Abstract
Issues:
Non-medical prescription opioid use (NMPOU) contributes substantially to the global burden of morbidity. However, no systematic assessment of the scientific literature on the associations between NMPOU and health outcomes has yet been undertaken.
Approach:
We undertook a systematic review evaluating health outcomes related to NMPOU based on ICD-10 clinical domains. We searched 13 electronic databases for original research articles until 1 July 2021. We employed an adaptation of the Oxford Centre for Evidence-Based Medicine ‘Levels of Evidence’ scale to assess study quality.
Key Findings:
Overall, 182 studies were included. The evidence base was largest on the association between NMPOU and mental and behavioural disorders; 71% (129) studies reported on these outcomes. Less evidence exists on the association of NMPOU with infectious disease outcomes (26; 14%), and on external causes of morbidity and mortality, with 13 (7%) studies assessing its association with intentional self-harm and 1 study assessing its association with assault (<1%).
Implications:
A large body of evidence has identified associations between NMPOU and opioid use disorder as well as on fatal and non-fatal overdose. We found equivocal evidence on the association between NMPOU and the acquisition of HIV, hepatitis C and other infectious diseases. We identified weak evidence regarding the potential association between NMPOU and intentional self-harm, suicidal ideation and assault.
Discussion and Conclusions:
Findings may inform the prevention of harms associated with NMPOU, though higher quality research is needed to characterise the association between NMPOU and the full spectrum of physical and mental health disorders.
Keywords: prescription drugs, opioid overdose, health outcomes, non-medical, systematic review
INTRODUCTION
The use of naturally-occurring, synthetic and semisynthetic opioids is common worldwide, with methadone, morphine and fentanyl all included on the World Health Organization’s List of Essential Medicines [1]. Strong evidence suggests that opioids are effective in the treatment of acute and cancer-related pain [2], though little evidence their use for chronic non-cancer pain exists, particularly in the long-term [3]. Nevertheless, rates of opioid prescribing increased precipitously in the United States and Canada over the past two decades [4,5]. In the United States and Canada, the increased availability of prescription opioids has contributed to a high prevalence of non-medical use: in 2015, for example, 12.5 million people in the United States engaged in non-medical prescription opioid use (NMPOU) amounting to 4.7% of the population [6]. This is of concern given that NMPOU is a primary driver of the opioid overdose epidemic affecting North America [5,7].
To date, efforts to respond to and reduce the health impacts of NMPOU have focused largely on its contribution to overdose risk [8]. However, the range of health harms associated with NMPOU remain poorly synthesized (e.g. only one scoping review on the topic exists [9]), despite a large evidence base spanning multiple scientific and clinical disciplines. This is of public health and public policy concern, given that clinicians, implementation scientists and policymakers require a comprehensive assessment of potential harms to inform clinical practice, interventions and policy responses.
We therefore conducted a systematic review of the scientific literature to assess the known associations between NMPOU and a range of clinical outcomes.
METHODS
We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. We searched electronic literature databases, listed in Table S1 (Supporting Information), for articles published as of 1 October 2018. We defined NMPOU as: use in the context of someone else’s prescription or the use of one’s own prescription outside of prescribed parameters (i.e. dose, frequency, mode of consumption or indication) [11]. We used International Classification of Diseases, 10th revision (ICD-10) for physical and mental health disorders potentially associated with NMPOU, using relevant Medical Subject Headings (MeSH) and keywords (see Table S1). Table 1 lists ICD-10 codes potentially associated with non-medical prescription opioid use. Hand searching of relevant academic meetings and reference lists was also done. All searches were conducted between 24 October 2015 and 15 October 2018.
Table 1.
International Classification of Diseases–10th revision codes potentially associated with non-medical prescription opioid use
| I. Infectious diseases |
| B15 – B19: Viral hepatitis |
| B20 – B24: Human Immunodeficiency Virus [HIV] |
| III. Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism |
| D65 – D69: Coagulation defects, purpura and other hemorrhagic conditions |
| V. Mental and behavioral disorders |
| F10 – F19: Mental and behavioral disorders due to psychoactive substance use |
| F30 – F39: Mood [affective] disorders |
| F40 - F48: Neurotic, stress-related and somatoform disorders |
| F00 – F99: Other psychiatric / mental health measures |
| XI. Diseases of skin and subcutaneous tissue |
| L00 – L99: Infections of the skin and subcutaneous tissue |
| XVI. Certain conditions originating in the perinatal period |
| P05 – P08: Disorders related to length of gestation and fetal growth |
| P90 – P96: Other disorders originating in the perinatal period |
| XVIII. Symptoms, signs and abnormal clinical and laboratory findings not elsewhere classified |
| R40-R46. Symptoms and signs involving cognition, perception, emotional state and behavior |
| R50-R69: General symptoms and signs |
| XIX. Injury, poisoning, and certain other consequences of external causes |
| T36 – T50: Poisoning by drugs, medicaments, and biological substances |
| XX. External causes of morbidity and mortality |
| X60-X64: Intentional self-harm |
| X85-Y09: Assault |
Eligible studies were: (i) peer-reviewed; (ii) measured current or past NMPOU; and (ii) were a randomised control trial, case-control, comparison study, cohort study, non-randomised trial, quasi-experimental study, time-series analysis or ecological study. Studies were excluded if they failed to isolate NMPOU, did not capture ICD-10 outcomes and were not written in English. Two of four investigators independently reviewed titles and abstracts in stage 1, and full texts in stage 2, classifying studies as “potentially relevant” or “irrelevant”. Disagreements were resolved by consensus.
We employed the Oxford Centre for Evidence-Based Medicine ‘Levels of Evidence’ [12] to assess the contribution of each study to the scientific evidence base. The ‘Levels of Evidence’ tool provides a rank based on study design, with studies designed to provide a higher level of evidence ranked lower (i.e. the highest rank is 1; the lowest is 5). For the current study, we adapted the tool to range from 1 to 4; this was done given that studies with the lowest level of evidence based on the tool (ranked at 5, and consisting of non-data driven expert opinion) were not eligible for inclusion in the systematic review because they did not present data. Two investigators independently scored studies, with a third author verifying, and consensus achieved where needed by a fourth author. Assessments of the level of evidence within the 1-4 range were not a basis for exclusion.
RESULTS
Study selection and characteristics
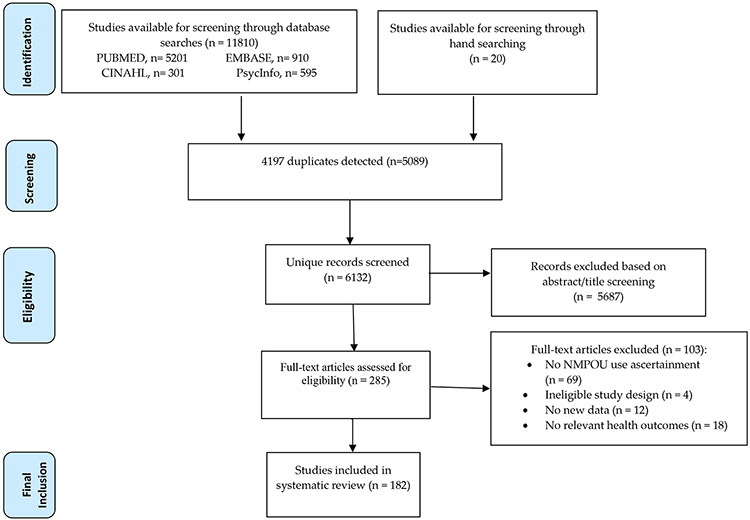
Overall, as shown in Figure 1, 11,810 studies were identified, yielding 285 full text articles assessed for eligibility, with 103 excluded. Ultimately, 182 studies met the inclusion criteria; three studies only included event count and not participant sample size [13-15]. A total of 53 (29%) studies employed cross-sectional, population-based and nationally representative observational designs [7,14,16-64]; 13 (7%) employed longitudinal, cohort-based observational designs [65-77]; 4 (2%) employed case control designs [78-81]; 6 (3%) were randomised control trials [82-87]; 54 (30%) employed cross-sectional data from convenience samples [73,88-140]; 10 (5%) employed longitudinal (including repeated cross-sectional) data from nationally representative studies [15,17,141-147]; and 42 (23%) retrospectively assessed individual-level data [13,37,148-187]. As shown in Figure 2, the majority of studies presented data from the USA (126, 69%). Other countries represented included Canada (22, 12%), Australia (13, 7%), France (4, 2%), India (4, 2%), Nordic countries (4, 2%), China (3, 2), the United Kingdom (2, 1%), Estonia (1, <1%), Iran (1, <1%) and Israel (1, <1%).
Figure 1:
PRISMA flow chart of study selection. NMPOU, non-medical prescription opioid use.
Figure 2:
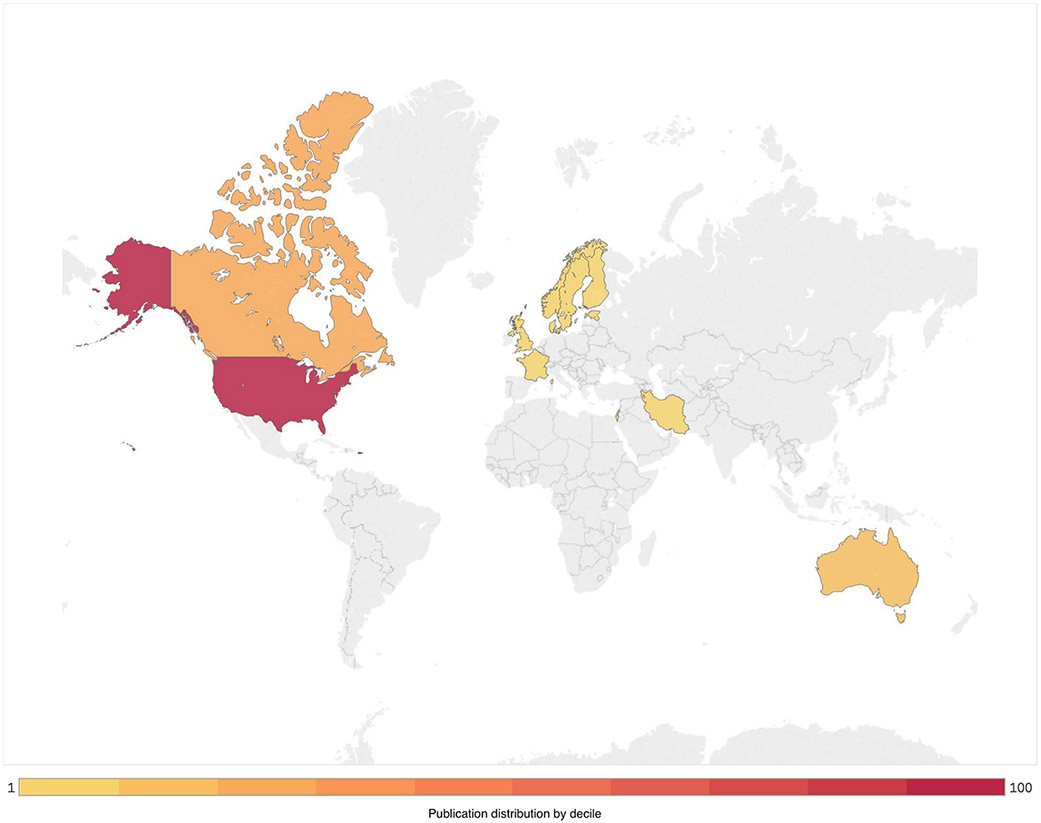
Distribution of publications on clinical outcomes associated with non-medical prescription opioid use
Levels of evidence assessment
The mean assessment score was 3.30 (SD = 1.01; interquartile range = 2-4), reflecting that the studies included in the systematic review generally used designs that provided a lower level of evidence. Seven studies (4%) received a score of 1 (the highest score); 53 (29%) received a score of 2; 7 (4%) received a score of 3; and 115 (63%) received a score of 4 (the lowest score). We detected no significant association between study quality and year of publication (R2 = > −0.01, P > 0.05), suggesting no significant change in quality over time.
Results of individual studies
Table 2 presents results from all eligible studies classified by ICD-10 code. As can be seen, studies investigated the association between NMPOU and outcomes across eight major clinical domains. The median number of studies investigating each clinical outcome was 15.0 (interquartile range 3.0-23.5).
Table 2.
Summary of 182 studies examining health outcomes of non-medical prescription opioid use, sorted by World Health Organization International Classification of Diseases (ICD 10) code [V. 2016]
| Study | Study design | Participant characteristics | NMPOU exposure | Study rank |
Outcome measure | Main finding and quantitative results |
|---|---|---|---|---|---|---|
|
I. Infectious and parasitic diseases
B15 – B19: Viral hepatitis [Hepatitis B] | ||||||
| Havens 2007, USA | Cross-sectional | 184 prescription opioid [PO] users recruited through street outreach and snowball sampling; median age: 30 years | Injection in past 30 days | 4 | HBV [self-reported] | There was no significant difference in HBV infection between those who inject PO and those who did not (P=0.184). |
| Wylie 2006, Canada | Cross-sectional 2003-2004 | 369 PWID recruited through street outreach | Injection in past 6 months 6 months | 4 | HBV serostatus [IMX HBV core IgG] | Morphine injection was not significantly associated with HBV serostatus (OR=1.2 [0.7–1.8]); overall HBV prevalence was 30%. |
| B15 – B19: Viral hepatitis [Hepatitis C] | ||||||
| Aitken 2008, Australia | Cross-sectional 2005-2006 | 316 street-recruited PWID | Injection drug use in previous 3 months | 4 | HCV serostatus | There was no significant difference between buprenorphine injectors and non-injectors in prevalence of HCV antibody (OR=1.2 [0.5-3.0]) or ribonucleic acid (OR=0.95 [0.4-2.1]). |
| Barry 2011, USA | Cross-sectional 2006 | 4122 veterans in the third wave of a follow-up survey, median age: 52 years | Past-year use of NMPOU | 4 | HCV | Veterans in care have a high prevalence of NMPOU which was associated with hepatitis C (AOR=1.5 [1.2-1.9]); HCV prevalence: 56.8% |
| Bruneau 2012, Canada | Prospective 2004-2009 | 246 HCV-negative PWID at baseline; mean age: 34.5 years | Past month injection drug use | 2 | HCV infection [EIA test] | Compared to non-PO injectors, PO injectors were more likely to become infected with HCV (aHR 1.87 [1.16-3.03]). |
| Chelleng 2008, India | Cross-sectional 2004-2005 | 143 PWIDs including female sex workers; median age: 24.7 years | Injection drug use in previous 6 months | 4 | HCV antibodies prevalence [ELISA] | Prevalence of anti-HCV antibodies was significantly lower among exclusive PO injectors compared to exclusive heroin injectors [n=13/31] vs 85.2% (41.9% [n=23/27]], p<0.05). |
| Das 2007, India | Cross-sectional | 254 PWIDs randomly sampled and enrolled; median age: 26 years | Injection drug use in previous 6 months | 4 | HCV serostatus [EIA] | Prevalence of HCV significantly lower among exclusive PO injectors compared to exclusive heroin injectors (23.9% [n=27/113] vs 45.4% [5/11], P <0.05). |
| Hadland 2014, Canada | Prospective 2005-2011 | 940 adolescents and young adults aged 14–26 recruited through street-based outreach and snowball sampling; mean age: 21.7 years | Substance use in past 6 months (injecting, non-injecting) | 2 | HCV serostatus and seroconversion | PO injection was significantly and positively associated with HCV serostatus at baseline (OR=8.69 [5.01-15.1]), after covariate adjustment PO injection was not positively associated with HCV seroconversion (aHR=0.94 [0.40-2.21]) |
| Han 2017, USA | Cross-sectional 2015 | 51200 respondents in the 2015 NSDUH identified as prescription opioid users | Lifetime and past-year NMPOU | 4 | HBV or HCV | Among adults with prescription opioid misuse, prevalence of HBV or HCV was 12.4% (95% CI, 8.32-18.19) |
| Havens 2007, USA | Cross-sectional | 184 prescription opioid [PO] users recruited through street outreach and snowball sampling; median age: 30 years | PO injection in past 30 days | 4 | HCV [self-reported] | More people who inject PO self-reported HCV compared to those who did not inject PO, (n=9 [14.8%] vs. n=2 [1.7%], P <0.05) |
| Havens2013, USA | Cross-sectional 2008-2010 | 392 PWIDs recruited through street outreach median age: 31 years | Past month (any drug use), lifetime (injection) | 4 | HCV [home access test] | Injecting prescription opioids was significantly associated with HCV seropositivity (AOR=2.22 [1.13-4.35]); prevalence of HCV infection was 54.8% |
| Iversen 2010, Australia | Cross-sectional 1998-2008 | 15,852 PWID recruited from needle and syringe programs; mean age: 31 years | Number of years injecting NMPOU | 4 | HCV serostatus [EIA test] | For those injecting ≤ 4 years, injection of methadone or buprenorphine (female: AOR=4.78 [2.63-8.70]; male: AOR=3.06[1.58-5.92]), and injection of other prescription opioids (women: AOR=2.20 [1.24-3.90]; men: AOR=1.77 [1.03-3.04]), were associated with HCV positive serostatus |
| Lankenau 2015, USA | Cross-sectional 2009-2011 | 162 PWIDs sampled in New York and Los Angeles; mean age: 21.4 years | Lifetime NMPOU injection | 4 | HCV [self-reported] | Lifetime prescription opioid injectors were nearly 3 times more likely to report being HCV positive than non-prescription opioid injectors (AIRR=2.69 [1.07-6.78], P <0.05) |
| Mahanta 2009, India | Cross-sectional 2004-2006 | 398 PWIDs recruited from drop-in centres; median age: 26 years | Injection NMPOU in previous 6 months | 4 | HCV serostatus [EIA] | Proxyvon-only injection was associated with reduced odds of HCV infection (AOR=0.42 [0.24-0.71] |
| Patra 2009, Canada | Prospective 2004-2005 | 582 participants from the most recent follow-up in the OPICAN study; mean age:35.2 years | NMPOU use in past 30 days | 4 | HCV [self-reported] | Among participants who primarily used prescription opioids, risk of HCV positivity was low (OR=0.35 [0.12-1.01]); self-reported HCV prevalence was 24% |
| Powell 2019, USA | Ecological 2004-2015 | 50 US states | Past-year median OxyContin misuse rate | 2 | HCV incidence rate | States with above-median OxyContin misuse before the reformulation experienced a 222% increase in HCV infection rates vs. 75% increase in states with below-median OxyContin misuse |
| Wylie 2006, Canada | Cross-sectional 2003-2004 | 369 PWID recruited through street outreach | Injection NMPOU in previous 6 months | 4 | HCV serostatus [AxSYM HCV] | Morphine injection was not significantly associated with HCV serostatus (OR=1.2 [0.8-1.9]); overall HCV prevalence was 53.7% |
| Zibbell 2014, USA | Cross-sectional 2012 | 123 PWID recruited using snowball sampling | Injection NMPOU in previous in past year | 4 | HCV serostatus [PCR] | Prescription opioid injection was significantly associated with HCV positivity (AOR=5.53 [1.92-15.91]) |
| B20 – B24: HIV | ||||||
| Barry 2011, USA | Cross-sectional 2006 | 4122 veterans in the third wave of a follow-up survey; median age 52 years | Past year NMPOU | 4 | HIV | Veterans in care have a high prevalence of NMPOU which was not associated with HIV status; HIV prevalence was 60% |
| Buttram 2014, USA | Clinical trial 2008-2010 | 515 MSM participating in a risk reduction intervention trial; mean age: 38.9 years | Past year and past 90 days injection NMPOU | 2 | HIV | Prescription opioid misuse in past 90 days was associated with lower odds of HIV-positive serostatus (OR=0.825 [0.553-1.232]); HIV seropositive prevalence: 48.5% |
| Black 2013, USA | Cross sectional 2007-2011 | 29,459 PO users recruited from 540 treatment facilities; mean age=33.1 years | Past 30 days NMPOU | 4 | HIV/AIDS prevalence [ASI-MV] | Prescription opioid injection was not significantly associated with increased odds of HIV/AIDS (OR=1.05 [0.73-1.51; 1.1% reported a positive HIV/AIDS test |
| Conrad 2015, USA | Cross-sectional | 135 HIV positive persons in a rural Indiana county; mean age: 35 years | Lifetime NMPOU injection | 4 | HIV | Among 135 persons with HIV infection, 80.0% are PWIDs and inject tablets of oxymorphone as their drug of choice |
| Novak 2016, Denmark, Germany, Great Britain, Sweden | Cross-sectional 2014 | 22070 survey respondents in the European Union ages 12-49 | Lifetime and past year NMPOU | 4 | HIV | NMPO users were more likely to be HIV positive (AOR=18.9 [10-34; P <0.001] that those not reporting NMPOU |
