Abstract
Globally, there is growing interest to recycle organic waste using insect larvae into high-quality frass fertilizer through circular economy approach. This paper presents the first comparative report on the nutrient concentrations, fertilizing indices, nutrient supply potentials and compost maturity of nine edible insect frass fertilizers. Our results revealed that frass fertilizers from all the insect species had adequate concentrations and contents of macronutrients [nitrogen (N), phosphorus (P), potassium (K)], secondary nutrients (calcium, magnesium, and sulphur) and micro-nutrients (manganese, copper, iron, zinc, boron, and sodium). The fertilizing indices of the frass fertilizers were above 3. However, black soldier fly (BSF) frass fertilizer had significantly higher N (20–130%) and K (17–193%) concentrations compared to others. The P concentration of Gryllus bimaculatus frass fertilizer was 3–800% higher compared to those of frass fertilizers from other insect species. The potential N and K supply capacities of BSF frass fertilizer was 19–78% and 16–190% higher, respectively. The P supply capacity of cricket frass fertilizer was 17–802% higher compared to others. The highest seed gemination rate (> 90%) and germination index (267%) were observed in seeds treated with BSF frass fertilizer. Frass fertilizer obtained from the other eight insect species showed medium to high phytotoxicity. These findings demonstrate that insect frass fertilizers are promising alternatives to existing commercial fertilizers (i.e., mineral, and organic) for improved soil health and crop yield.
Subject terms: Biogeochemistry, Environmental sciences
Introduction
Soil degradation and poor waste management are major challenges to environmental health, and food and nutrition security in sub-Saharan Africa (SSA)1–7. About 40% of soils in SSA are deficient in most nutrients required for crop growth, with 25% affected by aluminium toxicity, 18% prone to leaching and 8.5% characterized by phosphorus fixation8. Despite the challenges, most smallholder farmers use little (≤ 10 kg ha−1 year−1) or no mineral fertilizer due to the high-cost implications and limited access3. Even in situations where mineral fertilizers are widely used, their efficiency is hindered by low soil organic matter, micronutrient deficiencies and high soil acidity9–12. Although the use of organic fertilizer is acceptable and affordable to farmers13–16, there has been limited uptake in SSA due to poor quality, long production time as well as limited sources of organic matter on the farm10,17,18. Thus, there is need to explore alternative sources of organic fertilizers that are readily available, affordable and of good quality such as insect frass fertilizer.
The use of insects as bio-converters of low-value organic matter into affordable and high-quality food, feed, fibre and organic fertilizer products has rapidly attracted attention globally19–26. Several insect species are being mass produced at the International Centre of Insect Physiology and Ecology (icipe) including black soldier fly (BSF) (Hermertia illucens L.), two-spotted crickets (Gryllus bimaculatus De Geer) and Scapsipedus icipe Hugel and Tanga), silk moth (Bombyx mori L.), edible saturniid caterpillar [Gonimbrasia krucki Nudaurelia), mealworm (Tenebrio molitor L.), desert locust (Schistocerca gregaria Forsskål), African fruit beetle (Pachnoda sinuata L.) and rhinoceros beetle (Oryctes rhinoceros L.). Among these insects, only the frass fertilizer from H. illucens and T. molitor have been tested and proven to play a critical role in improving soil fertility, yield and the nutritional quality of different crops27–33.
Insect mass rearing using organic waste could contribute to addressing the challenges of poor waste management and low soil fertility in SSA2,4,6,34,35. Insect-mediated bioconversion of organic waste into organic fertilizers could reduce on land filling and return nutrients to agricultural lands. The bioconversion of organic waste into high value commercial products is a positive step towards sustainable waste recycling, whereby the income and other nonmonetary benefits obtained could act as incentives towards improved waste management and circular economy36,37. For example, it has been demonstrated that H. illucens larvae require only 5 weeks to recycle organic wastes into nutrient-rich mature and stable frass fertilizer compared to 8–24 weeks for conventional composting26. In Kenya, ex-ante macroeconomic estimates revealed that adoption of insect bioconversion technology can recycle between 2 and 18 million tonnes of waste into organic fertilizer worth approximately 9–85 million USD/year38.
Studies on the utilization of frass fertilizer from edible and commercial insects as organic fertilizer are still limited, except for H. illucens and T. molitor26,30,33,39–42. There is inadequate research on the fertilizer quality of frass fertilizer generated by other insect species. There is urgent need to explore the nutrient quality of different frass fertilizer products to ensure future diversification of suitable organic fertilizer products in the market that can replace or serve as important alternatives to the low quality organic fertilizers used in most African cropping systems15–17,43,44. The nutrient quality and fertilizing index of frass fertilizer generated by most insects are largely unknown, yet such information would guide recommendations for field application as organic fertilizers45.
The scanty research on the maturity and stability status of frass fertilizers obtained from most edible insects makes it difficult to determine whether they are mature or stable for field application46,47. Previous studies have shown that the application of immature and unstable compost causes nutrient immobilization and phytotoxicity which reduce seed germination, crop growth and yield48–51. This study hypothesized that the nutrient quality of frass fertilizer derived from different edible insect species are highly suitable as organic fertilizer compared to that of black soldier fly and mealworm, which have received adequate research attention and global acceptance as alternative fertilizers for soil amendment and crop production. Therefore, the current study was conceptualized to comparatively establish the nutrient concentrations, potential nutrient supply, fertilizing indices, maturity and stability of frass fertilizers generated from nine different insect species that are mass produced at icipe. This information would be a prerequisite to inform policy makers to develop and promote guidelines for their integration into already existing agro-input markets (i.e., fertilizer) and farming practices.
Results
Moisture, total organic carbon, and mineral nitrogen levels of edible insect frass fertilizer
The moisture content of frass fertilizer produced by the different insect species varied significantly (p ≤ 0.001) (Table 1). The moisture content ranged between 8 and 11% whereby, the O. rhinoceros and H. illucens had the lowest and highest values, respectively. Frass fertilizer from H. illucens, B. mori, and P. sinuata had significantly (p ≤ 0.001) higher moisture content than the frass fertilizer produced by other insects. The moisture content of frass fertilizer produced by T. molitor and S. icipe was significantly (p ≤ 0.001) higher than those of the rest, except H. illucens, B. mori and P. sinuata. Likewise, frass fertilizer from G. krucki and S. gregaria had significantly (p ≤ 0.001) higher moisture content than the frass fertilizer produced by O. rhinoceros (Table 1).
Table 1.
Selected characteristics of frass fertilizer produced by different edible insect species.
| Source of frass fertilizer | Moisture (%) | Total organic carbon (%) | Ammonium | Nitrates |
|---|---|---|---|---|
| (mg kg−1) | ||||
| Hermertia illucens | 11.2 ± 0.31a | 39.1 ± 0.12abc | 30.3 ± 4.03ab | 7.7 ± 4.01c |
| Tenebrio molitor | 10.2 ± 0.12b | 49.6 ± 0.11a | 0.01 ± 0.00c | 1.6 ± 0.07c |
| Scapsipedus icipe | 10.1 ± 0.09b | 42.0 ± 0.47ab | 0.01 ± 0.00c | 1.9 ± 0.19c |
| Bombyx mori | 11.1 ± 0.03a | 44.1 ± 10.3ab | 0.01 ± 0.00c | 0.01 ± 0.00c |
| Gryllus bimaculatus | 8.1 ± 0.07de | 42.7 ± 0.31ab | 0.01 ± 0.00c | 0.9 ± 0.09c |
| Gonimbrasia krucki | 9.3 ± 0.09c | 49.0 ± 0.29a | 0.80 ± 0.10bc | 0.01 ± 0.00c |
| Pachnoda sinuata | 11.0 ± 0.13a | 31.0 ± 0.32bc | 56.1 ± 6.56a | 35.3 ± 1.88b |
| Schistocerca gregaria | 8.6 ± 0.37cd | 40.3 ± 1.25ab | 0.01 ± 0.00c | 4.4 ± 0.99c |
| Oryctes rhinoceros | 7.6 ± 0.06e | 24.1 ± 0.75c | 55.3 ± 19.0a | 361.7 ± 12.2a |
| χ2-value | 440.3 | 44.5 | 104.3 | 6034.5 |
| df | 8 | 8 | 8 | 8 |
| p value | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
In the same column, means (± standard error) followed by the same letters are not significantly different at p ≤ 0.05, n = 3.
The concentration of total organic carbon was found to vary significantly (p ≤ 0.001) (Table 1). The total organic carbon concentration of the frass fertilizer samples was 24–50%, T. molitor and O. rhinoceros frass fertilizer had the lowest and highest values, respectively. Tenebrio molitor and G. krucki frass fertilizer had significantly (p ≤ 0.001) higher total organic carbon concentrations than the frass fertilizer produced by P. sinuata and O. rhinoceros.
There were significant (p ≤ 0.001) differences in the concentrations of ammonium and nitrates in frass fertilizer produced by different insects (Table 1). The ammonium concentration of the frass fertilizer samples ranged between 0.01 and 174 mg kg−1. The highest ammonium concentration was recorded in frass fertilizer produced by P. sinuata, and this was 1–5610 times higher than those of other treatments. Pachnoda sinuata and O. rhinoceros produced frass fertilizer with significantly (p ≤ 0.001) higher ammonium concentration than other insects, except H. illucens. Also, the ammonium concentration of H. illucens frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer from T. molitor, S. icipe, G. bimaculatus, B. mori and S. gregaria by 3030 times.
