Abstract
Sleep is important for immune function, metabolic function and physical repair. Sleep is more commonly disrupted in women compared with men and is disrupted by surgery, chemotherapy and cancer itself, making gynecologic oncology patients some of the highest risk of insomnia and sleep disruption. Insomnia and sleep disruption are linked to increased pain, poorer quality of life, depression, and anxiety which can all negatively affect patient outcomes. A number of environmental, behavioral, and pharmacological interventions have been investigated to improve patient sleep and aid in the recovery process. It is vital to understand and address patient sleep quality in order to give patients the highest quality care and improve outcomes.
Precis
Gynecologic cancer patients experience poor sleep due to gender, cancer, and treatment. Poor sleep alters immune function and impairs recovery. Interventions to improve sleep are available.
Introduction
Sleep is crucial to the maintenance of normal human physiology including the secretion of growth hormones, physical repair, and immune and metabolic function (1, 2). Having sleep disturbances or sleep disorders, such as insomnia, hypersomnia, or circadian rhythm sleep-wake disorders, can impair these functions, which negatively affects patient health, outcomes, and quality of life (3–7). It is especially important to address sleep in oncology patients, as 30–88% of these patients are affected by sleep disturbances, impacting their daily lives, disease course and even prognosis (4).
Sleep and sleep disturbances can be assessed in several ways including polysomnography, actigraphy, sleep quality indexes, or subjective reporting. Polysomnography, the gold standard for measuring sleep, is a method in which multiple patient variables are monitored overnight and often used in the official diagnosis of sleep disorders. However, it is often too intensive to be used widely in large studies assessing sleep as patients must spend the night in a sleep laboratory under observation (8, 9). Actigraphy utilizes devices that a patient can wear and assesses sleep by monitoring patient movement during the night (9). Actigraphy is also a previously validated method of assessing sleep, though it requires the purchase of monitors for patients to wear and is thus less commonly used in large hospital sleep studies. The most commonly used method in hospital studies is sleep quality indexes, examples of which include the Pittsburgh Sleep Quality Index (PSQI) and the patient-reported outcome measurement information system (PROMIS) measure for sleep(10, 11). These tools have previously been validated and are prevalent in sleep research, likely due to ease of administration as well as assessment of other variables such as daytime dysfunction, use of sleeping medication, pain, and subjective sleep quality (11, 12).
There are a number of external factors that can affect sleep in postoperative patients. Previous studies have shown that pain causes the largest reduction in sleep duration, as it is the most likely to persist throughout the night (13). Sleep and pain furthermore seem to have a bidirectional relationship where sleep disruption can cause hyperalgesia, and pain can conversely cause sleep disruption (14–16). In breast cancer patients, those who experienced poorer sleep preoperatively had higher postoperative pain scores when compared to those who reported good sleep (17). A proposed mechanism for this phenomenon is an elevation of IL-6 in post-surgical and cancer patients with sleep disturbances, which has previously been correlated to an increase in pain scores (15, 18). The implication is that decreased sleep will raise IL-6 levels, which then go on to impact the central nervous system and sleep center.
There is also evidence that decreased sleep quality and increased sleep disruption can affect a patient’s recovery time after surgery (13). Because sleep is vital for immune system functioning, any sleep disruption or limitation can cause a delay in healing (14, 15). One study looking at fast track hysterectomy patients showed that poor sleep quality on the first postoperative night was associated with longer hospital stays (16). It is well known that longer hospital stays can lead to an increased risk for nosocomial infections (19). Worsening sleep is also associated with increased risk of postoperative delirium, which can further contribute to worse outcomes (16). Even after leaving the hospital, poor sleep can still affect patients by decreasing their quality of life. Sleep deprivation is associated with decreased activities of daily living (ADL) performance and physical functioning post-discharge, as well as impaired cognitive abilities (2, 4). Patients may find themselves unable to function to the same level as they did before diagnosis or treatment. Furthermore, women are twice as likely to have sleep disturbances or insomnia at some point in their lifetime compared to men and sleep dysregulation is thought to have more severe consequences in female patients (20).
In this paper, we discuss the factors affecting and consequences of poor sleep in female oncologic and surgical patients, modifiable factors to improve patient outcomes, and possible interventions for improving sleep in these patients post-operatively.
Sleep in Female Cancer and Surgical Patients
Most of the literature surrounding sleep quality and disturbances in female surgical patients focuses on patients being treated for breast cancer. In fact, breast cancer patients have the highest number of overall complaints of insomnia compared to patients with other types of cancer (21). Other studies focus on patients undergoing hysterectomy or benign gynecologic conditions(22, 23). Estimates put the proportion of these surgical patients experiencing sleep disturbances at 20–70% before, during, and persisting up to 6 months after treatment (24). This indicates the importance of addressing sleep in this patient population.
Sleep disturbance is common among female surgical patients and its causes are multifactorial (Figure 1). Some of the more prevalent causes of sleep problems include disruption from pain, nocturia, or hot flashes (25). There is also evidence that elevation of cytokines, such as IL-6, can contribute to sleep problems as well (18). These disruptors are also known to alter sleep in perimenopausal women (22). Pain likely disrupts sleep via its persistence throughout the night, while nocturia and hot flashes are more likely to episodically wake a woman up in the middle of the night, possibly disrupting her ability to return to sleep. The reduced total sleep duration and multiple awakenings can then lead to poorer sleep quality throughout the diagnosis, treatment, and post-treatment periods (25).
