Abstract
Objective:
To assess survival among patients diagnosed with uterine carcinosarcoma (CS) who underwent sentinel lymph node (SLN) biopsy alone vs. systematic lymph node dissection (LND).
Methods:
We identified newly diagnosed CS patients who underwent primary surgical management from January 1996–December 2019. The SLN cohort underwent SLN biopsy alone with bilateral SLNs identified. The systematic LND cohort did not undergo SLN biopsy.
Results:
Ninety-nine patients underwent SLN biopsy, and 100 patients underwent systematic LND. There was no difference by age, stage, body mass index, myoinvasion (<50%, ≥50%), lymphovascular space invasion, or positive washings. Eighty-five SLN (85.9%) and 15 LND (15%) underwent minimally invasive surgery (P<0.001). The median total node count was four (range, 1–13) for SLN and 19 (range, 2–50) for LND (P<0.001). Nodal metastasis occurred in 23 (23.2%) SLN and in 22 (22%) LND (P=0.4). Postoperative therapy was administered to 85 (85.9%) SLN and 71 (71%) LND (P=0.02). Median follow-up was 33 months (range, 1–205) for SLN and 55.3 months (range, 1–269) for LND (P=0.001). The three-year progression-free survival (PFS) was 62.9% (SE 5.2%) for SLN and 52.3% (SE 5.3%) for LND (P=0.13). The three-year overall survival (OS) was 72.1% (SE 5.1%) for SLN and 71.6% (SE 4.6%) for LND (P=0.68). An isolated nodal recurrence occurred in two (2%) SLN and four (4%) LND (P=0.26).
Conclusions:
There is no difference in PFS or OS among CS patients who undergo SLN biopsy vs. systematic LND. SLN biopsy detects nodal metastasis without compromising oncologic outcomes.
Keywords: sentinel lymph node biopsy, systematic lymphadenectomy, uterine carcinosarcoma
Introduction:
Uterine cancer is the most common gynecologic malignancy in developed countries. In 2021, there was an estimated 14,480 new cases and 4,290 deaths in the United States [1]. Uterine carcinosarcoma (CS) is an aggressive subtype and comprises approximately 4% of all uterine cancers [2]. Even patients with early-stage CS experience a five-year relapse rate of 45% and five-year disease-related mortality of 30%[3]. Patients with apparent uterine-confined disease harbor a 61% risk of occult metastases. Prognosis has been demonstrated to be driven by myometrial invasion, presence of lymphovascular space invasion, adnexal/serosal involvement, positive cytology, and lymph node metastases[4].
Surgery is the standard treatment for newly diagnosed uterine CS and consists of total hysterectomy, bilateral salpingo-oophorectomy, peritoneal staging with biopsies of peritoneum and omentum, and lymph node evaluation. Peritoneal washings are recommended. However, they do not affect staging, and omental biopsies are commonly performed[5]. Traditionally, lymph node evaluation was performed via pelvic and para-aortic lymphadenectomy, which carries a substantial risk of morbidity to include lower extremity lymphedema, lymphocyst formation, and other possible organ damage [5–7]. Over the past decade, sentinel lymph node (SLN) mapping for uterine cancer has been demonstrated to improve detection of lymph node metastasis while decreasing the morbidity associated with more extensive lymphadenectomy [8, 9]. SLN mapping utilizing intracervical injection of the fluorophore indocyanine green has been proven safe [10]. Retrospective studies suggest that SLN biopsy may be the preferred surgical management for high-risk uterine cancer histologies [11].
To date, studies comparing exclusive SLN biopsy to systematic lymph node dissection (LND) for uterine CS are small or included a heterogenous group of high-risk histologies [2–5, 12]. Data regarding the impact of SLN mapping on oncologic outcomes in patients with uterine CS is sparse. In this study, we compared outcomes of patients with uterine CS who exclusively underwent SLN biopsy to those who underwent systematic LND without SLN biopsy.
