Abstract
Abundant fibrotic stroma is a typical feature of most solid tumors, and stromal activation promotes oncogenesis, therapy resistance, and metastatic dissemination of cancer cells. Therefore, targeting the tumor stroma in combination with standard-of-care therapies has become a promising therapeutic strategy in recent years. The leucine-rich repeat-containing protein 15 (LRRC15) is involved in cell–cell and cell–matrix interactions and came into focus as a promising anticancer target owing to its overexpression in mesenchymal-derived tumors such as sarcoma, glioblastoma, and melanoma and in cancer-associated fibroblasts in the microenvironment of breast, head and neck, lung, and pancreatic tumors. Effective targeting of LRRC15 using specific antibody–drug conjugates (ADC) has the potential to improve the outcome of patients with LRRC15-positive (LRRC15+) cancers of mesenchymal origin or stromal desmoplasia. Moreover, LRRC15 expression may serve as a predictive biomarker that could be utilized in the preclinical assessment of cancer patients to support personalized clinical outcomes. This review focuses on the role of LRRC15 in cancer, including clinical trials involving LRRC15-targeted therapies, such as the ABBV-085 ADC for patients with LRRC15+ tumors. This review spans perceived knowledge gaps and highlights the clinical avenues that need to be explored to provide better therapeutic outcomes in patients.
Introduction
Complex and dynamic interactions between cancer cells and the surrounding tumor microenvironment play an essential role in determining the malignant potential and the aggressiveness of cancer progression (1). Multiple autocrine and paracrine communications between tumor cells and their surrounding stromal partners including fibroblasts, stellate cells, endothelial cells, adipocytes, immune cells, and the extracellular matrix (ECM) help the tumor cells to modify their microenvironment in a way that supports their proliferation, growth, survival, and metastatic properties (2). These interactions are clinically important for cancers with abundant fibrotic stroma like pancreatic cancer, triple-negative breast cancer (TNBC), sarcoma, and ovarian cancer, and non–small cell lung cancer is associated with resistance to traditional chemotherapies and poor survival rates (3–5).
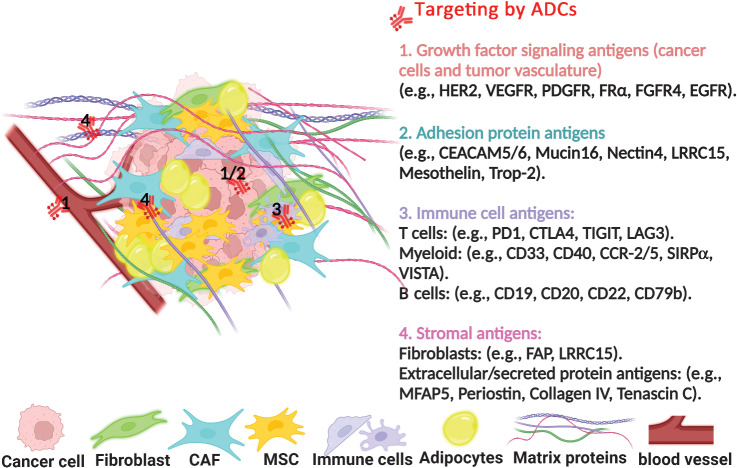
Several studies have revealed that activated stroma impairs the active uptake of traditional chemotherapeutic drugs and creates an immunosuppressive milieu in favor of tumor growth (6–9). Thus, there has been a growing interest to develop therapeutic approaches that target the tumor-stromal interaction to improve drug delivery. These approaches are currently being pursued preclinically and in early clinical trials in stroma-rich cancers to uncover new therapeutic interventions (Fig. 1).
Figure 1.
Current development in targeting the tumor-stromal interaction by ADCs is being pursued preclinically and in early clinical trials to discover novel therapeutic interventions. PDGFR, platelet-derived growth factor receptor; FRα, folate receptor α; CEACAM5/6, carcinoembryonic antigen-related cell adhesion molecules; Trop-2, trophoblast cell surface antigen 2; CTLA4, cytotoxic T-lymphocyte associated protein 4; TIGIT, T-cell immunoreceptor with immunoglobulin and ITIM domains; LAG3, lymphocyte activation gene-3; CD, cluster of differentiation; CCR-2/5, C-C chemokine receptor; SIRPα, signal regulatory protein α; VISTA, V-domain Ig suppressor of T-cell activation; FAP, fibroblast activation protein; MFAP5, microfibril associated protein 5.
One of the most studied components of the tumor microenvironment, cancer-associated fibroblasts (CAF), play a crucial role in increased tumorigenesis and resistance to therapy through a wide range of mechanisms including ECM deposition and remodeling, immunomodulation, promoting angiogenesis, and facilitating metabolic reprogramming of the tumor microenvironment (10–13). CAFs are activated in the tumor microenvironment (TME) by a variety of stimuli including inflammatory factors (IL1, IL6, and TNF), DNA damage (secondary to chemotherapy or radiotherapy), physiologic stress, and growth factors [FGF, platelet-derived growth factor (PDGF), and TGFβ; ref. 13]. Importantly, a TGFβ–driven CAF gene signature has been found to play an important role in cancer progression and immunotherapy treatment resistance (14, 15). Of interest, the protein leucine-rich repeat-containing 15 (LRRC15), has been found to be highly expressed in TGFβ–driven CAFs and serves as a marker of this CAF subpopulation in the TME (16). In addition to being expressed in CAFs, LRRC15 expression has also been found in the cells of multiple tumor types (17), Thus, LRRC15 has become a potential target for novel therapies directed at CAFs and the TME.
