Summary
Five decades ago, seminal studies positioned the brainstem locus coeruleus (LC) norepinephrine (NE) system as a key substrate for the regulation of wakefulness and sleep, and this picture has recently been elaborated thanks to methodological advances in the precise investigation and experimental modulation of LC structure and functions. This review presents and discusses findings that support the major role of the LC-NE system at different levels of sleep-wake organization, ranging from its involvement in the overall architecture of the sleep-wake cycle to its associations with sleep microstructure, while accounting for the intricate neuroanatomy surrounding the LC. Given the particular position held by the LC-NE system by being at the intersection of sleep-wake dysregulation and initial pathophysiological processes of Alzheimer’s disease (AD), we conclude by examining emerging opportunities to investigate LC-NE mediated relationships between sleep-wake alteration and AD in human aging. We further propose several research perspectives that could support the LC-NE system as a promising target for the identification of at-risk individuals in the preclinical stages of AD, and for the development of novel preventive interventions.
Keywords: Locus coeruleus, Norepinephrine, Sleep-wake regulation, Sleep microstructure, Human aging, Tau pathology, Alzheimer’s disease
Introduction
From young adulthood to older age, several macro- and microstructural aspects of sleep and wakefulness are altered so much that it may constitute one of the most evident behavioral and physiological correlates of human aging [1]. With increasing age, sleep becomes shallower and shorter, and the overall sleep-wake cycle appears more fragmented, as evidenced by the frequent intrusion of wakefulness periods during sleep (i.e. nocturnal awakenings) in older individuals [1]. Over the past decade, a growing body of evidence has established that such changes in wakefulness and sleep contribute to the pathophysiological mechanisms of neurodegenerative diseases including Alzheimer’s disease (AD), which often involves what could be considered as an exacerbated form of age-related sleep-wake dysregulation [2]. Interestingly, the majority of the nuclei controlling sleep and wakefulness are affected by neuropathological processes early in the course of AD, before the onset of cognitive symptoms [3]. Among them, the brainstem locus coeruleus (LC)-norepinephrine (NE) system is receiving increasing attention, owing to a series of recent advances in the precise investigation of its structure and function [4]. Crucially, these scientific breakthroughs provide researchers with a new theoretical framework which sets the LC-NE system as a nexus between early sleep-wake disruption in aging and AD pathogenesis.
Many questions remain unanswered, however, with regards to the exact contribution of the LC-NE system to the regulation of sleep and wakefulness and their associated features, especially in humans as illustrated by the scarce number of in vivo studies. Here, we will start by introducing the structure and functions of the LC-NE system in the context of the broader sleep-wake circuitry. We will then present evidence supporting the important role of the LC-NE system in the overall organization of the sleep-wake cycle. Next, we will discuss the involvement of the LC-NE system in sleep macrostructure, i.e. in non-pathologic transitions across sleep stages from non-rapid eye movement (NREM) to rapid-eye movement (REM) sleep, with an emphasis on the specific relationships between LC-NE and REM sleep. Recent findings of direct associations with microstructural aspects of sleep will also be considered. We will end with emerging questions and perspectives regarding the potential utility of investigating LC-NE mediated sleep-wake alteration in aging and AD.
The locus coeruleus-norepinephrine system: structure, functions, and proxy measures
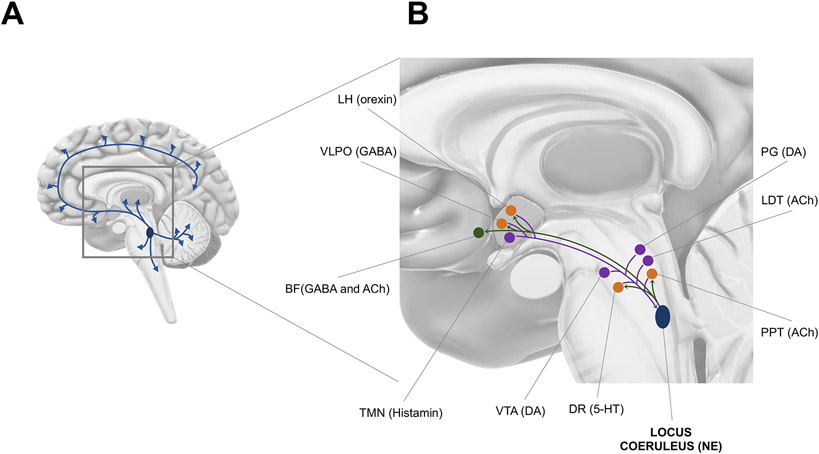
The LC is a nucleus located bilaterally in the dorsal area of the rostral pons, lateral to the fourth ventricle, extending from the lower level of the inferior colliculus to the motor nucleus of the trigeminal nerve [5]. The name locus coeruleus (Latin for ‘blue spot’) originates from the blue color observed during the initial histological investigation of this brain structure, which is due to the presence of neuromelanin granules within LC NE-containing neurons. Despite its small number of neurons and its modest size (~14.5 mm long and ~2.5 mm thick in adult humans) [6], the LC constitutes the primary source of NE for the central nervous system [4]. LC neurons possess immensely ramified axons that allow for extensive projections and release of NE over the whole brain, including the hippocampus, amygdala, thalamus, and neocortex (Fig. 1), with the exception of a few dopaminergic basal nuclei [7]. Mixed findings were reported with regards to age-related effects on LC-NE structural alteration in post-mortem investigations of non-pathological aging, whereas several in vivo studies suggest an inverted U-shape curve between MRI-assessed LC signal and increasing age, with the 5th decade as a tipping point [6,8]. By contrast, LC-NE neurodegeneration is clearly evident in neurodegenerative conditions, such as AD, with patients consistently displaying LC neuronal loss compared to controls, as early as in the prodromal stages of the disease [6].
Fig. 1. Afferences and efferences of the brainstem locus coeruleus (LC).
(A) The LC sends ubiquitous projections over most of the brain including the hippocampus, amygdala, thalamus, and neocortex.
(B) Relationships between the LC and other sleep-wake
centers. Purple dots  represent nuclei which send projections to the LC; green dots
represent nuclei which send projections to the LC; green dots  represent nuclei which receive inputs
from the LC; orange dots
represent nuclei which receive inputs
from the LC; orange dots  represent nuclei which both send and receive projections from the LC. LC
efferences (
represent nuclei which both send and receive projections from the LC. LC
efferences ( ) target
cholinergic and GABAergic neurons of the basal forebrain (BF), GABAergic neurons
of the ventrolateral preoptic area (VLPO) in the anterior hypothalamus,
orexinergic neurons of the lateral hypothalamus (LH), serotoninergic neurons of
the dorsal raphe (DR), and cholinergic neurons of the pedunculopontine tegmentum
(PPT) nucleus. LC afferences (
) target
cholinergic and GABAergic neurons of the basal forebrain (BF), GABAergic neurons
of the ventrolateral preoptic area (VLPO) in the anterior hypothalamus,
orexinergic neurons of the lateral hypothalamus (LH), serotoninergic neurons of
the dorsal raphe (DR), and cholinergic neurons of the pedunculopontine tegmentum
(PPT) nucleus. LC afferences (  ) arise from orexinergic neurons of the lateral hypothalamus (LH), GABAergic
neurons of the VLPO, histaminergic neurons of the tuberomammillary nucleus
(TMN), dopaminergic neurons of the ventral tegmental area (VTA), serotoninergic
neurons of the dorsal raphe (DR), cholinergic neurons of the pedunculopontine
tegmentum (PPT) and laterodorsal tegmentum (LDT), and dopaminergic neurons of
the periaqueductal grey matter (PG). All nuclei are positioned for illustrative
purpose and may not reflect their precise anatomical location. 5-HT=serotonin;
ACh=acetylcholine; BF=basal forebrain; DA=dopamine; DR=dorsal raphe; LC=locus
coeruleus; LDT=laterodorsal tegmentum; LH=lateral hypothalamus;
NE=norepinephrine; PG=periaqueductal grey matter; PPT=pedunculopontine
tegmentum; TMN=tuberomammillary nucleus; VLPO=ventrolateral preoptic area;
VTA=ventral tegmental area.