Figure 1:
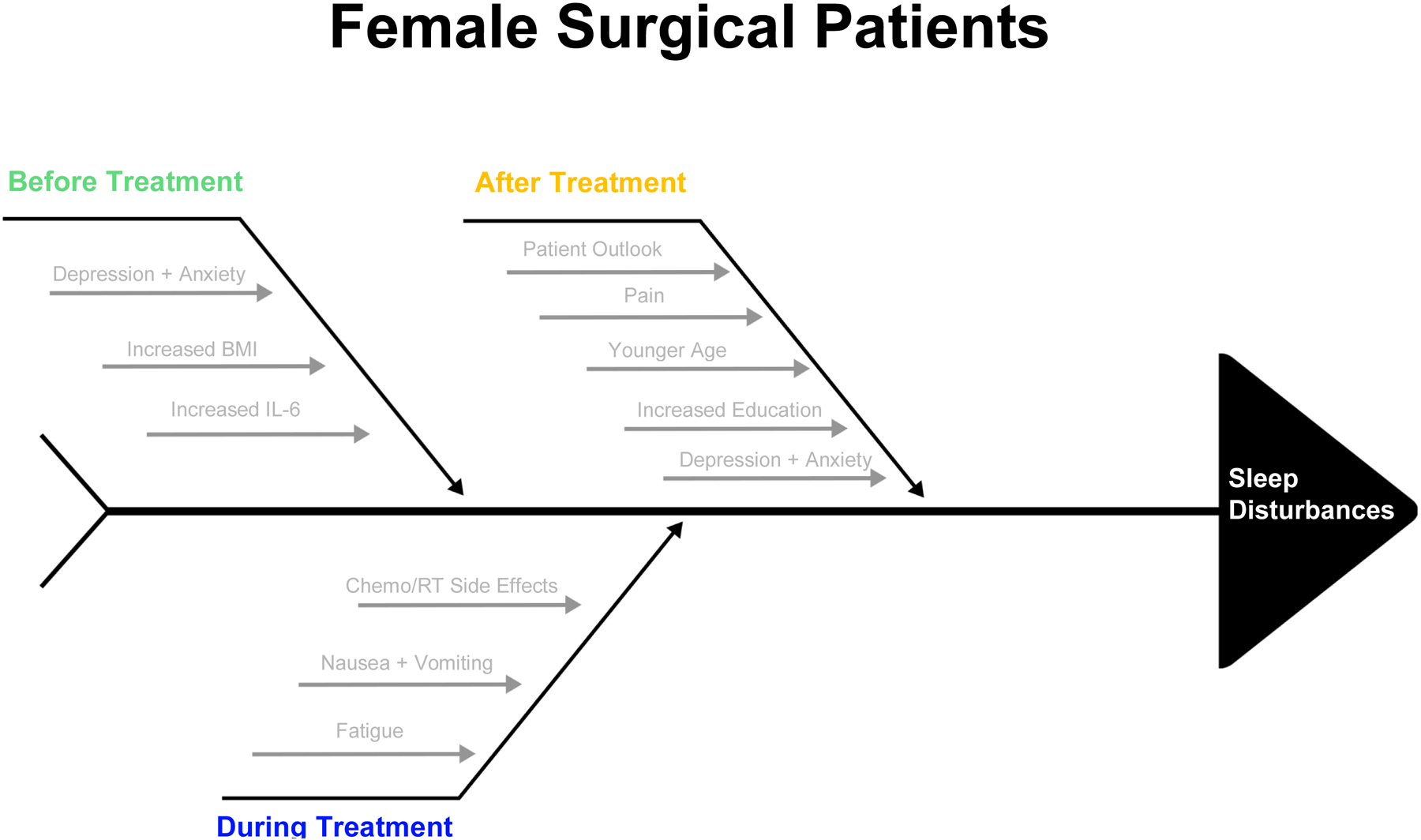
Factors Contributing to Sleep Disturbance in Female Surgical Patients
Before Treatment
The average breast cancer patient has significant sleep difficulty, indicated by a Pittsburgh Sleep Quality Index (PSQI) score ≥5 (12). Before starting treatment, the existing prevalence of sleep disturbance amongst breast cancer patients ranges from 33–88% (26). Two major associated factors with increased levels of sleep disturbances are higher levels of depressive symptoms and anxiety (26, 27). Predictors of depressive symptoms and anxiety preoperatively include fear of future diagnostic tests, undergoing lymph node dissection, higher BMI, and lower performance status (26). These factors may influence sleep through invasive or preoccupying thoughts that either prevent patients from falling asleep, interfere with restful sleep, or cause them to awaken throughout the night. In patients with noncancerous gynecologic conditions, major depressive disorder and higher pelvic pain impact questionnaire (PPIQ) scores also tended to have poorer sleep quality and shorter sleep duration compared to the general population (23).
During Treatment
Patient sleep may also be affected during treatment with chemotherapy or radiotherapy, most often due to side effects and the grueling process of undergoing treatments. In one study, the proportion of breast cancer patients who met the diagnostic criteria for insomnia was three times higher in patients receiving chemotherapy versus the general population (21). Cancer patients receiving chemo or radiotherapy also had higher levels of physical fatigue that persisted for the duration of their treatment (28). This fatigue was strongly correlated with poor sleep quality. Cancer-related and treatment-related fatigue likely contribute to poor quality sleep through both direct and indirect mechanisms. Other studies have linked poor sleep during chemotherapy and radiotherapy to chemo-induced nausea and vomiting, which may prevent sleep or interrupt it (17). In fact, nocturnal awakenings increase in frequency while patients are undergoing chemotherapy (29). These results are consistent with other studies which showed that sleep disturbances developed in as many as 65% of patients while they were receiving adjuvant chemotherapy and radiation therapy (26).