| Obadia 2001, France | Cross-sectional 1997 | 343 PWIDs recruited at 32 pharmacies, 4 needle exchange programs and 3 syringe vending machines; median age = 30 years | Injection NMPOU in previous 6 months | 4 | HIV | Compared to people who inject [self-reported] other drugs, buprenorphine-only injection was significantly associated with reduced odds of HIV (AOR=0.63 [0.41-0.94]) |
| Talu 2010, Estonia | Cross-sectional | 350 PWID recruited using respondent driven sampling mean age: 23.9 years | Injection NMPOU in past 28 days | 4 | HIV [HIV GACPAT] | Fentanyl injection was associated with increased risk of testing positive for HIV (AOR=2.89 [1.55-5.39]); HIV prevalence was 62% among fentanyl injectors (95% CI 56.97–67.03, P <0.001) |
| Wylie 2006, Canada | Cross-sectional 2003-2004 | 369 PWID recruited through street outreach | Injection NMPOU in previous 6 months | 4 | HIV serostatus [AxSYM HIV1/2 gO] | Morphine injection was not positively associated with HIV serostatus (OR=0.4 [0.1–1.1]); overall HCV prevalence was 7% |
|
III. Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism
D65 – D69: Coagulation defects, purpura and other haemorrhagic conditions | ||||||
| CDC 2013, USA | Case-control | Cases: 15 patients with TTP-like illnesses, Controls:28 patients recruited from a methadone clinic; median age: cases= 34 years, controls= 31 years | Injection NMPOU in previous 6 months | 4 | TTP-like illness | The cases of TTP-like illness were associated with dissolving and injecting tablets of Opana-ER, a recently reformulated extended-release form of oxymorphone intended for oral administration; 14 of the 15 case-patients, reported recent injection of reformulated Opana-ER (OR= 35.0 [3.9–312.1]) |
|
V. Mental and behavioural disorders
F10 – F19: Mental and behavioural disorders due to psychoactive substance use | ||||||
| Adams 2006, USA | Prospective | 11352 patients with chronic pain taking prescription opioids | Past year NMPOU | 2 | Withdrawal [withdrawal score] | Withdrawal score of hydrocodone was significantly higher than that of tramadol (P <0.01) |
| Back 2010, USA | Cross-sectional 2006 | 55,279 respondents in the NSDUH | Past year NMPOU | 4 | Dependence [DSM IV criteria] | Among the respondents, 13.2% reported prescription opioid dependence |
| Back 2011, USA | Cross-sectional | 24 non-treatment seeking individuals (12 men and 12 women) | Past year NMPOU | 4 | Dependence [DSM-IV criteria] | All recruited participants had PO dependence. The most common NMPO was with oxycontin (56%) |
| Barry 2011, USA | Cross-sectional 2006 | 4122 veterans in the third wave of a follow-up up survey; median age: 52 years | Past year NMPOU | 4 | Opioid use disorder | Veterans in care have a high prevalence of NMPOU which is associated with opioid use disorder [AOR 2.7 (1.9-3.9)]; opioid use disorder prevalence: 30.7% |
| Becker 2007, USA | Cross-sectional 2002-2004 | 6879 respondents in the NSDUH | Past year NMPOU | 4 | Dependence [DSM-IV criteria] | Of those with non-medical use, 12.9% met criteria for abuse or dependence |
| Becker 2011, USA | Retrospective 2006-2008 | 3238 respondents in the NSDUH | Past year NMPOU | 2 | Dependence [self-reported] | Past-year opioid analgesic abuse and/or dependence was associated with having a physician source of opioids (AOR=2.0 [1.5-2.7]); 20.3 % of participants had PO abuse/dependence |
| Blanco 2016, USA | Prospective 2001-2005 | 34653 sample of US adults participating in NESARC | Past-year NMPOU | 1 | Opioid use disorder | Prospective association between pain at wave 1 predicting PO use disorder at wave 2 (AOR=2.17 [1.35-3.48]). Predicted risk of developing PO use disorder at wave 2 was 0.44% for persons without and 0.62% for those with pain interference at wave 1 (increase of 41%) |
| Blanco 2016, USA | Prospective 2001-2005 | 34653 sample of US adults participating in NESARC | Past year NMPOU | 1 | Opioid tolerance, withdrawal | Pain at wave 1 was significantly associated with opioid tolerance (β=0.17, P=0.002) and withdrawal (β=0.24, P <0.001) at wave 2; association approached significance for taking opioids longer or at greater doses than prescribed (β=0.12, P=0.07) |
| Boyd 2009, USA | Prospective 2001-2005 | 34653 sample of US adults participating in NESARC | Lifetime and past year NMPOU | 1 | Dependence [DSM-IV criteria] | Non-medical use (at young age) in Wave 1 was associated with higher odds of a general substance or opioid abuse/dependence disorder at Wave 2 (AOR=3.42, 95% CI 1.45, 8.07) |
| Buttram 2014, USA | Clinical trial 2008-2010 | 515 MSM participating in a risk reduction intervention trial; mean age: 38.9 years | Past year and past 90 days injection NMPOU | 2 | Dependence [DSM-IVR criteria] | Non-medical prescription opioid use was associated with higher odds of substance dependence (OR=2.150 [1.253-3.689]; P=0.005); substance dependence prevalence: 62.1% |
| Cohen 2010, Ireland | Cross-sectional | 89 service users attending an outpatient methadone stabilisation and detoxification program | Lifetime NMPOU | 4 | Dependence [MAP] | Those with any history of codeine misuse were more likely to have a longer drug dependence history (P <0.05) |
| Cottler 2016, USA | Retrospective cohort 1981-1983, 2007 | 9985 participants in population-based sample | NMPOU more than 5 times in life | 2 | Alcohol abuse/dependence | Prevalence of alcohol abuse or dependence was greater in non-medical prescription opioid users (56.9%) compared to non-PO drug users (23.1%) and non-drug users (9.0%) |
| Edlund 2007, USA | Cross-sectional 1996-1997 | 9279 participants from a nationally representative study of the US civilian population | NMPOU in past 12 months | 4 | Problem opioid misuse including tolerance and/or physiologic problems [CIDI] | Users of prescribed opioids had significantly higher rates of any opioid misuse (AOR = 3.07 [2.05-4.60], P < 0.001) and problem opioid misuse (AOR = 6.11 [3.02-12.36], P < 0.001) |
| Edlund 2010, USA | Cross-sectional 2001-2004 | 36,605 enrollees in HealthCore and 9651 enrollees in Arkansas Medicaid all receiving chronic opioid therapy | Past six month NMPOU | 4 | Dependence/abuse [ICD-9-CM] | Participants with a diagnosis of 2 or more mental health disorders prior to the period of opioid use episode had increased odds of dependence/abuse in the period following opioid use episode (HealthCore patients: AOR=2.08 [1.69-2.55]; Medicaid patients: AOR=1.70 [1.21-2.39]) |
| Edlund 2014, USA | Cross-sectional 2000-2005 | 568,640 individuals with new chronic non cancer pain episode being prescribed opioids | NMPOU in the 18 months after the index date | 4 | Opioid use disorder [ICD-9-CM] | Individuals with pre-index mental health disorders had higher rates of opioid use disorders (AOR=3.12 [2.41-4.04], P <0.001) |
| Ghandour 2008, USA | Cross-sectional 2002-2003 | 7810 respondents in the 2002-2003 NSDUH | Past year NMPOU | 4 | Dependence [DSM-IV] | Among past year PO users, 8.3% met criteria for opioid dependence, also individuals belonging to the highest latent class had high probabilities of endorsing each of the seven symptoms of dependence (0.70–0.99) |
| Han 2017, USA | Cross-sectional 2015 | 51,200 respondents in the 2015 NSDUH identified as prescription opioid users | Lifetime and past year NMPOU | 4 | Opioid use | Among adults with prescription disorder opioid misuse, prevalence of past-year PO use disorder was 16.7% (95% CI 14.8-18.49) |
| Humeniuk 2003, Australia | Cross-sectional 1996-1997 | 365 heroin users recruited through snowball sampling; mean age: 28.9 years | Injection NMPOU in last 6 months | 4 | Dependence [SDS scale] | Participants injecting methadone were significantly more dependent on heroin than people not injecting methadone (mean SDS scores: 8.9 vs 6.39 [F=14.5, df=1, 362, P=0.000]) |
| Kouyanou 1997, London, UK | Cross-sectional | 125 patients receiving treatment for chronic pain; mean age: 41 years | Past year NMPOU | 4 | Dependence/abuse [DSM-III-R] | Among the sample of chronic pain sufferers, 3.2% and 4.8% met criteria for opioid abuse and dependence respectively |
| Martins 2007, USA | Cross-sectional 2002-2003 | 7810 respondents in the 2002-2003 NSDUH | Past year NMPOU | 4 | Withdrawal [self-reported], Tolerance [self-reported] | Prescription opioid dependence was significantly associated with withdrawal symptoms (AOR=3.76 [2.88-4.91]) and tolerance [AOR=9.90 [7.70-12.74]) |
| McCabe 2016, USA | Longitudinal observational 1993-2013 | 4072 high school seniors | Past year and past month NMPOU | 2 | SUD at age 35 | Medical use of PO without misuse during adolescence was not associated with SUD symptoms at age, while NMPOU was (AOR=2.61 [1.88-3.61]), compared to no medical or nonmedical use |
| McCabe 2012, USA | Cross-sectional 2009-2010 | 148 middle and high school students reporting past year NMPOU; mean age: 15.2 years | Past year NMPOU | 4 | SUD [CRAFT] | Among past year NMPO users, 35.1% screened positive for SUD based on the craft test |
| Miller 2004, USA | Retrospective 2000 | 534 patients admitted and discharged from an addiction detoxification unit; mean age: 40.37 years | Current NMPOU | 2 | Substance-related diagnosis [DSM criteria] | Users of NMPOs had a higher mean number of substance-related diagnosis than non-users, also opiate dependent patients were more likely to have a medical diagnosis (OR = 3.47 [2.00-5.99]) |
| Morasco 2008, USA | Randomised trial 2006-2007 | 127 patients recruited from a veteran’s affairs medical centre; mean age: 59.8 years | Lifetime NMPOU | 1 | SUD [ICD-9-CM] | Borrowing pain medication (misuse) was significantly associated with increased odds of SUD (AOR=6.62 [1.4-30.7]) |
| Morasco 2013, USA | Cross-sectional | 80 participants with chronic pain recruited from a veteran’s affairs medical centre; mean age: 54.9 years | Current opioid prescription and composite NMPOU risk measure | 4 | SUD [SCID] | Participants in the high prescription opioid misuse group had the highest rates of current substance use disorders (32% vs 20% and 0, P=0.009) |
| Morizio 2017, USA | Retrospective cohort study 2010-2015 | 923 patients admitted to a medical centre for heroin or non-heroin opioid overdose event; median age: 24 | Lifetime history of NMPOU | 2 | History of prescription opioid abuse | History of NMPOU was more prevalent among those with non-heroin opioid overdose (46.9%), compared to the heroin overdose group (21.9%), P <0.0001 |
| Reid 2002, USA | Retrospective 1997-1998 | Medical records of 50 veteran affairs and 48 primary care centre patients; veteran affairs patients median age: 54 years; primary care centre patients median age: 55 years | Lifetime history of NMPOU based on patient records | 2 | Substance use | Prescription opioid abuse was disorder [medical associated with a lifetime history records review] of substance use disorder (AOR=3.8 [1.4-10.8]) |
| Romach 1999, Canada | Cross-sectional | 339 long term codeine users recruited through newspaper adverts; mean age: 44 years | Past year codeine use | 4 | Dependence [DSM-IV] | Codeine dependence/abuse was present in 41% of long-term codeine users |
| Roux 2011, France | Prospective 1995-1996 | 235 HIV-infected opioid-dependent individuals; median age: 34 years | Injecting or sniffing PO in the previous 6 months | 2 | Withdrawal symptoms [self-report] | NMPOU was significantly associated with a reduced odds of experiencing opioid withdrawal symptoms (AOR=0.62 [0.36-0.89]) |
| Saha 2016, USA | Cross-sectional 2012-2013 | 36,309 respondents in the NESARC-III | Past-year and lifetime NMPOU | 4 | Any other substance use disorders | NMPOU was significantly related to having any other substance use disorder (12-month AOR=2.95 [2.54-3.44]; lifetime AOR = 3.07 [2.67-3.53]) |
| Schepis 2019, USA | Cross-sectional 2012-2013 | 14667 respondents in the NESARC-III | Persistent NMPOU (defined as past-year and prior) | 4 | Past-year substance use disorders | Persistent NMPOU significantly associated with higher past year prevalence of any substance use disorder (56.3% vs. 20.5%), alcohol use disorder (41.0% vs. 19.3%), and tobacco use disorder (48.4% vs. 24.6%) vs. no NMPOU |
| Schepis 2019, USA | Cross-sectional 2015-2016 | 114,043 respondents in the NSDUH | Past-year non-medical prescription fentanyl use | 3 | Past-year non-alcohol substance use disorders | Past-year non-medical prescription fentanyl use was associated with past-year non-alcohol substance use disorders (RR=20.64 [9.38-45.45]) compared to population controls |
| Schepis 2019, USA | Cross-sectional 2009-2014 | 103,920 adolescent respondents in in the NSDUH | Past 30 days NMPOU | 4 | Major depression in past year (DSM-IV) | NMPOU was significantly associated with major depression (AOR=1.91 [1.21-3.01]) |
| Wu 2008, USA | Cross-sectional 2005-2006 | 2675 adolescent participants in the 2005-2006 NSDUH | Past year NMPOU | 4 | Dependence [DSM-IV] | Among adolescents age 12 to 17, weekly use of NMPO was significantly associated with dependence (AOR=5.45 [3.97-7.49]) |
| Wu 2009, USA | Cross-sectional 2006 | 1290 adolescent participants in the 2006 NSDUH | Past year NMPOU | 4 | Dependence [DSM-IV] | Among adolescents age 12 to 17, 15.1% met DSM-IV criteria either for opioid abuse (7.4%) or dependence (7.7%) in the past year |
| Wu 2011, USA | Cross-sectional 2001-2002 | 2450 participants in the 2001-2002 National Institute on Alcohol Abuse and Alcoholism survey | Past year NMPOU | 4 | Substance use [DSM-IV] | Comparing prescription opioid disorder users vs users of non-opioid drugs, odds of any drug use disorder was increased among opioid users (AOR=1.31 [1.02-1.69]) |
| F30 – F39: Mood [affective] disorders | ||||||
| Ali 2015, USA | Cross-sectional 2008-2012 | 84,800 adolescents in the 2008-2012 NSDUH; mean age: 14.49 years | Lifetime NMPOU | 4 | Major depressive episode [Propensity scoring] | Adolescents who used prescription drugs non-medically were 33% to 35% more likely to experience major depressive episodes compared to their non-abusing counterparts (P < 0.05) |
| Barth 2013, USA | Case-control | Cases: 86 participants with current PO dependence; Controls:41 healthy participants in a larger study on stress; mean age: 35.4 years | Past 30 days NMPOU | 4 | Psychiatric disorders [ASI Lite] | Among individuals with prescription opioid dependence, prevalence of psychiatric disorders was: major depressive disorder 12.9%; panic disorder 20%; generalised anxiety disorder 14.1% |
| Barth 2014, USA | Cross-sectional | 307 individuals with non-alcoholic chronic pancreatitis ;mean age: 51 years | Past 30 days medication usage | 4 | Depressive symptoms [CESD] | A high opioid misuse measure score was associated with increased score on the depressive symptoms measurement scale (β = 0.38, P <0.0001) |
| Becker 2007, USA | Cross-sectional 2002-2004 | 6879 respondents in the NSDUH | Past year NMPOU | 4 | Depression [CIDI-SF] | Past-year non-medical use of prescription opioids was significantly associated with depressive symptoms [AOR=1.2 (1.01–1.5)] |
| Bohnert 2013, USA | Cross-sectional 2009 | 351 adults in a residential treatment program; mean age: 35.6 years | Past month NMPOU, Lifetime use | 4 | Depression [PHQ-9] | NMPOU was significantly associated with depression (AOR=1.06 [1.01-1.11], P=0.01) |
| Bouvier 2019, USA | Cross-sectional 2015-2016 | 199 young adults treatment program; mean age: 35.6 years | Past month NMPOU, Lifetime use | 3 | Diagnosed for depression | NMPOU use was significantly associated with a depressive disorder (AOR=1.51 [1.14-1.99], P <0.01) |
| Carrieri 2003, USA | Prospective 1995-2000 | 114 HIV-infected patients on buprenorphine maintenance treatment; mean age: 33.6 years | Buprenorphine injection misuse during the previous 6 months | 2 | Depression [CESD] | Depression was associated with injection misuse in the stabilised (ARR=1.04 [1.01-1.06]) and un-stabilised (ARR=1.05 [1.01-1.09]) phase of treatment |
| Edlund 2007, USA | Cross-sectional 1996-1997 | 9279 participants from a nationally representative study of the US civilian population | NMPOU in past 12 months | 4 | Depression | Depression was significantly [CIDI] associated with self-reported opioid misuse (OR = 2.23 [1.71-2.90], P < 0.001) |
| Edlund 2015, USA | Cross-sectional 2008-2012 | 112,600 adolescents aged 12–17, and 7100 adolescents aged 12–17 | Past year NMPOU | 4 | MDE [NCS-A] | MDE was associated with NMPOU among all adolescents (OR = 1.51, P <0.001); in the sample of adolescents with NMPO use, 20% reported past year MDE |
| Fink 2015, USA | Cross-sectional 2011-2012 | 113655 participants in the 2011 and 2012 NSDUH | Past year NMPOU | 4 | MDE [DSM-IV] | Among adolescent and adult respondents, 5.8% and 4.5% reported any past-year NMPO respectively, while 8.6% and 6.8% met criteria for past-year MDE respectively |
| Grattan 2012, USA | Cross-sectional 2008-2009 | 1334 patients on chronic opioid therapy for non-cancer pain who had no history of substance abuse | NMPOU in past 2 weeks | 4 | Depression [PHQ-8 score] | Prescription opioid misuse was significantly associated with moderate depression (OR=1.75 [1.05-2.91, P=0.031]) and severe depression (OR=2.42 [1.46-4.02, P=0.001]) |
| Green 2009, USA | Retrospective 2005-2008 | 29,906 respondent assessments from 220 treatment centres; mean age: 34.9 years | Past 30 days use of any prescription opioid | 2 | Depression [ASI-MV] | Prescription opioid abuse was associated with increased odds of depression in males (AOR=1.29 [1.09-1.53]) but not significant in females (AOR=1.15 [0.96-1.38]) |
| Mackesy-Amiti 2015, USA | Cross-sectional 2008-2010 | 570 PWIDs recruited through street outreach and respondent driven sampling; mean age: 22.2 years | Past year and lifetime prescription opioid injection | 4 | Substance-induced major depression [DSM-IV] | Past year PO misuse was significantly associated with past year substance-induced major depression (AOR=1.81 [1.16–2.83]) |