A variety of exciting anti-CAF therapies are under development with targets such as FGFR, Hedgehog signaling, and TGFβ (13). However, anti-CAF therapies have not come without their difficulties for anti–TGFβ therapies, which often had poor outcomes in cancer clinical trials, while a study by Özdemir and colleagues found that depletion of α-smooth muscle actin-positive (αSMA+) myofibroblasts resulted in multiple adverse outcomes leading to poor survival in a pancreatic ductal adenocarcinoma (PDAC) mouse model (18, 19). Taken together, this suggests that there is a need to develop therapies that target specific CAF subtypes such as the LRRC15-positive (LRRC15+) CAF subtype. Excitingly, LRRC15-targeting antibody–drug conjugate therapies such as ABBV-085 may offer more selective targeting of tumor-promoting CAFs in addition to targeting LRRC15-expressing cancer cells, thus leading to improved outcomes. ABBV-085 has been shown to be effective in preclinical models of several cancer types and prevented metastatic spread in an ovarian cancer xenograft model (17, 20–22). Recent results from a first-in-human phase I study showed that ABBV-085 appeared safe and tolerable at a dose of 3.6 mg/kg every 14 days, with preliminary antitumor activity noted in patients with osteosarcoma and undifferentiated pleomorphic sarcoma (UPS; NCT02565758; ref. 23).
Leucine-Rich Repeat Proteins: Integrators of Molecular Signaling
Leucine-rich repeats (LRR) are short (19–29 residue) sequence motifs present in a large number of proteins of different structure, function, and localization in bacteria, fungi, plants, and animals (24). The structural arrangement of the LRR motifs in repetitive stretches of varied length creates an adaptable framework for several protein–protein interactions (25). LRR domains generally organize themselves in a horseshoe structure, with the concave face comprising of parallel β-strands and the convex face with a variable region of secondary structures like helices. Additionally, the N-terminal part consists of a conserved 11-residue sequence rich in leucine (25). Proteins with the LRRs are found to be involved in versatile functions including adhesion, receptor-ligand binding, and target recognition (25–26). In addition, most of the studied proteins with LRR motifs have well recognized functions in the innate immune pathway (27), and in the nervous system development (28). Among the seven classes of LRR proteins, four types are well characterized within mammals (intracellular and extracellular; ref. 29). Reports suggested that the shortest known LRRs contain entirely of the 20-residue repeat motif without any other domains. Most of these LRR proteins are of gram-negative bacterial origin essential for virulence and playing a significant role in the initial stages of an infection (29). Two of the most well-defined classes include the Toll-like receptors (TLR) and NOD-like receptors (NLR) in mammals that senses the molecular determinants from varied group of viral, bacterial, fungal, and parasite-derived components through their LRR domain (30–31). To date, polymorphisms, or mutations in more than 30 LRR proteins have been shown to be involved in a wide variety of human diseases including multiple sclerosis and rheumatoid arthritis (MHC2TA; ref. 32), Legionnaires’ disease (TLR5; ref. 33) and Crohn's disease (NOD2; ref. 34). In addition, the LRR proteins are also important for human nervous system development (35) and mutations in genes encoding LRR proteins leads to several neurological disorders including epilepsy (LGI1; ref. 36), night blindness (NYX; ref. 37), congenital insensitivity to pain (TRKA/NGF; ref. 38), and Tourette's syndrome (SLITRK1; ref. 39). In addition to the typically recognized TLRs and NLRs, most of the 375 human LRR proteins still remain uncharacterized functionally. Therefore, several current studies focus to identify different human LRR proteins using the various computational and functional analyses.
Type-I LRRC15: Role in Health and Cancer
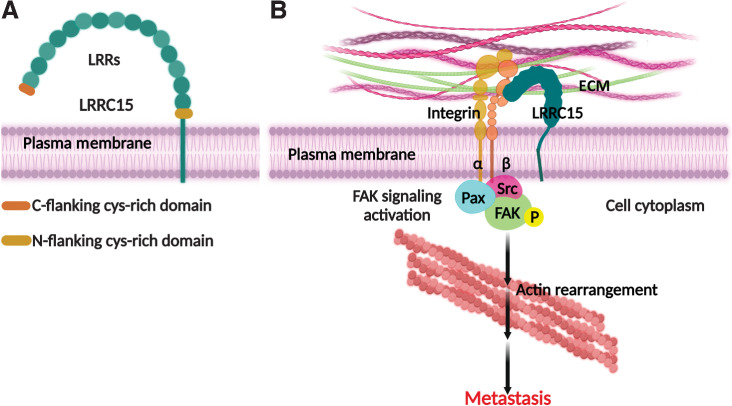
LRRC15 is a transmembrane protein (Fig. 2A) located on chromosome 3 at 3q29 that belongs to the LRR superfamily, which is involved in cell–cell and cell–ECM interactions (40, 41).
Figure 2.
A and B, Diagrammatical illustration of the structure of LRRC15 (A) and its role in promoting cancer metastasis through activation of focal adhesion kinase signaling (B). p-FAK, phosphorylated focal adhesion kinase; Src, steroid receptor coactivator; Pax, Paxillin.