Frass fertilizer from G. crucki and B. mori had the lowest nitrate concentration, while the O. rhinoceros produced frass fertilizer with the highest nitrate concentration, which was 10–36,170 times significantly (p ≤ 0.001) higher than those of frass fertilizer generated by other insects. Also, the nitrate concentration of P. sinuata frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer from other insects by 4.6–3530 times, except O. rhinoceros frass fertilizer.
Concentrations of macro and secondary nutrients in frass fertilizer produced by different insect species
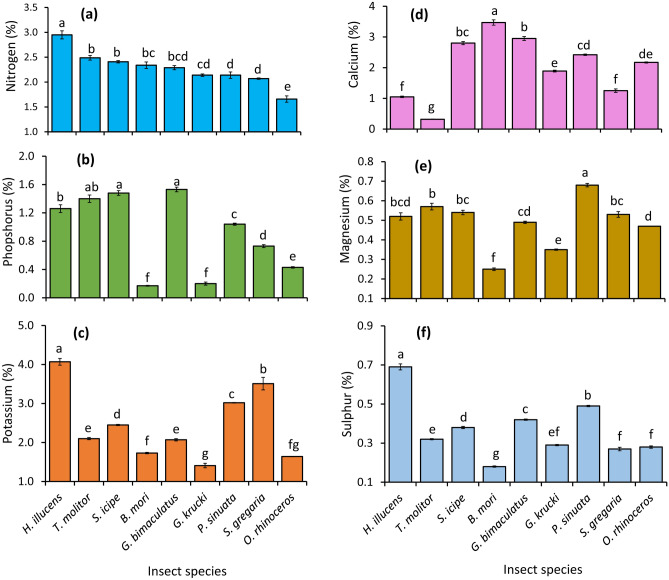
There were significant differences (p ≤ 0.001) in the concentration of total N in frass fertilizer produced by different insects (Fig. 1a). The highest total N concentration was recorded in frass fertilizer produced by the H. illucens, 20–130% significantly (p ≤ 0.001) higher than those of frass fertilizer produced by other insects. The total N of frass fertilizer produced by T. molitor and S. icipe was significantly (p ≤ 0.001) higher than those of G. krucki, P. sinuata, S. gregaria, O. rhinoceros. Also, the total N concentration of frass fertilizer produced by B. mori was significantly (p ≤ 0.001) higher than those of P. sinuata, S. gregaria and O. rhinoceros frass fertilizer by 10, 11 and 35%, respectively. Oryctes rhinoceros produced frass fertilizer with the lowest total N concentration, which was significantly lower than those of frass fertilizer generated by other insects by 25–130%. Apart from O. rhinoceros frass fertilizer, it was noted that frass fertilizer produced by other insects would supply more than 100 kg N ha−1 per season, if applied for crop production at the rate of 5 t ha−1 (Table 3).
Figure 1.
Total concentrations of nitrogen (a), phosphorus (b), potassium (c), calcium (d), magnesium (e) and sulphur (f) in frass fertilizer generated by different edible insects. Per panel, means (± standard error) followed by the same letters are not significantly different at p ≤ 0.05, n = 3.
Table 3.
Nutrients in frass fertilizers if applied at the rate of 5 t ha−1 for crop production.
| Source of frass fertilizer | Nitrogen | Phosphorus | Potassium | Calcium | Magnesium | Sulphur | Zinc | Manganese | Iron | Copper | Boron |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (kg ha−1) | |||||||||||
| Hermertia illucens | 147.5 ± 4.1 | 63.2 ± 2.7 | 203.7 ± 4.2 | 52.7 ± 2.2 | 26.2 ± 0.93 | 34.5 ± 0.76 | 0.84 ± 0.03 | 2.1 ± 0.18 | 39.0 ± 1.60 | 0.14 ± 0.00 | 0.16 ± 0.00 |
| Tenebrio molitor | 124.3 ± 2.3 | 69.8 ± 2.6 | 104.8 ± 1.5 | 15.8 ± 0.6 | 28.5 ± 0.87 | 15.8 ± 0.17 | 0.51 ± 0.00 | 0.9 ± 0.07d | 2.2 ± 0.27 | 0.06 ± 0.00 | 0.03 ± 0.00 |
| Scapsipedus icipe | 120.7 ± 1.2 | 74.2 ± 1.7 | 122.7 ± 0.7 | 139.8 ± 4.5 | 27.0 ± 0.58 | 19.0 ± 0.28 | 0.98 ± 0.01 | 2.2 ± 0.08 | 21.9 ± 0.98 | 0.15 ± 0.01 | 0.16 ± 0.00 |
| Bombyx mori | 117.2 ± 3.3 | 8.5 ± 0.3 | 86.5 ± 0.9 | 173.7 ± 7.4 | 12.7 ± 0.33 | 9.2 ± 0.17 | 0.08 ± 0.00 | 0.6 ± 0.03 | 4.4 ± 0.28 | 0.04 ± 0.00 | 0.59 ± 0.02 |
| Gryllus bimaculatus | 114.5 ± 2.1 | 76.7 ± 1.6 | 103.7 ± 1.4 | 147.3 ± 5.8 | 24.5 ± 0.29 | 20.8 ± 0.17 | 1.04 ± 0.01 | 1.8 ± 0.01 | 18.2 ± 0.42 | 0.15 ± 0.00 | 0.14 ± 0.01 |
| Gonimbrasia krucki | 107.0 ± 1.3 | 9.8 ± 1.1 | 70.3 ± 2.9 | 94.7 ± 2.5 | 17.3 ± 0.17 | 14.7 ± 0.17 | 0.07 ± 0.01 | 1.1 ± 0.18 | 9.1 ± 3.08 | 0.02 ± 0.00 | 0.18 ± 0.01 |
| Pachnoda sinuata | 106.8 ± 3.3 | 52.2 ± 0.7 | 151.2 ± 0.4 | 121.2 ± 2.1 | 34.2 ± 0.44 | 24.3 ± 0.17 | 0.98 ± 0.01 | 13.3 ± 0.25 | 108.5 ± 8.81 | 0.14 ± 0.00 | 0.25 ± 0.01 |
| Schistocerca gregaria | 103.3 ± 0.7 | 36.5 ± 1.0 | 175.3 ± 8.0 | 62.7 ± 4.9 | 26.3 ± 0.73 | 13.7 ± 0.44 | 0.59 ± 0.02 | 1.7 ± 0.10 | 15.9 ± 1.05 | 0.09 ± 0.01 | 0.12 ± 0.01 |
| Oryctes rhinoceros | 82.8 ± 2.2 | 21.5 ± 0.5 | 82.0 ± 0.3 | 108.5 ± 1.5 | 23.5 ± 0.00 | 14.0 ± 0.29 | 0.92 ± 0.02 | 22.3 ± 2.42 | 216.7 ± 2.44 | 0.15 ± 0.01 | 0.37 ± 0.01 |
Mean (± standard error), n = 3.
The concentration of total P in frass fertilizer produced by different insects was found to vary significantly (p ≤ 0.001) (Fig. 1b). The total P concentration ranged between 0.17 and 1.5%, whereby G. bimaculatus and B. mori produced frass fertilizer with highest and lowest P concentration, respectively. The total P concentration of frass fertilizer produced by G. bimaculatus and S. icipe was significantly (p ≤ 0.001) higher than those of frass fertilizer produced by other insects, except T. molitor. Hermertia illucens and T. molitor frass fertilizer had significantly (p ≤ 0.001) higher P concentration than other insect frass fertilizer samples, except G. bimaculatus and S. icipe. The P concentration of P. sinuata frass fertilizer was significantly (p ≤ 0.001) higher than frass fertilizer from S. gregaria, O. rhinoceros, G. krucki and B. mori by 1.4, 2.4, 5.2 and 6.1 folds, respectively. Also, S. gregaria frass fertilizer had significantly (p ≤ 0.001) higher P concentration when compared to those of O. rhinoceros, B. mori, and G. krucki. The P concentration of frass fertilizer produced by O. rhinoceros was 2 and 2.5 folds higher than those of frass fertilizer from G. krucki and B. mori, respectively. If applied for crop production, the frass fertilizer generated by other insects would supply 22–77 kg P ha−1 per season, except frass fertilizer from B. mori and G. krucki (Table 3).
There were significant differences in the concentration of total K in frass fertilizer produced by different insect species (Fig. 1c). The concentration of K in frass fertilizer produced by H. illucens was 17–193% higher (p ≤ 0.001) than those of frass fertilizer produced by other insects. The concentrations of K in frass fertilizer produced by S. gregaria, P. sinuata, and S. icipe were significantly (p ≤ 0.001) higher than those of frass fertilizer produced by other insects, except H. illucens. Also, the T. molitor and G. bimaculatus frass fertilizer had significantly (p ≤ 0.001) higher K concentration than those of frass fertilizer from B. mori, O. rhinoceros and G. krucki. The B. mori achieved significantly (p ≤ 0.001) higher frass fertilizer K concentration than G. krucki whose frass fertilizer had the lowest K concentration. Beside frass fertilizer obtained from B. mori, G. krucki and O. rhinoceros which contained less than 90 kg K ha−1 in every five tonnes of biomass, the frass fertilizer produced by other insects would supply between 104 and 204 kg K ha−1 per season if applied for crop production (Table 3).