| Martel 2014, USA | Cross-sectional | 82 patients with chronic pain being prescribed POs; mean age: 48.7 years | Past month NMPOU | 4 | Depression [self-report] | Depression (r = 0.25, P < 0.05) was significantly associated with prescription opioid misuse |
| Martins 2009, USA | Cross-sectional 2005-2006 | 8218 respondents in the NSDUH | Past year NMPOU | 4 | MDE [NCS-R and NCS-A] | Past year opioid users were significantly more likely to have past year MDE (AOR=1.4 [1.2-1.6]), compared to users of other illegal drugs |
| Martins 2012, USA | Prospective 2001-2002; 2004-2005 | 34653 respondents in the NESARC | NMPOU over lifetime, past-year, or since last interview | 2 | Major depressive disorder [DSM-IV] | Baseline lifetime NMPOU was associated with incident major depressive disorder (AOR=1.4 [1.1-1.9]) |
| Mason 2016, USA | Cross-sectional 2015 | 625 neurosurgery and orthopedic patients | NMPOU in the past 2 weeks | 4 | Depression [PROMIS] | Symptoms of depression symptoms experienced in the past week was significantly related to NMPOU in the past 2 weeks (AOR=1.16 [1.01-1.33]) |
| Morasco 2008, USA | Randomised trial 2006-2007 | 127 patients recruited from a veteran’s affairs medical centre; mean age: 59.8 years | Lifetime NMPOU | 1 | Depression [DSM-IV] | Participants reporting PO misuse were more likely to meet criteria for major depression (22% vs 3.7%, P=0.008) |
| Nugraheni 2020, USA | Cross-sectional 2016 | 40,632 respondents in the NSDUH | Past-year NMPOU | 4 | Major depressive episode | NMPOU associated with major depressive episode in past year (AOR=2.99 [2.47-3.62]) |
| Price 2011, USA | Cross-sectional 2008-2009 | 351 patients seeking treatment from a drug and alcohol treatment program; mean age: 35.8 years | NMPOU in past 30 days | 4 | Depressive symptoms [PHQ] | Prescription opioid misuse was associated with greater depressive symptoms (AOR=1.09 [1.01-1.18]) |
| Rigg 2015, USA | Cross-sectional 2010-2013 | 10201 respondents in the NSDUH; 18 years or older | Past-year NMPOU | 4 | Major depressive episode | NMPO users were not more likely to have experienced a major depressive episode in the past year compared to heroin users (AOR=0.774 [0.441-1.360] P-value=0.373) |
| Saha 2016, USA | Cross-sectional 2012-2013 | 36309 respondents in the NESARC-III | Past-year and lifetime NMPOU | 4 | Major depressive disorder | Past-year NMPOU was significantly related to having a major depressive disorder (AOR=1.26 [1.05-1.52]) and persistent depression (AOR=1.43 [1.06-1.92]) |
| Sobieraj 2012, USA | Cross-sectional | 1334 patients treated with chronic opioids | NMPOU in past 2 weeks | 4 | Depression [self-report] | Prescription opioid misuse was significantly associated with moderate depression (AOR=1.75 [1.05-2.91]) or severe depression (AOR=2.42 [1.46-4.02]) |
| Tang 2016, China | Cross-sectional 2012 | 18686 Chinese high school students age 11-20; mean age: 15.43 | Lifetime, past-year, and past-month NMPOU | 4 | Depressive symptoms [CES-D] | Depressive symptoms were more prevalent among participants reporting past-month NMPOU (16.6%) than non-users (8.8%); P-value <0.0001 |
| Wheeler 2019, USA | Cross-sectional | 208 African-American male prisoners | Lifetime NMPOU | 4 | Lifetime severe anxiety (ASI-V) | Lifetime NMPOU was associated with severe anxiety (AOR=3.97 [1.58-9.86]) |
| Wu 2011, USA | Cross-sectional 2001-2002 | 2450 participants in the 2001-2002 National Institute on Alcohol Abuse and Alcoholism survey | Past year NMPOU | 4 | Mood disorder [DSM-IV] | Comparing prescription opioid users vs users of non-opioid drugs, odds of any mood disorder wasincreased among opioid users (AOR=1.07 [0.82-1.40]), though relationship was not statistically significant |
| Zale 2015, USA | Cross-sectional | 24348 participants in the 2009 NSDUH | Past year NMPOU | 4 | Major depressive episode (MDE) [DSM-IV criteria] | Past year NMPOU was associated with past year MDE (AOR=2.44 [1.88-3.17], P <0.001) |
| F40 - F48: Neurotic, stress-related and somatoform disorders | ||||||
| Becker 2007, USA | Cross-sectional 2002-2004 | 6879 respondents in the NSDUH | Past year NMPOU | 4 | Panic disorder, social phobia [CIDI-SF] | Past-year non-medical use of prescription opioids was significantly associated with panic symptoms [AOR=1.2 (1.04–1.5)], and phobic symptoms [AOR=1.2 (1.1–1.4)] |
| Boyd 2014, USA | Cross-sectional 2009-2010 | 2627 secondary school students; mean age: 14.8 years | Lifetime NMPOU | 4 | Anxiety [YSR version of CBCL] | NMPOU associated with anxiety in 14.6% of sensation seekers and associated with anxiety in 3.3% of non-medical self-treaters |
| Feingold 2017, Israel | Cross-sectional | 544 chronic pain patients | NMPOU use in past 30 days | 4 | Anxiety [GAD-7] | Patients who screened positive for anxiety were significantly more likely to screen positive for PO misuse than those without anxiety (AOR=2.18 [1.37-4.17]) |
| Hall 2016, USA | Cross-sectional | 406 victimised women on probation or parole; mean age: 37.2 | Lifetime and past year NMPOU | 4 | Post-traumatic stress diagnostic scale | Participants who met diagnostic criteria for PTSD were more likely to report NMPOU (AOR=1.1 [0.61-1.8]) |
| Kerridge 2015, USA | Cross-sectional 2012-2013 | 36309 noninstitutionalised adults residing in house-holds and selected group quarters | Lifetime and past year NMPOU | 4 | PTSD [DSM-5] | Past year NMPOU was associated with PTSD in men: (AOR=1.47 [1.04–2.09]), and women: (AOR=1.41 [1.05–1.91]) |
| Mackesy-Amiti 2015, USA | Cross-sectional 2008-2010 | 570 PWIDs recruited through street outreach and respondent driven sampling; mean age: 22.2 years | Past year and lifetime prescription opioid injection | 4 | PTSD [DSM-IV] | Past year PO misuse was significantly associated with prior PTSD (AOR=2.45 [1.31–4.60]) |
| Martel 2014, USA | Cross-sectional | 82 patients with chronic pain being prescribed POs; mean age: 48.7 years | Past month NMPOU | 4 | Anxiety [self-report] | Anxiety (r =0.31, P <0.01) was significantly associated with prescription opioid misuse |
| Martins 2009, USA | Cross-sectional 2005-2006 | 8218 respondents in the NSDUH | Past year NMPOU | 4 | Anxiety [self-reported] | Past year opioid use was not significantly associated with past year anxiety disorder (AOR=1.2 [0.9-1.4]) |
| Martins 2012, USA | Prospective 2001-2002; 2004-2005 | 34653 respondents in the NESARC | NMPOU over lifetime, past-year, or since last interview | 2 | Generalised anxiety disorder (GAD) [DSM-IV] | Baseline lifetime NMPOU was associated with incident GAD (AOR=1.5 [1.1-2.1]) |
| Mason 2016, USA | Cross-sectional 2015 | 625 neurosurgery and orthopedic patients | NMPOU in the past 2 weeks | 4 | Anxiety | Symptoms of anxiety experienced symptoms in the past week were not [PROMIS] significantly related to NMPOU in the past 2 weeks (AOR=1.00 [0.89-1.11]) |
| Saha 2016, USA | Cross-sectional 2012-2013 | 36309 respondents in the NESARC-III | Past-year and lifetime NMPOU | 4 | PTSD | NMPOU was significantly related to having PTSD (12-month AOR=1.41 [1.13-1.75]; lifetime AOR = 1.30 [1.11-1.51]) |
| Smith 2016, USA | Cross-sectional 2004-2005 | 34653 respondents in the NESARC-III | Past-year and lifetime NMPOU | 4 | Past-year and lifetime PTSD diagnosis | Prevalence of a lifetime PTSD diagnosis was greater in those traumatic stress reporting NMPOU (19.8%) compared to non-users (9.3%). Prevalence of a past-year PTSD diagnosis was also greater in NMPO users (14.6%) compared to non-users (6.3%). Past-year PTSD diagnosis was associated with increased odds of past-year NMPOU (AOR=0.91, P <0.001) |
| Wu 2011, USA | Cross-sectional 2001-2002 | 2450 participants in the 2001-2002 National Institute on Alcohol Abuse and Alcoholism survey | Past year NMPOU | 4 | Anxiety [DSM-IV] | Comparing prescription opioid users vs users of non-opioid drugs, odds of any anxiety disorder was increased (AOR=1.28 [0.98-1.66]) though relationship was not statistically significant |
| Xiao 2019, China | Cross-sectional 2015 | 159640 adolescents enrolled in the School-Based Chinese Adolescents Health Survey | Past year NMPOU | 4 | Past-year sleep disturbances | Past year NMPOU use associated past month sleep disturbances among boys (AOR=2.07 [1.66-2.58]) and girls (AOR=2.16 [1.68-2.77]) |
| F00 – F99: Other psychiatric/mental health measures | ||||||
| Back 2010, USA | Cross-sectional 2006 | 55,279 respondents in the NSDUH | Past year NMPOU | 4 | Psychological distress | NMPOU was significantly associated with having serious psychological stress in females (AOR: 1.65 [1.24-2.18]) but not significant in males (AOR=1.26 [0.85-1.85]) |
| Becker 2011, USA | Retrospective 2006-2008 | 3238 respondents in the NSDUH | Past year NMPOU | 2 | Psychological distress [Kessler 6 inventory] | Serious psychological distress was present in 27.6% of participants |
| Boyd 2014, USA | Cross-sectional 2009-2010 | 2627 secondary school students; mean age: 14.8 years | Lifetime NMPOU | 4 | Conduct disorder | Nonmedical prescription opioid use was associated with conduct disorder in 46.3% of sensation seekers |
| Buttram 2014, USA | Clinical trial 2008-2010 | 515 MSM participating in a risk reduction intervention trial; mean age: 38.9 years | Past year and past 90 days injection NMPOU | 2 | Severe mental distress [GMDS] | Nonmedical prescription opioid use was associated with higher odds of severe mental distress (OR=1.72 [1.13-2.61]); P=0.011); severe mental distress prevalence: 57.9% |
| Chan 2020, USA | Cross-sectional 2016 | 11489 adolescent respondents in the NSDUH | Past year NMPOU | 4 | Past-year major depressive episode | Past year NMPOU was significantly associated with a past-year major depressive episode (AOR=1.60 [1.11-2.32]) |
| Darke 1996, Australia | Cross-sectional | 312 persons who inject drugs (PWID) recruited through street outreach; mean age: 28.8 years | Injection NMPOU in last 6 months | 4 | Psychological distress [GHQ score] | Methadone injectors had increased psychological distress (GHQ score: 10.2 vs. 7.8, P <0.05), and met diagnostic cut-off for psychopathology (67% vs 54%, P <0.05) |
| Fischer 2013, Canada | Cross-sectional 2010-2011 | 4023 adults and 3339 grade 7–12 public system students; students’ mean age: 15.9 years; adults’ mean age: 46.3 years | Past year NMPOU | 4 | Psychological distress [PHQ-2] | NMPOU was not significantly associated with increased odds of psychological distress among students (AOR=1.14 [0.76-1.70] nor among adults (AOR=1.63 [0.95-2.80] |
| Hruschak 2020, Australia | Cross-sectional 2014-2015 | 333 patients filling prescriptions at community pharmacies | Current NMPOU (Prescription Opioid Misuse Index) | 4 | Depression | Taking more medications than prescribed was significantly associated with depression (P <0.05) |
| Kozhimannil 2017, USA | Cross-sectional 2005-2014 | 8721 NSDUH respondents; women who were pregnant at the time of survey | Past-year, past-month NMPOU | 4 | Depression or anxiety | Among pregnant women, depression or anxiety in the past year was strongly associated with NMPOU in the past year (AOR=2.15 [1.52-3.04]). Past-year depression or anxiety was also associated with greater odds of past-month NMPOU (AOR=1.90 [1.10-3.30]) |
| Kerridge 2015, USA | Cross-sectional 2012-2013 | 36,309 non-institutionalised adults residing in house-holds and selected group quarters | Lifetime to past year NMPOU | 4 | Personality disorders [DSM-5] | Past year NMPOU was associated with any personality disorder in men: (AOR=1.93 [1.52–2.45]), and women: (AOR=1.45 [1.13–1.86]) |
| Mackesy-Amiti 2015, USA | Cross-sectional 2008-2010 | 570 PWIDs recruited through street outreach and respondent driven sampling; mean age: 22.2 years | Past year and lifetime prescription opioid injection | 4 | Antisocial personality disorder [DSM-IV] | Past year PO misuse was significantly associated with increased odds of antisocial personality disorder (AOR=2.15 [1.43–3.24]) |
| Martins 2007, USA | Cross-sectional 2002-2003 | 7810 respondents in the 2002-2003 NSDUH | Past year NMPOU | 4 | Mental health problems [CIDI] | Prescription opioid dependence was significantly associated with serious mental health problems (AOR=2.34 [2.04-2.69]) |
| Martins 2015, USA | Cross-sectional 2008-2010 | 4421 respondents in the NSDUH | Past year NMPOU | 4 | Psychological distress [Kessler 6] | NMPOU was significantly associated with past-year serious psychological distress (AOR=2.09 [1.89–2.31]) |
| Morizio 2017, USA | Retrospective cohort study 2010-2015 | 923 patients admitted to a medical centre for heroin or non-heroin opioid overdose event; median age: 24 | Lifetime history of NMPOU | 2 | History of bipolar schizophrenia | History of bipolar schizophrenia was more prevalent among those with non-heroin opioid overdose (7.3%), compared to the heroin overdose group (2.5%), P <0.0014 |
| Novak 2016, Denmark, Germany, Great Britain, Sweden | Cross-sectional 2014 | 22,070 survey respondents in the European Union ages 12-49 | Lifetime and past year NMPOU | 4 | Serious psychological distress [K9] | NMPO users were more likely to report non-specific psychological distress (AOR=3.2 [2.6-3.9]) |
| Ouellet 2012, USA | Cross-sectional 2002-2005 | 645 non-injecting heroin users recruited through street outreach and respondent driven methods | Lifetime history of NMPOU | 4 | Psychiatric disorder [self-reported] | Being diagnosed with a psychiatric disorder was associated with PO use prior to heroin initiation (AOR=2.2 [1.2-4.1) |
| Rigg 2015, USA | Cross-sectional 2010-2013 | 10,201 respondents in the NSDUH;18 years or older | Past-year NMPOU | 4 | Psychological distress | NMPO users were not more likely to have experienced psychological distress in the past year compared to heroin users (AOR=0.876 [0.537-1.428] P-value=0.596) |
| Romach 1999, Canada | Cross-sectional | 339 long term codeine users recruited through newspaper adverts; mean age: 44 years | Past year codeine use | 4 | Psychological problems [self-reported] | A fraction of the subjects identified depression (13%) and worry (12%) as psychological consequences of long term codeine use |
| Saha 2016, USA | Cross-sectional 2012-2013 | 36309 respondents in the NESARC-III | Past-year and lifetime NMPOU | 4 | Any personality disorder | NMPOU was significantly related to having any personality disorder (12-month AOR=1.70 [1.45-1.99]; lifetime AOR = 1.97 [1.75-2.22]) |
| Shield 2011, Canada | Cross-sectional 2008-2009 | 2030 participants in the 2008-2009 Center for Addiction and Mental Health monitor | NMPOU in past 12 months | 4 | Psychological distress [GHQ-12] | NMPOU was associated with increased odds of psychological distress in men (AOR=7.55 [2.87-19.88]) and women (AOR=4.21, 1.61-11.00]) |
| Sproule 1999, Canada | Cross-sectional | 399 regular users of codeine recruited through newspaper adverts; mean age: 43.5 years | Past year NMPOU | 4 | Mental health problems [self-report] | Significantly more subjects that were codeine dependent had sought help for a mental health problem (P <0.001) |
| Subramaniam 2009, USA | Cross-sectional | 94 adolescents (ages 14-17 years) with past year diagnosis of opioid use disorder; mean age:16.9 years | NMPOU in past 30 days | 4 | Psychiatric disorders [DICA-IV] | Prescription opioid users presented with higher rates of current ADHD (P=0.01) and manic episodes (P=0.04) |
| Tetrault 2007, USA | Cross-sectional 2003 | 55,230 participants in the 2003 NSDUH | Past year NMPOU | 4 | Serious mental illness [Kessler inventory] | Past year NMPOU was significantly associated with increased odds of serious mental illness in females (AOR=1.67 [1.29-2.17]) but not significantly associated with increased odds in males (AOR=1.25 [0.91-1.70]) |
| Tragesser 2013, USA | Cross-sectional | 606 students aged 18 to 52 taking psychology courses; mean age: 20.4 years | NMPOU in past 3 months | 4 | Borderline personality disorder [PAI-BOR] | Opioid misuse and dependence features were positively associated with heightened levels of borderline personality disorder features (P <0.001) |
| Uosukainen 2014, Finland | Cross-sectional 2001-2008 | 1227 clients seeking treatment for buprenorphine and amphetamine abuse; mean age: buprenorphine users 25.8 years, amphetamine users 27.6 years | Current and lifetime NMPOU | 4 | Psychotic symptoms [self-reported] | Buprenorphine abuse was associated with reduced odds of psychotic symptoms (AOR=0.33, [0.24-0.45], P <0.001) |
| Vietri 2014, USA | Cross-sectional 2010, 2011 | 25,864 participants in the 2010 and 2011 US National Health and Wellness Survey | NMPOU in the 3 months prior to the survey (modes of use: chewing, smoking, snorting, rectal, injecting) | 4 | Psychiatric conditions [self-reported] | PO abuse was significantly associated with self-reported psychiatric conditions (AOR=1.71 [1.28-2.27], P=0.0002) |
| Wang 2013, USA | Cross-sectional 2008-2009 | 75,964 participants in the 2008-2009 NSDUH | Past year NMPOU | 4 | Psychological distress [K6 scale] | Non-medical use of prescription opioids was associated with increased odds of psychological distress in urban (AOR=1.64 [1.41–1.89]) and rural residents (AOR=1.63 [1.23–2.17]) |
| Wasan 2007, USA | Prospective | 288 patients prescribed opioids for chronic pain; mean age: 50.5 years | Current NMPOU | 2 | Psychiatric morbidity [PDUQ] | Presence of psychiatric morbidities such as mood disorders, psycho-social stressors, and psychologic problems was associated with self-reported measures of prescription opioid misuse (COMM P <0.01, SOAPP P <0.05) |
|
XI. Diseases of skin and subcutaneous tissue
L00 – L99: Infections of the skin and subcutaneous tissue | ||||||
| Ambekar 2015, India | Cross-sectional | 902 male PWID recruited from harm reduction centres; mean age: 33.4 years | Injection PO use in past 3 months | 4 | Abscess, blocked veins [self-reported] | Prescription opioid injectors were significantly more likely to develop abscess or blocked veins ever in their injecting life (Abscess: RR=1.39 [1.05-1.85]; blocked veins: RR=2.513 [1.89-3.35]) |
