical regulatory bodies warning of potential drug-drug interactions between citalopram/escitalopram and CYP 2C19 inhibitors such as omeprazole.36, 37 Clinicians may consider stopping PPI therapy before citalopram/escitalopram initiation, or, if possible, use sertraline instead. Regardless of PPI use, in cases where citalopram/escitalopram cannot be avoided, we suggest conducting a baseline electrocardiogram to measure the QT-interval prior to SSRI initiation and monitoring electrocardiograms every 3 to 6 months in accordance with American Heart Association recommendations.38
To-date, drug-drug interactions in the hemodialysis population, have been understudied, and it is plausible that related harm may be underappreciated. Hemodialysis patients may be particularly susceptible to the fatal consequences of drug interaction-induced QT-prolongation due to several pharmacokinetic-, cardiac-, and dialysis treatment-related factors. For example, the uremic milieu is known to suppress CYP isoenzyme expression and activity,39 diminishing associated hepatic drug clearance and potentially increasing the impact of drug-drug interactions mediated by CYP inhibition.40 In addition, underlying structural heart abnormalities from conditions such as ischemic heart disease, left ventricular hypertrophy, and heart failure are highly prevalent in the hemodialysis population.41 Associated cardiac ion channel abnormalities may make hemodialysis patients particularly susceptible to fatal arrhythmias from QT-prolonging medications and associated drug-drug interactions.42, 43 Finally, hemodialysis patients are routinely exposed to non-drug pro-arrhythmic triggers, such as electrolyte abnormalities and hemodialysis treatment-related electrolyte and fluid shifts, increasing their vulnerability to fatal arrhythmias from QT-prolonging medications and drug-drug interactions involving these medications.42, 44 Concerted efforts to educate clinicians about known drug-related risks are needed.
Our findings must be considered in the context of study limitations. First, the four SSRI-PPI exposure groups were relatively small in size, limiting statistical power and making it difficult to draw definitive conclusions when comparing the exposure groups to one another. While our findings suggest that PPIs may enhance the risk of SCD associated with citalopram/escitalopram initiation, confirmation in future well-powered studies is needed. Second, because this study was observational, residual confounding may remain. Reassuringly, negative control outcome analysis yielded null results. In addition, the lack of an association between sertraline initiation and SCD among patients using vs. not using a PPI provides further evidence that PPI-related confounding by indication was minimal in our study. Third, because the SSRI-PPI exposure groups were constructed based upon SSRI new-use and the presence or absence of PPI use on the SSRI index date, PPI-related prevalent user bias may have impacted study results. Reassuringly, a sensitivity analysis considering the duration of PPI use at the time of SSRI initiation (>30 and ≤30 days) generated results consistent with primary analyses, indicating that prevalent user bias was minimal. Fourth, various PPIs were available over-the-counter in the U.S. during the study period, and it is possible that exposure misclassification related to PPI use status may have occurred. However, a sensitivity analysis restricting to individuals with the Medicare Part D low income subsidy, patients likely to obtain PPIs using their Part D benefit, produced consistent results. Fifth, while individual PPIs have different affinities for CYP 2C19,11, 12, 20 we were unable consider dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole separately in our analyses because of the relatively small number of patients using each PPI. Sixth, although we defined SCD using the established USRDS definition, it is possible that outcome misclassification may have occurred. Reassuringly, analyses considering broader cardiac outcomes yielded consistent results. Finally, our results may not generalize to excluded populations such as patients receiving peritoneal dialysis or to other combinations of QT-prolonging medications and potentially interacting drugs.
In conclusion, existing PPI use may further enhance the risk of SCD associated with citalopram/escitalopram initiation among individuals receiving maintenance hemodialysis. These findings suggest that drug-drug interactions may heighten the risk of serious medication-related adverse events in the hemodialysis population, emphasizing the need for future research in this area.
Supplementary Material
KEY POINTS.
Concurrent use of proton pump inhibitors with citalopram or escitalopram may potentiate the QT-prolonging effects of these selective serotonin reuptake inhibitors through pharmacodynamic and/or pharmacokinetic interactions.
Existing PPI use may elevate the risk of SCD associated with citalopram or escitalopram initiation among hemodialysis patients.