After Treatment
In patients who undergo surgical procedures, sleep problems tend to persist post-treatment (24, 27). Over half of patients who have undergone surgery for breast cancer still have insomnia at 6 months post-operatively (27). These results are often influenced by patient outlook; those who have more optimism tend to experience fewer negative emotions and a lower degree of hopelessness with resulting lower sleep disturbances.
Kim and Lee compared sleep and fatigue in women at 3 and 6 weeks post-hysterectomy. The patients reported significantly more sleep disturbance post-operatively that persisted up to at least 6 weeks in those who had a vaginal hysterectomy (30). However, patients who had an abdominal hysterectomy reported better sleep and less fatigue at 6 weeks compared to their baseline (30). Interestingly, it seems as though younger women are more likely to have a higher number of nocturnal awakenings during the night than older women (21, 30). One possible explanation for this is that a diagnosis requiring a hysterectomy at a younger age may induce more anxiety, depression, and stress leading to increased sleep disturbances (5). Furthermore, for patients who underwent a hysterectomy and then immediate surgical menopause have been shown to have worse sleep trajectories compared to women who had already undergone menopause at the time of their surgery (31). This is likely due to the sudden onset of menopause symptoms such as hot flashes, bladder dysfunction, and myalgias, which can interrupt sleep.
It is important to address sleep because of the bidirectional relationship that sleep has with symptoms such as pain and depression, as well as its effect on long term quality of life and survival. Sleep problems can lead to a decreased ability to perform daily tasks or work, which then leads to problems with physical functioning and daily quality of life (25). Patients may not be able to do everything that they could before their diagnosis or treatment, leading to a possible loss of independence. Poor sleep and sleep deprivation have further been linked to an increase in post-operative pain (25, 32). As previously discussed, pain has a bidirectional relationship with sleep and thus can also impact quality of life and physical functioning. Insomnia has further been linked to lowered immune and metabolic functions and may be related to an acceleration of cancer progression. In fact, Ren et al showed that just a 10% increase in patient sleep efficiency could result in a decrease in mortality as high as 32% (27). This emphasizes the need to examine and address the causes of sleep disturbances in this population (27).
Sleep in Gynecologic Oncology Patients
Sleep is often disturbed in gynecologic oncology patients, whether at diagnosis, during treatment, or post-treatment (Figure 2). More than half of survivors report experiencing sleep disturbances, poor sleep quality, or insomnia and sleep difficulties fall in the top 5 reported problems for these patients (33–35). It is important to study sleep in this population due to the rising incidence and mortality of gynecologic cancers (36, 37). Endometrial cancer incidence and mortality rates increased by 0.7% and 1.1% each year between 1999 and 2016, while ovarian cancer is the 5th highest cause of cancer death in women (38, 39). Furthermore, there is evidence that sleep dysfunction is worse in gynecologic cancer survivors at a prevalence of 40–55% compared to all cancer survivors at 30–50% (40). Given sleep’s effects on quality of life and patient outcomes in this population, it is vital to recognize and address sleep disturbances early on.
Figure 2:
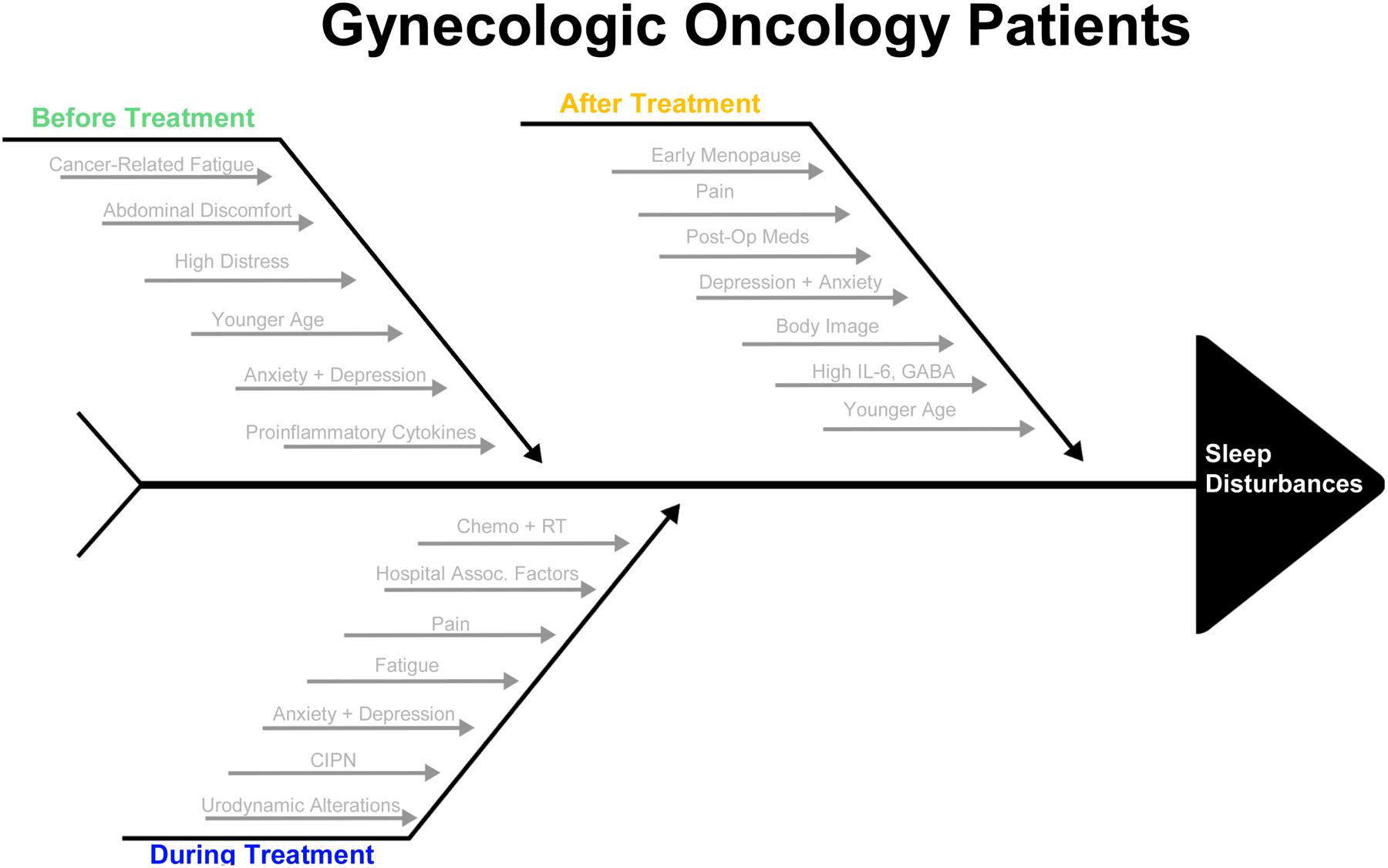
Factors Contributing to Sleep Disturbances in Gynecologic Oncology Patients
Before Treatment
Sleep disorders can arise before treatment begins in some types of gynecologic cancer, while in others they arise during or after treatment. For instance, patients with endometrial cancer often have sleep quality comparable to the healthy population due to earlier diagnosis and a lack of systemic symptoms for most patients (35). In contrast, ovarian cancer patients often report a higher sleep disorder rate compared to the general population before they undergo surgery, with some studies showing as many as two thirds of ovarian cancer patients having poor sleep quality, as measured by a PSQI ≥ 5, that can persist over a year post-diagnosis (5, 33, 35). The highest prevalence of this insomnia occurs at 3 months post-diagnosis (41).