Methods:
This retrospective study was approved by the Institutional Review Board (IRB# 17–546). All patients who underwent primary surgical staging for uterine CS at our institution from January 1996–December 2019 were identified. We included patients who underwent either a systematic LND or exclusive SLN biopsy. Any cases that underwent SLN biopsy followed by completion LND or cases that failed mapping on one or both sides were excluded to keep cohorts as homogeneous as possible. The decision to proceed with systematic LND or SLN biopsy was at each surgeon’s discretion. Utilization of SLN biopsy has evolved over time at our institution. Our published SLN algorithm, also designated in the National Comprehensive Cancer Network guidelines, has become more frequently applied to all uterine cancer patients over the past decade [8]. All specimens were reviewed by an expert gynecologic pathologist.
SLN biopsy was performed using indocyanine green alone for 87 patients (88%), isosulfan blue alone for 11 patients (11%), and methylene blue for one patient (1%), following our previously published standard institutional algorithms [12]. All SLNs were evaluated using an ultrastaging technique that included 5-μm sections 50-μm apart at two different levels for each node [13]. Routine hematoxylin and eosin staining were performed, as well as anticytokeratin AE1:AE3 immunohistochemical staining of all positive nodes. SLN findings were classified as micro-metastases or macro-metastases. For the purposes of this study, isolated tumor cells (ITCs) alone were not considered as nodal metastasis, were considered “negative”, and did not upstage patients.
Chemotherapy with or without radiotherapy was recommended postoperatively to the majority of patients with uterine CS, with the exception of select patients with stage I disease with negative peritoneal washings, no myometrial invasion, and no nodal metastases. The decision for post-surgical treatment was made on an individual basis and with shared decision-making between the patient and providers. Following initial treatment, patients were surveilled with follow-up visits every 3–6 months for the first 2–3 years and then every six months for the following 2–3 years, with computerized axial tomography scans performed every 3–6 months for the first two years and then every 6–12 months for the following 2–3 years. Recurrences are categorized as locoregional, isolated nodal, or distant/multifocal.
Clinicopathological and follow-up data were reviewed in each patient’s medical record. Statistical analyses were performed using SPSS software. The Kruskal-Wallis test was used to compare continuous variables. Fisher’s exact test or Chi-squared test were used for categorical variables. Progression-free survival (PFS) was calculated from the date of initial surgery to the date of progression, death, or last follow-up. Overall survival (OS) was calculated from the date of surgical staging to the date of death or last follow-up. Three-year PFS and OS were calculated using the Kaplan-Meier method, and the log-rank test was used to compare cohorts. A Cox proportional hazards model was used for assessment of independent prognostic factors or survival. All P-values were calculated as two-sided, and any P-value <0.05 was considered statistically significant.
Results:
One-hundred ninety-nine patients were identified. Of those, 99 patients underwent exclusive SLN mapping, and 100 patients underwent systematic LND. Of note, 81 out of 100 (81%) of the systematic LND patients underwent surgery before 2010, compared to only five out of 99 (5%) of patients in the exclusive SLN cohort. This reflected the evolution of our institutional standard.
Demographics and clinical characteristics of the study patients are summarized in Table 1. The median age was 65 years (range, 21–91) in the SLN cohort and 66 years (range, 36–87) in the LND cohort (P=0.95). There was no difference between cohorts in median body mass index, stage distribution, depth of myometrial invasion, presence of lymphovascular space invasion, rate of positive peritoneal washings, or lymph node positivity rate. The median total node count was four (range, 1–13) for SLN and 19 (range, 2–50) for LND (P<0.001). Nodal metastasis (micro-metastasis or macro-metastasis) was noted in 23 (23.2%) SLN patients and in 22 (22%) LND patients (P=0.4). ITCs alone were noted in 6 (6%) SLN cases.
Table 1.