Drug-drug interactions have the potential to heighten the risk of serious medication-related adverse events in the hemodialysis population, underscoring the importance of conducting comprehensive medication reviews prior to prescribing new medications.
PLAIN LANGUAGE SUMMARY.
Polypharmacy is common in the hemodialysis population and increases the likelihood that patients will be exposed to clinically significant drug-drug interactions. Concomitant use of proton pump inhibitors (PPIs) with citalopram or escitalopram may potentiate the QT-prolonging effects of these selective serotonin reuptake inhibitors. This retrospective cohort study of hemodialysis patients examined the differential risks of sudden cardiac death (SCD) associated with newly initiating citalopram or escitalopram vs. newly initiating sertraline in the presence and absence of existing PPI use. Compared with sertraline initiators not using a PPI, citalopram/escitalopram initiators using a PPI had the numerically highest risk of SCD, followed by citalopram/escitalopram initiators not using a PPI. Sertraline initiators using vs, not using a PPI had a similar risk of SCD. Existing PPI use may enhance the risk of SCD among hemodialysis patients newly initiating citalopram or escitalopram. These findings underscore the importance of conducting comprehensive medication reviews prior to prescribing new medications.
ACKNOWLEDGEMENTS
Some of the data reported here have been provided by the U.S. Renal Data System under Data Use Agreement 2018–23d to JEF. This manuscript underwent privacy review by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) officer and received clearance. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government. MMA, PHP, SMA, MAB, WCW, and JEF are supported by R01 HL152034 awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health
ETHICS STATEMENT
This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board (#18–0297). A waiver of consent was granted due to the study’s large size, data anonymity, and retrospective nature.
REFERENCES
- 1.St Peter WL. Management of Polypharmacy in Dialysis Patients. Semin Dial 2015; 28: 427–432. [DOI] [PubMed] [Google Scholar]
- 2.Frament J, Hall RK, Manley HJ. Medication Reconciliation: The Foundation of Medication Safety for Patients Requiring Dialysis. Am J Kidney Dis 2020; 76: 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rama M, Viswanathan G, Acharya LD, et al. Assessment of Drug-Drug Interactions among Renal Failure Patients of Nephrology Ward in a South Indian Tertiary Care Hospital. Indian J Pharm Sci 2012; 74: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Ramahi R, Raddad AR, Rashed AO, et al. Evaluation of potential drug- drug interactions among Palestinian hemodialysis patients. BMC Nephrol 2016; 17: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer J, Seeling A, Rupprecht H. Adverse Drug Events in Patients with Chronic Kidney Disease Associated with Multiple Drug Interactions and Polypharmacy. Drugs Aging 2020; 37: 359–372. [DOI] [PubMed] [Google Scholar]
- 6.Nelson SD, LaFleur J, Hunter E, et al. Identifying and Communicating Clinically Meaningful Drug-Drug Interactions. J Pharm Pract 2016; 29: 110–115. [DOI] [PubMed] [Google Scholar]
- 7.Beach SR, Kostis WJ, Celano CM, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry 2014; 75: e441–449. [DOI] [PubMed] [Google Scholar]
- 8.Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ 2013; 346: f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funk KA, Bostwick JR. A comparison of the risk of QT prolongation among SSRIs. Ann Pharmacother 2013; 47: 1330–1341. [DOI] [PubMed] [Google Scholar]
- 10.United States Renal Data System. 2020. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Google Scholar]
- 11.Li XQ, Andersson TB, Ahlstrom M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004; 32: 821–827. [DOI] [PubMed] [Google Scholar]
- 12.Zvyaga T, Chang SY, Chen C, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos 2012; 40: 1698–1711. [DOI] [PubMed] [Google Scholar]
- 13.Lazzerini PE, Bertolozzi I, Finizola F, et al. Proton Pump Inhibitors and Serum Magnesium Levels in Patients With Torsades de Pointes. Front Pharmacol 2018; 9: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2020 Researcher’s Guide to the USRDS Database. https://www.usrds.org/media/2482/2020_usrds_researcher_guide.pdf. Accessed January 17, 2022.
- 15.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158: 915–920. [DOI] [PubMed] [Google Scholar]
- 16.Woosley RL, Heise CW, Romero KA: QT drugs list. 2021. Available at: www.Crediblemeds.org. Accessed October 1, 2021.