In patients with ovarian cancer, insomnia affects 14–60% of patients, twice as many as patients without ovarian cancer, with symptoms persisting years after diagnosis (42). The most commonly cited risks of developing sleep disorders in the ovarian cancer population are abdominal discomfort and high distress at time of diagnosis (4, 5, 35). Abdominal discomfort is often a result of a diagnosis later in the course of disease, while high distress may be related to the general diagnosis of cancer, prognosis, loss of fertility, or anxiety about treatment. There are also three variables predictive of the development of clinically significant insomnia in ovarian cancer patients: younger age (<50 years old), higher unmet needs in the physical and daily living domains, and elevated anxiety (43). Specific subcategories of these variables may include loss of fertility, early menopause leading to symptoms such as hot flashes, and sexual disharmony (4). As discussed earlier, a higher incidence of insomnia in younger patients may be related to having a cancer diagnosis earlier in life, surgical menopause, as well as loss of fertility.
Poor sleep quality is also present in patients with cervical cancer. Tian et al compared sleep quality in cervical cancer patients before adjuvant therapy to the general female population and found that almost twice as many women with cervical cancer (52.6% compared to 27.6%) had poor sleep quality (4). These results indicate that no matter the type of gynecologic cancer, many women develop problems with sleep around the time of diagnosis.
Proposed mechanisms of the development of sleep disturbances include the release of cytokines such as tumor necrosis factor (TNF) or C-reactive protein (CRP), depression and distress, and cancer-related fatigue (35). IL-6 and other proinflammatory cytokines are thought to be the biggest mechanistic factors in play. In ovarian cancer patients, those who have advanced stage or invasive disease at diagnosis have elevated plasma IL-6, which is associated with a profound effect on the central nervous system and subsequent sleep impairment (44). This same IL-6 elevation is seen in multiple other types of cancers and linked to sleeping difficulties as well. In addition, there are multiple patient factors that increase IL-6, such as increased body mass index (BMI) and tobacco use, which, when combined with a cancerous process, can further exacerbate sleep disturbances (45).
During Treatment
Many gynecologic cancers are treated surgically, but neoadjuvant and adjuvant chemotherapy are also commonly used and these treatments affect sleep as well. King et al studied patient reported outcome measures among women with ovarian cancer undergoing palliative chemotherapy and found that sleep disturbances were prevalent at 24%, making it one of the most commonly occurring side effects (46). Other studies have found that as many as 80% of patients with gynecologic cancer had sleep disorders while they were staying in the hospital (47). This latter finding may be related to the process of undergoing treatment or due to hospital factors such as noise, light, or nursing interventions that disrupt or prevent sleep.
Combination chemo and radiotherapy is thought to have the greatest impact on sleep quality (35). Some of the most common treatment induced side effects include pain, fatigue, anxiety, and depression, all of which can affect sleep quality (4). This is particularly salient in patients who suffer from chemo-induced peripheral neurotoxicity, which can cause intense pain and sensory discomfort leading to disturbed sleep (4). As stated earlier, pain is the most likely symptom to persist throughout the night, thus making it a common cause of sleep onset latency or disruption. Radiotherapy can furthermore lead to urodynamic alterations that interrupt sleep during the night (4). These sleep disturbances can then persist after treatment has been completed (37).