Role in health
Early studies on LRRC15 revealed that it is induced by β-amyloid and that LRRC15 lacks obvious intracellular domains (17, 40). Northern blot analysis first demonstrated high expression of LRRC15 in the placenta while RNA in situ hybridization experiments conducted by Reynolds and colleagues showed that LRRC15 expression is restricted to the leading edge of migrating cytotrophoblast cells of the placenta (40, 42). Subsequent IHC analysis by Purcell and colleagues demonstrates that LRRC15 is not normally expressed in most tissues except for localized areas within hair follicles, tonsil, stomach (cardia and pylorus regions only), spleen (peritrabecular region), osteoblasts, and sites of wound healing (17). In addition, recent analysis of single-cell RNA sequencing (RNA-seq) data performed by Song and colleagues demonstrated LRRC15 expression in a subset of fibroblasts and lymphatic endothelial cells within the lung (43).
LRRC15 expression has been shown to be upregulated in C6 astrocytoma cells in response to proinflammatory cytokines (TNFα, IL1β, and IFNγ) and upregulated in fibroblasts in response to TGFβ (16, 17, 40). Interestingly, the expression of LRRC15 at sites of wound healing is in line with the important role that TGFβ–activated fibroblasts play in the wound healing process; however, whether LRRC15 plays any role in this process has yet to be determined (44). In line with other LRR proteins and given that normal tissue expression of LRRC15 is localized to areas that make up innate immune barriers such as the placenta, skin, activated fibroblasts in wounds, and lymphoid tissues such as the spleen; it appears that LRRC15 may play some role in innate immunity. This role in innate immunity is further suggested by data showing that LRRC15 impedes adenovirus infection and recent data showing that LRRC15 impedes SARS-CoV-2 viral entry by binding to the spike protein on the viral envelope (41, 43). It has also been suggested that LRRC15 may play a role in mineralized tissue biology and disease as LRRC15 has been found to be highly expressed in carious diseased pulpal tissue and Wang and colleagues has further demonstrated a role for LRRC15 in regulating the osteogenic differentiation of mesenchymal stems cells (45, 46).
Role in cancer
LRRC15 was reported to be highly expressed in CAFs within the stroma of numerous solid tumors and directly expressed in mesenchymal tumors such as glioblastoma, sarcomas, and melanoma (17). Based on our reports LRRC15 expression analysis by IHC of the ovarian tumors on the tissue microarray from chemo-naïve patients, showed both stromal and cellular staining (22). Besides published reports in other indications (e.g., sarcoma) where expression of LRRC15 on cancer and/or stromal fibroblasts was observed on treatment in naïve patient tumor samples by IHC (23). In addition, it was also reported that LRRC15 is regulated by TGFβ (17), all of which refers to the intrinsic nature of LRRC15 expression. In addition, LRRC15 expression has been shown in prostate cancer, cervical cancer, and soft-tissue sarcomas (STS; 21, 47, 48). LRRC15 has also been identified to have a role in a rare desmoplastic small round cell tumor originating from peritoneum in pediatric patients and in paratesticular desmoplastic small cell tumors (42, 49). These tumors are characterized by a chimeric protein EWS-WT1 (+KTS) that functions as an oncogenic transcription factor enhancing the expression of LRRC15. Importantly, LRRC15 exhibits high expression levels in solid tumors compared with normal tissues and has been shown to adhere to components of the ECM such as fibronectin (50) and collagen. The Cancer Genome Atlas (TCGA) analysis of LRRC15 expression across most cancer types showed genetic alteration commonly associated with amplification of the gene in the patient cohorts. RNA-seq expression analysis from TCGA (https://cancergenome.nih.gov/) breast cancer cohorts showed low baseline LRRC15 expression in normal tissue versus high differential LRRC15 expression among cancer and its adjacent normal tissues and thus suggests it may play a role in breast cancer progression (51, 52). In support of the role of LRRC15 in cancer progression multiple studies in different cancer types have demonstrated elevated LRRC15 expression in metastatic tumors (48, 52–56). Using microarray-based gene expression analysis, Klein and colleagues showed LRRC15 as one of many significantly upregulated genes in tumors metastasizing to the bone in patients with breast cancer (53). This is in line with previous work by Schuetz and colleagues demonstrating elevated expression of LRRC15 in invasive ductal carcinoma tumors compared to their matched-pair ductal carcinoma in situ counterparts (52). In a genome–wide gene expression profiling conducted by Bignotti and colleagues, LRRC15 was identified as one of 120 genes that were upregulated (≥2 fold) in omental metastases in comparison to unmatched ovarian serous carcinoma (54). In a recent study by Cui and colleagues, high LRRC15 expression correlated with metastasis, poor chemotherapeutic response, and shorter overall survival in patients with osteosarcoma (55).