| Darke 1996, Australia | Cross-sectional | 312 PWID recruited through street outreach; mean age: 28.8 years | Injection PO use in last 6 months | 4 | Thrombosis, abscess [self-reported] | Methadone injection was associated with abscesses and infections (OR=2.4 [1.3-4.4]), and thrombosis (OR=2.2 [1.1-4.6]); prevalence of abscesses and infections: 23%; prevalence of thrombosis: 16% |
| Degenhardt 2006, Australia | Cross-sectional 2001-2004 | 795 PWID recruited through street outreach; mean age: 33.8 years | Injection PO use in last 6 months | 4 | Injection site harms (difficulty finding veins, bruising/scarring) [self-reported] | After covariate adjustment, recent morphine injection was not significantly associated with injection site harms; prevalence of difficulty finding veins was 36%, prevalence of scarring or bruising was 27% |
| Jenkinson 2005, Australia | Cross-sectional 2002 | 156 respondents in the Melbourne arm of the Illicit Drug Reporting System; mean age: 30 years | Injection PO use in last 6 months | 4 | Injection-related health problems (overdose, bruising abscesses, scarring, thrombosis) [self-reported] | Buprenorphine injection was not significantly associated with increased odds of injection-related health problems (AOR=2.9 [0.98-8.8]) |
|
XVI. Certain conditions originating in the perinatal period
P05-P08: Disorders related to length of gestation and fetal growth | ||||||
| Almario 2009, USA | Retrospective 2000-2006 | 258 opiate-addicted women treated with methadone; mean age of mothers with preterm delivery: 28.7 years | Current NMPOU | 2 | Preterm delivery | NMPOU in mothers treated for addiction was not significantly associated with lower odds of preterm birth (AOR=0.9 [0.4-2.2]) |
| Liu 2010, Australia | Retrospective 2000-2006 | 215 opioid dependent mothers; median age: 27.2 years | Current NMPOU | 2 | IUGR [ICD-10-AM] | Risk of IUGR is higher in non-smoking opioid dependent mothers compared to non-smoking non-opioid dependent mothers (RR=3.48 [1.70-7.14]) |
| Maeda 2014, USA | Retrospective 1998-2011 | 113,105 hospitalisations for delivery identified in the nationwide inpatient sample | 2 | Stillbirth, IUGR preterm labor, [ICD-9-CM] | Maternal opioid abuse or dependence was associated with stillbirth (AOR=1.5 [1.3-1.8]), preterm labor (AOR=2.1 [2.0-2.3]), IUGR (AOR=2.7 [2.4-2.9]), maternal death during hospitalisation (AOR=4.6 [1.8-12.1]) | |
| P90 – P96: Other disorders originating in the perinatal period | ||||||
| Kelly 2011, Canada | Retrospective 2009-2010 | 482 live births that occurred at a Canadian health centre; mean age: 24.5 years | Oral use, snorting or injection of opioids (most commonly oxycodone) | 2 | NAS [Infant Finnegan scores] | Narcotic-exposed neonates experienced NAS 29.5% of the time; daily maternal use was associated with a higher rate of NAS (66.0%) |
| Lee 2015, USA | Retrospective 2007-2013 | 139 cases of NAS pooled from a large Medicaid health plan | In utero exposure to prescription opioids | 2 | NAS [ICD-9], hospital length of stay | In utero exposure to methadone or buprenorphine leads to an average NICU length of stay of 21 days for NAS treatment |
| McQueen 2015, Canada | Retrospective 2010-2011 | 131 infant/mother pairs with NAS symptoms and maternal substance abuse; mean age of mothers at delivery: 25.6 years | In utero exposure to prescription opioids | 2 | NICU admission; NAS [MFS tool] | Among the eligible sample of infants, 78.6% were admitted to the NICU and 72.5 % of eligible sample received pharmacologic treatment for NAS |
|
XVIII. Symptoms, signs, and abnormal clinical and laboratory findings not elsewhere classified
R40-R46. Symptoms and signs involving cognition, perception, emotional state and behaviour | ||||||
| Ashrafioun 2017, USA | Cross-sectional 2014 | 24,653 respondents in the NSDUG | Past-year NMPOU | 4 | Suicidal ideation [self-reported] | Past-year suicidal ideation was significantly associated with less than monthly (AOR=1.52 [1.21-1.91]), monthly to weekly (AOR=1.41 [1.04-1.93]), and weekly or more (AOR=1.62 [1.19-2.21]) NMPOU in the past year |
| Barman-Adhikari 2019, USA | Cross-sectional 2016-2017 | 1426 young adults experiencing homelessness | Past-month NMPOU | 4 | Lifetime suicidal ideation | Past-month NMPOU was associated with lifetime suicidal ideation (AOR=1.8 [1.1-3.0]) |
| Bohnert 2013, USA | Cross-sectional 2009 | 351 adults in a residential treatment program; mean age: 35.6 years | Past month NMPOU Lifetime use | 4 | Suicidal ideation [BSS] | Non-medical PO use was not significantly associated with suicidal ideation (AOR=1.01 [0.95-1.07]) |
| Epstein-Ngo 2014, USA | Prospective | 575 patients in an urban emergency department who screened positive for past 6 months drug use; mean age: 20 years | NMPOU in past 6 months | 2 | Aggression [TLFB-AM] | Prescription opioid misuse was more likely immediately prior to dating violence than non-dating violence (AOR=6.24 [2.81-13.88]) |
| Fischer 2013, Canada | Cross-sectional 2010-2011 | 4023 adults and 3339 grade 7–12 public system students; students’ mean age: 15.9 years; adults’ mean age: 46.3 years | Past year NMPOU | 4 | Suicidal ideation [self-reported] | Among students, NMPOU was significantly associated with suicidal ideation (AOR=2.13, [1.12-4.06]) |
| Guo 2016, China | Prospective longitudinal 2009-2010, 2011-2012 | 3273 adolescent students; mean age: 13.7 | Past-year NMPOU | 1 | Suicidal ideation | Baseline NMPOU was associated with suicidal ideation at one year follow-up (AOR=2.31 [1.30-4.11]) |
| Han 2017, USA | Cross-sectional 2015 | 51,200 respondents in the 2015 NSDUH identified as prescription opioid users | Lifetime and past-year NMPOU | 4 | Suicidal ideation | Among adults with prescription opioid misuse, prevalence of past-year suicidal ideation was 21.5% (95% CI 18.77-24.46) |
| Havens 2011, USA | Cross-sectional 2001-2004 | 800 felony probationers recruited into a HIV-prevention trial; median age: 32.3 years | Lifetime PO injection | 4 | Risky behaviour [self-reported] [reported] | Participants indicating lifetime injection of POs were 14.7 times more likely to have reported participating in risky injection behaviours (AOR=14.7 [7.7-28.1]) |
| Humeniuk 2003, Australia | Cross-sectional 1996-1997 | 365 heroin users recruited through snowball sampling; mean age: 28.9 years | Injection PO use in last 6 months | 4 | Risky behaviour [self-reported] | Methadone injectors were significantly more likely to use other drug types intravenously than non-injectors of methadone, both on a life-time scale (F =48.0, df=1, 362, P=0.000) and over the last 6 months (F=15.4, df=1, 362, P=0.000) |
| Kuramoto 2011, USA | Cross-sectional 2009 | 37,933 respondents in the NSDUH | Lifetime and past year NMPOU | 4 | Suicidal ideation [self-reported] | NMPOU was significantly associated with suicidal ideation (AOR=1.88 ([1.13-3.12]) |
| Lin 2015, USA | Cross-sectional | 1076 youths ages 12–18, presenting to primary care community health clinics in two urban settings | NMPOU in past 3 months | 4 | Delinquency [self-reported] | NMPOU was significantly associated with non-violent delinquency (OR=1.06 [1.01-1.12]) |
| Rigg 2019, USA | Cross-sectional 2012-2016 | 22,693 respondents in the NSDUH | Past year NMPOU | 4 | Suicide planning and attempts | No significant differences in suicidality between participants who engage in NMPOU use in urban (AOR=1.20 [0.67-2.13]) and rural settings (AOR=2.12 [0.43-10.55]) |
| Schepis 2019, USA | Cross-sectional | 17,608 adult respondents in the NSDUH | Past-year NMPOU | 4 | Suicidal ideation | NMPOU was significantly associated with suicidal ideation (AOR=1.84 [1.07-3.19]) |
| Tang 2016, China | Cross-sectional 2012 | 18,686 Chinese high school students age 11-20; mean age: 15.43 | Lifetime, past-year, and past-month NMPOU | 4 | Suicidal ideation | Suicidal ideation was more prevalent among participants reporting past-month NMPOU (34.9%) than non-users (21.2%); P-value <0.0001 |
| Wilkins 2021, USA | Cross-sectional 2019 | 13,667 high school students enrolled in the Youth Risk Behavior Survey | Current NMPOU | 4 | Past-year suicidal ideation, planning and attempts | Current NMPOU significantly associated with higher prevalence ratios for suicidal ideation (APR=2.30 [1.97-2.69]), planning (APR=2.33 [1.99-2.79]) and attempts (APR=3.21 [2.56-4.02]) |
| Young 2012, USA | Cross-sectional 2009-2010 | 2597 middle and high school students enrolled in two school districts, mean age: 14.8 years | NMPOU in past 12 months | 4 | Aggressive behaviour [YSR version of CBCL] | Sensation seeking non-medical use of prescription opioids was associated with rule breaking behaviour, high risk of substance dependence, and aggressive behaviour |
| R50-R69: General symptoms and signs | ||||||
| Becker 2007, USA | Cross-sectional 2002-2004 | 6879 respondents in the NSDUH | Past year NMPOU | 4 | General health status [self-reported] | Respondents meeting criteria for abuse/dependence were more likely to self-report fair/poor health (AOR 2.1 [1.4–3.0]) |
| Black 2013, USA | Cross sectional 2007-2011 | 29,459 PO users recruited from 540 treatment facilities; mean age=33.1 years | Past 30 days NMPOU | 4 | General health status (liver disease, ER visit) [ASI-MV criteria] | Prescription opioid injection was significantly associated with liver disease (OR=1.71 [1.53-1.90], P < 0.0001]) and visit to the ER in the past 30 days (OR=1.84 [1.71-1.98], P <0.0001). |
| Brunet 2015, Canada | Cross-sectional 2013 | 1238 HIV/HCV co-infected persons [800 in prevalence cohort, 582 in incidence cohort] | Injection PO use in past 6 months | 4 | General health status (liver fibrosis progression) [APRI score] | Prescription opioid injection was associated with faster progression to liver fibrosis [hazard odds ratio=1.20 (0.73-1.67)] though the association was not statistically significant |
| Catalano 2011, USA | Prospective | 912 students from 1st or 2nd grade to age 21; mean age: 16.2 years | Past year and lifetime NMPOU | 2 | General physical [self-reported] and mental health consequences [CIDI] | Ever use of NMPO was associated with a 7.9 greater odds of having a current drug use disorder, 2.1 greater odds of a mood disorder, 1.4 greater odds of poor/fair health, 2.6 greater odds of being violent |
| Choo 2014, USA | Retrospective 2011 | 426,010 DAWN-defined visits involving prescription opioid | NMPOU at emergency department presentation | 2 | ED presentation | Use of prescription opioids was associated with decreased odds of hospital admission in females (AOR=0.65 [0.54–0.77]) and males (AOR=0.62 [0.46–0.83]) |
| Fischer 2013, Canada | Cross-sectional 2010-2011 | 4023 adults and 3339 grade 7–12 public system students; students’ mean age: 15.9 years; adults’ mean age: 46.3 years | Past year NMPOU | 4 | Physical health [self-reported] | NMPOU was not significantly associated with self-reported poor/fair health among students (AOR=1.30 [0.69-2.46] nor among adults (AOR=1.41 [0.79-2.52]) |
| Green 2009, USA | Retrospective 2005-2008 | 29,906 respondent assessments from 220 treatment centres; mean age: 34.9 years | Past 30 day NMPOU | 2 | >5 ER visits in past 30 days [self-reported] | Prescription opioid abuse was associated with marginally increased odds of ER visits in males (AOR=1.06 [0.56-2.03]) and in females (AOR=1.75 [0.97-3.14]), though the associations were not statistically significant |
| Griffin 2015, USA | Clinical trial | 653 prescription opioid dependent patients; mean age: 33.2 years | Current NMPOU dependence | 2 | Health-related quality of life [SF-36] | Prescription opioid-dependent patients had worse physical and mental quality of life than a healthy population or a general population [mental scores=15.3 points vs healthy population and 12.3 points vs general population; physical scores=7.1points vs healthy population and 1.7 points vs general population] |
| Han 2017, USA | Cross-sectional 2015 | 51,200 respondents in the 2015 NSDUH identified as prescription | Lifetime and past-year NMPOU | 4 | Hypertension | Among adults with prescription opioid misuse, prevalence of hypertension was 6.0% (95% CI, 5.06-7.03) |
| Hartwell 2014, USA | Case-control | 68 non-treatment seeking individuals recruited through advertisements (33 PO dependent cases and 35 healthy controls) | NMPOU in the one month prior to baseline visit | 3 | Sleep quality [PSQI and ISI] | Poor sleep quality was identified in 80.6% of the PO dependent group, as compared to 8.8% of the control group (P <0.001) |
| Jeevanjee 2014, USA | Cross-sectional 2007-2008 | 258 HIV positive individuals recruited from homeless shelters, free meal programs, and single room occupancy hotels; mean age: 48 years | NMPOU use in past 90 days | 4 | Treatment adherence [self-reported] | PO misuse was significantly associated with an increased odds of incomplete adherence to treatment (AOR=1.47 [1.06-2.03]) |
| Mason 2016, USA | Cross-sectional 2015 | 625 neurosurgery and orthopedic patients | NMPOU in the past 2 weeks | 4 | Pain and depression [PROMIS] | Interaction between pain and depression experienced in the past week was significantly associated with NMPOU (AOR=0.96, [0.92-0.99]) |
| McCabe 2013, USA | Prospective 2009-2011 | 2050 middle and high school students from two public school districts | Past year NMPOU | 1 | Physical pain [YSR], pain [YSR] | NMPOU was not significantly associated with increased odds of physical pain in the previous 6 months during year 2 of study [OR=2.0, 0.7-5.6] |
| Morizio 2017, USA | Retrospective cohort study 2010-2015 | 923 patients admitted to a medical centre for heroin or non-heroin opioid overdose event; median age: 24 | Lifetime history of NMPOU | 2 | Hospital admission due to overdose | 84.6% of patients in the non-heroin opioid overdose group required hospital admission due to overdose compared to 72.2% of the heroin overdose group, P<0.0001 |
| Nielsen 2013, Australia | Cross-sectional 2011 | 141 participants recruited from opioid substitution treatment programs; mean age: 40.8 years | NMPOU in past 28 days | 4 | Pain [Brief Pain Inventory] | Any illicit PO use was associated with pain (OR=1.85 [0.78-4.39], though the association was not statistically significant |
| Patra 2009, Canada | Prospective 2004-2005 | 582 participants from the most recent follow-up in the OPICAN study; mean age: 35.2 years | NMPOU in past 30 days (non-injecting) | 4 | Personal health status [self-reported] | Among participants who majorly use prescription opioids, 55% reported poor or fair health status in past 30 days |
| Price 2011, USA | Cross-sectional 2008-2009 | 351 patients seeking treatment from a drug and alcohol treatment program; mean age:35.8 years | NMPOU in past 30 days | 4 | Physical functioning [SF-12] | Prescription opioid misuse was associated with lower physical functioning; as physical functioning increased (AOR=0.94 [0.91-0.98]), likelihood of NMPOU decreased |
| Schepis 2014, USA | Prospective 2001-2002, 2004-2005 | 34,653 participants in the NESARC | Lifetime NMPOU | 2 | Health related quality of life [SF-12] | NMPOU initiation was generally associated with poorest longitudinal outcomes for health related quality of life |
| Stein 2015, USA | Cross-sectional 2012-2013 | 328 primary care patients treated with buprenorphine; mean age: 38.7 years | NMPOU use in previous month | 4 | Pain [Brief Pain Inventory] | NMPOU was associated with moderate/severe chronic pain (OR=1.55 [0.29-8.23]), though the association was not statistically significant |
| Tang 2016, China | Cross-sectional 2012 | 18,686 Chinese high school students age 11-20; mean age: 15.43 | Lifetime, past-year, and past-month NMPOU | 4 | Poor sleep [CPSQI] | Past-year and lifetime NMPOU was significantly associated with poor sleep among participants (AOR=1.47 [1.17-1.85]; AOR=1.43 [1.28-1.60]) |
| Tetrault 2007, USA | Cross-sectional 2003 | 55,230 participants in the 2003 NSDUH | Past year NMPOU | 4 | >5 ER visits in past 1 year [self-reported] | Past year NMPOU was not significantly associated with increased odds of ER visits in females (AOR=1.77 [0.98-3.21]) nor reduced odds in males (AOR=0.74 [0.31-1.78]) |
| Vietri 2014, USA | Cross-sectional 2010, 2011 | 25864 participants in the 2010 and 2011 US National Health and Wellness Survey | NMPOU in the 3 months prior to the survey (modes of use: chewing, smoking, snorting, rectal, injecting) | 4 | Work productivity and general health [WPAI:GH] | PO tampering and abuse was associated with greater loss of productivity and increased use of health care (P <0.05) |
|
XIX. Injury, poisoning and certain other consequences of external causes
T36 – T50: Poisoning by drugs, medicaments and biological substances | ||||||
| Alexander 2004, USA | Retrospective 1997-2002 | 1,175,781 opioid-related emergency medical services patient encounters; mean age=35.4 year | Current NMPOU at patient encounter | 2 | Non-fatal overdose | Patients diagnosed as having poisoning or overdose increased by 47% over the study period |
| Black 2013, USA | Cross sectional 2007-2011 | 29,459 PO users recruited from 540 treatment facilities; mean age=33.1 years | Past 30 days NMPOU (oral route:90%; snorting:36%; injection:32%) | 4 | Non-fatal overdose | Prescription opioid injection was significantly associated with non-fatal overdose (OR: 1.32 [1.22-1.42], P <0.0001) |
| Bohnert 2013, USA | Cross-sectional 2009 | 351 adults in a residential treatment program; mean age: 35.6 years | Past month NMPOU, Lifetime use | 4 | Overdose, Addiction Severity Index, ASI] | Non-medical PO use was associated with past history of overdose (AOR=2.10 [1.10-4.02], P=0.03) |
| Bonar 2014, USA | Cross sectional 2008-2009 | 326 patients recruited from substance use disorder treatment centres; mean age: 35.1 years | Past 30 days NMPOU | 4 | Overdose | Past 30 days heavy NMPOU was associated with lifetime overdose (OR=2.99 [1.53-5.84]) |