After Treatment
The majority of sleep problems emerge after surgical intervention. Post-operative PSQI scores increase significantly compared to patient baselines with almost 70% of patients having poor sleep quality (35). The most commonly affected areas include subjective sleep quality, sleep disturbances, and daytime dysfunction (35). Mechanisms often include sleep fragmentation and reduced sleep time due to surgical stress, pain, and post-operative medications (35).
Sleep disturbances also occur after completion of non-surgical treatments such as radiotherapy and chemotherapy. According to Ilhan et al, who looked at cervical cancer patients, radiotherapy is associated with poor sleep quality due to urological symptoms such as nocturia and can have effects on daytime dysfunction, sleep latency, and sleep quality (35). Chemotherapy patients have a rate of insomnia 3x higher than the general population (21). Tian et al compared the prevalence of poor sleep quality before and after adjuvant therapy to the general female population and found that there was an increase in the prevalence of poor sleep quality after adjuvant therapy (4). The highest prevalence of sleep disturbances occurs in gynecologic cancer survivors who received combination therapy (34).
Sleep duration improves around 6 months post-surgery, though the quality of sleep does not (5, 35, 37). However, there are factors that can hinder this improvement. For instance, depressive symptoms predict a decline in sleep over time in ovarian cancer patients (5). This connection is also observed in gynecologic oncology patients who undergo a hysterectomy and/or an oophorectomy; PSQI scores in these patients have a positive correlation with anxiety and depression (47). Furthermore, a disturbed body image after treatment is also a main predictor of poor sleep quality in patients who receive radical surgery (47). The possible proposed mechanisms for this observed phenomenon include GABA system or serotonin receptor functioning, discomfort, as well as elevations in IL-6 impacting the central nervous system (18).
While more than 50% of women recover their well-being within 6 months, there is still a large proportion who continue to have symptoms and poor well-being affecting their sleep more than 18 months after treatment (37). This then has subsequent impacts on quality of life. Studies have shown that as many as 40% of women may have this issue (37, 48). Major predictive factors of this phenomenon include younger age, a history of anxiety, or a history of depression (37). A greater number or severity of stressors at the time a patient undergoes surgery is also associated with a poorer quality of life a year after diagnosis (49).
Interventions to Improve Sleep
Before Treatment
Sleep interventions before treatment with chemotherapy, radiotherapy, or surgery focus on methods to improve sleep hygiene and mitigation of symptoms that may be contributing to sleep disturbances (Table 1). The National Cancer Institute (NCI) makes a number of both non-pharmacological and pharmacological suggestions for improving sleep (7). Non-pharmacological recommendations include Cognitive Behavioral Therapy for Insomnia (CBT-I), a regular sleep schedule, relaxing before bedtime, or limiting caffeine intake. Pharmacological recommendations include medications to treat sleep-wake cycle disturbances, such as zolpidem, benzodiazepines, or melatonin, though they suggest that any medications be used in combination with lifestyle changes with the goal of only using medications over the short-term (50).
Table 1:
Interventions to Improve Sleep
| Non-pharmacologic | Pharmacologic | |
|---|---|---|
| Before Treatment/Survivorship | Cognitive Behavioral Therapy for Insomnia | Zolpidem |
| Regular sleep schedule | Benzodiazepines | |
| Limit caffeine intake | Melatonin | |
| Increased physical activity | ||
| During Chemotherapy/Radiation | Cognitive Behavioral Therapy | Zolpidem |
| Exercise Therapy | Benzodiazepines | |
| Proactive treatment toxicity management | Melatonin | |
| After Surgery | Eye masks | Zolpidem |
| Ear plugs | Dexmedetomidine | |
| Regulating fluid intake | Melatonin | |
| Ambient noise limitation | Diphenhydramine |
During Treatment
Sleep disturbances are common in gynecologic cancer patients who are undergoing chemotherapy and radiotherapy (51). The most effective strategies shown to combat these effects are CBT and exercise therapy (52). These two interventions, particularly when combined, have been shown to reduce fatigue in patients with ovarian cancer undergoing chemotherapy (52). Furthermore, management of the side effects of chemotherapy or radiotherapy, such as nausea, is also essential to improving sleep, as these symptoms have previously been shown to be associated with poorer quality of sleep (53).
After Treatment
Sleep interventions in the immediate postoperative period include environmental and pharmacological interventions. One of the most studied environmental interventions is the use of eye masks and ear plugs. These function to block light and noise, which is particularly salient because noise is one of the most cited causes of awakenings in hospitalized patients (2, 14). This intervention has been shown to improve sleep depth, sleep length, and reduce the number of awakenings throughout the night (2, 16). Mahran et al compared the use of eye masks to routine care with low light, low noise, and limited awakenings for medications and found significantly improved sleep quality and pain scores in the intervention group (15). In this study, the pain reduction was evident on the first postoperative day, indicating a rapid effect. Given the low cost of earplugs and eye masks, this may be a relatively inexpensive method of combating some modifiable factors affecting sleep postoperatively.
In addition to eye masks and earplugs, or in the case that these interventions could not be implemented, there are other environmental changes that have been shown to be beneficial. As discussed earlier, noise and light are two of the most common sleep disruptors, with nursing activities during the night rounding out the list (2, 14). Reducing nighttime noise and light on the wards, as well as clustering nursing activities in order to have minimal interruptions throughout the night have all been shown to improve sleep quality and duration (14, 16). Furthermore, exposure to daytime light has been shown to be important for sleep regulation, particularly in elderly patients, allowing them to sleep longer (2).