) arise from orexinergic neurons of the lateral hypothalamus (LH), GABAergic
neurons of the VLPO, histaminergic neurons of the tuberomammillary nucleus
(TMN), dopaminergic neurons of the ventral tegmental area (VTA), serotoninergic
neurons of the dorsal raphe (DR), cholinergic neurons of the pedunculopontine
tegmentum (PPT) and laterodorsal tegmentum (LDT), and dopaminergic neurons of
the periaqueductal grey matter (PG). All nuclei are positioned for illustrative
purpose and may not reflect their precise anatomical location. 5-HT=serotonin;
ACh=acetylcholine; BF=basal forebrain; DA=dopamine; DR=dorsal raphe; LC=locus
coeruleus; LDT=laterodorsal tegmentum; LH=lateral hypothalamus;
NE=norepinephrine; PG=periaqueductal grey matter; PPT=pedunculopontine
tegmentum; TMN=tuberomammillary nucleus; VLPO=ventrolateral preoptic area;
VTA=ventral tegmental area.
Three families of receptors (α1-, α2-, and β-adrenergic receptors), with either excitatory (α1, β) or inhibitory (α2) effects on cell signaling, have been identified as widespread binding sites for the action of NE [9]. Given the neuromodulatory properties of NE, the primary role of the LC-NE system is to modulate its targets in order to induce and/or maintain behavioral states and state-dependent cognitive processes [4]. The LC-NE system is therefore involved in regulating a broad range of brain functions and processes [10], including arousal, attention, autonomic activity, emotional regulation, memory, sensory processing, nociception, or stress. The LC-NE system fulfills its functions through two modes of functioning, defined by either tonic or phasic discharge patterns [11]. During wakefulness, tonic LC-NE activity is state-dependent and covaries with arousal levels [12,13]. In addition to tonic discharge rates, phasic bursts of LC-NE neurons are elicited when confronted with novel or salient stimuli, and this phasic LC activity is mirrored by frequency increases in electroencephalography (EEG) and behavioral markers of attention and alertness [14]. Beside electrophysiological recordings, the activity of the LC-NE system can be indirectly assessed through proxy measures derived from LC-NE neuronal activity, such as extracellular levels of NE [15] or variations in pupil size [16].
The locus coeruleus-norepinephrine system and the anatomy of the sleep-wake circuitry
The LC-NE system is part of the reticular formation, a network of nuclei composing the ascending and descending pathways. While the latter deals with regulation of sensory and motor aspects (e.g. nociception, muscular tonus) and will not be considered here, the position of the LC-NE system in the former is the focus of the present section. In addition to the LC, the ascending arousal system includes the pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei, the raphe nucleus, and the ventral tegmental area (VTA), releasing acetylcholine (Ach), serotonin (5-HT) and dopamine, respectively. Together with the basal forebrain (Ach), the hypothalamus (histamine and orexin), and the action of fast neurotransmitters (glutamate and GABA), these wakefulness-promoting systems are opposed to the sleep-promoting action of GABAergic and galaninergic neurons of the preoptic area, melanin-concentrating hormone (MCH) neurons of the hypothalamus, and GABAergic neurons of the parafacial zone. Altogether, this intricate sleep-wake circuitry constitutes the neurobiological underpinning of sleep and wakefulness regulation (for a detailed visualization of the sleep-wake circuitry and pathways, see [17]).
More than 40 years ago, retrograde tracing studies suggested that LC-NE neurons were topographically organized according to their target projection sites [4,18], and this modular architecture was later confirmed with the use of viral-genetic approaches [7]. Within the sleep-wake neurobiological network, LC-NE neurons were found to project to cholinergic and GABAergic neurons of the basal forebrain, GABAergic neurons of the ventrolateral preoptic area (VLPO) in the anterior hypothalamus, orexinergic neurons of the lateral hypothalamus, serotoninergic neurons of the dorsal raphe, and cholinergic neurons of the PPT nucleus [3,17,19] (Fig. 1).
Likewise, afferent projections to LC-NE neurons were initially quantified with retrograde labeling techniques [20], and the picture was recently refined by viral tracing methods, showing that the LC receives connections from more than 100 brain regions [7]. Among the sleep-wake circuitry, LC-NE neurons receive inputs from orexinergic neurons of the lateral hypothalamus, GABAergic neurons of the VLPO and ventral lateral hypothalamus, histaminergic neurons of the tuberomammillary nucleus (TMN), dopaminergic neurons of the VTA, serotoninergic neurons of the dorsal raphe, cholinergic neurons of the PPT and LDT, and dopaminergic neurons of the periaqueductal grey matter [3,17,19] (Fig. 1).
Importantly, the existence of GABAergic LC neurons located in the dendritic field surrounding the LC nucleus (termed pericerulear or peri-LC region), and also found intertwined with LC-NE neurons, was recently identified as serving an important inhibiting function to locally regulate LC-NE tonic and phasic activity [21].
This complex afferent-efferent organization, complemented by a local gain mechanism based on GABAergic inhibition of LC-NE activity, allows LC-NE neurons to integrate information from multiple sources and act as a broadcasting center for the whole brain, which constitutes a crucial feature to support the many roles of the LC over multiple timescales [10]. Particularly for sleep-wake regulation, the anatomical interconnections with several sleep- and wake-promoting nuclei place the LC as an important contributor to the onset and maintenance of sleep and wakefulness states, as well as to their associated behavioral and electrophysiological properties.
The locus coeruleus-norepinephrine system and the sleep-wake cycle
Early electrophysiological, pharmacological, and lesion studies
Early electrophysiological studies on the activity of the LC-NE system across the sleep-wake cycle in rodents, cats, and monkeys, established that tonic LC activity progressively decreases when animals switch from engaged, behaviorally active states (~3Hz) to quieter, resting conditions (~1Hz), to slow wave sleep (< 1Hz) [22,23]. During sleep, LC-NE neurons were found to anticipate sleep-to-wake transitions, as they display bursts of activity in the seconds preceding spontaneous or sensory-evoked awakenings [12]. This state-dependent LC-NE neuronal discharge pattern was further corroborated by reports showing that NE levels in the pons, amygdala, and hippocampus are high during wakefulness, lower during quiet wake, and lowest during sleep [24,25]. Likewise, a novel experimental paradigm that allows to track pupil diameter across wakefulness and sleep states in rodents revealed that pupil size was progressively smaller when shifting from wakefulness to sleep, mirroring the gradual silencing of LC-NE neurons across behavioral states [26].
Accordingly, a series of pharmacological studies investigating the impact of modulating LC-NE activity on sleep-wake periods showed that injection of α2-adrenergic receptor agonists into the LC area, such as clonidine or dexmedetomidine, or combined α1- and β-adrenergic receptor blockade, suppressed LC-NE activity and induced dose-dependent sedative states in rats [27-29]. In contrast, activating LC-NE neurons through yohimbine, an α2-adrenergic receptor antagonist, increased wakefulness [27].
Unlike pharmacological studies, early lesion studies in rodents, rabbits, and cats provided conflicting evidence about the consequences of LC damages on sleep-wake states, with some reporting increased wakefulness and reduced drowsiness [30,31] while others found acute suppressed wakefulness [32] or limited to no effect on time spent in wakefulness or sleep states [33]. Of important note, the coverage and accuracy of LC lesions in the aforementioned studies were inherently linked to the technique used (e.g. electrolytic-, neurotoxic-, cryo-lesion). Thus, these inconsistencies may be tied to the extent of LC injuries and to robust compensatory responses within surviving LC neurons that help sustain NE release and post-synaptic NE uptake, as long as no more than 90% of the LC is damaged [34], a threshold that was usually not reached in those studies. More recently, specific lesioning of more than 95% of LC-NE neurons in rats did not affect the total duration of wakefulness per 24h [35,36], but significantly compromised the promoting effect of exposure to a novel environment on the maintenance of wakefulness [36].