In our study to identify genes that promote metastasis to bowel in high-grade serous ovarian cancer, we executed RNA-seq of ovarian cancer primary tumors (PT) and their corresponding bowel metastases from 21 patients with high-grade serous ovarian, fallopian tube, and primary peritoneal cancer (56). LRRC15 expression was found to be significantly increased in bowel metastases compared with their matched PTs, indicating that LRRC15 could be a marker of high-grade serous ovarian cancer progression (56). In our attempt to understand the function of LRRC15 in ovarian cancer metastasis we found that knockdown of LRRC15 reduces the metastatic dissemination of ovarian cancer cells in an in vivo xenograft model (56). We further demonstrate that LRRC15 expression leads to anoikis resistance and promotes adhesion and invasion through ECM that mimics omentum using various complementary models of ovarian cancer (22). Mechanistically, it was found that LRRC15 promotes metastasis upon interaction with fibronectin and β1 integrin, leading to activation of focal adhesion kinase signaling (Fig. 2B) and suggests that it can be implicated as a potential antitumor target, a concept further supported by the development of ABBV-085, an LRRC15–antibody targeted drug conjugate (17, 21). In addition to its potential role in integrin signaling, LRRC15 has recently been shown to promote TNBC tumor migration and invasion by regulating the Wnt/β-catenin signaling pathway (57). In a study by Yang and colleagues, high LRRC15 expression in CAFs of TNBC cell lines was associated with increased cytoplasmic and nuclear β-catenin, which drove cancer cell migration and invasion (57). Mechanistically, LRRC15 expression was demonstrated to decrease the expression of Axin1, a protein member of the destruction complex that promotes the degradation of β-catenin, leading to increased β-catenin levels (57). However, the precise mechanism by which LRRC15 downregulates Axin1 has yet to be elucidated. In addition, the authors elucidated a second mechanism for how LRRC15-expressing CAFs promote TNBC cancer cell migration. In their study, they found that LRRC15 expressing CAFs promoted matrix metalloproteinase (MMP) expression in TNBC cell lines, which further mediated migration and invasion (57). However, the impact of LRRC15 on MMP expression may be context dependent as LRRC15 expression in astrocytes was not associated with MMP overexpression (50).
Additional research on LRRC15-expressing fibroblasts in the lung suggests that LRRC15 may also play a bimodal role in fibroblast collagen deposition (58). Work by Loo and colleagues demonstrates that low LRRC15 expression in fibroblasts was associated with increased collagen production whereas high LRRC15 expression was not (58). However, the potential implications of these results on the role of LRRC15-expressing CAFs in tumor progression warrants further study. Furthermore, a recent investigation using a lentiviral-based DNA barcoded and clonally tracked human carcinoma OVCAR5 orthotopic xenotransplant model further established the role of LRRC15 in promoting diverse clonal growth and dissemination dynamics of tumor initiating cells from intraovarian and intraoviductal injection sites (59). This innovative in vivo barcoded clonal competition assay demonstrated that rapid promotion of lethal system wide OVCAR5 clones through various metastatic routes (peritoneal vs. blood) was linked to their LRRC15 expression levels (59). As previously mentioned, a study using a murine PDAC progression model, found a population of TGFβ programmed LRRC15+ CAFs that surround tumor islets and are absent in normal pancreatic tissues (16). Importantly, an analogous population of LRRC15+ CAFs were also reported in human pancreatic tumors and this LRRC15+ CAF signature was correlated with poor response to anti–programmed cell death 1-ligand 1 (PD-L1) therapy in multiple solid tumor types (16). These results suggest that combining therapies that target LRRC15-expressing CAFs with immunotherapy could lead to improved patient outcomes. Excitingly, the combination of the LRRC15-targeting antibody–drug conjugate (ADC) ABBV-085 with immunotherapy (anti–PD-1) was shown to enhance antitumor activity in preclinical cancer models (17). Thus, LRRC15-targeting therapies such as ABBV-085 could have exciting therapeutic potential in patients who are resistant to immunotherapy.
Clinical Translation: Targeted Therapy of LRRC15
ADCs
Targeted therapy using ADCs is currently being developed based on the principle to increase tumor drug delivery simultaneously reducing off-target toxicities. ADCs are generally comprised of a cancer-targeting mAb linked to a cytotoxic payload to elicit an antitumor effect (60–61). In the context of stromal targeting ADCs, the antibody must be specific for the target cell surface antigen that is selectively expressed in the TME. The linkers are designed to be either cleavable or noncleavable depending on the chemical design. Following antigen binding and internalization noncleavable linkers require lysosomal trafficking and subsequent degradation for the payload to be released and permeate into the cell where it can then perform its cytotoxic function (62). The drug-antibody ratio (DAR) needs cautious consideration during the development of ADCs, as a higher cytotoxic payload per unit of the antibody may lead to greater antitumor efficacy but may also increase adverse toxicity. Attaching large linker-drugs can make the ADC unstable with different pharmacokinetic properties, attenuated half-life, increased plasma clearance and systemic toxicity. The rapid clearance of high DAR species will also result in fewer ADC molecules reaching the target of interest and reduce the efficacy. The payload should be highly cytotoxic to be able to kill tumor cells at the intracellular concentrations attainable following tissue distribution. Usually an average of 3 to 4 payloads per antibody molecule is achievable without compromising the biophysical and pharmacokinetic properties (60). mAbs have proven activity in cancer treatment, with recently approved drugs like trastuzumab deruxtecan, sacituzumab govitecan, and enfortumab vedotin targeting HER2, Trop2, and Nectin4 respectively, providing meaningful clinical benefit in different cancer indications. Current advancements implement numerous ADCs into preclinical and clinical studies in both solid tumor and in hematologic cancers (63, 64). Besides, combination therapies are also explored in several clinical trials nowadays, like combining with the standard-of-care therapies (NCT03187210/NCT01476410/NCT01771107/NCT03959085) and with checkpoint inhibitors (NCT01896999/NCT02684292/NCT02605915/NCT02572167/NCT02581631). Nonetheless the development of ADCs still faces challenges, including selection of patients’ and/or biomarker assessment to exploit ADCs as its best.