There are also interventions specifically related to patient care in the hospital that can be used to mitigate sleep disturbances and are recommended by the NCI. These include regulating fluid intake to avoid awakening during the night to urinate, encouraging patients to void before going to bed, avoiding caffeine or other stimulants, helping the patients position themselves comfortably, and encouraging the patient to be active and out of bed as much as possible during the day (50).
Pharmacological interventions for sleep disruptions in the hospital have been studied less, as first line interventions typically involve different subsets of the aforementioned non-pharmacological methods (50). Previously proposed medications include Zolpidem, and melatonin (16, 50). Medications such as benzodiazepines are not used as often due to considerations for the risk of tolerance, dependence, or withdrawal (50). Antihistamines can also induce drowsiness, but have been shown to reduce sleep quality (54). Existing studies have shown that Zolpidem may reduce postoperative pain and fatigue, as well as improving patient quality of life while decreasing narcotic consumption (16). Dexmedetomidine may decrease the prevalence of postoperative delirium in the days following a surgery (16). While these medications are generally deemed safe to use in this patient population, they are typically meant to be used as shorter-term more immediate management and in conjunction with long-term lifestyle changes (50). This is because the medications, while improving sleep quality, do not re-create normal sleep architecture. More investigation is needed into the benefits of this type of intervention in gynecologic cancer patients (18).
As discussed earlier, patients may still suffer from sleep disturbances after their discharge from the hospital. These disturbances are less likely to be related to the same factors as sleep in the hospital, but nonetheless there are interventions that may aid these patients. Similarly to how daylight exposure helps patients staying in the hospital, bright light therapy has been shown to improve sleep disturbances and health related quality of life in patients with ovarian and endometrial cancer (55). In ovarian cancer patients, an increase in physical activity is associated with improved sleep outcomes (48). There has also been evidence that CBT, particularly CBT-I, is an effective way to improve sleep efficiency, total wake time, and time to sleep onset in patients who have undergone surgery for breast or gynecologic cancer (50, 56). Other proposed methods include yoga and exercise, such as walking (50). These methods are particularly salient for patients who have undergone surgically induced menopause after a hysterectomy but cannot utilize hormone therapy due to the nature of their cancer (57).
Conclusion
Sleep is a vital factor for recovery and quality of life, particularly in female oncology patients who have a plethora of environmental and physical barriers to undisturbed sleep. Poor sleep can lead to increased pain, depression, anxiety, and worse outcomes (5, 35). Conversely, good sleep in survivors is linked with a more positive affect and fewer episodes of anxiety or depression (40). Because of the difference sleep quality poses to outcomes and patient quality of life, patient sleep at diagnosis, during treatment, and after treatment must be addressed. As such, it is vital that providers routinely assess their patients for sleep disturbances with scales such as the General Sleep Disturbance Scale (GSDS) in addition to counseling their patients on methods to improve sleep hygiene (7, 51). There are several successful interventions that have previously been investigated including earplugs, eye masks, environmental changes, and CBT that may aid these patients in achieving improved sleep, which subsequently helps their recovery. Many of these interventions are easily implemented at a low cost, or no cost, and may benefit patients with gynecologic cancers.
Acknowledgements:
Dr. Barber is supported by NICHD (K12 HD050121-12), the NIA (P30AG059988-01A1) and the GOG Foundation.
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- 1.Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14(7):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubose J, Hadi K. Improving inpatient environments to support patient sleep. Int J Qual Health Care. 2016;28(5):540–33. [DOI] [PubMed] [Google Scholar]
- 3.Carrera-Hernandez L, Aizpitarte-Pejnaute E, Zugazagoitia-Ciarrusta N, Goni-Viguria R. Patients’ perceptions of sleep in a Critical Care Unit. Enferm Intensiva (Engl Ed). 2018;29(2):53–63. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Chen GL, Zhang HR. Sleep status of cervical cancer patients and predictors of poor sleep quality during adjuvant therapy. Supportive Care in Cancer. 2014;23:1401–8. [DOI] [PubMed] [Google Scholar]
- 5.Clevenger L, Schrepf A, DeGeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J, Thaker PM, Mendez L, Sood AK, Slavich GM, Lutgendorf SK. Sleep Disturbance, Distress, and Quality of Life in Ovarian Cancer Patients during the First Year Post Diagnosis. Cancer. 2013;119(17):3234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercadante S, Girelli D, Casuccio A. Sleep disorders in advanced cancer patients: prevalence and factors associated. Supportive Care in Cancer. 2004;12:355–9. [DOI] [PubMed] [Google Scholar]
- 7.National Comoprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Survivorship. 2021. [Accessed March 3, 2022].