The concentration of total Ca in frass fertilizer samples produced by different insects varied significantly (p ≤ 0.001) (Fig. 1d). The concentration of Ca ranged between 0.3 and 3.5%; B. mori and T. molitor frass fertilizer had the highest and lowest Ca concentrations, respectively. The Ca concentration of T. molitor frass fertilizer was significantly (p ≤ 0.001) higher than those of other insect frass fertilizer samples by 1.2–12 folds. Also, G. bimaculatus, S. icipe and P. sinuata achieved significantly (p ≤ 0.001) higher frass fertilizer Ca concentration than other insects, except T. molitor and O. rhinoceros. The concentrations of Ca in frass fertilizer produced by P. sinuata, O. rhinoceros and G. krucki were significantly (p ≤ 0.001) higher than those of frass fertilizer from S. gregaria, H. illucens and T. molitor. At the application rate of 5 t ha−1, frass fertilizer from all insects would supply 16–174 kg Ca ha−1. The Ca content of frass fertilizer from T. molitor was exceptionally low (Table 3).
The concentration of Mg in frass fertilizer produced by different insects also varied significantly (p ≤ 0.001) (Fig. 1e). The P. sinuata produced frass fertilizer with the highest Mg concentration, which was significantly (p ≤ 0.001) higher than those of other insect frass fertilizer samples by 1.2–2.7 folds. The Mg concentration of frass fertilizer produced by T. molitor frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer from G. bimaculatus, O. rhinoceros, G. krucki and B. mori by 16, 21, 63 and 128%, respectively. Likewise, the Mg concentrations of frass fertilizer produced by S. gregaria and S. icipe were significantly (p ≤ 0.001) higher than those of frass fertilizer from O. rhinoceros, G. krucki and B. mori by 13–15%, 51–54% and 112–116%, respectively. The Mg concentration of frass fertilizer from O. rhinoceros was significantly (p ≤ 0.001) higher than those of G. krucki and B. mori by 1.3 and 1.9 folds, respectively. The lowest Mg concentration was recorded in frass fertilizer produced by B. mori. The frass fertilizer produced by all the insect species would supply 13–34 kg Mg ha−1 per season if used applied fertilizer at a rate of 5 t ha−1 (Table 3).
There were significant differences in the concentration of total S in frass fertilizer produced by different insects (p ≤ 0.001) (Fig. 1f). Hermertia illucens produced frass fertilizer with the highest S concentration, significantly (p ≤ 0.001) higher than those of frass fertilizer from other insects by 1.4–3.8 folds. The concentrations of S in frass fertilizer from the P. sinuata, G. bimaculatus and S. icipe were significantly (p ≤ 0.001) higher than those of other insect frass fertilizer samples, except H. illucens. It was noted that the S concentration of frass fertilizer from P. sinuata was significantly higher than those of frass fertilizer produced by G. bimaculatus and S. icipe and T. molitor by 17, 29 and 53%, respectively. Also, T. molitor produced frass fertilizer with significantly higher S concentration than the frass fertilizer produced by O. rhinoceros, S. gregaria and B. mori whose frass fertilizer had the lowest S concentration. The frass fertilizer from all the insects would supply 14–35 kg S ha−1 per season, except S. icipe (Table 3).
Concentrations of micronutrients in frass fertilizer produced by different insect species
There were significant (p ≤ 0.001) differences in the total concentrations of Mn, Fe, Zn, Cu, B, Na, and Al in frass fertilizer produced by different insects (Table 2). The Mn concentration ranged between 128 and 4600 mg kg−1, O. rhinoceros and B. mori produced frass fertilizer with the lowest and highest Mn concentrations, respectively. The concentrations of Mn in frass fertilizer produced by O. rhinoceros and P. sinuata were significantly (p ≤ 0.001) higher than those of frass fertilizer generated by other insects by 1.7–35 folds and 6.5–21 folds, respectively.
Table 2.
Concentrations of micronutrients in frass fertilizers produced by different insect species.
| Source of frass fertilizer | Manganese | Iron | Zinc | Copper | Boron | Sodium | Aluminium (%) |
|---|---|---|---|---|---|---|---|
| (mg kg−1) | |||||||
| Hermertia illucens | 409 ± 36.2c | 7803 ± 319.5c | 168.0 ± 5.03d | 26.9 ± 0.50b | 32.6 ± 0.66d | 5263.3 ± 153.0b | 0.59 ± 0.020c |
| Tenebrio molitor | 176 ± 14.2c | 436 ± 54.1d | 102.7 ± 0.33f | 12.6 ± 0.07d | 5.8 ± 0.45f | 170.7 ± 8.1g | 0.03 ± 0.003e |
| Scapsipedus icipe | 431 ± 16.4c | 4380 ± 196.0cd | 196.0 ± 2.00ab | 30.4 ± 1.07a | 31.8 ± 0.19d | 680.3 ± 20.4ef | 0.28 ± 0.006d |
| Bombyx mori | 128 ± 5.6c | 887 ± 56.2d | 16.1 ± 0.60g | 8.9 ± 0.35e | 118.0 ± 3.61a | 39.8 ± 3.0g | 0.07 ± 0.004e |
| Gryllus bimaculatus | 359 ± 2.5c | 3643 ± 84.1cd | 208.0 ± 1.53a | 29.9 ± 0.18ab | 28.5 ± 1.12de | 957.7 ± 12.3e | 0.25 ± 0.003d |
| Gonimbrasia krucki | 225 ± 35.0c | 1825 ± 615.7d | 13.8 ± 2.68g | 4.3 ± 0.50f | 35.3 ± 0.97d | 1546.7 ± 56.1d | 0.13 ± 0.033e |
| Pachnoda sinuata | 2660 ± 50.3b | 21,767 ± 1762.9b | 195.3 ± 2.73b | 28.6 ± 0.60ab | 49.0 ± 1.91c | 7626.7 ± 148.5a | 1.77 ± 0.059b |
| Schistocerca gregaria | 335 ± 18.9c | 3187 ± 211.7d | 117.0 ± 3.51e | 17.8 ± 0.98c | 23.5 ± 1.43e | 566.7 ± 13.3f | 0.30 ± 0.017d |
| Oryctes rhinoceros | 4460 ± 484.5a | 43,333 ± 2488.2a | 183.0 ± 3.22c | 30.6 ± 1.08a | 73.2 ± 1.90b | 2320.0 ± 76.4c | 2.94 ± 0.027a |
| χ2 value | 684.1 | 1470.2 | 5994.4 | 1794.4 | 3161.7 | 8883.3 | 11,847.0 |
| df | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| p value | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
In the same column, means (± standard error) followed by the same letters are not significantly different at p ≤ 0.05, n = 3.
The concentration of Fe in O. rhinoceros frass fertilizer was 2–99 folds higher (p ≤ 0.001) than those of frass fertilizer produced by insects (Table 2). The concentration of Fe in P. sinuata frass fertilizer was significantly (p ≤ 0.001) higher than those of other insect frass fertilizers, except O. rhinoceros. The concentration of Fe in H. illucens frass fertilizer was 2.5, 4, 9, and 17 times higher (p ≤ 0.001) than those of frass fertilizer produced by S. gregaria, G. krucki, B. mori, and T. molitor, respectively. Gryllus bimaculatus produced frass fertilizer with 1.1–15 times higher (p ≤ 0.001) Zn concentration than other insects, except S. icipe. The concentrations of Zn in frass fertilizer produced by T. molitor and G. krucki were significantly (p ≤ 0.001) lower than those of frass fertilizer produced by other insects.
The concentration of Cu ranged between 4 and 31 mg kg−1 whereby, G. krucki and O. rhinoceros produced frass fertilizer with highest and lowest values, respectively (Table 2). The concentration of Cu in O. rhinoceros frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer produced by other insects, except P. sinuata, S. icipe and G. bimaculatus. Hermertia illucens frass fertilizer had 51, 114, 202 and 526% (p ≤ 0.001) higher Cu concentration than the frass fertilizer produced by S. gregaria, T. molitor, G. krucki and B. mori, respectively. Furthermore, S. gregaria produced frass fertilizer with 1.4, 2 and 4 times higher (p ≤ 0.001) Cu concentration than frass fertilizer from T. molitor, B. mori, and G. krucki, respectively.
The concentrations of B in frass fertilizer produced by B. mori and O. rhinoceros were significantly (p ≤ 0.001) higher than those in frass fertilizer from other insects by 1.6–20 folds and 1.5–13 folds, respectively (Table 2). Also, P. sinuata produced frass fertilizer with significantly (p ≤ 0.001) higher B concentration than other insects, except B. mori and O. rhinoceros. The concentrations of B in frass fertilizer produced by G. krucki, S. icipe and H. illucens were significantly (p ≤ 0.001) higher than those of frass fertilizer from S. gregaria and T. molitor. Tenebrio molitor produced frass fertilizer with a significantly (p ≤ 0.001) lower concentration of B than other insects.