Saper and colleagues proposed the so-called ‘flip-flop’ mechanism to describe the transitions between wakefulness and sleep [37]. According to this model, two mutually inhibitory circuits are driving the onset and maintenance of wakefulness and sleep, forming a bi-stable switch which supports consolidated periods and helps preventing intermediate states: wakefulness is considered to be driven mainly by the influences of monoaminergic neurons, i.e. LC-NE, serotoninergic neurons from raphe nuclei, histaminergic neurons from TMN, and cholinergic neurons of PPT and LDT, while sleep is promoted through the inhibitory action on this arousal circuit by GABAergic and galaninergic neurons located in the VLPO and median preoptic nuclei [38]. Importantly, the development of novel methods covering pharmacogenetics, chemogenetics, and optogenetics, was instrumental in enabling new experimental manipulations aimed at unraveling the precise role of the LC-NE system within this framework.
Shedding new light on the Blue Spot
In 2010, Carter et al. demonstrated that photoinhibition of LC-NE neurons during the active period caused a reduction in time spent in wakefulness as well as an increase in wake-to-sleep transitions. Conversely, photoactivation of LC-NE neurons during the inactive period produced immediate sleep-to-wake transitions [39]. Moreover, using a dual optogenetic approach, they further showed that photoactivation of orexinergic neurons of the lateral hypothalamus, which send dense projections to the LC [40], concomitant with photoinhibition of LC-NE activity prevented sleep-to-wake transitions, while simultaneous photoactivation of both nuclei significantly increased the probability of awakenings [41]. Hence, the LC-NE system also serves as a necessary and sufficient gateway for the effect of upstream arousal-regulating neuronal ensembles [41,42]. More recently, the role of LC-NE activity in sleep-to-wake transitions was further expanded to awakenings triggered by external perturbators, and it was shown that the probability to transition from sleep to wakefulness in response to auditory stimuli was increased after photoactivation of LC-NE neurons and decreased after their photoinhibition in rats [43]. Altogether, these compelling optogenetic findings demonstrate that LC-NE activity is crucially involved in the regulation of both endogenous and sensory-evoked transitions from sleep to wake states (Fig. 2).
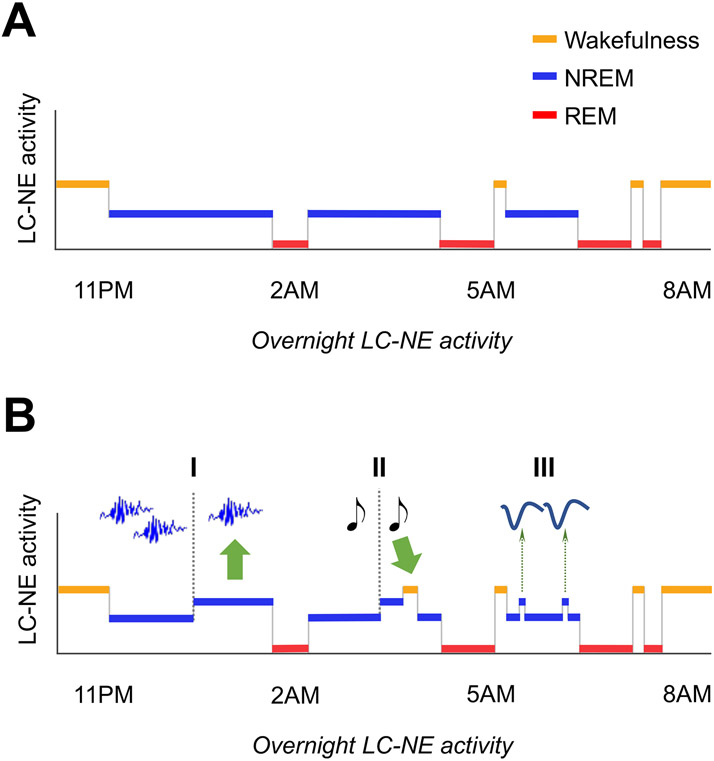
Fig. 2. Overnight LC-NE activity vs. sleep macro- and microstructure.
(A) LC-NE activity and sleep macrostructure. LC-NE tonic firing rate is highest during wakefulness, lower during NREM sleep, and virtually silent during REM sleep.
(B) LC-NE activity and sleep microstructure. Elevated LC-NE activity (I) reduces spindle occurrence and (II) increases likelihood of sensory-evoked awakenings; (III) Learning-dependent increase in LC-NE activity is phase-locked to the rising phase of NREM slow oscillations. Separation into I/II/III segments is for visual representation only. Figure overall layout is inspired by Van Someren (2020).
Importantly, while the LC consists mainly of NE-ergic neurons, other chemical compounds, including wake-promoting neurotransmitters such as dopamine, are produced or co-released by LC neurons [44]. A recent study therefore used optogenetics combined with LC-NE cell-type specific selective knockdown of dopamine beta hydroxylase, a necessary enzyme for NE synthesis: after this specific genetic disruption of NE production in mice, the duration of wakefulness was reduced and optogenetically-driven sleep-to-wake transitions immediately following stimulations of LC-NE neurons were abolished [45], supporting the essential role of NE for LC-mediated regulation of wakefulness periods.
Additional influences: importance of circadian and homeostatic factors
It is well established that the organization of the sleep-wake cycle is regulated by overarching circadian (‘process C’) and homeostatic (‘process S’) factors [46], and that alteration of these two processes in the course of human aging underlie the age-related changes in sleep and wake phenotypes [1]. Crucially, a series of evidence suggest that the circadian and homeostatic processes may directly or indirectly involve the LC-NE system [13].
Trans-synaptic retrograde tracing revealed that the LC receives indirect input from the suprachiasmatic nucleus (SCN) of the hypothalamus, the central pacemaker responsible for the generation of circadian rhythms, through critical relay nuclei including the dorsomedial hypothalamic nucleus (DMH) [47]. In that same study, Aston-Jones et al. reported that tonic LC-NE activity itself displays a certain degree of circadian variations: in rats maintained in free-running conditions under constant darkness for at least three days, LC-NE neurons were found to discharge faster during the active circadian period compared to the inactive circadian period. In addition, they showed that such circadian fluctuations in LC-NE activity was abolished after DMH lesioning [47]. Although systematic assessments of LC-NE activity across multiple circadian times within the same animals were lacking in that study, the existence of circadian rhythmicity within the LC-NE system was further corroborated by reports of circadian influences on NE content within the LC and SCN [48] and on the number of α- and β-receptors in the rat brain [49], as well as circadian variations in steady-state pupil size [50,51] and in glucose metabolism in the LC area [52] in humans. Overall, these findings suggest that, as part of the SCN-DMH-LC circuit, the LC-NE system is under strong circadian influence and, in turn, contribute to the circadian regulation of the sleep-wake cycle [53].
With regards to homeostatic influences, the increase in slow wave sleep after sleep deprivation, which constitutes a gold standard marker of the wake-dependent build-up of sleep need, was largely dampened by lesioning the LC-NE system in rats [54-56]. Interestingly, release of NE during wakefulness strongly promotes synaptic potentiation [57], which has been directly related to the amount of slow-wave activity (0.5-4Hz) during the following night [58,59]. Moreover, in vivo microdialysis experiments in mice showed that NE levels in the prefrontal cortex increase during prolonged wakefulness, and that LC-NE neurons projecting to the medial prefrontal cortex were particularly affected by neural fatigue [60], which may contribute to the specific cognitive impairment resulting from sleep deprivation (i.e. vigilance, working memory).
The locus coeruleus-norepinephrine system and sleep macrostructure
Akin to the discrepancy observed in LC-NE discharge rate between wakefulness and sleep, the activity of the LC-NE system is differentially regulated across sleep stages. In 1975, Hobson et al. described that transitions from NREM to REM sleep were anticipated by an increase in activity within a pool of cholinergic neurons located in the gigantocellular tegmental field (FTG), concomitant with a silencing of neurons from the posteroventral LC [61]. Therefore, the authors proposed a computational model revolving around a reciprocal inhibitory interplay between REM-OFF LC-NE and REM-ON cholinergic FTG neurons to regulate the onset and offset of REM episodes [62]. Accordingly, early electrophysiological observations indicated that reduced, but existent tonic LC-NE activity occurred during NREM sleep, while LC-NE neurons were virtually silent during REM sleep [12,22] (Fig. 2). Likewise, REM sleep has been associated with the lowest level of extracellular NE in the amygdala and in the pons [25] and with the highest degree of pupil constriction [26], reflecting almost complete inactivity of the LC-NE system.