| Brands 2004, Canada | Retrospective 1997-1999 | 178 new admission patients in a methadone maintenance treatment program; mean age: 34.5 years | Current PO injection | 2 | Overdose | Among those who inject prescription opioids-only, 46.5% reported ever overdosing on opioids |
| Bretteville-Jensen 2015, Norway | Cross sectional 2006-2013 | 1355 street-recruited PWID; mean age: 37 years | PO injection in past 4 weeks | 4 | Non-fatal overdose | Illicit use of buprenorphine was associated with an increased risk of non-fatal overdose, [OR=1.132, S.E=0.298] |
| Calcaterra 2013, USA | Retrospective 1999-2009 | All overdose deaths in the US among 15–64 year olds from 1999 to 2009 | Death certificate mention of PO | 2 | Fatal overdose | Age adjusted death rate related to pharmaceutical opioids increased almost 4-fold from 1999 to 2009 (1.54/100,000 p-y [95% CI 1.49-1.60] to 6.05/100,000 p-y [95% CI 5.95-6.16; P <0.001) |
| CDC 2011, USA | Retrospective 1999-2008 | 36,450 deaths that were attributed to drug overdose | Death certificate mention of PO | 2 | Fatal overdose | Opioid pain relievers were involved in 14,800 deaths (73.8%) of the 20,044 prescription drug overdose deaths |
| CDC 2013, USA | Retrospective 1999-2010 | 15,323 deaths among women that were attributed to drug overdose | Death certificate mention of PO | 2 | Fatal overdose | Deaths from opioid pain relievers increased fivefold for women and 3.6 times for men between 1999 and 2010 |
| CDC 2000, USA | Retrospective 1996-1999 data | 484 decedents where heroin or opiate intoxication was listed as a cause of death; median age: 40 years | Death certificate mention of PO | 2 | Fatal overdose | Opiate overdose death rate increased from 3.1 per 100,000 population in 1990 to 6.6 in 1999, an increase of 112.9% (P <0.001) |
| Challoner 1990, Canada | Retrospective 1977-1987 | 57 cases of overdose on pentazocine; median age: 28 years | Recent ingestion of pentazocine | 2 | Overdose | Reasons for overdose were attempted suicide (35 cases) excessive therapeutic use (11 cases) and drug abuse to obtain a “high” (11 cases) |
| Cheng 2020, Canada | Cross-sectional 2013-2016 | 599 people who report opioid use in past 6 months | Past six month NMPOU | 3 | Non-fatal overdose in past six months | No significant differences in non-fatal overdose risk among those who did and did not acquire opioids from physicians |
| Clayton 2019, USA | Cross-sectional 2017 | 14756 respondents in the Youth Risk Behavior Survey (Grades 9-12) | Lifetime NMPOU | 2 | Past-year suicide planning and attempt | NMPOU associated with past-year suicide planning (AOR=2.41 [2.15-2.71]) and attempt (AOR=3.45, [2.86-4.17]) |
| Clayton 2019, USA | Cross-sectional 2017 | 3697 respondents in the Virginia Youth Survey (Grades 9-12) | Past-year and current NMPOU | 4 | Past-year suicide planning and attempt | Current NMPOU associated with past year suicide attempt (APR=2.18 [1.21-3.93]) |
| Darke 1996, Australia | Cross-sectional | 312 PWID recruited through street outreach; mean age: 28.8 years | Injection PO use in last 6 months | 4 | Overdose [self-reported] | Ever injecting methadone was associated with overdose (OR=2.2 [1.4-3.5]); also injectors of methadone in previous 6 months were more likely to overdose (OR=2.5 [1.4-4.6]) |
| Darke 2002, Australia | Cross sectional 1996-2000 | 788 PWID interviewed in Sydney; mean age: 28.6 years | Injection PO use in previous 6 months | 4 | Overdose | Among the methadone injectors, 72% had ever overdosed |
| Degenhardt 2009, Australia | Retrospective 1985-2006 | 42,676 people who entered opioid pharmacotherapy program | PO dispensations from the New South Wales Pharmaceutical Drugs of Addiction System linked to overdose events | 2 | Overdose | Drug overdose and trauma were the major contributors of death |
| Dhalla 2011, Canada | Cross sectional 2006 | Family physician of participants who were eligible for prescription drug coverage; other details from office of the chief coroner | Administrative prescription data on PO dispensation | 4 | Mortality related to opioid use | The number of opioid-related deaths increased across prescribing-volume quintiles (P <0.001) |
| Fernandes 2015, USA | Retrospective 2003-2012 | 358 Montana residents aged 18–64 years who died from unintentional PO poisoning | PO enrolment via Medicaid | 2 | Overdose | Age-adjusted mortality rate per 100,000 from opioid poisoning in Montana Medicaid adults was eight times higher than the rate for non-Medicaid Montana adults (38.2 [CI (30.7–45.7]) vs. 4.7 (CI (4.1–5.3)]) |
| Fischer 2004, Canada | Cross sectional 2002 | 651 illicit opioid users recruited from 5 Canadian cities; mean age: 34.8 years | Injection PO use in past 30 days | 4 | Overdose | Non-injection administration of hydromorphone in the past 30 days was a predictor of overdose episodes (OR=2.73 [1.37–5.46]) |
| Fischer 2008, Canada, USA | Cross-sectional 2004-2005 | 448 participants of which 304 (62.8%) were classified as prescription only users | Injection PO use in past 30 days | 4 | Overdose [self-reported], Emergency room use [self-reported] | PO use was associated with lower odds of ER use (OR=0.88, [0.41-1.87]) and associated with increased odds of overdose (OR=1.16 [0.32-4.18]), though the associations were not statistically significant |
| Green 2011, USA | Retrospective 1997-2007 | 2900 adult with accidental/undetermined drug intoxication deaths | Post mortem toxicology report of NMPOU | 2 | Fatal overdose | From 2001, intoxication deaths involving any prescription opioid increased from previous years, (linearly: 25.14, P <0.001) |
| Hakkinen 2010, Finland | Retrospective 2000-2008 | 12,891 subjects on which a post mortem toxicological analyses was performed | Post mortem toxicology report of NMPOU | 2 | Fatal overdose | Proportion of fatal prescription opioid poisonings out of all fatal drug poisonings increased from 9.5% (52 cases) in 2000 to 32.4% (179 cases) in 2008, being 22.3% over the whole period |
| Hall 2008, USA | Cross sectional 2006 | 295 residents who died of unintentional pharmaceutical overdoses; mean age: 39 years | Post mortem toxicology report of NMPOU | 4 | Fatal overdose [post mortem records] | Pharmaceutical diversion was associated with 186 (63.1%) deaths, while 63 (21.4%) were accompanied by evidence of doctor shopping |
| Han 2015, USA | Retrospective 2003-2013 | 472,200 persons aged 18 through 64 years who participated in the 2003-2013 NSDUH. Mortality data from 2003-2013 National Vital Statistics System’s Multiple Cause of Death Files | NMPOU in past 12 months | 2 | Fatal overdose | Among adults aged 18 through 64 years, overdose death rates involving prescription opioids increased from 4.5 per 100 000 (CI 4.42-4.61) in 2003, to 7.8 per 100 000 (CI:7.64-7.89) in 2013 (absolute difference: 3.3; [CI: 3.09-3.41]) |
| Hardt 2013, USA | Retrospective 1999-2005 | 169 drug-positive, pregnancy-associated non-natural deaths | 2 | Mortality related to opioid use | At least one prescription drug was found in 91 of the 169 cases (54%) of drug-positive, pregnancy-associated non-natural deaths | |
| Hassanian-Moghaddam 2013, Iran | Retrospective 2009-2010 | 525 patients referred with pure tramadol intoxication | 2 | Non-fatal overdose | The mean dose ingested by patients experiencing apnea was 2125 ± 1360 mg (range, 200-4600 mg) with 359 (68.4%) engaged in deliberate self-poisoning | |
| Havens 2011, USA | Cross sectional | 400 rural drug users median age: 31 years | Past 30-day PO injection | 4 | Non-fatal overdose | Rural drug users with history of overdose were more likely to have injected with prescription opioids |
| Iversen 2017, Australia | Cross-sectional 2014 | 1488 Australian NSP survey respondents | PO injection in past 6 months | 4 | Non-fatal overdose [self-reported] | PO injection in the previous 6 months was associated with non-fatal overdose in the past year (AOR=1.81 [1.36-2.42]) |
| Jenkins 2011, USA | Cross sectional 2009 | 443 participants at syringe exchanges; median age: 38 years | PO injection in past 4 months | 4 | Non-fatal overdose | Injection of prescription type opioid was not significantly associated with increased odds of non-fatal overdose (OR=1.84 [0.70–4.80]) |
| Kerr 2007, Canada | Prospective 1996-2004 | 1587 injection drug users median age: 33.4 years | PO injection in previous 6 months | 2 | Non-fatal overdose | Non-fatal overdose was significantly associated with non-injection opiate use (AOR=1.16 [1.03-1.3], p=0.014) but not significantly associated with morphine injection (AOR=1.01 [0.84-1.22], P=0.878) |
| Lake 2015, Canada | Prospective 2005-2013 | 1614 adult illicit drug users recruited through self-referral and street outreach; median age: 41.6 years | PO injection in previous 6 months | 1 | Non-fatal overdose | Prescription opioid use was independently associated with non-fatal overdose (AOR: 1.61 [1.32–1.95]) |
| Lake 2015, Canada | Prospective 2005-2014 | 1660 adult illicit drug users recruited through self-referral and street outreach; median age: 41.1 years | PO injection in previous 6 months | 2 | Non-fatal overdose | Exclusive prescription opioid injection was not significantly associated with increased odds of non-fatal overdose (AOR:1.17 [0.74–1.86]) |
| Lanier 2012, USA | Case-control 2008-2009 | Cases: 278 decedents who met the study criteria, Controls: 1308 Utah BRFSS respondents; median age: 41 years | Past year NMPOU | 3 | Mortality related to opioid use | Oxycodone was the most frequent opioid cause of death among cases, while hydrocodone was the most frequently reported opioid among comparison group |
| Madadi 2013, Canada | Retrospective 2006-2008 | 2330 drug-related deaths history retrieved from the Office of the Chief Coroner; median age: 44 years | Mention of POs in Coroner’s reports | 2 | Mortality related to opioid use | Oxycodone was involved in approximately one-third of opioid-related deaths (35%) |
| Maloney 2009, Australia | Retrospective case-control 2004-2008 | 1500 opioid-dependent individuals; mean age: males 37.2 years, females 35.3 years | Lifetime NMPOU | 2 | Non-fatal overdose | Ever injecting opioids was associated with non-fatal overdose [OR: 11.90 (4.18–33.33)] and 37% reported non-fatal overdose |
| Morizio 2017, USA | Retrospective cohort study 2010-2015 | 923 patients admitted to a medical centre for heroin or non-heroin opioid overdose event; median age: 24 | Lifetime history of NMPOU | 2 | Fatal overdose | 4.2% of patients who experienced non-heroin opioid overdose event had a discharge status of ‘died’, compared to 4.5% of the heroin overdose group, P >0.0001 |
| Pabayo 2013, Canada | Prospective | 1931 PWID recruited via self-referral, word of mouth and street outreach | PO injection in past 30 days | 2 | Non-fatal overdose | Morphine injection was associated with non-fatal overdose experience during follow-up (OR = 1.81 [1.24-2.64]) |
| Roux 2008, France | Prospective 2004, 2005 | 111 stabilised patients receiving office-based buprenorphine; median age: 38 years | Injection PO use in last 30 days | 2 | Overdose [self-reported] | PO injection was significantly associated with experiencing overdose (OR=2.4 [1.1-5.3], P=0.04) |
| Roxburgh 2017, Australia | Retrospective cohort study 2007-2014 | 8382 clients of a medically supervised injecting centre | On-site PO injection | 2 | Non-fatal overdose | Rate of oxycodone overdose was 3.1 times (95% CI, 3.0-3.2) lower than the rate of heroin overdose (4.1 vs. 12.7 overdoses per 1000 injections) |
| Schrager 2014, USA | Cross-sectional 2009-2011 | 575 participants in a mixed-methods investigation into prescription drug misuse among high risk adults; mean age: 20.9 years | NMPOU in past 12 months | 4 | Overdose [self-reported] | Participants with overdose history were more likely to be intensive or active users rather than limited users (intensive: OR = 7.0, P <0.001; active: OR = 4.2, P <0.01) and were also more likely to be intensive than reduced users (OR=2.9, P <0.001) |
| Silva 2013, USA | Cross sectional 2009-2011 | 596 nonmedical users of prescription drugs; mean age: 21 years | NMPOU by injection or snorting in past 90 days | 4 | Non-fatal overdose | Ever snorting or sniffing drugs (OR 2.51 [1.48-4.27], P <0.001) and injection drug use (OR 1.68 [1.03-2.74], P <0.05) were associated with increased non-fatal overdose risk |
| Talu 2010, Estonia | Cross-sectional | 350 PWID recruited using respondent driven sampling mean age: 23.9 years | Lifetime PO injection | 4 | Overdose [self-reported] | Fentanyl injection was associated with higher risk of lifetime overdose (AOR= 3.02 [1.65–5.54]) |
| Tucker 2015, Canada | Prospective 2005-2013 | 1911 PWID; median age: 41 years | Methadone injection in the previous 6 months at baseline | 1 | Non-fatal overdose | Methadone injection was independently and positively associated with non-fatal overdose [AOR=1.67, (1.00–2.79)] |
|
XX. External causes of morbidity and mortality
X60-X64: Intentional self-harm | ||||||
| Ashrafioun 2017, USA | Cross-sectional 2014 | 24,653 respondents in the NSDUH | Past-year NMPOU | 4 | Suicide attempts [self-reported] | Past-year suicide attempts were significantly associated with weekly or more NMPOU (AOR=2.03 [1.11-3.71]) |
| Ashrafioun 2019, USA | Cross-sectional 2015-2017 | 45,074 respondents in the NSDUH | Past-year NMPOU | 4 | Suicidal ideation, planning and attempts | Past-year suicidal ideation (AOR=1.67 [1.29-2.15]), planning (AOR=1.83 [1.35-2.48]) and attempts (AOR=1.61, [1.02-2.55]) associated with NMPOU |
| Baiden 2019, USA | Cross-sectional 2017 | 8803 adolescents in the Youth Risk Behavior Surveillance System | Past-year NMPOU | 4 | Suicidal ideation, planning, and attempts | Past-year suicidal ideation (AOR=1.50 [1.25-1.82]), planning (AOR=1.44 [1.19-1.75]) and attempts (AOR=1.58, [1.25-2.01]) associated with NMPOU |
| Davis 2020, USA | Cross-sectional Not reported | 889 college students | Past-year NMPOU | 2 | Suicidal ideation | Suicidal ideation significantly associated with NMPOU (AOR=2.21 [1.42-3.42]) |
| Guo 2016, China | Prospective longitudinal 2009-2010, 2011-2012 | 3273 adolescent students; mean age: 13.7 | Past-year NMPOU | 1 | Suicide attempts [self-reported] | Baseline NMPOU was associated with reported suicide attempts at one year follow-up (AOR=2.31 [1.30-4.11]) |
| Hakansson 2011, Sweden | Retrospective 2001-2006 | 1404 persons who attempted suicide; mean age of attempt repeaters: 33.5 years | Lifetime history of NMPOU | 2 | Suicide attempt [self-reported] | Suicide attempt repetition was associated with opioid analgesics use (OR=1.52 [1.13–2.05]) |
| Kim-Godwin 2019, USA | Cross-sectional 2016 | 42,625 respondents in the NSDUH | Current NMPOU | 4 | Suicidal attempt, planning or ideation | Current NMPOU was significantly associated with suicide ideation (AOR=2.19 [1.44-3.33]) and suicide planning (AOR=1.98 [1.14-3.45]) |
| Kelley 2019, USA | Cross-sectional Not reported | 212 military veterans | Past-year NMPOU | 4 | Past 2-week suicidality (6-item scale) | NMPOU not associated with suicidality (AOR=1.03 [0.89-1.18]) |
| Lin 2015, USA | Cross-sectional | 1076 youths ages 12–18, presenting to primary care community health clinics in two urban settings | NMPOU in past 3 months | 4 | Suicidal attempt or ideation | PO misuse was significantly associated with suicidal thoughts or attempts (OR=2.33 [1.18–4.59]) |
| Quinn 2019, USA | Retrospective 1994-2009 | 12228 respondents in the National Longitudinal Study of Adolescent Health | Lifetime NMPOU | 4 | Suicidality (planning and attempts) | Lifetime NMPOU associated with suicidality among males (AOR=2.10 [1.54-2.87]) and females (AOR=3.15 [2.31-4.29]) |
| Rigg 2015, USA | Cross-sectional 2010-2013 | 10201 respondents in the NSDUH; 18 years or older | Past year NMPOU | 4 | Suicidal ideation or attempt | NMPO users were not more likely to have experienced suicidal ideation and/or attempts in the past year compared to heroin users (AOR=0.959 [0.539-1.706] P-value=0.887) |
| Roux 2008, France | Prospective 2004, 2005 | 111 stabilised patients receiving office-based buprenorphine; median age: 38 years | Injection PO use in last 30 days | 2 | Suicide attempt or ideation[self-reported] | PO injection was significantly associated with suicidal ideation or attempt (AOR=2.6 [1.2-5.7], P=0.02) |
| Samples 2019, USA | Cross-sectional 2015-2016 | 86,186 respondents in the NSDUH | Past-year NMPOU | 4 | Past year suicidal ideation, planning and attempts | Prescribed PO use associated with a significantly lower odds of suicidal ideation (AOR=0.57 [0.45-0.72]) and planning (AOR=0.53 [0.35-0.80]) but not attempts, compared to past-year NMPOU |
| X85-Y09: Assault | ||||||
| Martins 2009, USA | Cross-sectional 2005-2006 | 8218 respondents in the NSDUH | Past year NMPOU | 4 | Assault [self-reported] | Past year opioid users were significantly more likely to have attacked someone in the past year (AOR=1.5 [1.3-1.7]), compared to users of other illegal drugs. |
aHR, adjusted hazard ratio; AIRR, adjusted incidence risk ratio; ARR, adjusted risk ratio; BRFSS, Behavioral Risk Factor Surveillance System; CI, confidence interval; COMM, Current Opioid Misuse Measure; DSM, Diagnostic and Statistical Manual; ER, emergency room; HBV, hepatitis C virus; HCV, hepatitis C virus; IUGR, intrauterine growth restriction; MDE, Major Depressive Episode; MSM, men who have sex with men; NAS, Neonatal Abstinence Syndrome; NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; NICU, Neonatal Intensive Care Unit; NMPOU, non-medical prescription opioid use; NSDUH, National Survey on Drug Use and Health; OR, odds ratio; p-y, person years; PO, prescription opioid; PWID, persons who inject drugs; RR, risk ratio; SDS, Severity of Dependence Scale; SOAPP, Screener and Opioid Assessment for Patients with Pain; SUD, substance use disorder; TTP, Thrombotic Thrombocytopenic Purpura.