ABBV-085, a humanized anti-LRRC15 ADC
ABBV-085 is an anti-LRRC15 humanized IgG1 antibody (Ab1), consisting of hydrophobic interaction chromatography (HIC)–enriched E2 wherein approximately two antimitotic monomethyl auristatin E (MMAE) payloads are conjugated per antibody through a protease cleavable valine–citrulline (vc) linker (17). The LRRC15-specific mAb in ABBV-085 is used to deliver the MMAE drug payload at high levels in the TME. Once delivered to the LRRC15+ stroma, the cell-permeable MMAE drug diffuses into the adjacent tumor cells where it can cause a targeted-bystander response to kill the proliferating cancer cells and eventually lead to tumor reduction. Purcell and colleagues showed an increased growth inhibitory response by ABBV-085 specifically on highly proliferative cells within a tumor stroma compared with normal LRRC15+ mesenchymal stem cells or stromal fibroblasts, which have reduced proliferative rate, thus alleviating the associated toxicity within areas of the normal tissue having LRRC15 expression. More importantly, efficacy of ABBV-085 monotherapy was observed in both the LRRC15 cancer-positive/stromal-positive and LRRC15 cancer-negative/stromal-positive cancer models. In vivo xenograft models of many solid tumor types including sarcoma, breast, head and neck, lung, pancreatic, and glioblastoma (with ≥2+ LRRC15 positivity) showed response to ABBV-085 treatment demonstrating ABBV-085′s broad preclinical activity in multiple solid tumor indications (17). Promising antitumor efficacy was achieved when ABBV-085 was used in combination therapy with other standard-of-care cancer therapeutics. The major observations raise the question to clinically identify whether LRRC15 cancer-negative/stromal-positive tumors (like head and neck, breast) or LRRC15 cancer-positive/stromal-positive tumors (like sarcoma) demonstrate preferential sensitivity to ABBV-085 treatment and whether the appropriate clinical application of ABBV-085 as a monotherapy or in the combination setting will benefit the patients. A follow-up study was executed using the ABBV-085 monotherapy in LRRC15+ patient-derived xenograft (PDX) models of STS (21), including the highly chemo-refractive UPS PDX with poor survival rate and treatment options (65). Administration of ABBV-085 monotherapy attenuated tumor growth in the STS PDX models with positive LRRC15 expression. Having known that tumor-associated macrophages (TAM) are associated with the antitumor efficacy of ADCs (66), IHC analysis showed strong infiltration of TAM in the TME of the UPS PDX model in the ABBV-085–treated cohort (21). Since the importance of TAM infiltration in STS towards the prognostic impact and in resistance to PD-1 inhibition is well established (67–68), assessing the therapeutic efficacy of combinatorial drug therapy of ABBV-085 with the programmed cell death protein 1 (PD-1) or PD-L1 immune checkpoint inhibitors (currently in phase II clinical trial), specifically in the STS subtypes is of interest. Our studies have assessed the efficacy of ABBV-085 in the high-grade ovarian serous cancer using both the prevention and intervention models of ovarian cancer xenograft. ABBV-085 treated cohort showed a significant regression of the metastatic spread and tumor growth. These studies led to the testing of ABBV-085 efficacy in two preclinical PDX models of ovarian cancer with high LRRC15 expression, which shows regression of tumor in the prevention models (22).
ABBV-085 has completed a phase I first-in-human safety study in sarcomas and other advanced solid tumors (NCT02565758; ref. 23). A nonrandomized, multicenter, phase I, open-label, dose-escalation study was performed to determine the pharmacokinetics and safety of ABBV-085 and evaluate the suggested phase II dose, either as a monotherapy or in combination with standard care of therapies in patients with advanced solid tumors including sarcomas, squamous cell carcinoma of the head and neck, and breast carcinoma (NCT02565758). As reported by Demetri and colleagues at American Society of Clinical Oncology (ASCO) 2019, the study at that point, had enrolled a total of 78 patients, which included 27 patients with advanced sarcomas (UPS; n = 10), osteosarcoma (n = 10), and other sarcomas (n = 7). Patients were treated in the monotherapy dose-escalation (from 0.3 to 4.8 mg/kg dosed every 2 weeks) and then dose-expansion was performed at 3.6 mg/kg (23). ABBV-085 was stated to be well tolerated with a favorable safety profile relative to other MMAE ADCs. Grade ≥3 treatment-emergent adverse events reported in 56 (72%) patients with the most common being anemia (14%, n = 11). Neuropathy and blurred vision were noted and were reported to be manageable by reducing dose-intensity. Among the 27 patients with sarcoma treated at the RP1bD, 4 had confirmed partial responses (15%), 8 confirmed stable diseases (30%), 11 had progressive disease (41%), and 2 were not evaluable (7%); with the median response duration of 7.6 months [95% confidence interval (CI): 5.6–9.2, confirmed responders]. In UPS 4 of 10 patients had more than 30% tumor shrinkage with two confirmed responses that were durable (280 days and 392 days at data cut-off). In osteosarcoma 2 of 10 patients had confirmed responses. While no response greater than stable disease was reported in LRRC15 stromal-positive/cancer-negative indications. ABBV-085 therapy was therefore reported to be generally well tolerated with a manageable safety profile and encouraging signs of antitumor activity in hard-to-treat sarcoma indications. These preliminary clinical findings suggest that LRRC15 may be a promising new therapeutic target in select solid cancer indications. In addition, other LRRC15-targeting ADCs have been assessed preclinically. The ADC LRRC15-PNU consists of an LRRC15 ADC conjugated to the anthracycline derivative PNU-159682 was assessed in osteosarcoma preclinical models as anthracyclines are the standard of care in osteosarcoma (69). LRRC15-PNU demonstrated promising results in osteosarcoma with cure rates between 40% and 100% in osteosarcoma xenograft models (69). These results suggest that LRRC15 ADCs can be tailored to deliver different payloads to the TME to specifically target varying cancer types.