As for the investigation of the sleep-wake cycle, conflicting evidence arose from lesion studies assessing the impact of damaging the LC on NREM and REM duration [30,33,35]. In addition, neither optogenetic inhibition nor stimulation of LC-NE neurons yielded significant changes on the duration of REM episodes or the probability to transition from NREM to REM sleep [39]. These latter observations led to the hypothesis that the LC-NE system would be involved in the modulation of REM sleep rather than directly contributing to its genesis [17,38]; a causal role that has now been principally attributed to mutually inhibitory cell groups located in the mesopontine tegmentum: the REM-ON glutamatergic and GABAergic cells of the sublaterodorsal tegmental nucleus (also termed subcoeruleus region in humans) and the REM-OFF GABAergic neurons of the ventrolateral periaqueductal grey matter and lateral pontine tegmentum [63]. It is important to note, however, that the effort to identify the neurobiological circuit underlying REM sleep control is still ongoing, and the contributions of additional nuclei, are continuously being unveiled [64,65].
The LC-NE system during REM sleep: a silence that speaks volume
The complete silencing of LC-NE neurons observed during REM sleep episodes is a unique condition for the brain [66]. Among the many purposes attributed to REM sleep, it is therefore understandable that some of them directly relate to the (dys)function of the LC-NE system [63]. For instance, it was proposed that the temporary NE-free milieu inherent to REM sleep constitutes a prerequisite for the upregulation of NE receptors after the continuous exposure to NE during wakefulness and NREM sleep [67]. Others proposed that the silence of LC-NE neurons during REM sleep acts as a critical process to maintain an optimal level of brain excitability, based on a series of observations demonstrating that elevated concentrations of NE follow specific REM sleep deprivation and that the resulting excess of NE induces aberrant neuronal excitability through modulation of Na-K ATPase activity [68,69].
Importantly, the activity pattern of the LC-NE system during sleep has also been recently linked to the consolidation of memories [70,71], with a particular emphasis on the sleep-dependent processing of emotional memories [72]. Within that framework, the absence of LC-NE neuronal activity during REM sleep is proposed to provide synapses with a suitable neuromodulatory environment that allows for neuronal depotentiation, which is otherwise blocked by the effect of NE, and subsequent plastic rewiring of memory schemas in the hippocampus [70], while the timely discharge of LC-NE neurons during NREM sleep would promote plasticity during memory replay (discussed in more details in the following section) [73]. With regards to consolidation of emotional memories, LC-NE neuronal silence during REM episodes is thought to support the integration of the content of a given emotional event in memory networks while disconnecting and downplaying its associated arousal in limbic structures, so that the event can be subsequently recalled without triggering the original emotional reaction [66]. Particular attention has therefore been allocated towards elucidating the emotional function of LC-NE silence during REM sleep, as its disruption –or an endogenous predisposition to be disrupted– has been further postulated to strongly contribute to certain psychiatric conditions, such as post-traumatic stress disorder (PTSD) [74] and insomnia [66]. Correspondingly, novel experimental findings in humans showed that the sleep-dependent adaptation to stressful stimuli or self-conscious emotions is impaired after restless REM episodes, reflecting abnormal activity of the LC-NE system during REM sleep, in healthy individuals and insomnia patients [75,76] as well as in PTSD [77].
Altogether, these elements provide a strong rationale to further investigate the causes, correlates, and consequences of abnormal LC-NE activity during critical time windows of supposed silence, especially given the foreseeable clinical applications among several LC-NE-associated psychiatric and neurological disorders, but also in non-pathological aging which typically involves an increase in time spent in lighter sleep stages at the expense of REM sleep.
The locus coeruleus-norepinephrine system and sleep microstructure
While investigating LC-NE unit activity across the sleep-wake cycle, Aston-Jones & Bloom already noted that, during NREM sleep, tonic LC-NE activity displayed consistent fluctuations around spindles (trains of transient 12-16Hz waves): LC-NE neurons became almost silent in the seconds preceding the onset of a spindle, substantially increased firing during spindle activity, and decreased discharge again after the spindle offset [12]. These findings were recently refined and expanded by fiber photometry and optogenetic studies in rodents demonstrating that troughs in extracellular NE concentration during NREM sleep concurred with spindle activity [78,79], and that stimulation of LC-NE neurons reduced spindle occurrence [43,79] (Fig. 2) and impaired sleep-dependent memory consolidation [80,81], while their inhibition increased spindle density and interfered with their temporal distribution, specifically through altered NE signaling in the thalamus [79]. In addition to the interplay with spindle activity, electrophysiological recordings in rats further showed that LC-NE neurons display a learning-dependent increase in activity during NREM sleep [82], and that this activity is phase-locked to the rising phase of NREM cortical slow oscillations [83] (Fig. 2).
Overall, these findings point at an overarching function for LC-NE neurons to provide memory circuits with an optimal neuromodulatory background; a dual task which involves balancing between promoting synaptic potentiation during NREM sleep and synaptic depotentiation during REM sleep [66]. Importantly, the interplay between LC-NE activity and sleep microstructure might be particularly relevant in human aging, as the preservation of those microstructural aspects have been linked to preserved cognitive performance in older adults [84].
The locus coeruleus-norepinephrine system and sleep-wake disruption in human aging and Alzheimer’s disease
The vast majority of the findings described so far are based on animal studies, which provide inherent advantages when characterizing the consequences of experimental manipulation of LC-NE neurons on wakefulness and sleep. However, early evidence from human studies also contributed to identify and characterize the important role of the LC-NE system for sleep-wake regulation mechanisms. Almost 50 years ago, studies in Parkinson’s disease or progressive supranuclear palsy (PSP) patients suggested that damage to the LC may underlie the observed disruption of wakefulness and sleep periods, including alteration of EEG features across sleep stages, increased nocturnal awakenings, and suppression of REM episodes [85,86]. A single case study in a young adult with cerebral palsy further reported that electrical stimulation of the LC led to an increase of wakefulness at the expense of REM sleep [87]. Although limited, these initial observations hint at the translational potential of animal findings and support the relevance of investigating LC-NE-mediated sleep-wake alterations in conditions associated with LC injuries in humans. Here, we argue that Alzheimer’s disease (AD) provides a valuable research perspective, given that the LC-NE system holds a crucial position by being at the intersection of initial AD-related pathophysiological processes [88] and sleep-wake dysregulation. In this final section, we will gather evidence supporting that the LC-NE system constitutes a strong neurobiological candidate underlying the sleep-wake disturbances commonly observed along the progression of AD, and we will examine emerging opportunities to test this assumption that are feasible due to recent advances in the assessment of LC-NE structural and functional properties in vivo.
Sleep-wake regulation and AD pathogenesis: identifying a new locus of action
As illustrated by a growing body of evidence over the past decade, sleep-wake dysregulation has emerged as a potent modifiable factor to slow down the characteristic pathophysiological processes of the disease, i.e. the accumulation of beta-amyloid (Aβ) and tau misfolded proteins together with neurodegeneration, as early as during the preclinical stages of the disease [2]. Indeed, important discoveries established that the disruption of sleep and wakefulness constitutes a core mechanism of early AD pathogenesis: the physiological dynamics of both Aβ and tau proteins, encompassing their release and clearance are regulated by the sleep-wake cycle [89-91]. Conversely, both Aβ and tau pathology per se induce alteration of the sleep-wake cycle in AD mouse models [92,93], supporting bidirectionality in the relationships between sleep-wake disturbances and AD-related neuropathological processes [2].