Overall, the evidence base was largest with respect to the association between NMPOU and ‘mental and behavioural disorders’ with over half of all studies (129; 71%) reporting on these outcomes. The second highest quantity of evidence existed for the association between NMPOU and ‘injury, poisoning, and certain other consequences of external causes’, with 44 studies (24%) reporting specifically on fatal and non-fatal overdose. Conversely, evidence was limited for the association between NMPOU and ‘external causes of morbidity and mortality’, with only 13 studies (7%) assessing its association with intentional self-harm and only 1 study assessing its association with assault (<1%). Outcomes that were investigated by a higher than average number of studies were ‘Viral hepatitis (HCV [hepatitis C virus] and HBV [hepatitis B virus])’ (18; 10%), ‘Human Immunodeficiency Virus (HIV)’ (8; 4%), ‘Symptoms and signs involving cognition, perception, emotional state and behavior’ (17; 9%) and ‘General symptoms and signs’ (22; 12%).
B15 – B24: Viral hepatitis (Hepatitis B and C) and HIV
Studies assessing viral hepatitis infection (ICD-10 B15-B19; n = 18) found mixed results. Specifically, three studies found no association between NMPOU and hepatitis B or hepatitis C transmission [106,120,127], while one study by Patra et al. found that participants who reported exclusively injecting PO were significantly less likely to be HCV-seropositive compared to participants that injected other substances [118]. While the number of studies investigating HIV transmission (ICD-10 B20-B24) and NMPOU was small (n = 8, 4%), the evidence was equivocal, with 3 studies (38%) reporting no association between NMPOU and HIV transmission [22,106,153], two studies (25%) reporting that exclusive NMPOU was associated with a reduced odds of HIV acquisition compared with participants that used other drugs [84,96], and two studies (25%) reporting that fentanyl injection was associated with a significantly increased risk of testing positive for HIV [44,104].
F10-F19: Mental and behavioural disorders due to psychoactive substance use
Among the 35 studies reporting on mental and behavioural disorders due to psychoactive substance use (see Table 1, Section V), there was agreement (20/20; 100%) regarding an association between NMPOU and concurrent or subsequent opioid use disorder (OUD), dependence or withdrawal. Among this subgroup of studies, 7 (35%) identified those engaging in NMPOU as specifically having a higher risk of experiencing substance use disorders compared to users of other drugs (including street opioids). For example, Miller et al. found that among patients discharged from a detoxification unit East Lansing, Michigan (n = 534), those that reported prescription opioid use had a signfiicantly higher odds of also reporting a medical diagnosis (adjusted odds ratio = 3.47, P < 0.05) [152].
F30 – F39: Mood [affective] disorders
Further, 27 studies (15%) examined the association between NMPOU and depressive symptomatology. An association between NMPOU and depression was reported across the vast majority of papers (>95%) examining clinical outcomes related to mood or affective disorders (ICD F30-F39). For example, Ali et al. (2015) found that among adolescents enrolled in the National Survey on Drug Use and Health between 2008 and 2012 (n = 84,800, mean age 14.5 years), those that engaged in NMPOU were 33% more likely to report a major depressive episode [23]. Four of the 13 studies (31%) examining neurotic, stress-related and somatoform disorders (ICD-10 F40-F48) investigated associations between NMPOU and post-traumatic stress disorders and all identified significant associations [7,25,109,125]. One study from Becker et al. [34] found that reporting past year NMPOU was significantly associated with reporting symptoms of panic and phobia among National Survey on Drug Use and Health respondents (n = 6,879; both adjusted odds ratios = 1.2, P < 0.05). Ten studies (5%) assessed the relationship between NMPOU and anxiety, with 7 (70%) identifying a significant association [7,33,113,126,129,137,143], two studies (20%) reporting a null finding [43,123], and one (10%) limited to reporting on the prevalence of generalized anxiety disorder (i.e. 14%) among individuals engaged in NMPOU [78].
R40-R46. Symptoms and signs involving cognition, perception, emotional state and behavior
A small subset of studies assessed the association of NMPOU with ‘symptoms and signs involving cognition, perception, emotional state and behavior’ (n = 16; 9%). Of note, 10 studies (63%) investigated suicidal ideation, with 7 studies reporting that NMPOU was associated with a significantly higher odds [16,21,39,41,48,138,146], and one study by Bohnert et al. reporting no significant association between NMPOU and suicidal ideation [133]. Only two studies (1%) investigated associations between NMPOU and aggression; one study of youth found that NMPOU was associated with intimate partner violence though not with other forms of violence [68], while another study found that NMPOU was associated with higher scores on the Aggressive Behavior Sub-Scale of the Child Behavior Checklist [46].
R50-R69: General symptoms and signs
There was scant evidence on the direct association between NMPOU and pain. Indeed, only three studies investigated this topic, undertaken in the USA (Michigan: n = 2050 high school students; and southeastern New England: n = 328 primary care patients) and Australia (n = 141 participants in opioid substitution programs in Sydney and Newcastle); none identified a significant association between NMPOU and pain [69,102,130]. However, one study by Blanco et al. reported that, among adults enrolled in the US National Epidemiologic Survey on Alcohol and Related Conditions (n = 34,653), reporting pain in the first wave of data collection was significantly associated with NMPOU in the subsequent wave (adjusted odds ratio = 2.17, P < 0.05) [145].
T36 – T50: Poisoning by drugs, medicaments, and biological substances
All of the 44 studies reporting on injury and poisoning reported on poisoning by drugs, medicaments and biological substances (ICD T36–T50); specifically, opioid overdose. NMPOU was found to be significantly associated with prior, subsequent and a lifetime history of non-fatal opioid overdose, with 13 studies (3%) reporting specifically on overdose-related mortality; this included analyses of population time trends in the United States undertaken by Calcaterra et al. and the US Centers on Disease Control indicating increases in the opioid overdose mortality rate since 1990 [167,169].
DISCUSSION
We report the findings of a systematic review that found voluminous and consistent evidence of an association between NMPOU and OUD, as well as on fatal and non-fatal overdose. Strong evidence supported an association between NMPOU and some mental disorders, particularly depression, consistent with previous reviews in this area [188]. A smaller set of studies reported equivocal evidence on the association between NMPOU and HIV and hepatitis transmission. Finally, only scant evidence was identified on the association between NMPOU and intentional self-harm, suicidal ideation and assault.
The results of this systematic review provide further confirmatory evidence that NMPOU is implicated in the high incidence of overdose-related morbidity and mortality observed in North America. However, despite a large body of scientific literature assessing the influence of injection drug use on a range of health and social harms [189,190], the current review highlights the paucity of evidence demonstrating that injection NMPOU is associated with a higher risk of HIV or HCV transmission compared with the injection of other drugs. Collectively, these findings have important implications for policy and practice. First, while they confirm that interventions to prevent overdose should be tailored specifically to NMPO-using subpopulations, they suggest that efforts to prevent transitions into injection drug use and the disease transmission risk that often follows should be tailored to a broader range of subpopulations beyond those engaging in non-injection NMPOU. For instance, data from multiple North American settings has demonstrated that crystal methamphetamine is the drug primarily implicated in injection initiation events [191,192]; furthermore, injection cocaine use is an identified risk factor for disease transmission (likely related to its short half-life) [193]. Given the heterogeneous distribution of injection-related risks among subpopulations, ensuring that frontline harm reduction and related health services are appropriately tailored to those at greatest risk is needed to reduce injection-related morbidity and mortality.
Given the established link between NMPOU and OUD, and that opioid agonist therapies (OAT) are recognised as the gold standard for management of OUD [194], expanding access to patient-oriented OAT modalities should be prioritised by treatment systems in North America and other settings experiencing heightened opioid-related overdose mortality. Indeed, the United States has lagged behind other settings (e.g. Western Europe) in achieving appropriate coverage of OAT such as methadone and buprenorphine, with only an estimated 15 per 100,000 PWID in the United States that access OAT [195], compared with 90 per 100,000 PWID in France [196]. Beyond coverage, recent advances have been made in Canada in refining clinical guidelines for the provision of OAT for OUD in light of the extensive evidence base on this subject as well as the increasingly diverse set of pharmacological tools used in OAT [197]. Further, there is a need to expand access to harm reduction interventions to support low-threshold entry into treatment for structurally vulnerable subpopulations engaging in NMPOU and/or with OUD (i.e. street-involved youth and individuals experiencing homelessness); this is in line with technical guidelines jointly developed by the UN Office on Drugs and Crime, the Joint United Nations Programme on HIV/AIDS, and the World Health Organization to reduce harms associated with the use of opioids and other illegal drugs [198]. To address the disproportionate burden of overdose mortality among populations engaging in NMPOU, expanded distribution of naloxone (an opioid antagonist used to reverse opioid overdose events) and the implementation of supervised consumption sites should be undertaken, given the high level of evidence demonstrating their effectiveness in overdose prevention [199,200]. These should be coupled with syringe services programs, given their cumulative impact on reducing infectious disease transmission among people who use NMPOU by injection [201].
The findings of this review also suggest future directions for research. While a consensus exists regarding the association between NMPOU and overdose, less is known regarding the relationship between its use and psychiatric conditions such as anxiety disorders. While six studies assessed the association between these conditions, findings were equivocal, and the direction of causality in all studies was not determined. This suggests the potential that NMPOU is intensified by anxiety, symptomatic of anxiety, or both; alternately, given that only a minority of studies (2/6; 33%) identified a significant association between anxiety and NMPO use, alternative pathways may be present. While outside the scope of this review, evidence suggests that the endogenous opioid system may mediate the association between opioid use and psychiatric comorbidities via variation in resting levels of endogenous opioids (i.e. individuals with lower resting levels may be at higher risk of developing psychiatric comorbidities or symptoms) [202]. Delineating the complex links between these conditions is particularly important in light of a recent meta-regression analysis that estimated the global prevalence of anxiety disorders at 7.3% [203], with individuals having experienced conflict and those in rural settings at significantly greater risk of experiencing anxiety disorders. If, as the results of this systematic review imply, anxiety disorders are associated with NMPOU, increasing access to OAT may, beyond its capacity to reduce the severity of OUD, also address key risk factors for the initiation or continuation of NMPOU, including co-morbid mental health conditions. That is because retention in OAT has been shown to be significantly associated with a subsequent reduction in the severity of psychiatric comorbidities among individuals experiencing both OUD and psychiatric comorbidities [204]. Importantly, the inverse approach (i.e. treating psychiatric comorbidities prior to enrolment in OAT) was not shown to be significantly associated with reductions in OUD severity [204].
This study has limitations. First, while we restricted the systematic review to scientific peer-reviewed literature, we did so in an effort to ensure a standard of scientific rigor commensurate with clinical practice and the development of effective policy; however, this resulted in the exclusion of potentially important ‘gray literature’ findings. Relatedly, we restricted to studies that assessed clinical outcomes that fell within ICD-10 classifications, which was done to improve the clinical relevance of this systematic review, but which likely resulted in additional health outcomes related to NMPOU not being captured. Second, it is possible that certain published studies were overlooked during the search, though this concern was likely mitigated by the fact that this process was duplicated, with additional verification by a third author. Third, we only assessed English-language literature because of linguistic limitations on our team; it is likely that peer-reviewed studies on the topic of NMPOU were published in other languages but not included in the review. Fourth, we did not undertake a meta-analysis as an additional step after systematic assessment of the scientific literature given the diversity of outcomes and methodologies employed by included studies, which limited meaningful meta-analytic assessment. Fifth, Although studies focused on HCV and HIV (L00-L99; ‘Infections of the skin and subcutaneous tissues’), others can arise from the injection of opioids intended for oral consumption (e.g. nerve damage, foreign body pulmonary embolisation and pulmonary hypertension, thrombophlebitis, deep vein thrombosis, endocarditis, septic arthritis, osteomyelitis and septicaemia); these may also be a result of NMPOU. Finally, we assessed study quality using a standardised checklist, though author subjectivity may have influenced the quality score.
CONCLUSIONS
In sum, we report on the findings of a systematic review assessing the peer-reviewed evidence regarding clinical outcomes associated with NMPOU. We note areas of general consensus as well as those wherein evidence is equivocal, weak, or non-existent. These findings should inform current efforts to address the ongoing use of—and harms associated with—NMPOU in a range of settings internationally.
Supplementary Material
KEY POINT SUMMARY.
We identified 182 peer-reviewed studies that assessed health outcomes associated with non-medical prescription opioid use until 1 July 2021
A large body of evidence exists on the association between non-medical prescription opioid use and mental and behavioural disorders as well as on fatal and non-fatal overdose.
Equivocal evidence exists on its association with the acquisition HIV, hepatitis C and other infectious diseases; weak evidence exists on its potential association between NMPOU and intentional self-harm, suicidal ideation, and assault
This is the first systematic review identifying the range of health outcomes associated with non-medical prescription opioid use
These results can support policy and clinical decision-making with respect to preventing opioid-related outcomes, and can inform efforts to bolster the evidence base on outcomes for which little data currently exist
Acknowledgments
We thank Kristy Scarfone and Sufiat Fusigboye for additional assistance in the systematic search.
Competing Interests and Funding Source
No competing interests declared. DW is supported by the Canadian Institutes of Health Research via a New Investigator Award, the Ontario Ministry of Research, Innovation and Science via an Early Career Researcher Award, and the St. Michael’s Hospital Foundation. Brandon Marshall is supported by the National Institute of General Medical Science (P20-GM125507) and the National Institute of Allergy and Infectious Diseases (P30-AI042853). SH is supported by the National Institute on Drug Abuse (K23-DA045085 and R01-DA046527-02S1) and the Thrasher Research Fund Early Career Award. AISc was supported by a Canadian Institutes of Health Research Fellowship. Benedikt Fischer acknowledges research support from the Hugh Green Foundation Chair In Addiction Research, Faculty of Medical and Health Sciences, University of Auckland, as well as from the Canadian Institutes of Health Research (CIHR SAF-94814) for the present work. Additional support for this study was provided by the Canadian Institutes of Health Research via the Canadian Research Initiative on Substance Misuse (CIHR SMN-139150). No funders had any role in the the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication. In addition, we confirm the independence of all authors from funders and also confirm that all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis and interpretation.