- 8.Bloch K Polysomnography: a systematic review. Technol Health Care. 1997;5(4):285–395. [PubMed] [Google Scholar]
- 9.Marino M, Li Y, Rueschman MN, Wikelman JW, Ellenbogen JM Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep. 2013;36(11):1747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buysse DJ, Reynolds CF , Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL, Pilkonis PA. Development of Short Forms from the PROMIS Sleep Disturbance and Sleep-Related Impairment Item Banks. Behav Sleep Med. 2012;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13. [DOI] [PubMed] [Google Scholar]
- 13.Grossman MN, Anderson SL, Worku A, Marsack W, Desai N, Tuvilleja A, Ramos J, Francisco MA, Lafond C, Balachandran JS, Mokhlesi B, Farnan JM, Meltzer DO, Arora VM. Awakenings? Patient and Hospital Staff Perceptions of Nighttime Disruptions and Their Effect on Patient Sleep. J Clin Sleep Med. 2017;13(2):301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locihova H, Axmann K, Padysakova H, Fejfar J. Effect of the use of earplugs and eye mask on the quality of sleep in intensive care patients: a systematic review. J Sleep Res. 2018;27(3):e12607. [DOI] [PubMed] [Google Scholar]
- 15.Mahran GS, Leach MJ, Abbas AM, Ghoneim AM. Effect of Eye Masks on Pain and Sleep Quality in Patients Undergoing Cardiac Surgery: A Randomized Controlled Trial. Crit Care Nurse. 2020;40(1):27–35. [DOI] [PubMed] [Google Scholar]
- 16.Su X , Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. 2018;31(1):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JP, Lu SF, Guo LN, Ren CG, Zhang ZW. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: A prospective cohort study. Medicine (Baltimore). 2019;98(44):e17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Penedo F, Lubaroff DM, Sood AK, Lutgendorf SK. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun. 2012;26(7):1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauck K, Zhao X. How dangerous is a day in the hospital? A model of adverse events and length of stay for medical inpatients. Med Care. 2011;48:1068–75. [DOI] [PubMed] [Google Scholar]
- 20.Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, HEckler C, Purnell JQ, Janelsins MC, Morrow GR. Prevalence, Demographics, and Psychological Associations of Sleep Disruption in Patients with Cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones HJ, Zak R, Lee KA. Sleep Disturbances in Midlife Women at the Cusp of the Menopausal Transition. J Clin Sleep Med. 2018;14(7):1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh JK, Learman LA, Nakagawa S, Gregorich SE, Kupperman M. Sleep problems among women with noncancerous gynecologic conditions. J Psychosom Obstet Gynaecol. 2014;35(1):29–35. [DOI] [PubMed] [Google Scholar]
- 24.Thomas KM, Bower J, Hoyt MA, Sepah S. Disrupted sleep in breast and prostate cancer patients undergoing radiation therapy: The role of coping processes. Psychooncology. 2010;19(7):767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortner BC, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24(5):471–80. [DOI] [PubMed] [Google Scholar]
- 26.Van Onselen C, Paul SM, Lee K, Dunn L, Aouizerat BE, West C, Dodd M, Cooper B, Miaskowski C. Trajectories of Sleep Disturbance and Daytime Sleepiness in Women Before and After Surgery for Breast Cancer. J Pain Symptom Manage. 2013;45(2):244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Y, Li S, Zhou S, Wang Y, Li L, Zhang J, Yang Y, He J, Zhu X. Optimism outweighs neuroticism and anxiety sensitivity to predict insomnia symptoms in women after surgery for breast cancer. Supportive Care in Cancer. 2018;17:2903–9. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt ME, Wiskemann J, Schneeweiss A, Potthoff K, Ulrich CM, Steindorf K. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer. 2018;142(6):1148–57. [DOI] [PubMed] [Google Scholar]
- 29.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8):2261–9. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, Lee KA. Sleep and fatigue symptoms in women before and 6 weeks after hysterectomy. J Obstet Gynecol Neonatal Nurs. 2018;38(3):344–52. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Tsai Y, Chang T, Chen L. Associations among menopausal symptoms, sleep and fatigue in Taiwanese women with endometrial cancer. European Journal of Cancer Care. 2016;26(5):e12559. [DOI] [PubMed] [Google Scholar]
- 32.Orbach-Zinger S, Fireman S, Ben-Haroush A, Karoush T, Klein Z, Mazarib N, Artyukh A, Chen R, Ioscovich A, Eidelman LA, Landau R. Preoperative sleep quality predicts postoperative pain after planned caesaren delivery. Eur J Pain. 2017;21(5):787–94. [DOI] [PubMed] [Google Scholar]
- 33.Sandadi SFH, Broderick MJ, Waggoner SE, Miller JA, von Gruenigen VE. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecologic Oncology. 2011;123(2):351–5. [DOI] [PubMed] [Google Scholar]
- 34.Westin SN, Sun CC, Tung CS, Lacour RA, Meyer LA, Urbauer DL, Frumovitz MM, Lu KH, Bodurka DC. Survivors of gynecologic malignancies: Impact of treatment on health and well-being. J Cancer Surviv. 2016;10(2):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilhan TT, Ucar MG, Gul A, Ilhan TS, Yavas G, Celik C. Sleep quality of endometrial cancer survivors and the effect of treatments. Turk J Obstet Gynecol. 2017;14(4):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nock NL, Dimitropoulos A, Zanotti KM, Waggoner S, Nagel C, Golubic M, Michener CM, Kirwan JP, Alberts J. Sleep, Quality of Life and Depression in Endometrial Cancer Survivors with Obesity Seeking Weight Loss. Support Care Center. 2020;28(5):2311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beesley VL, Webber K, Nagle CM, DeFazio A, Obermair A, Williams M, Friedlander M, Webb PM. When will I feel normal again? Trajectories and predictors of persistent symptoms and poor wellbeing after primary chemotherapy for ovarian cancer. Gynecologic Oncology. 2020;159(1):179–86. [DOI] [PubMed] [Google Scholar]
- 38.CDC. Uterine Cancer Incidence and Mortality - United States 1999–2016 2018. [Available from: https://www.cdc.gov/mmwr/volumes/67/wr/mm6748a1.htm. [DOI] [PMC free article] [PubMed]
- 39.American Cancer Society. Ovarian Cancer Statistics: How Common is Ovarian Cancer: American Cancer Society; [Available from: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html] [Accessed March 3, 2022].