Crucially, landmark post-mortem studies in humans revealed that, beside its close connection with sleep-wake regulation described throughout this review, the LC is among the first sites of tau pathology across the lifespan [88], such that the vast majority of individuals harbor abnormally phosphorylated tau in the LC by the age of 40 [94]. In addition, the consequences of accumulated tau burden within the LC were found to be specifically expressed in AD cases compared to other tauopathies (i.e. PSP, cortico-basal degeneration), as substantial LC neuronal loss appeared as a phenotype exclusive to AD [95].
Combined, these observations lend support to a theoretical framework in which the LC-NE system would constitute a bridge connecting sleep-wake dysregulation and initial AD-related pathophysiological processes (Fig. 3). In mice, chronic sleep disruption or intermittent short sleep for three days a week during one month in mice was sufficient to produce profound alterations in LC-NE morphology, as evidenced by a drastic reduction of LC-NE neuronal counts and axonal projections [96]. Moreover, in a mouse model of tauopathy, repeated exposures to shortened sleep accelerated tau accumulation within LC-NE neurons and its progression to the entorhinal cortex, hippocampus, and amygdala, and advanced the onset of neurobehavioral deficits [97]. Remarkably, these effects were long-lasting, with structural alteration of the LC-NE system and cognitive impairments persisting one year after the chronic sleep disruption protocol [98]. These animal findings suggest that the negative consequences of early-in-life sleep-wake dysregulation precipitate and sustain AD-related processes within the LC-NE system.
Fig. 3. Emerging framework.
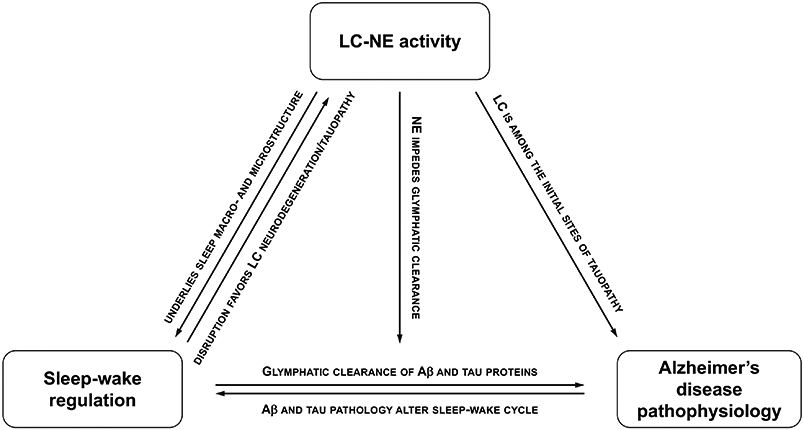
Schematic representation of the LC-NE system constituting a bridge that connects sleep-wake dysregulation and initial AD-related pathophysiological processes.
Bringing similar research questions into human studies has long been hindered by the considerable challenge to image the LC-NE, due to its deep location and its small size. However, recent advances in MRI methods now allow to investigate LC-NE structural and functional integrity with relatively short acquisition times to accommodate clinical studies [99]. Importantly, the benefits of such sequences are exponentially amplified when implemented at ultra-high field (i.e. ≥ 7 Tesla MRI), given the sub-millimeter resolution available to identify and characterize the LC [100]. In that context, it was established that MRI-assessed LC-related contrast, supposedly reflecting neuronal and fiber projection density [101], starts declining slowly in the 5th decade of life in cognitively unimpaired older individuals [8], while it is markedly reduced and correlated with Aβ pathology in AD patients [102] and with tau pathology in asymptomatic older individuals [103].
Interestingly, a parallel may be drawn between deterioration of the LC-NE system and the unfolding of sleep-wake disruption in late life; a relationship that appears even more striking during the course of AD. Sleep disturbances are common in AD patients, starting as early as in the preclinical stages under the form of exacerbated age-related sleep-wake disruption [2], but their prevalence and magnitude increase with disease severity [104] which often precipitate institutionalization [105]. Of note, most of these disturbances pertain to sleep-wake dimensions that are tightly linked to the function of LC-NE system based on evidence from animal models: fragmented sleep with more frequent nocturnal arousals and awakenings, insomnia, poorer REM sleep integrity, and reduction in spindle density and slow-wave activity [104]. Yet, while the significant degeneration of the LC-NE system observed at histological investigation of AD brains was suggested to contribute to the disruption of the sleep-wake cycle commonly experience by AD patients [95], no direct assessments of sleep-wake measures were available in these post-mortem studies. Similarly, except for two recent studies discussed below, no in vivo studies correlated LC-NE properties with sleep-wake measurements in healthy aging or AD, leaving important gaps in the understanding of the interplay between alteration of the LC-NE system, sleep-wake dysregulation, and AD-related pathophysiological processes in humans. Hereafter, we propose research perspectives to address these unanswered questions, and that would pinpoint the LC-NE system as a new target connecting early sleep-wake disruption to AD pathogenesis.
Emerging opportunities and perspectives
Before addressing the context of AD, an important first step for the field will be to characterize the relationships between LC-NE structural/functional properties and sleep-wake regulation across the human lifespan. Experimental protocols should therefore take advantage of the aforementioned MRI methods, ideally at ultra-high field, to establish the links between fine-grained LC-related MRI metrics and subjective (e.g. sleep questionnaires) as well as objective (e.g. EEG, actigraphy) sleep-wake dimensions in different age groups. For example, aligning with previous work in animals, it would be informative to assess whether the structural integrity of the LC-NE system or its resting-state functional activity patterns [76,106], is associated with the relative duration or distribution of EEG-derived sleep stages in humans. For that purpose, particular attention should be allocated to examine non-linear associations [107,108] as well as the influence of confounding factors such as depression or anxiety levels, which are directly related to both the LC-NE system [109] and sleep-wake quality [66]. In addition, the inherent difficulty to record human sleep in MRI, a fortiori in older individuals, may tend to force comparison of EEG-derived sleep features acquired outside the scanner with LC functional characteristics measured during wakefulness. Existing studies using simultaneous EEG/fMRI during sleep usually collect only incomplete sleep cycles during which naturally occurring REM sleep is not reached while inside the scanner.
In the context of AD, additional animal work relating glymphatic clearance of toxic proteins to fluctuations of LC-NE activity across the sleep-wake cycle would provide valuable insights into the potential pathways linking the LC-NE system to AD pathogenesis through sleep-wake dysregulation. Indeed, in their landmark work on the role of the sleep-wake cycle for metabolite clearance in mice, Xie et al. demonstrated that elevated NE levels impaired glymphatic function by restricting the interstitial space volume [89], but no direct assessment of LC-NE activity was available in that study. In turn, existing optogenetic studies modulating LC-NE activity across the sleep-wake cycle have not evaluated its impact on glymphatic clearance, nor did they investigate the long-term effect of chronic LC-NE photoactivation/photoinhibition on Aβ or tau protein burden in AD mouse models. In humans, clear-cut evidence and methods to measure such a sleep-dependent glymphatic clearance system are still lacking [110], although one recent study by Fultz et al. provides support to the existence of a temporally ordered sequence of events during sleep in the human brain, linking the occurrence of slow waves to CSF flow through hemodynamic oscillations [111].
Importantly, the relationships between alteration of the LC-NE system and sleep-wake disturbances should be investigated across the different stages of the disease, and their combined contribution to forecast cognitive trajectories should be evaluated. One recent study by Elman et al. provides preliminary evidence in that direction, indicating that reduced MRI-assessed LC-NE contrast is associated with worse self-reported metrics of daytime functioning among 481 older men from the Vietnam Era Twin Study of Aging, and that both lower LC-NE structural integrity and increased daytime dysfunction act as independent predictors of increased odds of conversion to mild cognitive impairment [112]. We showed that lower integrity of the middle-to-caudal LC, as measured with a 7T LC-NE MRI sequence, is associated with more frequent self-reported nocturnal awakenings in 72 cognitively unimpaired older adults, particularly in individuals with higher plasma levels of total tau protein burden [113], a biomarker of increased risk for cognitive decline and incident dementia. Additional longitudinal designs with repeated cognitive, sleep-wake, and LC MRI assessments in cognitively impaired and unimpaired older individuals will be crucial to shed light on the temporal ordering of events, and will contribute to understanding the isolated protective effects of preserved sleep-wake regulation [114] and LC-NE integrity [115] for cognitive performance in aging and AD.