REFERENCES :
- 1.World Health Organization. WHO Model List of Essential Medicines: 20th Edition. Anonymous, editor: World Health Organization; 2017. [Google Scholar]
- 2.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58–e68. [DOI] [PubMed] [Google Scholar]
- 3.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Annals Int Med 2015;162:276–86. [DOI] [PubMed] [Google Scholar]
- 4.Mazer-Amirshahi M, Mullins PM, Rasooly I, den Anker J, Pines JM. Rising opioid prescribing in adult US emergency department visits: 2001–2010. Acad Emerg Med 2014;21:236–43. [DOI] [PubMed] [Google Scholar]
- 5.Fischer B Prescription opioid use, harms and interventions in Canada: A review update of new developments and findings since 2010. Pain Physician 2015;18:E605–E14. [PubMed] [Google Scholar]
- 6.Hughes A, Williams M, Lipari R, Bose J, Copello E, Kroutil L. Prescription drug use and misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. NSDUH Data Review. 2017. [Google Scholar]
- 7.Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, et al. Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. J Clin Psychiatry 2016;77:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med 2011;12(Suppl 2):S26–S35. [DOI] [PubMed] [Google Scholar]
- 9.Lalic S, Jokanovic N, Ilomäki J, Gisev N, Lloyd B, Lubman DI, et al. Harms associated with extramedical use of prescription opioid analgesics in Australia: A scoping review. Res Social Adm Pharm 2019;15:925–35. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voon P, Kerr T. “Nonmedical” prescription opioid use in North America: a call for priority action. Subst Abuse Treat Prev Policy 2013;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B. Oxford Centre for Evidence-based Medicine-levels of evidence (March 2009). Centre for Evidence Based Medicine Website. 2015. [Google Scholar]
- 13.Alexander JL, Burton JH, Bradshaw JR, Colin F. Suspected opioid-related emergency medical services encounters in a rural state, 1997-2002. Prehosp Emerg Care 2004;8:427–30. [DOI] [PubMed] [Google Scholar]
- 14.Choo EK, Douriez C, Green T. Gender and Prescription Opioid Misuse in the Emergency Department. Acad Emerg Med 2014;21:1493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell D, Alpert A, Pacula RL. A Transitioning epidemic: How the opioid crisis is driving the rise in hepatitis C. Health Aff (Millwood) 2019;38:287–94. [DOI] [PubMed] [Google Scholar]
- 16.Ashrafioun L, Bishop TM, Conner KR, Pigeon WR. Frequency of prescription opioid misuse and suicidal ideation, planning, and attempts. J Psychiatr Res. 2017;92:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA 2015;314:1468–78. [DOI] [PubMed] [Google Scholar]
- 18.Kozhimannil KB, Graves AJ, Levy R, Patrick SW. Nonmedical use of prescription opioids among pregnant US women. Womens Health Issues 2017;27:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigg KK, Monnat SM. Comparing characteristics of prescription painkiller misusers and heroin users in the United States. Addict Behav 2015;51:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KZ, Smith PH, Cercone SA, McKee SA, Homish GG. Past year non-medical opioid use and abuse and PTSD diagnosis: Interactions with sex and associations with symptom clusters. Addict Behav 2016;58:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang D, Li P, Guo L, Xu Y, Gao X, Deng J, et al. The prevalences of and association between nonmedical prescription opioid use and poor sleep among Chinese high school students. Sci Rep 2016;6:30411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry DT, Goulet JL, Kerns RK, Becker WC, Gordon AJ, Justice AC, et al. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain 2011;152:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali MM, Dean D Jr., Lipari R, Dowd WN, Aldridge AP, Novak SP. The mental health consequences of nonmedical prescription drug use among adolescents. J Ment Health Policy Econ 2015;18:3–15. [PubMed] [Google Scholar]
- 24.Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addict Behav 2010;35:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerridge BT, Saha TD, Chou SP, Zhang H, Jung J, Ruan WJ, et al. Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Drug Alcohol Depend 2015;156:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins S, Kim J, Chen L-Y, Levin D, Keyes K, Cerdá M, et al. Nonmedical prescription drug use among US young adults by educational attainment. Soc Psychiatry Psychiatr Epidemiol 2015;50:713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shield KD, Ialomiteanu A, Fischer B, Mann RE, Rehm J. Non-medical use of prescription opioids among Ontario adults: data from the 2008/2009 CAMH Monitor. Can J Public Health 2011;102:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE. Gender and non-medical use of prescription opioids: results from a national US survey. Addiction 2008;103:258–68. [DOI] [PubMed] [Google Scholar]
- 29.Vietri J, Joshi AV, Barsdorf AI, Mardekian J. Prescription opioid abuse and tampering in the United States: Results of a self-report survey. Pain Med 2014;15:2064–74. [DOI] [PubMed] [Google Scholar]
- 30.Wang KH, Becker WC, Fiellin DA. Prevalence and correlates for nonmedical use of prescription opioids among urban and rural residents. Drug Alcohol Depend 2013;127:156–62. [DOI] [PubMed] [Google Scholar]
- 31.Wu L-T, Ringwalt CL, Yang C, Reeve BB, Pan J-J, Blazer DG. Construct and differential item functioning in the assessment of prescription opioid use disorders among American adolescents. J Am Acad Child Adolesc Psychiatry 2009;48:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L-T, Woody GE, Yang C, Blazer DG. Subtypes of nonmedical opioid users: Results from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend 2010;112:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L-T, Woody GE, Yang C, Blazer DG. How Do prescription opioid users differ from users of heroin or other drugs in psychopathology: Results from the national epidemiologic survey on alcohol and related conditions. J Addict Med 2011;5:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among US adults: Psychiatric, medical and substance use correlates. Drug Alcohol Depend 2008;94:38–47. [DOI] [PubMed] [Google Scholar]
- 35.Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW. Tobacco smoking, nicotine dependence, and patterns of prescription opioid misuse: Results from a nationally representative sample. Nicotine Tob Res 2015;17:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edlund MJ, Forman-Hoffman VL, Winder CR, Heller DC, Kroutil LA, Lipari RN, et al. Opioid abuse and depression in adolescents: Results from the National Survey on Drug Use and Health. Drug Alcohol Depend 2015;152:131–8. [DOI] [PubMed] [Google Scholar]
- 37.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain 2007;129:355–62. [DOI] [PubMed] [Google Scholar]
- 38.Fink DS, Hu R, Cerda M, Keyes KM, Marshall BDL, Galea S, et al. Patterns of major depression and nonmedical use of prescription opioids in the United States. Drug Alcohol Depend 2015;153:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer B, Ialomiteanu A, Boak A, Adlaf E, Rehm J, Mann RE. Prevalence and key covariates of non-medical prescription opioid use among the general secondary student and adult populations in Ontario, Canada. Drug Alcohol Rev 2013;32:276–87. [DOI] [PubMed] [Google Scholar]
- 40.Ghandour LA, Martins SS, Chilcoat HD. Understanding the patterns and distribution of opioid analgesic dependence symptoms using a latent empirical approach. Int J Methods Psychiatr Res 2008;17:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuramoto SJ, Chilcoat HD, Ko J, Martins SS. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among US adults. J Stud Alcohol Drugs 2012;73:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins SS, Ghandour LA, Chilcoat HD. Profile of dependence symptoms among extramedical opioid analgesic users. Addict Behav 2007;32:2003–19. [DOI] [PubMed] [Google Scholar]
- 43.Martins SS, Storr CL, Zhu H, Chilcoat HD. Correlates of extramedical use of OxyContin (R) versus other analgesic opioids among the US general population. Drug Alcohol Depend 2009;99:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak SP, Hakansson A, Martinez-Raga J, Reimer J, Krotki K, Varughese S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016;16:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCabe SE, West BT, Teter CJ, Cranford JA, Ross-Durow PL, Boyd CJ. Adolescent nonmedical users of prescription opioids: Brief screening and substance use disorders. Addict Behav 2012;37:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young A, McCabe SE, Cranford JA, Ross-Durow P, Boyd CJ. Nonmedical use of prescription opioids among adolescents: subtypes based on motivation for use. J Addict Dis 2012;31:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan K, Moller M, Marsack-Topolewski C, Winston P, Jennings R, Prifti A. Age differences in non-medical prescription opioid use and psychological distress. Subst Use Misuse 2020;55:1808–16. [DOI] [PubMed] [Google Scholar]
- 48.Schepis TS, Simoni-Wastila L, McCabe SE. Prescription opioid and benzodiazepine misuse is associated with suicidal ideation in older adults. Int J Geriatr Psychiatry 2019;34:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim-Godwin Y, Lee MH. Suicidal ideation, plan, and attempts and nonmedical prescription opioid use among U.S. adults. Arch Psychiatr Nurs 2019;33:9–15. [DOI] [PubMed] [Google Scholar]
- 50.Schepis TS, McCabe VV, Boyd CJ, McCabe SE. The epidemiology of prescription fentanyl misuse in the United States. Addict Behav 2019;96:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohm MK, Clayton HB. Nonmedical use of prescription opioids, heroin use, injection drug use, and overdose mortality among adolescents. J Adolesc Health 2020;66:S130–S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nugraheni SE, Hastings JF. Relationship between religious support and major depressive episode for adult non-medical prescription opioid users and non-users. Subst Use Misuse 2020;55:564–71. [DOI] [PubMed] [Google Scholar]
- 53.Schepis TS, McCabe SE. Prescription opioid misuse in US older adults: associated comorbidities and reduced quality of life in the National Epidemiologic Survey of Alcohol and Related Conditions-III. J Clin Psychiatry 2019;80:19m12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkins NJ, Clayton H, Jones CM, Brown M. Current prescription opioid misuse and suicide risk behaviors among high school students. Pediatrics 2021;147:e2020030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao D, Guo L, Zhao MJ, Zhang S, Li WY, Zhang WH, et al. Effect of sex on the association between nonmedical use of opioids and sleep disturbance among chinese adolescents: A cross-sectional study. Int J Environ Res Public Health 2019;16:4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigg KK, Nicholson HL. Prescription opioid misuse among African-American adults: A rural-urban comparison of prevalence and risk. Drug Alcohol Depend 2019;197:191–6. [DOI] [PubMed] [Google Scholar]
- 57.Quinn K, Frueh BC, Scheidell J, Schatz D, Scanlon F, Khan MR. Internalizing and externalizing factors on the pathway from adverse experiences in childhood to non-medical prescription opioid use in adulthood. Drug Alcohol Depend 2019;197:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashrafioun L, Heavey S, Canarapen T, Bishop TM, Pigeon WR. The relationship between past 12-month suicidality and reasons for prescription opioid misuse. J Affect Disord 2019;249:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baiden P, Graaf G, Zaami M, Acolatse CK, Adeku Y. Examining the association between prescription opioid misuse and suicidal behaviors among adolescent high school students in the United States. J Psychiatr Res 2019;112:44–51. [DOI] [PubMed] [Google Scholar]
- 60.Samples H, Stuart EA, Olfson M. Opioid use and misuse and suicidal behaviors in a nationally representative sample of US adults. Am J Epidemiol 2019;188:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schepis TS, Wilens TE, McCabe SE. Prescription drug misuse: Sources of controlled medications in adolescents. J Am Acad Child Adolesc Psychiatry 2019;58:670–680.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clayton HB, Bohm MK, Lowry R, Ashley C, Ethier KA. Prescription opioid misuse associated with risk behaviors among adolescents. Am J Prev Med 2019;57:533–9. [DOI] [PubMed] [Google Scholar]
- 63.Chan KTK, Marsack-Topolewski C. Gender differences in adolescent opioid misuse and major depressive episodes. Child Adolesc Social Work J 2020;37:397–409. [Google Scholar]
- 64.Hruschak V, Hildenbrand AK, Cochran G. Psychiatric comorbidity and co-occurring opioid misuse: Depression mediates the relationship between post-traumatic stress disorder and opioid misuse in community pharmacy settings. Subst Abuse 2020;41:77–84. [DOI] [PubMed] [Google Scholar]
- 65.Catalano RF, White HR, Fleming CB, Haggerty KP. Is nonmedical prescription opiate use a unique form of illicit drug use? Addict Behav 2011;36:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend 2007;87:39. [DOI] [PubMed] [Google Scholar]
- 67.Roux P, Villes V, Blanche J, Bry D, Spire B, Feroni I, et al. Buprenorphine in primary care: risk factors for treatment injection and implications for clinical management. Drug Alcohol Depend 2008;97:105–13. [DOI] [PubMed] [Google Scholar]
- 68.Epstein-Ngo QM, Walton MA, Chermack ST, Blow FC, Zimmerman MA, Cunningham RM. Event-level analysis of antecedents for youth violence: comparison of dating violence with non-dating violence. Addict Behav 2014;39:350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: Results from a longitudinal study. Pain 2013;154:708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roux P, Carrieri PM, Cohen J, Ravaux I, Spire B, Gossop M, et al. Non-medical use of opioids among HIV-infected opioid dependent individuals on opioid maintenance treatment: The need for a more comprehensive approach. Harm Reduct J 2011;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrieri M-P, Rey D, Loundou A, Lepeu G, Sobel A, Obadia Y, et al. Evaluation of buprenorphine maintenance treatment in a French cohort of HIV-infected injecting drug users. Drug Alcohol Depend 2003;72:13–21. [DOI] [PubMed] [Google Scholar]
- 72.Pabayo R, Alcantara C, Kawachi I, Wood E, Kerr T. The role of depression and social support in non-fatal drug overdose among a cohort of injection drug users in a Canadian setting. Drug Alcohol Depend 2013;132:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lake S, Wood E, Buxton J, Dong H, Montaner J, Kerr T. Prescription opioid use and non-fatal overdose in a cohort of injection drug users. Am J Drug Alcohol Abuse 2015;41:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hadland SE, DeBeck K, Kerr T, Feng C, Montaner JS, Wood E. Prescription opioid injection and risk of hepatitis C in relation to traditional drugs of misuse in a prospective cohort of street youth. BMJ Open 2014;4:e005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruneau J, Roy É, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction 2012;107:1318–27. [DOI] [PubMed] [Google Scholar]
- 76.Tucker D, Milloy MJ, Hayashi K, Nguyen P, Kerr T, Wood E. Factors associated with illicit methadone injecting in a Canadian setting. Am J Addict 2015;24:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain 2007;23:307–15. [DOI] [PubMed] [Google Scholar]
- 78.Barth KS, Maria MM, Lawson K, Shaftman S, Brady KT, Back SE. Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence. Am J Addict 2013;22:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Centers for Disease C, Prevention. Thrombotic thrombocytopenic purpura (TTP)-like illness associated with intravenous Opana ER abuse--Tennessee, 2012. MMWR Morb Mortal Wkly Rep 2013;62:1–4. [PMC free article] [PubMed] [Google Scholar]
- 80.Hartwell EE, Pfeifer JG, McCauley JL, Moran-Santa Maria M, Back SE. Sleep disturbances and pain among individuals with prescription opioid dependence. Addict Behav 2014;39:1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lanier WA, Johnson EM, Rolfs RT, Friedrichs MD, Grey TC. Risk factors for prescription opioid-related death, Utah, 2008-2009. Pain Med 2012;13:1580–9. [DOI] [PubMed] [Google Scholar]
- 82.Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage 2006;31:465–76. [DOI] [PubMed] [Google Scholar]
- 83.Bonar EE, Ilgen MA, Walton M, Bohnert AS. Associations among pain, non-medical prescription opioid use, and drug overdose history. Am J Addict 2014;23:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buttram ME, Kurtz SP, Surratt HL, Levi-Minzi MA. Health and social problems associated with prescription opioid misuse among a diverse sample of substance-using MSM. Subst Use Misuse 2014;49:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Griffin ML, Bennett HE, Fitzmaurice GM, Hill KP, Provost SE, Weiss RD. Health-related quality of life among prescription opioid-dependent patients: results from a multi-site study. Am J Addict 2015;24:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Griffin ML, Dodd DR, Potter JS, Rice LS, Dickinson W, Sparenborg S, et al. Baseline characteristics and treatment outcomes in prescription opioid dependent patients with and without co-occurring psychiatric disorder. Am J Drug Alcohol Abuse 2014;40:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morasco BJ, Dobscha SK. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen Hosp Psychiatry 2008;30:93–9. [DOI] [PubMed] [Google Scholar]
- 88.Brunet L, Moodie E, Cox J, Gill J, Cooper C, Walmsley S, et al. Opioid use and risk of liver fibrosis in HIV/hepatitis C virus-coinfected patients in Canada. HIV Med 2016;17:36–45. [DOI] [PubMed] [Google Scholar]
- 89.Chelleng P, Borkakoty B, Chetia M, Das H, Mahanta J. Risk of hepatitis C infection among injection drug users in Mizoram, India. Indian J Med Res 2008;128:640–6. [PubMed] [Google Scholar]
- 90.Darke S, Ross J, Hall W. Prevalence and correlates of the injection of methadone syrup in Sydney, Australia. Drug Alcohol Depend 1996;43:191–8. [DOI] [PubMed] [Google Scholar]
- 91.Darke S, Topp L, Ross J. The injection of methadone and benzodiazepines among Sydney injecting drug users 1996-2000: 5-year monitoring of trends from the Illicit Drug Reporting System. Drug Alcohol Rev 2002;21:27–32. [DOI] [PubMed] [Google Scholar]
- 92.Das H, Borkakoty B, Mahanta J, Medhi G, Chelleng P. Hepatitis C virus infection and risk behaviors among injection drug users of Nagaland. IndianJ Gastroenterol 2007;26:253–4. [PubMed] [Google Scholar]
- 93.Humeniuk R, Ali R, McGregor C, Darke S. Prevalence and correlates of intravenous methadone syrup administration in Adelaide, Australia. Addiction 2003;98:413–8. [DOI] [PubMed] [Google Scholar]
- 94.Jeevanjee S, Penko J, Guzman D, Miaskowski C, Bangsberg DR, Kushel MB. Opioid analgesic misuse is associated with incomplete antiretroviral adherence in a cohort of HIV-infected indigent adults in San Francisco. AIDS Behav 2014;18:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins LM, Banta-Green CJ, Maynard C, Kingston S, Hanrahan M, Merrill JO, et al. Risk factors for nonfatal overdose at Seattle-area syringe exchanges. J Urban Health 2011;88:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction 2001;96:267–72. [DOI] [PubMed] [Google Scholar]
- 97.Price AM, Ilgen MA, Bohnert AS. Prevalence and correlates of nonmedical use of prescription opioids in patients seen in a residential drug and alcohol treatment program. J Subst Abuse Treat 2011;41:208–14. [DOI] [PubMed] [Google Scholar]
- 98.Romach MK, Sproule BA, Sellers EM, Somer G, Busto UE. Long-term codeine use is associated with depressive symptoms. J Clin Psychopharmacol 1999;19:373–6. [DOI] [PubMed] [Google Scholar]
- 99.Silva K, Schrager SM, Kecojevic A, Lankenau SE. Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug Alcohol Depend 2013;128:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sobieraj D Depressive symptoms are associated with opioid misuse in patients treated with chronic opioids. Formulary 2012;47:382–3. [Google Scholar]
- 101.Sproule BA, Busto UE, Somer G, Romach MK, Sellers EM. Characteristics of dependent and nondependent regular users of codeine. J Clin Psychopharmacol 1999;19:367–72. [DOI] [PubMed] [Google Scholar]