Future Perspectives
Multidrug resistance (MDR) including both inherent and acquired is the major reason for chemotherapy failure resulting in metastatic dissemination and relapse. Multiple factors like inactivation of drugs, accelerated drug efflux, and adaptations in the target cells is previously considered to be the reason behind MDR in cancer (70). However, the surge in research arrived when reports showed that tumor-stromal interaction is mostly responsible for the non–cell-autonomous mechanisms of resistance and targeting the tumor stroma, in combination with conventional standard of care, can become a promising treatment option (71). These led to the development of antifibrotic/antistromal therapies for diagnostic and therapeutic purposes. The future of ADCs seems promising, as the efficacy depends on the level of target expression, which is a key parameter in predicting the probability of patient benefit. LRRC15 is an important and potentially exploitable target in solid tumor therapy. The differential expression of LRRC15 within selected tumors of mesenchymal origin as opposed to nonmalignant cells enables target specificity and efficacy with low toxicity. LRRC15 may be exploitable for both diagnostic and theranostic purposes in future. Identification LRRC15-expressing tumor tissues will help in determining whether LRRC15 can be a better predictive marker and therapeutic target in the tumor types involved including both the LRRC15 cancer-positive/stromal-positive and LRRC15 cancer-negative/stromal-positive cancer models. Future study of LRRC15 as a predictive biomarker in defined tumor types can be implied and either the radiological or real-time imaging during surgery can open better functional analysis and clinical outcomes respectively. The antitumor efficacy of ABBV-085 either as monotherapy or with the standard-of-care therapy are currently at an interesting point in their development and have the potential to improve efficacy in a clinically defined class of tumors with LRRC15 positivity, to achieve better outcomes. With the development of ABBV-085 as an antistromal target, the degree of stromal/fibrotic cell death is not known and requires further investigation. Moreover, delineating the combination of ABBV-085 treatment with immune-checkpoint inhibitors could be an effective therapy in specific circumstances, similar to the successes as found with combinations with conventional cytotoxic chemotherapies, thus providing further therapeutic options for patients with LRRC15+ cancers. TAM infiltration in TME leading to PD-1 inhibition opens the future window to assess whether the therapeutic efficacy of ABBV-085 is increased in combination with the PD-1/PD-L1 immune-checkpoint inhibitors (phase II CT) in a defined set of solid tumors. There is clearly much yet to study about the optimal application of ADCs in the treatment of mesenchymal cancers, specifically in establishing the best combination modalities for treating LRRC15-expressing cancers in the future.
To move forward, analysis on the expression of LRRC15 in relation to recurrent and minimal residual disease (MRD) to date is missing. It is widely believed that the MRD are cells that escape resection during surgery survive in patients associated with poor survival. Therefore, identification of biomarkers of MRD is very critical specifically if they are therapeutic targets such as LRRC15 and may lead to effective treatment. Despite the extensive breadth of current knowledge, concerning the optimal sequence and practice of treatment combinations including both the stromal-targeting agents and the cancer-targeting agents’ prevalence of analytical gaps still prevents such schemes from being accessible to patients. As we continue to develop an improved understanding of the multifaceted complex interactions between a heterogeneous milieu of cellular components in the microenvironment, we will be able to advance stroma-targeting strategies for more effective anticancer treatments. Improved tailoring of treatments to specific patient subgroups and development of novel therapeutic combinations may drive successful clinical outcomes in the future.
Acknowledgments
This work was supported in part by grants from the Minnesota Ovarian Cancer Alliance (MOCA), NIH P50CA136393, and Department of Experimental Pathology and Laboratory Medicine Discretionary Funds, and the Mayo Clinic (Rochester, MN; to V. Shridhar). The figures were created with BioRender.com.
Authors' Disclosures
J.W. Purcell reports other support from AbbVie during the conduct of the study and other support from AbbVie outside the submitted work; in addition, J.W. Purcell has a patent for ANTI-huLRRC15 ANTIBODY DRUG CONJUGATES AND METHODS FOR THEIR USE issued to AbbVie. No disclosures were reported by the other authors.