The concentration of Na in frass fertilizer produced by different insects is presented in Table 2. Pachnoda sinuata and B. mori produced frass fertilizer with highest and lowest Na concentration, respectively. It was noted that the concentration of Na in P. sinuata frass fertilizer was 1.5–192 folds higher (p ≤ 0.001) than those of frass fertilizer produced by other insects. Hermertia illucens, O. rhinoceros and G. krucki produced frass fertilizer with significantly (p ≤ 0.001) higher Na concentrations than other insects, except the P. sinuata.
The highest concentration of Al was recorded in frass fertilizer produced by the O. rhinoceros, significantly (p ≤ 0.001) higher than those of other insect frass fertilizer samples by 1.7–42 folds. Frass fertilizer from P. sinuata and H. illucens had significantly (p ≤ 0.001) higher Al concentrations than other insects’ frass fertilizer, except O. rhinoceros. Also, the concentrations of Al in frass fertilizer from S. gregaria, G. bimaculatus and S. icipe were significantly (p ≤ 0.001) higher than those of frass fertilizer produced by G. krucki, B. mori, and T. molitor which had the lowest concentration.
At the application rate of 5 t ha−1, frass fertilizer from eight insect species would supply less than 1 kg ha−1 of Cu, B and Zn. Out of the nine insect species, only G. bimaculatus frass fertilizer would supply at least 1 kg Zn ha−1 (Table 3). Apart from P. sinuata and O. rhinoceros, frass fertilizer from the other insects would supply less than 5 kg of Mn ha−1. Frass fertilizer from the various insects would supply 16–217 kg Fe ha−1 if applied at a rate of 5 t ha−1, except for T. molitor, B. mori and G. krucki (Table 3).
Fertilizing index of frass fertilizer produced by different insect species
The fertilizing indices of frass fertilizer produced by different insects ranged between 3.9 and 4.8, whereby, G. krucki and H. illucens produced frass fertilizer with the lowest and highest values, respectively. The fertilizing indices of frass fertilizer produced by other insects were than those of B. mori and G. krucki frass fertilizer.
Maturity status of frass fertilizer produced by different insect species
The pH and electrical conductivity (EC) of frass fertilizer produced by different insects varied significantly (p ≤ 0.001) (Table 4). The pH ranged between 4.6 and 8.3, whereby G. krucki and P. sinuata produced frass fertilizer with lowest and highest pH values, respectively. The pH of frass fertilizer produced by P. sinuata and B. mori were significantly (p ≤ 0.001) higher than those of frass fertilizer from other insects. Furthermore, the O. rhinoceros, H. illucens and S. icipe produced frass fertilizer with significantly (p ≤ 0.001) higher pH values than the T. molitor, G. bimaculatus, S. gregaria and G. krucki.
Table 4.
Compost maturity indices of frass fertilizer produced by different edible insect species.
| Source of frass fertilizer | pH | Electrical conductivity (mS cm−1) | Ammonium/nitrate ratio | C/N ratio | Seed germination rate | Gemination index |
|---|---|---|---|---|---|---|
| (%) | ||||||
| Hermertia illucens | 7.5 ± 0.09c | 16.0 ± 1.58b | 3.9 ± 3.83b | 13.2 ± 0.39b | 93.3 ± 3.3a | 267.1 ± 91.7a |
| Tenebrio molitor | 6.5 ± 0.05e | 6.7 ± 0.63f | 0.006 ± 0.00b | 20.0 ± 0.35ab | 56.7 ± 14.5c | 27.9 ± 6.2e |
| Scapsipedus icipe | 7.4 ± 0.02c | 11.5 ± 0.07cde | 0.005 ± 0.00b | 17.4 ± 0.35ab | 63.3 ± 3.3bc | 31.2 ± 1.6e |
| Bombyx mori | 8.1 ± 0.02a | 10.0 ± 0.69def | 1.00 ± 0.00b | 19.1 ± 4.94ab | 60.0 ± 5.8bc | 29.5 ± 2.8e |
| Gryllus bimaculatus | 6.6 ± 0.05e | 9.0 ± 0.34ef | 0.12 ± 0.00b | 18.6 ± 0.32ab | 76.7 ± 3.3abc | 37.8 ± 1.6de |
| Gonimbrasia krucki | 4.6 ± 0.04f | 15.5 ± 0.23b | 79.7 ± 10.1a | 22.9 ± 0.14a | 86.7 ± 3.3ab | 47.8 ± 6.2cd |
| Pachnoda sinuata | 8.3 ± 0.03a | 25.1 ± 0.18a | 1.58 ± 0.009b | 14.6 ± 0.56b | 96.7 ± 3.3a | 66.1 ± 8.5bc |
| Schistocerca gregaria | 6.9 ± 0.03d | 13.9 ± 0.16bc | 0.003 ± 0.00b | 19.5 ± 0.47ab | 23.3 ± 6.7d | 5.8 ± 1.6f |
| Oryctes rhinoceros | 7.9 ± 0.02b | 13.2 ± 0.25bcd | 0.15 ± 0.05b | 14.6 ± 0.85b | 93.3 ± 6.7a | 73.9 ± 12.4b |
| χ2-value | 5195.2 | 330.4 | 424.4 | 26.5 | 105.9 | 1602.0 |
| df | 8 | 8 | 8 | 8 | 8 | 8 |
| p value | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
C/N ratio = ratio of total organic carbon to total nitrogen. In the same column, means (± standard error) followed by the same letters are not significantly different at p ≤ 0.05, n = 3.
All insects produced frass fertilizer with EC values greater than 6 mS cm−1 (Table 4). The highest EC was recorded in frass fertilizer produced by O. rhinoceros, significantly (p ≤ 0.001) higher than other frass fertilizer samples by 1.7–3.8 folds. Also, the EC of frass fertilizer from H. illucens and G. krucki was significantly (p ≤ 0.001) higher than those of S. icipe, B. mori, G. bimaculatus and T. molitor.
The ratios of ammonium to nitrate, and total organic carbon to total nitrogen (C/N ratio) also varied significantly among frass fertilizer samples (p ≤ 0.001) (Table 4). The ratios of ammonium to nitrates ranged between 0.01 and 79.5, frass fertilizer from S. gregaria and G. krucki had the highest and lowest values, respectively. Frass fertilizer from T. molitor, S. icipe, B. mori, G. bimaculatus, S. gregaria and O. rhinoceros achieved ammonium to nitrate ratios of ≤ 1. The C/N ratios of 13–23 were observed during experiments, whereby the H. illucens and G. krucki produced frass fertilizer with the lowest and highest C/N ratios, respectively. Gonimbrasia krucki frass fertilizer had significantly (p ≤ 0.001) higher C/N ratio than frass fertilizer fertilizers produced by P. sinuata, O. rhinoceros and H. illucens.
There were significant differences (p ≤ 0.001) in seed germination rate and germination indices of seeds exposed to frass fertilizer produced by various insect species (Table 4). Frass fertilizer from P. sinuata achieved the highest (97%) seed germination rate, 4 folds higher (p ≤ 0.001) than the value achieved using S. gregaria frass fertilizer. Furthermore, the seed germination rates of frass fertilizer produced by P. sinuata, O. rhinoceros and H. illucens were significantly (p ≤ 0.001) higher than that of the S. gregaria, B. mori, S. icipe and T. molitor. The seed germination rate of G. krucki frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer from S. gregaria and T. molitor (3.7 and 1.5 folds, respectively).
Hermertia illucens frass fertilizer achieved the highest germination index (267%), significantly (p ≤ 0.001) higher than the values obtained using other frass fertilizer samples by 3.7–46 folds (Table 4). The lowest germination index (6%) was obtained using frass fertilizer produced by S. gregaria. The germination index of O. rhinoceros frass fertilizer was significantly (p ≤ 0.001) higher than those of frass fertilizer produced by S. gregaria, T. molitor, B. mori, S. icipe, G. bimaculatus, and G. krucki by 12.7, 2.6, 2.5, 2.4, 2.0 and 1.6 folds, respectively. Pachnoda sinuata frass fertilizer achieved significantly (p ≤ 0.001) higher germination index than the other insect frass fertilizer samples, except for H. illucens, O. rhinoceros and G. krucki (Table 4).