Of note, the specificity of the LC-NE system in the aforementioned relationships will have to be tested against the isolated role of other wake-promoting neuronal populations that also exhibit marked neurodegeneration in AD, such as the orexinergic cells of the lateral hypothalamus or the histaminergic neurons from the TMN [95,116]. In particular, as the LC-NE system has been suggested to act as an effector of the wake-promoting inputs from the orexinergic neurons [41], the structural and functional connections between the LC and these hypothalamic neurons warrant further attention to disentangle their respective contributions to age- and AD-related sleep-wake disruption [117].
Finally, interventional studies interfering with sleep-wake regulation and/or modulation of LC-NE activity through pharmacological means or non-invasive stimulation techniques will be critical to address causality in the aforementioned relationships. Indirectly bolstering LC-NE function with transcutaneous vagus nerve stimulation has been proposed to harbor some positive effects on subjective sleep quality in one study of older individuals [118], but objective evaluation of its quantitative impact and its long-term effects on the sleep-wake cycle, potentially in combination with other sleep-wake interventions such as sleep hygiene education or cognitive behavioral therapy for insomnia, remains to be performed. In turn, the deleterious effect of fragmented sleep-wake periods on the structural integrity of the LC-NE system proposed in animal studies [97,98] remain to be established in humans. Studies including clinical sleep interventions in populations with different degrees of sleep-wake fragmentation, such as elderly individuals, frequent nappers, or sleep apnea patients, would thus provide valuable knowledge for further development and evaluation of preventive interventions in populations at higher risk of cognitive decline and AD trajectories.
Practice Points.
The brainstem locus coeruleus constitutes the primary source of norepinephrine for the central nervous system, and is an important arousal-promoting nucleus of the sleep-wake circuitry.
Locus coeruleus-norepinephrine tonic firing rate fluctuates across the sleep-wake cycle: highest during wakefulness, lower during non-rapid eye movement sleep, and virtually silent during rapid eye movement sleep; locus-coeruleus norepinephrine activity is also influenced by both homeostatic and circadian factors.
During sleep, locus coeruleus-norepinephrine activity regulates sleep-to-wake transitions and is phase-locked to sleep microstructural elements such as spindles and slow waves; its silence during rapid eye movement sleep appears critical for neuronal depotentiation, brain excitability, and emotional regulation.
The locus coeruleus-norepinephrine system holds a crucial position at the intersection between initial Alzheimer’s disease-related pathophysiological processes and sleep-wake regulation in human aging.
Preliminary findings based on recent MRI developments bring support to in vivo investigations of locus coeruleus-norepinephrine-mediated sleep-wake dysregulation in Alzheimer’s disease pathogenesis.
Research Agenda.
Quantitative in vivo studies in humans to better characterize the associations between locus coeruleus-norepinephrine structural/functional properties and sleep-wake regulation across the lifespan.
Animal studies aiming to clarify the links between glymphatic clearance of toxic proteins and locus coeruleus-norepinephrine activity across the sleep-wake cycle.
Longitudinal studies in individuals across the Alzheimer’s disease continuum to evaluate the contribution of locus coeruleus-norepinephrine-mediated sleep-wake alterations to neuropathophysiological and cognitive trajectories.
Interventional studies, in animal models and in humans, aiming at modulating locus coeruleus-norepinephrine activity to address causality in the aforementioned relationships and to inform on novel preventive strategies among clinical sleep populations.
Funding
M.V.E. is supported by Wallonia-Brussels International (SOR/2020/479197) and BrightFocus Foundation (A20211016F). E.K. and G.V. are supported by Fonds de la Recherche Scientifique-FNRS Belgium (F.R.S.-FNRS, F.4513.17 & T.0242.19), Fondation Recherche Alzheimer (SAO-FRA 2019/0025), and University of Liège (ULiège). H.I.L.J. receives funding from the National Institute on Aging (R01AG062559, R01AG068062, R21AG074220).
Abbreviations:
- 5-HT
serotonin
- ACh
acetylcholine
- AD
Alzheimer’s disease
- Aβ
beta-amyloid
- BOLD
blood-oxygen level dependent
- DMH
dorsomedial hypothalamic nucleus
- EEG
electroencephalography
- FTG
gigantocellular tegmental field
- LC
locus coeruleus
- LDT
laterodorsal tegmental nucleus
- MCH
melanin-concentrating hormone
- NE
norepinephrine
- NREM
non-rapid eye movement
- PPT
pedunculopontine nucleus
- PSP
progressive supranuclear palsy
- PTSD
post-traumatic stress disorder
- REM
rapid-eye movement
- SCN
suprachiasmatic nucleus
- TMN
tuberomammillary nucleus
- VLPO
ventrolateral preoptic area
- VTA
ventral tegmental area
Footnotes
Conflicts of interest: the authors do not have any conflicts of interest to disclose.
References
- [1].Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron 2017;94:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Egroo M, Narbutas J, Chylinski D, Villar González P, Maquet P, Salmon E, et al. Sleep–wake regulation and the hallmarks of the pathogenesis of Alzheimer’s disease. Sleep 2019;42:1–13. [DOI] [PubMed] [Google Scholar]
- [3].Lew CH, Petersen C, Neylan TC, Grinberg LT. Tau-driven degeneration of sleep- and wake-regulating neurons in Alzheimer’s disease. Sleep Med Rev 2021;60:101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 2020;21:644–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR, et al. Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol 2010;518:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beardmore R, Hou R, Darekar A, Holmes C, Boche D. The Locus Coeruleus in Aging and Alzheimer’s Disease: A Postmortem and Brain Imaging Review. J Alzheimer’s Dis 2021;83:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 2015;524:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu KY, Marijatta F, Hämmerer D, Acosta-Cabronero J, Düzel E, Howard RJ. Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neurosci Biobehav Rev 2017;83:325–55. [DOI] [PubMed] [Google Scholar]
- [9].Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res 2006;326:541–51. [DOI] [PubMed] [Google Scholar]
- [10].Chandler DJ, Jensen P, McCall JG, Pickering AE, Schwarz LA, Totah NK. Redefining Noradrenergic Neuromodulation of Behavior: Impacts of a Modular Locus Coeruleus Architecture. J Neurosci 2019;39:8239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Devilbiss DM. Consequences of tuning network function by tonic and phasic locus coeruleus output and stress: Regulating detection and discrimination of peripheral stimuli. Brain Res 2019;1709:16–27. [DOI] [PubMed] [Google Scholar]
- [12].Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1981;1:876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aston-Jones GSPD, Gonzalez M, Doran S. Role of the locus coeruleus-norepinephrine system in arousal and circadian regulation of the sleep–wake cycle. In: Ordway GA, Schwartz MA, Frazer A, editors. Brain Norepinephrine, Cambridge: Cambridge University Press; 2007, p. 157–95. [Google Scholar]
- [14].Holland N, Robbins TW, Rowe JB. The role of noradrenaline in cognition and cognitive disorders. Brain 2021;144:2243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mather M Noradrenaline in the aging brain: Promoting cognitive reserve or accelerating Alzheimer’s disease? Semin Cell Dev Biol 2021;116:108–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Joshi S, Gold JI. Pupil Size as a Window on Neural Substrates of Cognition. Trends Cogn Sci 2020;24:466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saper CB, Fuller PM. Wake–sleep circuitry: an overview. Curr Opin Neurobiol 2017;44:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 1979;187:703–24. [DOI] [PubMed] [Google Scholar]
- [19].Samuels E, Szabadi E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr Neuropharmacol 2008;6:235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog. Brain Res, vol. 88, 1991, p. 47–75. [DOI] [PubMed] [Google Scholar]