References
- 1. Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater 2007;3:413–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol 2012;9:454–67. [DOI] [PubMed] [Google Scholar]
- 4. Giacchetti S, Porcher R, Lehmann-Che J, Hamy AS, de Roquancourt A, Cuvier C, et al. Long-term survival of advanced triple-negative breast cancers with a dose-intense cyclophosphamide/anthracycline neoadjuvant regimen. Br J Cancer 2014;110:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gajra A, Jatoi A. Non–small-cell lung cancer in elderly patients: a discussion of treatment options. J Clin Oncol 2014;32:2562–9. [DOI] [PubMed] [Google Scholar]
- 6. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol 2015;15:669–82. [DOI] [PubMed] [Google Scholar]
- 7. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berchtold S, Grünwald B, Krüger A, Reithmeier A, Hähl T, Cheng T, et al. Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Lett 2015;356:721–32. [DOI] [PubMed] [Google Scholar]
- 10. Madar S, Goldstein I, Rotter V. ' Cancer associated fibroblasts'–more than meets the eye. Trends Mol Med 2013;19:447–53. [DOI] [PubMed] [Google Scholar]
- 11. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 12. Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: Where we are and where we need to go. Cancers 2016;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RMet al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020;20:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MVet al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012;22:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoon H, Tang CM, Banerjee S, Delgado AL, Yebra M, Davis Jet al. TGF-β1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis 2021;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 2020;10:232–53. [DOI] [PubMed] [Google Scholar]
- 17. Purcell JW, Tanlimco SG, Hickson J, Fox M, Sho M, Durkin L, et al. LRRC15 is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res 2018;78:4059–72. [DOI] [PubMed] [Google Scholar]
- 18. Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TRet al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teixeira AF, Ten Dijke P, Zhu HJ. On-target anti-TGF-β therapies are not succeeding in clinical cancer treatments: What are remaining challenges? Front Cell Dev Biol 2020;8:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hingorani P, Roth ME, Wang Y, Zhang W, Gill JB, Harrison DJet al. ABBV-085, antibody-drug conjugate targeting LRRC15, is effective in osteosarcoma: a report by the pediatric preclinical testing consortium. Mol Cancer Ther 2021;20:535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ben-Ami E, Perret R, Huang Y, Courgeon F, Gokhale PC, Laroche-Clary Aet al. LRRC15 targeting in soft-tissue sarcomas: biological and clinical implications. Cancers 2020;12:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ray U, Jung DB, Jin L, Xiao Y, Dasari S, Sarkar Bhattacharya S, et al. Targeting LRRC15 inhibits metastatic dissemination of ovarian cancer. Cancer Res 2021Oct 15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demetri GD, Luke JJ, Hollebecque A, Powderly JD, 2nd, Spira AI, Subbiah V, et al. First-in-human phase I study of ABBV-085, an antibody-drug conjugate targeting LRRC15, in sarcomas and other advanced solid tumors. Clin Cancer Res 2021;27:3556–66. [DOI] [PubMed] [Google Scholar]
- 24. Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 2001;11:725–32. [DOI] [PubMed] [Google Scholar]
- 25. Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 1994;19:415–21. [DOI] [PubMed] [Google Scholar]
- 26. Buchanan SG, Gay NJ. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol 1996;65:1–44. [DOI] [PubMed] [Google Scholar]
- 27. Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 2004;198:249–66. [DOI] [PubMed] [Google Scholar]
- 28. Chen Y, Aulia S, Li L, Tang BL. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Rev 2006;51:265–74. [DOI] [PubMed] [Google Scholar]
- 29. Kajava AV. Structural diversity of leucine-rich repeat proteins. J Mol Biol 1998;277:519–27. [DOI] [PubMed] [Google Scholar]
- 30. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 31. Peter ME, Kubarenko AV, Weber AN, Dalpke AH. Identification of an N-terminal recognition site in TLR9 that contributes to CpG-DNA-mediated receptor activation. J Immunol 2009;182:7690–7. [DOI] [PubMed] [Google Scholar]
- 32. Swanberg M, Lidman O, Padyukov L, Eriksson P, Akesson E, Jagodic M, et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet 2005;37:486–94. [DOI] [PubMed] [Google Scholar]
- 33. Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med 2003;198:1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 35. Matsushima N, Tachi N, Kuroki Y, Enkhbayar P, Osaki M, Kamiya M, et al. Structural analysis of leucine-rich-repeat variants in proteins associated with human diseases. Cell Mol Life Sci 2005;62:2771–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet 2002;30:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 2000;26:319–23. [DOI] [PubMed] [Google Scholar]
- 38. Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 1996;13:485–8. [DOI] [PubMed] [Google Scholar]
- 39. Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science 2005;310:317–20. [DOI] [PubMed] [Google Scholar]
- 40. Satoh K, Hata M, Yokota H. A novel member of the leucine-rich repeat superfamily induced in rat astrocytes by beta-amyloid. Biochem Biophys Res Commun 2002;290:756–62. [DOI] [PubMed] [Google Scholar]
- 41. O'Prey J, Wilkinson S, Ryan KM. Tumor antigen LRRC15 impedes adenoviral infection: implications for virus-based cancer therapy. J Virol 2008;82:5933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reynolds PA, Smolen GA, Palmer RE, Sgroi D, Yajnik V, Gerald WL, et al. Identification of a DNA-binding site and transcriptional target for the EWS-WT1(+KTS) oncoprotein. Genes Dev 2003;17:2094–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song J, Chow RD, Pena-Hernandez M, Zhang L, Loeb SA, So EYet al. LRRC15 is an inhibitory receptor blocking SARS-CoV-2 spike-mediated entry in trans. bioRxiv 2021 Nov 24[Epub ahead of print].