Multivariate analysis of compost quality parameters
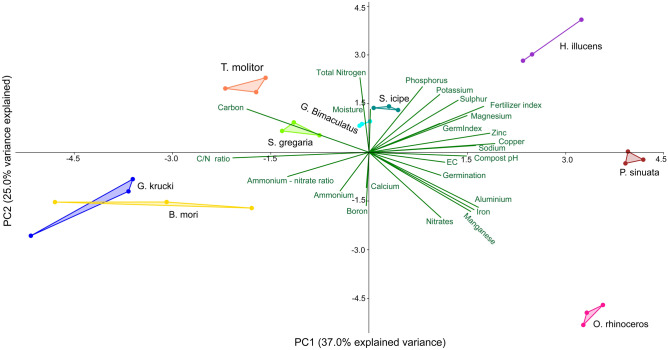
The principal component analysis (PCA) revealed that nutrient concentration, fertilizing index, and compost maturity were highly affected by the species of insects used to produce the frass fertilizer (Fig. 3). The first two components of the PCA accounted for 62% of the total variance whereby, PC1 accounted for 37% and PC2 accounted for 25%. It was noted that total phosphorus, potassium, sulphur, magnesium zinc, copper sodium, germination index and fertilizing were positively correlated, but negatively correlated with ammonium and ammonium to nitrate ratio (Fig. 3). The pH, electrical conductivity, germination rate, aluminium, iron, manganese, and nitrates were also highly correlated, but negatively correlated with total organic carbon. Total nitrogen, moisture content and total organic carbon were also highly correlated.
Figure 3.
Biplot graphs based on the principal component (PC) analysis of parameters that measure the quality of frass fertilizer composts produced by different edible insect species (n = 3).
Discussion
Nutrient levels of frass fertilizer generated by different insect species and potential for fertilizer use
Analysis of frass fertilizer from the nine insect species indicates high potential for use as high-quality and affordable alternative source of fertilizer compared to the scarce, costly and poor-quality organic fertilizers present in most regions of SSA14,17. The fertilizing index values of above three achieved by frass fertilizer produced by all the nine insect species (Fig. 2) also confirm the high suitability of insect frass fertilizer as a sustainable source of plant nutrients and quality fertilizer input for sustainable soil health management45.
Figure 2.
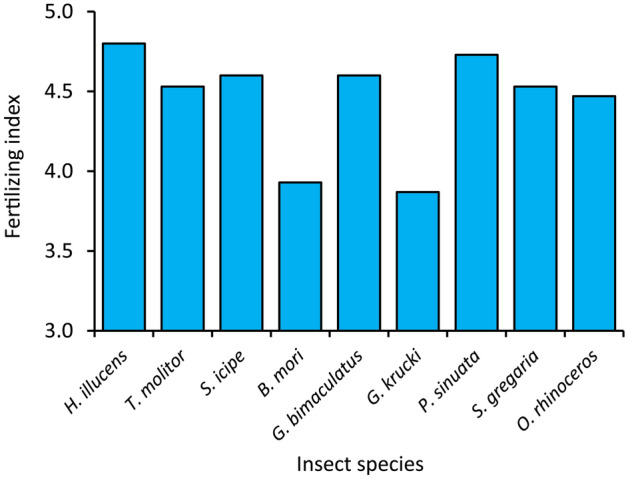
Fertilizing indices of frass fertilizer produced by different edible insect species, n = 3.
The high quantities of nitrogen, phosphorus, potassium, and micronutrients that would be supplied for crop production per season show that insect frass fertilizers could be relied on exclusively to cater for the nutrient demands of the key crop food and cash crop grown in SSA. For example, previous studies have reported higher yields and nutritional quality of maize, tomatoes, kales, French beans, cow peas, chilli pepper, shallots and barley grown using H. illucens frass fertilizer and meal worm frass fertilizer compared to conventional fertilizers27,28,42,52,53. Furthermore, soil amendment with frass fertilizer from H. illucens and T. molitor was found to suppress soil borne pathogens, stimulate soil microbial activity, reduce soil acidity and salinity, improve nitrogen mineralization, and increase availability of nutrients in the soil29–31,33,53, thus improving the soil quality for plant growth. It is therefore anticipated that adoption of insect fertilizer would contribute to improving food security by in SSA reversing the worrying trends in soil degradation, nutrient mining and declining crop productivity1,8,14.
The differences in nutrient concentrations and nutrient supply potential observed using frass fertilizer generated by different insect species highlights the variations in bioconversion and nutrient recycling efficiencies23. This could be largely attributed to differences in the nutritional quality of substrates used in rearing the various insect species39,40,54,55. For example, the higher concentration nitrogen, phosphorus and potassium in frass fertilizer produced by H. illucens, T. molitor, G. bimaculatus and S. icipe could be largely attributed to the high nutrient levels in the brewery spent grain, potato peels, wheat bran, soy bean and other substrates used to rear these insects26,56,57. In comparison however, G. krucki and B. mori were exclusively fed on tree branches with lower nutritional quality, thus producing frass fertilizer with less fertilizer potential. Nevertheless, the fertilizing indices of frass fertilizer produced by the two insect species were also above three, indicating potential for use as organic fertilizers45. There is inadequate research on the agronomic performance of most frass fertilizers assessed during the study. Therefore, field application of frass fertilizers would require prior agronomic studies to establish the optimal soil amendment rates for high nutrient release and synchrony for crop uptake, nutrient use efficiency, and crop yield and nutritional quality.
We found that the concentrations N, P, K, Ca, Mg, and S (> 1%) and those of Mn, Zn, Cu, Fe and B (> 0.1%) in frass fertilizers produced by all the nine insect species were within the recommended standards according to the Kenya Bureau of Standards guidelines for optimal commercial organic fertilizer58. Also, the nutrient concentrations in the various insect frass fertilizers meet the required international standards and guidelines for quality organic fertilizers in the United States, Canada and the European Union59.
It should be noted that the waste degradation efficiencies and composting periods of insect species differ, and thus the quantity of frass fertilizer available. For example, the H. illucens larvae have a high waste degradation efficiency (55–80%)60,61 and require a short bioconversion time, and thus could produce higher amounts of organic fertilizer than other insect species. On the other hand, the T. molitor, P. sinuata and O. rhinoceros take 4–6 weeks to convert organic waste into frass fertilizer, compared to 2 weeks for H. illucens larvae26, while S. gregaria and crickets can produce frass fertilizer daily but in less quantities compared to H. illucens larvae.
Maturity status of frass fertilizer generated by different insect species
Compost maturity refers to the degree of completeness of composting and absence of phytotoxic compounds and plant or animal pathogens that could negatively affect seed germination, plant growth and soil health46,47. In other words, compost maturity indicates the suitability of compost for field application as a fertilizer, using biological, physical and chemical indicators62–64. The values of C/N ratio (13–24) and ammonium concentration (0.01–56 mg kg−1) achieved during the study were within the ranges recommended by Goyal et al.63 (< 25) and Bernal et al.62 (< 400 mg kg−1), respectively, for quality compost production. Apart from G. krucki frass fertilizer, the pH of other insect frass fertilizer samples was within the acceptable range (6–8) for mature compost46.
The ratios of ammonium/nitrate of frass fertilizer produced by T. molitor, S. icipe, G. bimaculatus, P. sinuata, S. gregaria and O. rhinoceros were within the critical value (< 0.16) recommended by Bernal et al.62 for mature compost. On the other hand, the ratios of ammonium/nitrate recorded in frass fertilizer from H. illucens, B. mori and G. krucki (1–80) are comparable to the values reported by Guo et al.65 (1–60) and Beesigamukama et al.26 (0.3–34) for mature compost. This implies that the frass fertilizer produced by all the nine insect species can release adequate of quantities of nutrients once applied in the soil, thus high potential for improving soil and crop productivity. It is important to note that for all the frass fertilizer samples, the values of moisture content, ammonium concentration (Table 1), C/N and pH (Table 4), obtained were within the ranges recommended for mature and stable compost in Kenya58, the United States, Canada and European Union59.
The high seed germination rate (> 90%) and germination index (267%) achieved using H. illucens frass fertilizer indicate absence of phytotoxicity (Table 4), thus capacity to support optimal plant growth48. In comparison however, the germination index values of < 50% obtained using frass fertilizer from T. molitor, S. icipe, B. mori, G. krucki and S. gregaria indicate high phytotoxicity, thus low capacity to support crop growth without further treatment49. The germination index values of 50–80% achieved using frass fertilizer generated by P. sinuata and O. rhinoceros indicate moderate phytotoxicity and minimal suitability for crop production. Composts with high and moderate phytotoxicity have been reported to impair seed germination and radical elongation48,49.
The high and moderate levels of phytotoxicity observed in the above insect frass fertilizers could be attributed to the high salt (cation) concentration (Table 2) and high electrical conductivity (Table 3, Fig. 3)49,66. The values of electrical conductivity recorded in all frass fertilizers were above the allowable limit of < 4 mS cm−167. The low germination index and high electrical conductivity observed in all frass fertilizers except for H. illucens frass fertilizer, highlight the need for further composting and leaching to eliminate all the phytotoxic substances and excess salts, respectively. Future studies will be necessary to determine the time required to achieve full compost maturity and stability of frass fertilizer produced by the different insect species to improve suitability for field application.
Conclusion
Here, we present the first comparative report on the quality of frass fertilizer generated by nine edible insect species to offer specific guidelines on their effective use for improved soil health and crop productivity. The high nutrient levels, fertilizing indices and potential nutrient supply capacities of various frass fertilizer products indicate their suitability as sustainable alternative sources of plant nutrients. It is anticipated that the availability and adoption of insect frass fertilizers would significantly reduce overreliance on the unaffordable commercial mineral fertilizers as well as poor quality organic fertilizers. Frass fertilizer products from the H. illucens and the two cricket species (G. bimaculatus and S. icipe) were the best in terms of nutrient concentration, and potential supply capability. However, the frass fertilizer from all the insects, except H. illucens would require further composting to improve their maturity and stability. Further agronomic studies to establish the optimal amendment rates of the various frass fertilizers to ensure high nutrient release and synchrony for crop uptake, improved yield, and nutritional quality of food crops are crucial.