- 102.Stein M, Herman D, Bailey G, Straus J, Anderson B, Uebelacker L, et al. Chronic pain and depression among primary care patients treated with buprenorphine. J Gen Int Med 2015;30:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subramaniam GA, Stitzer MA. Clinical characteristics of treatment-seeking prescription opioid vs. heroin-using adolescents with opioid use disorder. Drug Alcohol Depend 2009;101:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Talu A, Rajaleid K, Abel-Ollo K, Rüütel K, Rahu M, Rhodes T, et al. HIV infection and risk behaviour of primary fentanyl and amphetamine injectors in Tallinn, Estonia: Implications for intervention. Int J Drug Policy 2010;21:56–63. [DOI] [PubMed] [Google Scholar]
- 105.Tragesser SL, Jones RE, Robinson RJ, Stutler A, Stewart A. Borderline personality disorder features and risk for prescription opioid use disorders. J Pers Disord 2013;27:427–41. [DOI] [PubMed] [Google Scholar]
- 106.Wylie JL, Shah L, Jolly AM. Demographic, risk behaviour and personal network variables associated with prevalent hepatitis C, hepatitis B, and HIV infection in injection drug users in Winnipeg, Canada. BMC Public Health 2006;6:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health 2014;104:2226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fischer B, Brissette S, Brochu S, Bruneau J, el-Guebaly N, Noel L, et al. Determinants of overdose incidents among illicit opioid users in 5 Canadian cities. CMAJ 2004;171:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mackesy-Amiti ME, Donenberg GR, Ouellet LJ. Prescription opioid misuse and mental health among young injection drug users. Am J Drug Alcohol Abuse 2015;41:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lankenau S, Kecojevic A, Silva K. Associations between prescription opioid injection and Hepatitis C virus among young injection drug users. Drugs Educ Prev Policy 2015;22:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ouellet L, Boodram B, Broz D. Prescription opioid misuse and heroin initiation by young urban and suburban non-injecting heroin users in Chicago. 140th American Public Health Association Annual Meeting and Exposition (APHA 2012); 2012. Oct 272012. [Google Scholar]
- 112.Barth KS, Balliet W, Pelic CM, Madan A, Malcolm R, Adams D, et al. Screening for current opioid misuse and associated risk factors among patients with chronic nonalcoholic pancreatitis pain. Pain Med 2014;15:1359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boyd CJ, Young A, McCabe SE. Psychological and drug abuse symptoms associated with nonmedical use of opioid analgesics among adolescents. Subst Abus 2014;35:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer B, Patra J, Cruz MF, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Revi 2008;27:625–32. [DOI] [PubMed] [Google Scholar]
- 115.Bretteville-Jensen AL, Lillehagen M, Gjersing L, Andreas JB. Illicit use of opioid substitution drugs: Prevalence, user characteristics, and the association with non-fatal overdoses. Drug Alcohol Depend 2015;147:89–96. [DOI] [PubMed] [Google Scholar]
- 116.Havens JR, Oser CB, Knudsen HK, Lofwall M, Stoops WW, Walsh SL, et al. Individual and network factors associated with non-fatal overdose among rural Appalachian drug users. Drug Alcohol Depend 2011;115:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Havens JR, Lofwall MR, Frost SDW, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural appalachian injection drug users. Am J Public Health 2013;103:e44–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patra J, Fischer B, Maksimowska S, Rehm J. Profiling poly-substance use typologies in a multi-site cohort of illicit opioid and other drug users in Canada-a latent class analysis. Addict Res Theory 2009;17:168–85. [Google Scholar]
- 119.Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2011;23(5):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend 2007;87:98–102. [DOI] [PubMed] [Google Scholar]
- 121.Ambekar A, Rao R, Mishra AK, Agrawal A. Type of opioids injected: does it matter? A multicentric cross-sectional study of people who inject drugs. Drug Alcohol Rev 2015;34:97–104. [DOI] [PubMed] [Google Scholar]
- 122.Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 2011;36:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mason MJ, Golladay G, Jiranek W, Cameron B, Silverman JJ, Zaharakis NM, et al. Depression moderates the relationship between pain and the nonmedical use of opioid medication among adult outpatients. J Addict Med 2016;10:408–13. [DOI] [PubMed] [Google Scholar]
- 124.Iversen J, Dertadian G, Geddes L, Maher L. High risk injecting behaviour among people who inject pharmaceutical opioids in Australia. Int J Drug Policy 2017;42:1–6. [DOI] [PubMed] [Google Scholar]
- 125.Hall MT, Golder S, Higgins GE, Logan TK. Nonmedical prescription opioid use among victimized women on probation and parole. Addict Behav 2016;53:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feingold D, Brill S, Goor-Aryeh I, Delayahu Y, Lev-Ran S. Misuse of prescription opioids among chronic pain patients suffering from anxiety: A cross-sectional analysis. Gen Hosp Psychiatry 2017;47:36–42. [DOI] [PubMed] [Google Scholar]
- 127.Aitken CK, Aitken CK, Higgs PG, Hellard ME. Buprenorphine injection in Melbourne, Australia—an update. Drug Alcohol Rev 2008;27:197–9. [DOI] [PubMed] [Google Scholar]
- 128.Cohen DP, Unoh E, Barry H, O'Connor JJ. Codeine misuse among service users on a methadone treatment programme. Ir J Med Sci 2010;179:465. [DOI] [PubMed] [Google Scholar]
- 129.Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The association between negative affect and prescription opioid misuse in patients with chronic pain: The mediating role of opioid craving. J Pain 2014;15:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nielsen S, Larance B, Lintzeris N, Black E, Bruno R, Murnion B, et al. Correlates of pain in an in-treatment sample of opioid-dependent people. Drug Alcohol Rev 2013;32:489–94. [DOI] [PubMed] [Google Scholar]
- 131.Mahanta J, Borkakoty B, Das HK, Chelleng PK. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care 2009;21:1420–4. [DOI] [PubMed] [Google Scholar]
- 132.Lin LA, Walton MA, Blow FC. Prescription opioid misuse among youth in primary care: A comparison of risk factors. Drug Alcohol Depend 2015;146:e180. [Google Scholar]
- 133.Bohnert AS, Eisenberg A, Whiteside L, Price A, McCabe SE, Ilgen MA. Prescription opioid use among addictions treatment patients: nonmedical use for pain relief vs. other forms of nonmedical use. Addict Behav 2013;38:1776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction 2005;100:197–205. [DOI] [PubMed] [Google Scholar]
- 135.Davis RE, Doyle NA, Nahar VK. Association between prescription opioid misuse and dimensions of suicidality among college students. Psychiatry Res 2020;287:112469. [DOI] [PubMed] [Google Scholar]
- 136.Kelley ML, Bravo AJ, Votaw VR, Stein E, Redman JC, Witkiewitz K. Opioid and sedative misuse among veterans wounded in combat. Addict Behav 2019;92:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wheeler PB, Stevens-Watkins D, Moody M, Dogan J, Lewis D. Culturally relevant risk and protective factors for nonmedical use of prescription opioids among incarcerated African American men. Addict Behav 2019;93:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barman-Adhikari A, Hsu HT, Brydon D, Petering R, Santa Maria D, Narendorf S, et al. Prevalence and correlates of nonmedical use of prescription drugs (NMUPD) among Young adults experiencing homelessness in seven cities across the United States. Drug Alcohol Depend 2019;200:153–60. [DOI] [PubMed] [Google Scholar]
- 139.Bouvier BA, Kinnard EN, Yedinak JL, Li Y, Elston B, Green TC, et al. Prevalence and correlates of depressive symptomology among young adults who use prescription opioids non-medically. J Psychoactive Drugs 2019;51:441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng T, Small W, Nosova E, Hogg R, Hayashi K, Kerr T, et al. Overdose risk and acquiring opioids for nonmedical use exclusively from physicians in Vancouver, Canada. Subst Use Misuse 2020;55:1912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boyd CJ, Teter CJ, West BT, Morales M, McCabe SE. Non-medical use of prescription analgesics: a three-year national longitudinal study. J Addict Dis 2009;28:232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iversen J, Wand H, Gonnermann A, Maher L. Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998–2008. Int J Drug Policy 2010;21:471–6. [DOI] [PubMed] [Google Scholar]
- 143.Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med 2012;42:1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schepis TS, Hakes JK. The association between nonmedical use of prescription medication status and change in health-related quality of life: results from a Nationally Representative Survey. Drug Alcohol Depend 2014;142:161–7. [DOI] [PubMed] [Google Scholar]
- 145.Blanco C, Wall MM, Okuda M, Wang S, Iza M, Olfson M. Pain as a predictor of opioid use disorder in a nationally representative sample. Am J Psychiatry 2016;173:1189–95. [DOI] [PubMed] [Google Scholar]
- 146.Guo L, Xu Y, Deng J, Huang J, Huang G, Gao X, et al. Association between nonmedical use of prescription drugs and suicidal behavior among adolescents. JAMA Pediatrics 2016;170:971–8. [DOI] [PubMed] [Google Scholar]
- 147.McCabe SE, Veliz P, Schulenberg JE. Adolescent context of exposure to prescription opioids and substance use disorder symptoms at age 35: A national longitudinal study. Pain 2016;157:2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morasco BJ, Turk DC, Donovan DM, Dobscha SK. Risk for prescription opioid misuse among patients with a history of substance use disorder. Drug Alcohol Depend 2013;127:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med 2012;10:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Uosukainen H, Ilomaeki J, Kauhanen J, Tacke U, Fohr J, Tiihonen J, et al. Factors associated with buprenorphine compared to amphetamine abuse among clients seeking treatment in Finland. J Subst Abuse Treat 2014;46:561–6. [DOI] [PubMed] [Google Scholar]
- 151.Hassanian-Moghaddam H, Farajidana H, Sarjami S, Owliaey H. Tramadol-induced apnea. Am J Emerg Med 2013;31:26–31. [DOI] [PubMed] [Google Scholar]
- 152.Miller NS, Greenfeld A. Patient characteristics and risks factors for development of dependence on hydrocodone and oxycodone. Am J Ther 2004;11:26–32. [DOI] [PubMed] [Google Scholar]
- 153.Black RA, Trudeau KJ, Cassidy TA, Budman SH, Butler SF. Associations between public health indicators and injecting prescription opioids by prescription opioid abusers in substance abuse treatment. J Opioid Manag 2013;9:5–17. [DOI] [PubMed] [Google Scholar]
- 154.Degenhardt L, Degenhardt L, Black E, Degenhardt L, Black E, Breen C, et al. Trends in morphine prescriptions, illicit morphine use and associated harms among regular injecting drug users in Australia. Drug Alcohol Rev 2006;25:403–12. [DOI] [PubMed] [Google Scholar]
- 155.Dhalla IA, Mamdani MM, Gomes T, Juurlink DN. Clustering of opioid prescribing and opioid-related mortality among family physicians in Ontario. Can Fam Physician 2011;57:e92–6. [PMC free article] [PubMed] [Google Scholar]
- 156.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend 2010;112:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain 2014;30:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA 2008;300:2613–20. [DOI] [PubMed] [Google Scholar]
- 159.Kouyanou K, Pither CE, Wessely S. Medication misuse, abuse and dependence in chronic pain patients. J Psychosom Res 1997;43:497–504. [DOI] [PubMed] [Google Scholar]
- 160.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy temporal trends and obstetrical outcomes. Anesthesiology 2014;121:1158–65. [DOI] [PubMed] [Google Scholar]
- 161.Almario CV, Seligman NS, Dysart KC, Berghella V, Baxter JK. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. Am J Obstet Gynecol 2009;201:326.e1-6. [DOI] [PubMed] [Google Scholar]
- 162.Centers for Disease C, Prevention. Unintentional opiate overdose deaths--King County, Washington, 1990-1999. MMWR Morb Mortal Wkly Rep 2000;49:636–40. [PubMed] [Google Scholar]
- 163.Hakansson A, Bradvik L, Schlyter F, Berglund M. Variables associated with repeated suicide attempt in a criminal justice population. Suicide Life Threat Behav 2011;41:517–31. [DOI] [PubMed] [Google Scholar]
- 164.Lee J, Hulman S, Musci M Jr., Stang E. Neonatal abstinence syndrome: Influence of a combined inpatient/outpatient methadone treatment regimen on the average length of stay of a Medicaid NICU Population. Popul Health Manag 2015;18:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Becker WC, Tobin DG, Fiellin DA. Nonmedical use of opioid analgesics obtained directly from physicians: prevalence and correlates. Arch Intern Med 2011;171:1034–6. [DOI] [PubMed] [Google Scholar]
- 166.Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend 2004;73:199. [DOI] [PubMed] [Google Scholar]
- 167.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999-2009. Drug Alcohol Depend 2013;131:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Centers for Disease C, Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- 169.Centers for Disease C, Prevention. Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999-2010. MMWR Morb Mortal Wkly Rep 2013;62:537–42. [PMC free article] [PubMed] [Google Scholar]
- 170.Challoner KR, McCarron MM, Newton EJ. Pentazocine (Talwin) intoxication: report of 57 cases. J Emerg Med 1990;8:67–74. [DOI] [PubMed] [Google Scholar]
- 171.Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone - Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015;64:443–4 2p. [PMC free article] [PubMed] [Google Scholar]
- 172.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009;105:9–15. [DOI] [PubMed] [Google Scholar]
- 173.Fernandes JC, Campana D, Harwell TS, Helgerson SD. High mortality rate of unintentional poisoning due to prescription opioids in adults enrolled in Medicaid compared to those not enrolled in Medicaid in Montana. Drug Alcohol Depend 2015;153:346–9. [DOI] [PubMed] [Google Scholar]
- 174.Green TC, Grau LE, Carver HW, Kinzly M, Heimer R. Epidemiologic trends and geographic patterns of fatal opioid intoxications in Connecticut, USA: 1997-2007. Drug Alcohol Depend 2011;115:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Green TC, Serrano JMG, Licari A, Budman SH, Butler SF. Women who abuse prescription opioids: Findings from the Addiction Severity Index-Multimedia Version® Connect prescription opioid database. Drug Alcohol Depend 2009;103:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hakkinen M, Launiainen T, Vuori E, Ojanpera I. Comparison of fatal poisonings by prescription opioids. Forensic Sci Int 2012;222:327–31. [DOI] [PubMed] [Google Scholar]
- 177.Hardt N, Wong TD, Burt MJ, Harrison R, Winter W, Roth J. Prevalence of prescription and illicit drugs in pregnancy-associated non-natural deaths of Florida mothers, 1999-2005. J Forensic Sci 2013;58:1536–41. [DOI] [PubMed] [Google Scholar]
- 178.Kelly L, Dooley J, Cromarty H, Minty B, Morgan A, Madden S, et al. Narcotic-exposed neonates in a First Nations population in northwestern Ontario: incidence and implications. Can Fam Physician 2011;57:e441–7. [PMC free article] [PubMed] [Google Scholar]
- 179.Liu A, Sithamparanathan S, Jones M, Cook C, Nanan R. Growth restriction in pregnancies of opioid-dependent mothers. Arch Dis Child Fetal Neonatal Ed 2010;95:F258–F62. [DOI] [PubMed] [Google Scholar]
- 180.Madadi P, Hildebrandt D, Lauwers AE, Koren G. Characteristics of opioid-users whose death was related to opioid-toxicity: a population-based study in Ontario, Canada. PLoS One 2013;8:e60600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maloney E, Degenhardt L, Darke S, Nelson EC. Are non-fatal opioid overdoses misclassified suicide attempts? Comparing the associated correlates. Addict Behav 2009;34:723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McQueen K, Murphy-Oikonen J, Desaulniers L. Maternal substance use and neonatal abstinence syndrome: A descriptive study. Matern Child Health J 2015;19:1756–65. [DOI] [PubMed] [Google Scholar]
- 183.Reid MC, Engles-Horton LL, Weber MAB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med 2002;17:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schrager SM, Kecojevic A, Silva K, Jackson Bloom J, Iverson E, Lankenau SE. Correlates and consequences of opioid misuse among high-risk young adults. J Addict 2014;2014:156954-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cottler LB, Hu H, Smallwood BA, Anthony JC, Wu LT, Eaton WW. Nonmedical opioid pain relievers and all-cause mortality: A 27-year follow-up from the Epidemiologic Catchment Area Study. Am J Public Health 2016;106:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morizio KM, Baum RA, Dugan A, Martin JE, Bailey AM. Characterization and management of patients with heroin versus nonheroin opioid overdoses: experience at an academic medical center. Pharmacotherapy 2017;37:781–90. [DOI] [PubMed] [Google Scholar]
- 187.Roxburgh A, Darke S, Salmon AM, Dobbins T, Jauncey M. Frequency and severity of non-fatal opioid overdoses among clients attending the Sydney Medically Supervised Injecting Centre. Drug Alcohol Depend 2017;176:126–32. [DOI] [PubMed] [Google Scholar]
- 188.Fischer B, Lusted A, Roerecke M, Taylor B, Rehm J. The prevalence of mental health and pain symptoms in general population samples reporting nonmedical use of prescription opioids: a systematic review and meta-analysis. J Pain 2012;13:1029–44. [DOI] [PubMed] [Google Scholar]
- 189.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet 2008;372:1733–45. [DOI] [PubMed] [Google Scholar]
- 190.Kerr T, Small W, Johnston C, Li K, Montaner JS, Wood E. Characteristics of injection drug users who participate in drug dealing: Implications for drug policy. J Psychoactive Drugs 2008;40:147–52. [DOI] [PubMed] [Google Scholar]
- 191.Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, et al. Crystal methamphetamine and injecting initiation among street-involved youth in a Canadian setting. CMAJ 2013;185:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hamida AB, Rafful C, Jain S, Sun S, Gonzalez-Zuniga P, Rangel G, et al. Non-injection drug use and injection initiation assistance among people who inject drugs in Tijuana, Mexico. J Urban Health 2018;95:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tyndall MW, Spittal PM, Laliberte N, Li K, O'Shaughnessy MV, Schechter MT. Intensive injection cocaine use as a primary risk factor of HIV seroconversion among polydrug users in Vancouver. AIDS 2002;17:887. [DOI] [PubMed] [Google Scholar]
- 194.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev 2016;5:CD011117. [DOI] [PubMed] [Google Scholar]
- 195.Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy 2014;25:53–60. [DOI] [PubMed] [Google Scholar]
- 196.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: A systematic review of global, regional, and national coverage. Lancet 2010;375:15. [DOI] [PubMed] [Google Scholar]
- 197.Bruneau J, Ahamad K, Goyer M-È, Poulin G, Selby P, Fischer B, et al. Management of opioid use disorders: A national clinical practice guideline. CMAJ 2018;190:E247–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users: 2012 revision. Geneva: WHO/UNODC/UNAIDS; 2012. [Google Scholar]
- 199.McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Magwood O, Salvalaggio G, Beder M, Kendall C, Kpade V, Daghmach W, et al. The effectiveness of substance use interventions for homeless and vulnerably housed persons: a systematic review of systematic reviews on supervised consumption facilities, managed alcohol programs, and pharmacological agents for opioid use disorder. PloS One 2020;15:e0227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav 2013;17:2878–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bresin K, Gordon KH. Endogenous opioids and nonsuicidal self-injury: A mechanism of affect regulation. Neurosci Biobehav Rev 2013;37:374–83. [DOI] [PubMed] [Google Scholar]
- 203.Baxter A, Scott K, Vos T, Whiteford H. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol Med 2013;43:897–910. [DOI] [PubMed] [Google Scholar]
- 204.Gossop M, Marsden J, Stewart D. Remission of psychiatric symptoms among drug misusers after drug dependence treatment. J Nervous Mental Disease 2006;194:826–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.