- [21].Breton-Provencher V, Sur M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 2019;22:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci 1980;77:3033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chu N -s., Bloom FE. Norepinephrine-Containing Neurons: Changes in Spontaneous Discharge Patterns during Sleeping and Waking. Science 1973;179:908–10. [DOI] [PubMed] [Google Scholar]
- [24].Kalen P, Rosegren E, Lindvall O, Bjorklund A. Hippocampal Noradrenaline and Serotonin Release over 24 Hours as Measured by the Dialysis Technique in Freely Moving Rats: Correlation to Behavioural Activity State, Effect of Handling and Tail-Pinch. Eur J Neurosci 1989;1:181–8. [DOI] [PubMed] [Google Scholar]
- [25].Shouse MN, Staba RJ, Saquib SF, Farber PR. Monoamines and sleep: Microdialysis findings in pons and amygdala. Brain Res 2000;860:181–9. [DOI] [PubMed] [Google Scholar]
- [26].Yüzgeç Ö, Prsa M, Zimmermann R, Huber D. Pupil Size Coupling to Cortical States Protects the Stability of Deep Sleep via Parasympathetic Modulation. Curr Biol 2018;28:392–400.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Sarro GB, Ascioti C, Froio F, Libri V, Nisticò G. Evidence that locus coeruleus is the site where clonidine and drugs acting at α1- and α2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol 1987;90:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Correa-Sales C, Rabin BC, Maze M. A Hypnotic Response to Dexmedetomidine, an α2 Agonist, Is Mediated in the Locus Coerüleus in Rats. Anesthesiology 1992;76:948–52. [DOI] [PubMed] [Google Scholar]
- [29].Berridge C, España R Synergistic sedative effects of noradrenergic α1- and β-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 2000;99:495–505. [DOI] [PubMed] [Google Scholar]
- [30].Braun CMJ, Pivik RT. Effects of locus coeruleus lesions upon sleeping and waking in the rabbit. Brain Res 1981;230:133–51. [DOI] [PubMed] [Google Scholar]
- [31].Cespuglio R, Gomez M, Faradji H, Jouvet M. Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroencephalogr Clin Neurophysiol 1982;54:570–8. [DOI] [PubMed] [Google Scholar]
- [32].Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res 1973;58:157–77. [DOI] [PubMed] [Google Scholar]
- [33].Caballero A, De Andrés I. Unilateral lesions in locus coeruleus area enhance paradoxical sleep. Electroencephalogr Clin Neurophysiol 1986;64:339–46. [DOI] [PubMed] [Google Scholar]
- [34].Berridge CW, Schmeichel BE, España RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev 2012;16:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of Saporin-Induced Lesions of Three Arousal Populations on Daily Levels of Sleep and Wake. J Neurosci 2007;27:14041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, et al. Locus Ceruleus and Anterior Cingulate Cortex Sustain Wakefulness in a Novel Environment. J Neurosci 2010;30:14543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Saper CB, Chou TC, Scammell TE. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726–31. [DOI] [PubMed] [Google Scholar]
- [38].Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron 2010;68:1023–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[39].Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 2010;13:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 1999;415:145–59. [PubMed] [Google Scholar]
- [41].Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci 2012;109:E2635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carter ME, de Lecea L, Adamantidis A. Functional wiring of hypocretin and LC-NE neurons: Implications for arousal. Front Behav Neurosci 2013;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[43].Hayat H, Regev N, Matosevich N, Sales A, Paredes-Rodriguez E, Krom AJ, et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci Adv 2020;6:eaaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Oh J, Petersen C, Walsh CM, Bittencourt JC, Neylan TC, Grinberg LT. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry 2019;24:1284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yamaguchi H, Hopf FW, Li S-B, de Lecea L. In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness. Nat Commun 2018;9:5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: A reappraisal. J Sleep Res 2016;25:131–43. [DOI] [PubMed] [Google Scholar]
- *[47].Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci 2001;4:732–8. [DOI] [PubMed] [Google Scholar]
- [48].Semba J, Toru M, Mataga N. Twenty-Four Hour Rhythms of Norepinephrine and Serotonin in Nucleus Suprachiasmaticus, Raphe Nuclei, and Locus Coeruleus in the Rat. Sleep 1984;7:211–8. [DOI] [PubMed] [Google Scholar]
- [49].Wirz-Justice A, Kafka MS, Naber D, Wehr TA. Circadian rhythms in rat brain alpha- and beta-adrenergic receptors are modified by chronic imipramine. Life Sci 1980;27:341–7. [DOI] [PubMed] [Google Scholar]
- [50].Van Egroo M, Gaggioni G, Cespedes-Ortiz C, Ly JQM, Vandewalle G. Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men. Clocks & Sleep 2019;1:240–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Daguet I, Bouhassira D, Gronfier C. Baseline Pupil Diameter Is Not a Reliable Biomarker of Subjective Sleepiness. Front Neurol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, et al. Regional Brain Glucose Metabolism During Morning and Evening Wakefulness in Humans: Preliminary Findings. Sleep 2004;27:1245–54. [DOI] [PubMed] [Google Scholar]
- [53].Szabadi E Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Front Neurol 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].González MM, Valatx JL, Debilly G. Role of the locus coeruleus in the sleep rebound following two different sleep deprivation methods in the rat. Brain Res 1996;740:215–26. [DOI] [PubMed] [Google Scholar]
- [55].Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci 2004;24:5410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci 2005;25:4503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain 2010;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vyazovskiy V V, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, et al. Cortical Firing and Sleep Homeostasis. Neuron 2009;63:865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huber R, Tononi G, Cirelli C. Exploratory Behavior, Cortical BDNF Expression, and Sleep Homeostasis. Sleep 2007;30:129–39. [DOI] [PubMed] [Google Scholar]
- *[60].Bellesi M, Tononi G, Cirelli C, Serra PA. Region-Specific Dissociation between Cortical Noradrenaline Levels and the Sleep/Wake Cycle. Sleep 2016;39:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hobson J, McCarley R, Wyzinski P. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 1975;189:55–8. [DOI] [PubMed] [Google Scholar]
- [62].McCarley R, Hobson J. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science 1975;189:58–60. [DOI] [PubMed] [Google Scholar]
- [63].Peever J, Fuller PM. The Biology of REM Sleep. Curr Biol 2017;27:R1237–48. [DOI] [PubMed] [Google Scholar]
- [64].Valencia Garcia S, Brischoux F, Clément O, Libourel PA, Arthaud S, Lazarus M, et al. Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat Commun 2018;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kroeger D, Bandaru SS, Madara JC, Vetrivelan R. Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience 2019;406:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev 2020;51:physrev.00046.2019. [DOI] [PubMed] [Google Scholar]
- [67].Siegel JM, Rogawski MA. A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res Rev 1988;13:213–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Khanday MA, Somarajan BI, Mehta R, Mallick BN. Noradrenaline from Locus Coeruleus Neurons Acts on Pedunculo-Pontine Neurons to Prevent REM Sleep and Induces Its Loss-Associated Effects in Rats. Eneuro 2016;3:ENEURO.0108–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Amar M, Mallick BN. Rapid Eye Movement Sleep Deprivation Associated Increase in Na-K ATPase Activity in the Rat Brain is Due to Noradrenaline Induced α1-Adrenoceptor Mediated Increased α-Subunit of the Enzyme. Neurochem Res 2015;40:1747–57. [DOI] [PubMed] [Google Scholar]
- [70].Poe GR. Sleep Is for Forgetting. J Neurosci 2017;37:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sara SJ. Sleep to Remember. J Neurosci 2017;37:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Goldstein AN, Walker MP. The Role of Sleep in Emotional Brain Function. Annu Rev Clin Psychol 2014;10:679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sara SJ. Locus Coeruleus in time with the making of memories. Curr Opin Neurobiol 2015;35:87–94. [DOI] [PubMed] [Google Scholar]