Materials and methods
Source of frass fertilizer compost samples
The frass fertilizer samples were sourced from insect colonies at Animal Rearing and Containment Unit (ARCU) at icipe (01° 13′ 25.3″ S, 36°53′ 49.2″ E, 1600 m asl), Nairobi, Kenya. Hermertia illucens frass fertilizer was obtained by feeding the larvae on a mixture of Irish potato peels and brewery spent grain for two weeks following procedures described by Beesigamukama et al.26. Schistocerca gregaria frass fertilizer was obtained from a colony of locusts fed on diet consisting of wheat and barley seedlings and wheat bran in a room maintained at 30 ± 4 °C, 40–50% relative humidity and a photoperiod of 12:12 L:D68.
The cricket frass fertilizer samples were obtained by feeding neonates on a diet consisting wheat bran, soy bean, sweet potato vines and weeds for a period of 2 months and 3 months for G. bimaculatus and S. icipe, respectively, following procedures described by Magara et al.56. Frass fertilizer sample of the P. sinuata and O. rhinoceros were obtained by feeding them on fresh cattle dung from a dairy farm for a period of 4–5 weeks. The pelletized faeces of the beetles were collected and stored for further analysis and utilization.
Tenebrio molitor frass fertilizer was obtained by feeding mealworms on wheat bran and chayote (Sechium edule) for a period of five weeks, following procedures described by Thévenot et al.69. Bombyx mori frass fertilizer was obtained by feeding silkworms on the leaves of mulberry tree (Morus spp.) for a period of six weeks, following the procedures described by Hailu70 and Nguku et al.71. For G. krucki, the frass fertilizer was obtained by feeding them on the Brazilian pepper tree leaves (Schinus terebinthifolia Raddi) for a period of 5 weeks. The frass fertilizer samples collected from various insect species were air-dried for five days pending laboratory analysis. Frass fertilizer products from crickets, beetles, S. gregaria, B. mori and G. krucki were in pelletized form, while that of the H. illucens and T. molitor were in powder form.
Determination of frass fertilizer quality
The fertilizer quality of the frass fertilizer generated by the different edible insects was assessed by determining the concentrations of macro—(total organic carbon [C], nitrogen [N], phosphorus [P] and potassium [K]), secondary—(calcium [Ca], magnesium [Mg] and sulphur [S]), and micro-nutrients (manganese [Mn], iron [Fe], zinc [Zn], copper [Cu], boron [B], sodium [Na] and aluminium [Al]) using standard laboratory methods described in “Concentrations of micronutrients in frass fertilizer produced by different insect species” section. Nutrient concentrations were determined using air-dried frass fertilizer samples. Furthermore, the C/N ratio and concentrations of C, N, P and K were used to determine the fertilizing index of frass fertilizers for soil and crop productivity using Eq. (1)45. On 5.0-point scale, only frass fertilizers with a fertilizing index of greater than 3.0 were considered suitable for use as organic fertilizer45
| 1 |
where Si represents the score value of analytical data. The Si scale ranges from 1 to 5; per parameter, a score of 1 represented the lowest concentration/value while 5 represented the highest. Wi represents the weighing factor of the ith fertility parameter. The scores of Wi were based on scientific knowledge on the roles of the nutrients and parameters in improving soil productivity. Consequently, Wi scores of 5, 3, 3, 1 and 3 were used for C, N, P, K and C/N ratio, respectively, according to Saha et al.45.
The concentrations of nutrients in different frass fertilizers were used to determine the amount of nutrients (N, P, K, Ca, Mg, S, Mn, Zn, Cu, Fe and B) that would be released for crop production per season per hectare (kg ha−1). The organic fertilizer application rate of 5 t ha−1 which is recommended in study country (Kenya) was used in the calculations (Eq. 2)51,72–75
| 2 |
where 5000 represents organic fertilizer application rate of 5000 kg ha−1 (dry weight).
Compost maturity and stability were assessed to determine if the frass fertilizer generated by various insects species were ready for use as organic fertilizers (freedom from compounds that could negatively affect seed germination and/or plant growth)46. Compost maturity and stability were determined using pH (6–8), ammonium concentration (< 400 mg kg−1), ammonium to nitrate ratio (< 0.16)46,62, electrical conductivity (< 4 mS cm−1)67 and C/N ratio (< 20)63, following the procedures described in “Concentrations of micronutrients in frass fertilizer produced by different insect species” section. Compost phytotoxicity tests were performed by determining seed germination index (> 80%), following standard procedures48,49. All tests compost maturity and stability were carried out using fresh frass fertilizer samples.
Laboratory analysis methods
Laboratory-based analysis of frass fertilizer pH and EC was carried out using aqueous extracts of 1:10 (weight/volume) compost to distilled water. The contents were then shaken for 30 min at 180 revolutions min−1 on an orbital and linear shaker (MI0103002, Foure’s scientific, China). The pH and EC were then read directly using a pH (AD1000, Adwa, Romania) and EC meter (AVI, Labtech, India), respectively76. The nitrate and ammonium were extracted from frass fertilizer using 0.5 M potassium sulphate at a ratio of 1:10 (weight/volume). Thereafter, the entire content of compost-potassium sulphate mixture was shaken for 1 h using an orbital and linear shaker (KOS–3333/KCS–3333, MRC, UK) as described above. The solution was later filtered through a Whatman No. 1 filter paper and the filtrate was used for further analyses. Furthermore, the nitrate and ammonium concentrations were determined by colorimetric methods as described by Okalebo et al.76.
Total organic carbon was determined using the wet oxidation method77 while total N, P, K, Ca, Mg, Mn, Fe, Zn, Cu, B, Na and Al were extracted using acid digestion method76. A sample weight of 0.3 g was used in the digestion. The nutrients were extracted using 10 ml of digestion mixture made by dissolving 0.42 g of selenium powder and 14 g of lithium sulphate in 350 ml of 30% hydrogen peroxide and 420 ml of concentrated sulphuric acid. The digestion process was carried out in 250 ml digestion tubes at temperatures of 360 °C for three hours to obtain a colourless solution (digestate) that was later used during the analysis of the different nutrients. Total N in the digestate was determined using the Kjeldahl distillation and titration method78. Total phosphorus was determined using the Ultraviolet–visible (UV–Vis) spectroscopy method76 by complexing the digestate with the solution containing sulphuric acid, ammonium molybdate, antimony potassium tartrate and ascorbic acid. Total K and Na were determined using flame photometry76.
The total concentrations of Ca, Mg, Mn, Fe, Zn and Cu in the digestates were determined using atomic absorption spectrometry (AAS) (iCE 3300 AA system, Thermo scientific, China) and element-specific wavelengths of 422.7, 285.2, 248.3, 248.3, 213.9, 324.7 nm, respectively76. The total concentrations of B and Al were determined using inductively coupled plasma-atomic emission spectrometry (249.77 nm) and dithionite–citrate method followed by AAS, respectively79.
The concentration of sulphur in frass fertilizer samples was extracted by heating 0.5 g in a muffle furnace at 450 °C for 2 h76. After cooling, 5 ml of 6 N HCl were added to the residue and the mixture was digested at 150 °C using for 25 min. After cooling, contents were transferred quantitatively to 100 ml volumetric flask, and topped up to the mark with distilled water. The extracts were then filtered through the extract through Whatman No. 40 filter paper. The total concentration of S in the filtrate was determined using the turbidimetric method at 420 nm, following procedures described in Okalebo et al.76.
Phytotoxicity test on frass fertilizer extracts
Seed germination index was determined by growing cabbage seeds in the various insect frass fertilizer extracts. Ten cabbage seeds were randomly selected and placed on petri dishes lined with filter paper moistened with 10 ml of 10% insect frass fertilizer extracts for 96 h at 25 °C in a dark chamber. The same procedure was repeated using distilled water as a positive control. After 96 h, germinated seeds were counted, and their radicle lengths measured. Germination index (GI) was calculated using Eq. (3)48. Frass fertilizer samples with GI values below 50% were considered highly phytotoxic, while values between 50 and 80% were moderately phytotoxic; and values above 80% indicated no phytotoxicity48,49
| 3 |
where RSG (%) represents the relative seed germination calculated as:
RRG (%) represents the relative root growth calculated as:
Data analysis
Before analysis, all data were checked for normality using the Shapiro–Wilk test. Normally distributed data was analysed using one-way analysis of variances, while data that was not normally distributed was analysed using generalised liner model (GLM) followed by analysis of deviances. Computation of least squares means was done using “lsmeans” package, followed by mean separation using adjusted Tukey’s method at p ≤ 0.05, implemented using “cld” function from the “multicompView” package. Principal component analysis was performed using the “prcomp” function from the “ggbiplot” package to examine the relationship among the frass fertilizer quality parameters. All the statistical analyses were conducted using R software version 4.0.380.