- 40.Armbruster SD, Song J, Gatus L, Lu KH, Basen-Engquist KM. Endometrial cancer survivors’ sleep patterns before and after a physical activity intervention: a retrospective cohort analysis. Gynecologic Oncology. 2018;149(1):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross TL, DeFazio A, Friedlander M, Grant P, Nagle CM, Williams M, Webb PM, Beesley VL. Insomnia and its association with quality of life in women with ovarian cancer. Gynecologic Oncology. 2020;158(3):760–8. [DOI] [PubMed] [Google Scholar]
- 42.Palagini L, Miniati M, Massa L, Folesani F, Marazziti D, Grassi L, Riemann D. Insomnia and circadian sleep disorders in ovarian cancer: Evaluation and management of underestimated modifiable factors potentially contributing to morbidity. Journal of Sleep Research. 2021:e13510. [DOI] [PubMed] [Google Scholar]
- 43.Price MA, Zachariae R, Butow PN, deFAzio A, Chauhan D, Espie CA, Friedlander M, Webb PM. Australian Ovarian Cancer Study - Quality of Life Study Investigators. Prevalence and predictors of insomnia in women with invasive ovarian cancer: anxiety a major factor. Eur J Cancer. 2009;45(18):3262–70. [DOI] [PubMed] [Google Scholar]
- 44.Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, deGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, Cole S, Sood AK, Lubaroff DM. Interleukin-6, Cortisol, and Depressive Symptoms in Ovarian Cancer Patients. J Clin Oncol. 2008;26(29):4820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kacel EL, Kirsch JL, Sannes TS, Patidar S, Postupack R, Jensen S, Wong S, Garey S, Dodd S, Ulfig CM, McCrae CS, Robinson ME, Castagno J, Schultz G, Pereira DB. Interleukin-6 and Body Mass Index, Tobacco Use, and Sleep in Gynecologic Cancers. Health Psychol. 2020;38(10):866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King MT, Stockler MR, Butow P, O’Connell R, Voysey M, Oza AM, Gillies K, Donovan HS, Mercieca-Bebber R, Martyn J, Sjoquist K, Friedlander ML. Development of the Measure of Ovarian Symptoms and Treatment Concerns: Aiming for Optimal Measurement of Patient-Reported Symptom Benefit with Chemotherapy for Symptomatic Ovarian Cancer. Int J Gynecol Cancer. 2014;24:865–73. [DOI] [PubMed] [Google Scholar]
- 47.Aquil A, Kherchi O, Azmaoui N, Mouallif M, Guerroumi M, Chokri A, Jayakumar AR, Benider A, Elgot A. Body image dissatisfaction and lower self-esteem as major predictors of poor sleep quality in gynecological cancer patients after surgery: cross-sectional study. BMC Women’s Health. 2021;21:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevinson C, Steed H, Faught W, Tonkin K, Vallance JK, Ladha AB, Schepansky A, Capstick V, Courneya KS. Physical Activity in Ovarian Cancer Survivors: Associations with Fatigue, Sleep, and Psychosocial Functioning. Int J Gynecol Cancer. 2009;19:73–8. [DOI] [PubMed] [Google Scholar]
- 49.Garvin L, Slavich GM, Schrepf A, Davis LZ, Thaker PH, Goodheart MJ, Cole SW, Sood AK, Lutgendorf SK. Chronic difficulties are associated with poorer psychosocial functioning in the first year post-diagnosis in epithelial ovarian cancer patients. Psychooncology. 2021;30(6):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.PDQ Supportive and Palliative Care Editorial Board. PDQ Sleep Disorders: National Cancer Institute; [updated November 19, 2021.] [Accessed March 3, 2022]
- 51.Pozzar RA, Hammer MJ, Paul SM, Cooper BA, Kober KM, Conley YP, Levine JD, Miaskowski C. Distinct sleep disturbance profiles among patients with gynecologic cancer receiving chemotherapy. Gynecologic Oncology. 2021;163(2):419–26. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q, Li F, Zhang H, Yu X, Cong Y. Effects of nurse-led home-based exercise and cognitive behavioral therapy on reducing cancer-related fatigue in patients with ovarian cancer during and after chemotherapy: A randomized controlled trial. International Journal of Nusing Studies. 2018;78:52–60. [DOI] [PubMed] [Google Scholar]
- 53.Blom K, Efverman A. Sleep During Pelvic-Abdominal Radiotherapy for Cancer: A Longitudinal Study With Special Attention to Sleep in Relation to Nausea and Quality of LIfe. Cancer Nursing. 2020;44(4):333–44. [DOI] [PubMed] [Google Scholar]
- 54.Ramakrishnan K, Scheid DC. Treatment Options for Insomnia. Am Fam Physician. 2007;76(4):517–26. [PubMed] [Google Scholar]
- 55.Fox RS, Baik SH, McGinty H, Garcia SF, Reid KJ, Bovbjerg K, Fajardo P, Wu LM, Shahabi S, Ong JC, Zee PC, Penedo FJ. Feasibility and Preliminary Efficacy of a Bright Light Intervention in Ovarian and Endometrial Cancer Survivors. Special Issue: Sleep Science. 2020;23:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padron A, McCrae CS, Robinson ME, Waxenberg LB, Antoni MH, Berry RB, Castagno J, Schultz G, Kacel EL, Ulfig C, Garey S, Patidar S, Sannes T, Tinastic L, Wong S, Pereira DB. Impacts of Cognitive Behavioral Therapy for Insomnia and Pain on Sleep in Women with Gynecologic Malignancies: A Randomized Controlled Trial. Behav Sleep Med. 2021:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santoro N, Epperson CN, Mathews SB. Menopausal Symptoms and Their Management. Endocrinol Metab Clin North Am. 2016;44(3):497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]