- [74].Vanderheyden WM, Poe GR, Liberzon I. Trauma exposure and sleep: Using a rodent model to understand sleep function in PTSD. Exp Brain Res 2014;232:1575–84. [DOI] [PubMed] [Google Scholar]
- *[75].Wassing R, Lakbila-Kamal O, Ramautar JR, Stoffers D, Schalkwijk F, Van Someren EJW. Restless REM Sleep Impedes Overnight Amygdala Adaptation. Curr Biol 2019;29:2351–2358.e4. [DOI] [PubMed] [Google Scholar]
- [76].Gong L, Shi M, Wang J, Xu R, Yu S, Liu D, et al. The Abnormal Functional Connectivity in the Locus Coeruleus-Norepinephrine System Associated With Anxiety Symptom in Chronic Insomnia Disorder. Front Neurosci 2021;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lipinska G, Thomas KG. The interaction of REM fragmentation and night-time arousal modulates sleep-dependent emotional memory consolidation. Front Psychol 2019;10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kjaerby C, Andersen M, Hauglund N, Ding F, Wang W, Xu Q, et al. Dynamic fluctuations of the locus coeruleus-norepinephrine system underlie sleep state transitions. BioRxiv 2020:2020.09.01.274977. [Google Scholar]
- [79].Osorio-Forero A, Cardis R, Vantomme G, Guillaume-Gentil A, Katsioudi G, Devenoges C, et al. Noradrenergic circuit control of non-REM sleep substates. Curr Biol 2021;31:5009–5023.e7. [DOI] [PubMed] [Google Scholar]
- *[80].Swift KM, Gross BA, Frazer MA, Bauer DS, Clark KJD, Vazey EM, et al. Abnormal Locus Coeruleus Sleep Activity Alters Sleep Signatures of Memory Consolidation and Impairs Place Cell Stability and Spatial Memory. Curr Biol 2018;28:3599–3609.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Novitskaya Y, Sara SJ, Logothetis NK, Eschenko O. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn Mem 2016;23:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: Brain stem-cortex interplay for memory consolidation? Cereb Cortex 2008;18:2596–603. [DOI] [PubMed] [Google Scholar]
- *[83].Eschenko O, Magri C, Panzeri S, Sara SJ. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb Cortex 2012;22:426–35. [DOI] [PubMed] [Google Scholar]
- [84].Djonlagic I, Mariani S, Fitzpatrick AL, Van Der Klei VMGTH, Johnson DA, Wood AC, et al. Macro and micro sleep architecture and cognitive performance in older adults. Nat Hum Behav 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mouret J Differences in sleep in patients with parkinson’s disease. Electroencephalogr Clin Neurophysiol 1975;38:653–7. [DOI] [PubMed] [Google Scholar]
- [86].Gross RA, Spehlmann R, Daniels JC. Sleep disturbances in progressive supranuclear palsy. Electroencephalogr Clin Neurophysiol 1978;45:16–25. [DOI] [PubMed] [Google Scholar]
- [87].Kaitin KI, Bliwise DL, Gleason C, Nino-Murcia G, Dement WC, Libet B. Sleep disturbance produced by electrical stimulation of the locus coeruleus in a human subject. Biol Psychiatry 1986;21:710–6. [DOI] [PubMed] [Google Scholar]
- [88].Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol 2012;25:708–14. [DOI] [PubMed] [Google Scholar]
- [89].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019;363:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med 2014;211:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Holth JK, Mahan TE, Robinson GO, Rocha A, Holtzman DM. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann Clin Transl Neurol 2017;4:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of -Amyloid in Mice with Alzheimer’s Disease Pathology. Sci Transl Med 2012;4:150ra122–150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories From 1 to 100 Years. J Neuropathol Exp Neurol 2011;70:960–9. [DOI] [PubMed] [Google Scholar]
- *[95].Oh J, Eser RA, Ehrenberg AJ, Morales D, Petersen C, Kudlacek J, et al. Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimer’s Dement 2019;15:1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhu Y, Fenik P, Zhan G, Somach R, Xin R, Veasey S. Intermittent Short Sleep Results in Lasting Sleep Wake Disturbances and Degeneration of Locus Coeruleus and Orexinergic Neurons. Sleep 2016;39:1601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhu Y, Zhan G, Fenik P, Brandes M, Bell P, Francois N, et al. Chronic Sleep Disruption Advances the Temporal Progression of Tauopathy in P301S Mutant Mice. J Neurosci 2018;38:10255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[98].Owen JE, Zhu Y, Fenik P, Zhan G, Bell P, Liu C, et al. Late-in-Life Neurodegeneration after Chronic Sleep Loss in Young Adult Mice. Sleep 2021;0:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kelberman M, Keilholz S, Weinshenker D. What’s That (Blue) Spot on my MRI? Multimodal Neuroimaging of the Locus Coeruleus in Neurodegenerative Disease. Front Neurosci 2020;14:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Priovoulos N, Jacobs HIL, Ivanov D, Uludağ K, Verhey FRJ, Poser BA. High-resolution in vivo imaging of human locus coeruleus by magnetization transfer MRI at 3T and 7T. Neuroimage 2018;168:427–36. [DOI] [PubMed] [Google Scholar]
- [101].Priovoulos N, van Boxel SCJ, Jacobs HIL, Poser BA, Uludag K, Verhey FRJ, et al. Unraveling the contributions to the neuromelanin-MRI contrast. Brain Struct Funct 2020;225:2757–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Betts MJ, Cardenas-Blanco A, Kanowski M, Spottke A, Teipel SJ, Kilimann I, et al. Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aβ levels. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2019;11:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jacobs HIL, Becker JA, Kwong K, Engels-Domínguez N, Prokopiou PC, Papp KV., et al. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci Transl Med 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gagnon J-F, Lafrenière A, Rauchs G, Petit D, Carrier J. Sleep in Normal Aging, Alzheimer’s Disease, and Mild Cognitive Impairment. Handb. Behav. Neurosci, vol. 30, 2019, p. 677–92. [Google Scholar]
- [105].Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev 2015;19:29–38. [DOI] [PubMed] [Google Scholar]
- [106].Jacobs HIL, Priovoulos N, Poser BA, Pagen LHG, Ivanov D, Verhey FRJ, et al. Dynamic behavior of the locus coeruleus during arousal-related memory processing in a multi-modal 7T fMRI paradigm. Elife 2020;9:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jacobs HIL, Müller-Ehrenberg L, Priovoulos N, Roebroeck A. Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: a 7T MRI study. Neurobiol Aging 2018;69:167–76. [DOI] [PubMed] [Google Scholar]
- [108].Liu KY, Acosta-Cabronero J, Cardenas-Blanco A, Loane C, Berry AJ, Betts MJ, et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol Aging 2019;74:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Grueschow M, Stenz N, Thörn H, Ehlert U, Breckwoldt J, Brodmann Maeder M, et al. Real-world stress resilience is associated with the responsivity of the locus coeruleus. Nat Commun 2021;12:2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mestre H, Mori Y, Nedergaard M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci 2020;43:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019;366:628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Elman JA, Puckett OK, Beck A, Fennema-Notestine C, Cross LK, Dale AM, et al. MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimer’s Dement 2021:alz.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[113].Van Egroo M, van Hooren RWE, Jacobs HIL. Associations between locus coeruleus integrity and nocturnal awakenings in the context of Alzheimer’s disease plasma biomarkers: a 7T MRI study. Alzheimers Res Ther 2021;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shi L, Chen S-J, Ma M-Y, Bao Y-P, Han Y, Wang Y-M, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 2018;40:4–16. [DOI] [PubMed] [Google Scholar]
- [115].Mather M, Harley CW. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn Sci 2016;20:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Berteotti C, Liguori C, Pace M. Dysregulation of the orexin/hypocretin system is not limited to narcolepsy but has far-reaching implications for neurological disorders. Eur J Neurosci 2021;53:1136–54. [DOI] [PubMed] [Google Scholar]
- [117].Giorgi FS, Galgani A, Puglisi-Allegra S, Busceti CL, Fornai F. The connections of Locus Coeruleus with hypothalamus: potential involvement in Alzheimer’s disease. J Neural Transm 2021;128:589–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bretherton B, Atkinson L, Murray A, Clancy J, Deuchars S, Deuchars J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging 2019;11:4836–57. [DOI] [PMC free article] [PubMed] [Google Scholar]