Acknowledgements
We are grateful for the financial support provided by the Canadian International Development Research Centre (IDRC) and the Australian Centre for International Agricultural Research (ACIAR) (INSFEED-Phase 2: Cultivate Grant No: 108866-001), Bill & Melinda Gates Foundation (INV-032416), the Curt Bergfors Foundation Food Planet Prize Award, Norwegian Agency for Development Cooperation, the Section for research, innovation, and higher education (CAP-Africa: Grant number: RAF-3058 KEN-18/0005), the Netherlands Organization for Scientific Research, WOTRO Science for Global Development (NWO-WOTRO) (ILIPA-W 08.250.202), the Rockefeller Foundation (SiPFeed-2018 FOD 009), the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors. We remain indebted to Faith N. Wamurango, Shem Ondiaka, Joshua Wambua, Isiah E. Rachami, and Judy Gitonga for maintaining the different insect colonies and their substantial contribution during insect rearing.
Author contributions
D.B., S.S. and C.M.T. conceived the experiments, D.B. and C.M.T. conducted the experiments, D.B. analyzed the data. C.M.T. acquired the funding. All authors reviewed the manuscript.
Data availability
All relevant data are presented in the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dennis Beesigamukama, Email: dbeesigamukama@icipe.org.
Chrysantus M. Tanga, Email: ctanga@icipe.org
References
- 1.Wortmann CS, et al. Diagnosis of crop secondary and micro-nutrient deficiencies in sub-Saharan Africa. Nutr. Cycl. Agroecosyst. 2019;113:127–140. doi: 10.1007/s10705-018-09968-7. [DOI] [Google Scholar]
- 2.Jambeck J, et al. Challenges and emerging solutions to the land-based plastic waste issue in Africa. Mar. Policy. 2018;96:256–263. doi: 10.1016/j.marpol.2017.10.041. [DOI] [Google Scholar]
- 3.FAO. The state of food and agriculture. Leveraging food systems for inclusive rural transformation. www.fao.org/publications (2017). 10.2307/2938399
- 4.Okot-Okumu J. Solid waste management in African Cities—East Africa. Waste Manag. Integr. Vis. 2012;57–72:3–20. [Google Scholar]
- 5.Cobo JG, Dercon G, Cadisch G. Nutrient balances in African land use systems across different spatial scales: A review of approaches, challenges and progress. Agric. Ecosyst. Environ. 2010;136:1–15. doi: 10.1016/j.agee.2009.11.006. [DOI] [Google Scholar]
- 6.Muniafu M, Otiato E. Solid Waste Management in Nairobi, Kenya. A case for emerging economies. J. Lang. Technol. Entrep. Africa. 2010;2:342–350. doi: 10.4314/jolte.v2i1.52009. [DOI] [Google Scholar]
- 7.Gachimbi LN, et al. Nutrient balances at farm level in Machakos (Kenya), using a participatory nutrient monitoring (NUTMON) approach. Land Use Policy. 2005;22:13–22. doi: 10.1016/j.landusepol.2003.07.002. [DOI] [Google Scholar]
- 8.Tully K, Sullivan C, Weil R, Sanchez P. The state of soil segradation in Sub-Saharan Africa: Baselines, trajectories, and solutions. Sustainability. 2015 doi: 10.3390/su7066523. [DOI] [Google Scholar]
- 9.Vanlauwe B, et al. Integrated soil fertility management in sub-Saharan Africa: Unravelling local adaptation. Soil J. 2015;1:491–508. doi: 10.5194/soil-1-491-2015. [DOI] [Google Scholar]
- 10.Ebanyat P, De Ridder N, De Jager A. Drivers of land use change and household determinants of sustainability in smallholder farming systems of Eastern Uganda. Popul. Environ. 2010;31:474–506. doi: 10.1007/s11111-010-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kihara J, et al. Understanding variability in crop response to fertilizer and amendments in sub-Saharan Africa. Agric. Ecosyst. Environ. 2016;229:1–12. doi: 10.1016/j.agee.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liverpool-Tasie LSO, Omonona BT, Sanou A, Ogunleye WO. Is increasing inorganic fertilizer use for maize production in SSA a profitable proposition? Evidence from Nigeria. Food Policy. 2017;67:41–51. doi: 10.1016/j.foodpol.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seleiman MF, et al. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass Bioenergy. 2013;59:477–485. doi: 10.1016/j.biombioe.2013.07.021. [DOI] [Google Scholar]
- 14.Stewart ZP, Pierzynski GM, Middendorf BJ, Vara Prasad PV. Approaches to improve soil fertility in sub-Saharan Africa. J. Exp. Bot. 2020;71:632–641. doi: 10.1093/jxb/erz446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusinamhodzi L, Dahlin S, Corbeels M. Living within their means: Reallocation of farm resources can help smallholder farmers improve crop yields and soil fertility. Agric. Ecosyst. Environ. 2016;216:125–136. doi: 10.1016/j.agee.2015.09.033. [DOI] [Google Scholar]
- 16.Rusinamhodzi L, Corbeels M, Giller KE. Diversity in crop residue management across an intensification gradient in southern Africa: System dynamics and crop productivity. Field Crop. Res. 2016;185:79–88. doi: 10.1016/j.fcr.2015.10.007. [DOI] [Google Scholar]
- 17.Ndambi OA, Pelster DE, Owino JO, de Buisonjé F, Vellinga T. Manure management practices and policies in Sub-Saharan Africa: Implications on manure quality as a fertilizer. Front. Sustain. Food Syst. 2019;3:1–14. doi: 10.3389/fsufs.2019.00029. [DOI] [Google Scholar]
- 18.Tumuhairwe JB, Tenywa JS, Otabbong E, Ledin S. Comparison of four low-technology composting methods for market crop wastes. Waste Manag. 2009;29:2274–2281. doi: 10.1016/j.wasman.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 19.van Huis A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013;58:563–583. doi: 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- 20.Makkar HPS, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014;197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- 21.Cheseto X, Baleba SB, Tanga CM, Kelemu S, Torto B. Chemistry and sensory characterization of a bakery edible insects. Foods. 2020;9:1–27. doi: 10.3390/foods9060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Huis A. Edible insects are the future? Proc. Nutr. Soc. 2016;75:294–305. doi: 10.1017/S0029665116000069. [DOI] [PubMed] [Google Scholar]
- 23.Oonincx DGAB, Van Broekhoven S, Van Huis A, Van Loon JJA. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE. 2015;10:1–20. doi: 10.1371/journal.pone.0144601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngoka BM, Kioko EN, Raina SK, Mueke JM, Kimbu DM. Semi-captive rearing of the African wild silkmoth Gonometa postica (Lepidoptera: Lasiocampidae) on an indigenous and a non-indigenous host plant in Kenya. Int. J. Trop. Insect Sci. 2007;27:183–190. doi: 10.1017/S1742758407883160. [DOI] [Google Scholar]
- 25.Magara HJO, et al. Edible crickets (Orthoptera) around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021;7:1–23. doi: 10.3389/fnut.2020.537915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beesigamukama D, et al. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021;119:183–194. doi: 10.1016/j.wasman.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Beesigamukama D, et al. Exploring black soldier fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020;11:1–17. doi: 10.3389/fpls.2020.574592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anyega AO, et al. Black soldier fly-composted organic fertilizer enhances growth, yield, and nutrient quality of three key vegetable crops in Sub-Saharan Africa. Front. Plant Sci. 2021;12:1–14. doi: 10.3389/fpls.2021.680312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beesigamukama D, et al. In situ nitrogen mineralization and nutrient release by soil amended with black soldier fly frass fertilizer. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-94269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poveda J, et al. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019;142:110–122. doi: 10.1016/j.apsoil.2019.04.016. [DOI] [Google Scholar]
- 31.Houben D, Daoulas G, Dulaurent AM. Assessment of the short-term fertilizer potential of mealworm frass using a pot experiment. Front. Sustain. Food Syst. 2021;5:1–7. doi: 10.3389/fsufs.2021.714596. [DOI] [Google Scholar]
- 32.Gärttling D, Kirchner SM, Schulz H. Assessment of the n- and p-fertilization effect of black soldier fly (Diptera: Stratiomyidae) by-products on maize. J. Insect Sci. 2020;20:1–11. doi: 10.1093/jisesa/ieaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houben D, Daoulas G, Faucon MP, Dulaurent AM. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somorin TO, Adesola S, Kolawole A. State-level assessment of the waste-to-energy potential (via incineration) of municipal solid wastes in Nigeria. J. Clean. Prod. 2017;164:804–815. doi: 10.1016/j.jclepro.2017.06.228. [DOI] [Google Scholar]
- 35.Friedrich E, Trois C. Current and future greenhouse gas (GHG) emissions from the management of municipal solid waste in the eThekwini Municipality—South Africa. J. Clean. Prod. 2016;112:4071–4083. doi: 10.1016/j.jclepro.2015.05.118. [DOI] [Google Scholar]