- 17.Celexa® (citalopram hydrobromide tablets) [package insert]. Madison, NJ; Allergan USA, Inc.; 2019. [Google Scholar]
- 18.Lexapro® (escitalopram oxalate tablets) [package insert]. Madison, NJ; Allergan USA, Inc.; 2020. [Google Scholar]
- 19.Zoloft® (sertraline hydrochloride tablets) [package insert]. New York, NY; Roerig, a Division fo Pfizer Inc., Inc.; 2016. [Google Scholar]
- 20.Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf 2014; 37: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System ESRD Analytical Methods. 2020. Avaible at: https://adr.usrds.org/2020/end-stage-renal-disease/esrd-analytical-methods. Accessed October 1, 2021.
- 22.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation 2009; 38: 1228–1234. [Google Scholar]
- 23.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999. [Google Scholar]
- 24.Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czaja AS, Valuck RJ, Anderson HD. Comparative safety of selective serotonin reuptake inhibitors among pediatric users with respect to adverse cardiac events. Pharmacoepidemiol Drug Saf 2013; 22: 607–614. [DOI] [PubMed] [Google Scholar]
- 26.Assimon MM, Brookhart MA, Flythe JE. Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals Receiving Maintenance Hemodialysis. J Am Soc Nephrol 2019; 30: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu WT, Tsai CT, Chou YC, et al. Cardiovascular Outcomes Associated With Clinical Use of Citalopram and Omeprazole: A Nationwide Population-Based Cohort Study. J Am Heart Assoc 2019; 8: e011607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Asbroeck PJ, Huybrechts W, De Soir R. Case report, aetiology, and treatment of an acquired long-QT syndrome. Acta Clin Belg 2014; 69: 132–134. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Ma J, Huang L, et al. Fluoroquinolones increase the risk of serious arrhythmias: A systematic review and meta-analysis. Medicine (Baltimore) 2017; 96: e8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malling D, Poulsen MN, Sogaard B. The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects. Br J Clin Pharmacol 2005; 60: 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha A, Coelho EB, Sampaio SA, et al. Omeprazole preferentially inhibits the metabolism of (+)-(S)-citalopram in healthy volunteers. Br J Clin Pharmacol 2010; 70: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozano R, Bibian C, Quilez RM, et al. Clinical relevance of the (S)-citalopram-omeprazole interaction in geriatric patients. Br J Clin Pharmacol 2014; 77: 1086–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Food and Drug Administration. In Vitro Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed January 17, 2022.
- 34.United States Food and Drug Administration. Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed January 17, 2022.
- 35.United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 2011. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump. Accessed October 1, 2021.
- 36.United States Food and Drug Administration. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. 2012. https://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Accessed October 1, 2021.
- 37.New Zealand Medicines and Medical Devices Safety Authority. Interaction Between Omeprazole and Citalopram/Escitalopram. https://www.medsafe.govt.nz/profs/PUArticles/September2014InteractionBetweenOmeprazoleCitalopramEscitalopram.htm. Accessed October 1, 2021.
- 38.Tisdale JE, Chung MK, Campbell KB, et al. Drug-Induced Arrhythmias: A Scientific Statement From the American Heart Association. Circulation 2020; 142: e214–e233. [DOI] [PubMed] [Google Scholar]
- 39.Deri MT, Kiss AF, Toth K, et al. End-stage renal disease reduces the expression of drug-metabolizing cytochrome P450s. Pharmacol Rep 2020; 72: 1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung CK, Shen DD, Thummel KE, et al. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int 2014; 85: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makar MS, Pun PH. Sudden Cardiac Death Among Hemodialysis Patients. Am J Kidney Dis 2017; 69: 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assimon MM, Wang L, Pun PH, et al. Use of QT Prolonging Medications by Hemodialysis Patients and Individuals Without End-Stage Kidney Disease. J Am Heart Assoc 2020; 9: e015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007; 87: 425–456. [DOI] [PubMed] [Google Scholar]
- 44.Gussak I, Gussak HM. Sudden cardiac death in nephrology: focus on acquired long QT syndrome. Nephrol Dial Transplant 2007; 22: 12–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.