- 44. Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2013;2:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cooper PR, McLachlan JL, Simon S, Graham LW, Smith AJ. Mediators of inflammation and regeneration. Adv Dent Res 2011;23:290–5. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Liu Y, Zhang M, Lv L, Zhang X, Zhang P, et al. LRRC15 promotes osteogenic differentiation of mesenchymal stem cells by modulating p65 cytoplasmic/nuclear translocation. Stem Cell Res Ther 2018;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bierkens M, Krijgsman O, Wilting SM, Bosch L, Jaspers A, Meijer GA, et al. Focal aberrations indicate EYA2 and hsa-miR-375 as oncogene and tumor suppressor in cervical carcinogenesis. Genes Chromosomes Cancer 2013;52:56–68. [DOI] [PubMed] [Google Scholar]
- 48. Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815–25. [DOI] [PubMed] [Google Scholar]
- 49. Cliteur VP, Szuhai K, Baelde HJ, van Dam J, Gelderblom H, Hogendoorn PC. Paratesticular desmoplastic small round cell tumour: an unusual tumour with an unusual fusion; cytogenetic and molecular genetic analysis combining RT-PCR and COBRA-FISH. Clin Sarcoma Res 2012;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Satoh K, Hata M, Shimizu T, Yokota H, Akatsu H, Yamamoto T, et al. Lib, transcriptionally induced in senile plaque-associated astrocytes, promotes glial migration through extracellular matrix. Biochem Biophys Res Commun 2005;335:631–6. [DOI] [PubMed] [Google Scholar]
- 51. Satoh K, Hata M, Yokota H. High lib mRNA expression in breast carcinomas. DNA Res 2004;11:199–203. [DOI] [PubMed] [Google Scholar]
- 52. Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M, et al. Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res 2006;66:5278–86. [DOI] [PubMed] [Google Scholar]
- 53. Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N, et al. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett 2009;276:212–20. [DOI] [PubMed] [Google Scholar]
- 54. Bignotti E, Tassi RA, Calza S, Ravaggi A, Bandiera E, Rossi E, et al. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol 2007;196:245. [DOI] [PubMed] [Google Scholar]
- 55. Cui J, Dean D, Wei R, Hornicek FJ, Ulmert D, Duan Z. Expression and clinical implications of leucine-rich repeat containing 15 (LRRC15) in osteosarcoma. J Orthop Res 2020;38:2362–72. [DOI] [PubMed] [Google Scholar]
- 56. Mariani A, Wang C, Oberg AL, Riska SM, Torres M, Kumka J, et al. Genes associated with bowel metastases in ovarian cancer. Gynecol Oncol 2019;154:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang Y, Wu H, Fan S, Bi Y, Hao M, Shang J. Cancer-associated fibroblast-derived LRRC15 promotes the migration and invasion of triple-negative breast cancer cells via Wnt/β-catenin signalling pathway regulation. Mol Med Rep 2022;25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Loo L, Waller MA, Cole AJ, Stella AO, Moreno CL, Denes CEet al. LRRC15 suppresses SARS-CoV-2 infection and controls collagen production. bioRxiv 2021Nov 10 [Epub ahead of print].
- 59. Aalam SMM, Tang X, Song J, Bakkum-Gamez J, Sherman ME, Ray U, et al. Barcoded Competitive Clone-Initiating Cell (BC-CIC) analysis reveals differences in ovarian cancer cell genotype and niche specific clonal fitness during growth and metastasis in vivo. bioRxiv 2021April 11 [Epub ahead of print].
- 60. Lambert JM, Morris CQ. Antibody-drug conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Ther 2017;34:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Diamantis N, Banerji U. Antibody-drug conjugates–an emerging class of cancer treatment. Br J Cancer 2016;114:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bakhtiar R. Antibody drug conjugates. Biotechnol Lett 2016;38:1655–64. [DOI] [PubMed] [Google Scholar]
- 63. Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol 2021;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B 2020;10:1589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Savina M, Le Cesne A, Blay JY, Ray-Coquard I, Mir O, Toulmonde M, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med 2017;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li F, Ulrich M, Jonas M, Stone IJ, Linares G, Zhang X, et al. Tumor-associated macrophages can contribute to antitumor activity through FcγR-mediated processing of antibody-drug conjugates. Mol Cancer Ther 2017;16:1347–54. [DOI] [PubMed] [Google Scholar]
- 67. Toulmonde M, Adam J, Bessede A, Ranchère-Vince D, Velasco V, Brouste V, et al. Integrative assessment of expression and prognostic value of PDL1, IDO, and kynurenine in 371 primary soft tissue sarcomas with genomic complexity. J Clin Oncol 2016;34:11008-. [Google Scholar]
- 68. Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Cesne L A, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol 2018;4:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Slemmons KK, Mukherjee S, Meltzer P, Purcell JW, Helman LJ. LRRC15 antibody-drug conjugates show promise as osteosarcoma therapeutics in preclinical studies. Pediatr Blood Cancer 2021;68:e28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marin JJ, Sanchez de Medina F, Castaño B, Bujanda L, Romero MR, Martinez-Augustin O, et al. Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab Rev 2012;44:148–72. [DOI] [PubMed] [Google Scholar]
- 71. Qu Y, Dou B, Tan H, Feng Y, Wang N, Wang D. Tumor microenvironment-driven non-cell-autonomous resistance to antineoplastic treatment. Mol Cancer 2019;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]