- 36.Chia SY, Tanga CM, van Loon JJ, Dicke M. Insects for sustainable animal feed: Inclusive business models involving smallholder farmers. Curr. Opin. Environ. Sustain. 2019;41:23–30. doi: 10.1016/j.cosust.2019.09.003. [DOI] [Google Scholar]
- 37.Bortolini S, et al. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020;262:121289. doi: 10.1016/j.jclepro.2020.121289. [DOI] [Google Scholar]
- 38.Abro Z, Kassie M, Tanga C, Beesigamukama D, Diiro G. Socio-economic and environmental implications of replacing conventional poultry feed with insect-based feed in Kenya. J. Clean. Prod. 2020;265:121871. doi: 10.1016/j.jclepro.2020.121871. [DOI] [Google Scholar]
- 39.Lalander C, Diener S, Zurbrügg C, Vinnerås B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens) J. Clean. Prod. 2019;208:211–219. doi: 10.1016/j.jclepro.2018.10.017. [DOI] [Google Scholar]
- 40.Klammsteiner T, Turan V, Juárez MF-D, Oberegger S, Insam H. Suitability of black soldier fly frass as soil amendment and implication for organic waste hygienization. Agronomy. 2020;10:1578. doi: 10.3390/agronomy10101578. [DOI] [Google Scholar]
- 41.Menino R, et al. Agricultural value of Black Soldier Fly larvae frass as organic fertilizer on ryegrass. Heliyon. 2021;7:e05855. doi: 10.1016/j.heliyon.2020.e05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beesigamukama D, et al. Nitrogen fertilizer equivalence of black soldier fly frass fertilizer and synchrony of nitrogen mineralization for maize production. Agronomy. 2020;10:1–9. doi: 10.3390/agronomy10091395. [DOI] [Google Scholar]
- 43.Rufino MC, et al. Competing use of organic resources, village-level interactions between farm types and climate variability in a communal area of NE Zimbabwe. Agric. Syst. 2011;104:175–190. doi: 10.1016/j.agsy.2010.06.001. [DOI] [Google Scholar]
- 44.Rufino MC, et al. Manure as a key resource within smallholder farming systems: Analysing farm-scale nutrient cycling efficiencies with the NUANCES framework. Livest. Sci. 2007;112:273–287. doi: 10.1016/j.livsci.2007.09.011. [DOI] [Google Scholar]
- 45.Saha JK, Panwar N, Singh MV. An assessment of municipal solid waste compost quality produced in different cities of India in the perspective of developing quality control indices. Waste Manag. 2010;30:192–201. doi: 10.1016/j.wasman.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 46.Bernal MP, Alburquerque JA, Moral R. Composting of animal manures and chemical criteria for compost maturity assessment A review. Bioresour. Technol. 2009;100:5444–5453. doi: 10.1016/j.biortech.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Bernal MP, et al. Current approaches and future trends in compost quality criteria for agronomic, environmental, and human health benefits. Adv. Agron. 2017;144:143–233. doi: 10.1016/bs.agron.2017.03.002. [DOI] [Google Scholar]
- 48.Emino ER, Warman PR. Biological assay for compost quality. Compost Sci. Util. 2004;12:342–348. doi: 10.1080/1065657X.2004.10702203. [DOI] [Google Scholar]
- 49.Teresa M, Remigio B. A review on the use of phytotoxicity as a compost quality indicator. Dyn. Soil Dyn. Plant Glob. Sci. Books. 2011;5:36–44. [Google Scholar]
- 50.Luo Y, et al. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018;71:109–114. doi: 10.1016/j.wasman.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Musyoka MW, et al. Nitrogen release and synchrony in organic and conventional farming systems of the Central Highlands of Kenya. Nutr. Cycl. Agroecosyst. 2019;113:283–305. doi: 10.1007/s10705-019-09978-z. [DOI] [Google Scholar]
- 52.Tanga CM, et al. Performance of black soldier fly frass fertiliser on maize (Zea mays L.) growth, yield, nutritional quality, and economic returns. J. Insects Food Feed. 2021 doi: 10.3920/jiff2021.0012. [DOI] [Google Scholar]
- 53.Quilliam RS, et al. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed. 2020;6:315–322. doi: 10.3920/JIFF2019.0049. [DOI] [Google Scholar]
- 54.Oonincx DGAB, van Huis A, van Loon JJA. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed. 2015;1:131–139. doi: 10.3920/JIFF2014.0023. [DOI] [Google Scholar]
- 55.Isibika A, Vinnerås B, Kibazohi O, Zurbrügg C, Lalander C. Pre-treatment of banana peel to improve composting by black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae. Waste Manag. 2019;100:151–160. doi: 10.1016/j.wasman.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Magara HJO, et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2018 doi: 10.1093/jee/toy397. [DOI] [PubMed] [Google Scholar]
- 57.van Broekhoven S, Oonincx DGAB, van Huis A, van Loon JJA. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015;73:1–10. doi: 10.1016/j.jinsphys.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Kenya Bureau of Standards. Organic fertilizer-Specification. Kenya Standard KS 2290:2017 (2017).
- 59.Brinton, W. Compost quality standards and guidelines: an international view. Woods End Research Laboratory, Inc., USA. https://woodsend.com//wp-content/uploads/2016/06/Brinton2000-International-Compost-Standards.pdf (2000).
- 60.Diener S, Studt Solano NM, Roa Gutiérrez F, Zurbrügg C, Tockner K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz. 2011;2:357–363. doi: 10.1007/s12649-011-9079-1. [DOI] [Google Scholar]
- 61.Lalander CH, Fidjeland J, Diener S, Eriksson S, Vinnerås B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015;35:261–271. doi: 10.1007/s13593-014-0235-4. [DOI] [Google Scholar]
- 62.Bernal MP, Paredes C, Sánchez-Monedero MA, Cegarra J. Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour. Technol. 1998;63:91–99. doi: 10.1016/S0960-8524(97)00084-9. [DOI] [Google Scholar]
- 63.Goyal S, Dhull SK, Kapoor KK. Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresour. Technol. 2005;96:1584–1591. doi: 10.1016/j.biortech.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Khan N, et al. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 2014;168:245–251. doi: 10.1016/j.biortech.2014.02.123. [DOI] [PubMed] [Google Scholar]
- 65.Guo R, et al. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012;112:171–178. doi: 10.1016/j.biortech.2012.02.099. [DOI] [PubMed] [Google Scholar]
- 66.Beesigamukama D, et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE. 2020;15:e0238154. doi: 10.1371/journal.pone.0238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang GF, Wong JWC, Wu QT, Nagar BB. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004;24:805–813. doi: 10.1016/j.wasman.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Cheseto X, et al. Potential of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) as an unconventional source of dietary and therapeutic sterols. PLoS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0127171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thévenot A, et al. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018;170:1260–1267. doi: 10.1016/j.jclepro.2017.09.054. [DOI] [Google Scholar]
- 70.Hailu A. Assessment of growth and performance of silk worms (Bombyx mori L.) on mulberry leaves at Jimma, South West Ethiopia. J. Biol. Agric. Healthc. 2016;6:10–16. [Google Scholar]
- 71.Nguku EK, Muli EF, Raina SK. Larvae, cocoon and post-cocoon characteristics of Bombyx mori L. (Lepidoptera: Bombycidae) fed on mulberry leaves fortified with Kenyan royal jelly. Int. J. Appl. Sci. Environ. Manag. 2007;11(4):85–89. [Google Scholar]
- 72.Adamtey N, et al. Productivity, profitability and partial nutrient balance in maize-based conventional and organic farming systems in Kenya. Agric. Ecosyst. Environ. 2016;235:61–79. doi: 10.1016/j.agee.2016.10.001. [DOI] [Google Scholar]
- 73.Musyoka MW, Adamtey N, Muriuki AW, Cadisch G. Effect of organic and conventional farming systems on nitrogen use efficiency of potato, maize and vegetables in the Central highlands of Kenya. Eur. J. Agron. 2017;86:24–36. doi: 10.1016/j.eja.2017.02.005. [DOI] [Google Scholar]
- 74.Mucheru-Muna M, et al. Enhancing maize productivity and profitability using organic inputs and mineral fertilizer in central Kenya small-hold farms. Exp. Agric. 2014;50:250–269. doi: 10.1017/S0014479713000525. [DOI] [Google Scholar]
- 75.Micheni, A., Tuwei, P., Mugwe, J. & Kiruiro, E. Integration of organic and inorganic soil fertility improvement inputs for improved crop yields in Central Highlands of Kenya. In 12thISCO Conference, Beijing. 1, 362–367 (2002).
- 76.Okalebo, J. R., Gathua, K. W. & Woomer, P. L. Laboratory Methods of Soil and Plant Analysis: A Working Manual 2nd edn (SACRED Africa, 2002).
- 77.Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. Methods of soil analysis Part 2: Chemical and microbiological properties. Am. Soc. Agron. 1982;9:539–579. [Google Scholar]
- 78.Jackson ML. Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd.; 1973. [Google Scholar]
- 79.Carter MR, Gregorich EG. Soil Sampling and Methods of Analysis. 2. CRC Press; 2008. [Google Scholar]
- 80.R Core Team. R: A Language and Environment for Statistical Computing. (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are presented in the paper.