Demographics and clinical characteristics of patients with uterine carcinosarcoma (n=199)
| Exclusive SLN cohort n=99 (%)* | Systematic LND n=100 (%)* | P- value | |
|---|---|---|---|
| Age, years [Median (range)] | 65 (21–91) | 66 (36–87) | 0.95 |
| BMI [Median (range)] | 30 (14–57) | 27.5 (16–48) | 0.33 |
| Length of surgery [Median (range)] | 142.5 (69–300) | 194 (92–417) | <0.001 |
| Procedure | |||
| Laparotomy | 14 (14.1) | 85 (85.0) | <0.001 |
| MIS | 85 (85.9) | 15 (15.0) | |
| Stage | |||
| I | 58 (58.6) | 58 (58.0) | 0.48 |
| II | 6 (6.1) | 11 (11.0) | |
| III | 29 (29.3) | 28 (28.0) | |
| IV | 6 (6.1) | 3 (3.0) | |
| Myometrial invasion (%)** | |||
| <50 | 62 (63.3) | 67 (67.0) | 0.66 |
| ≥50 | 36 (36.7) | 33 (33.0) | |
| LVSI*** | |||
| Present | 43 (43.9) | 48 (49.0) | 0.45 |
| Absent | 55 (56.1) | 49 (50.0) | |
| Suspicious | 0 | 1 (1.0) | |
| Peritoneal washings**** | |||
| Positive | 25 (27.2) | 18 (19.4) | 0.40 |
| Negative | 66 (71.7) | 73 (78.5) | |
| Suspicious | 1 (1.1) | 2 (2.2) | |
| Total node counts [Median (range)] | 4 (1–13) | 19 (2–50) | <0.001 |
| Paraaortic dissection | |||
| Yes | 0 (0) | 85 (85.0) | <0.001 |
| No | 99 (100.0) | 15 (15.0) | |
| Any lymph node positive | |||
| Yes | 23 (23.2) | 22 (22.0) | 0.87 |
| No | 76 (76.8) | 78 (78.0) |
Data are expressed as n (%) unless otherwise specified
Not evaluable in one SLN case.
Not evaluable in one SLN case and two LND cases.
Not performed in all cases. If not performed, it was not included for statistical reasons.
BMI, body mass index; LND, lymph node dissection; LVSI, lymphovascular space invasion; MIS, minimally invasive surgery; SLN, sentinel lymph node
Postoperative therapy consisting of chemotherapy with or without radiotherapy was utilized in 85 (85.9%) patients who underwent SLN mapping compared to 71 (71%) of those who underwent systematic LND (P=0.02) (Table 2). The type of postoperative therapy administered was as follows: no postoperative therapy or radiotherapy alone in 14 (14.1%) SLN patients and 29 (29%) LND patients and chemotherapy with or without radiotherapy in 85 (85.9%) SLN patients and 71 (71%) LND patients(P=0.02). Carboplatin plus paclitaxel doublet therapy was the most common chemotherapy regimen administered (81%) followed by ifosfamide containing chemotherapy regimens (12%).
Table 2.
Adjuvant treatment, recurrence patterns, and survival outcomes of patients with uterine carcinosarcoma (n=199)
| Exclusive SLN cohort n=99 (%)* | Systematic LND n=100 (%)* | P- value | |
|---|---|---|---|
| Adjuvant therapy | |||
| CT ± RT | 85 (85.9) | 71 (71) | 0.02 |
| None/RT alone | 14 (14.1) | 29 (29) | — |
| Radiotherapy received, detailed | |||
| IVRT only | 70 (88.6) | 42 (68.9) | 0.01 |
| EBRT only | 8 (10.1) | 15 (24.6) | — |
| EBRT+IVRT | 1 (1.3) | 4 (6.6) | — |
| Recurrence rates | |||
| None | 62 (62.6) | 49 (49) | 0.26 |
| Locoregional | 8 (8.1) | 12 (12) | — |
| Nodal | 2 (2.0) | 4 (4.0) | — |
| Peritoneal/distant | 27 (27.3) | 35 (35.0) | — |
| Follow up time, months [Median (range)] | 33 (1–205) | 55.3 (1–269) | 0.001 |
| Time to recurrence** [Median (range)] | 11 (1–60) | 12 (1–99) | 0.95 |
| 3-year PFS(±SE) rates | 62.9 (±5.2) | 52.3 (±5.3) | 0.13 |
| 3-year OS(±SE) rates | 72.1 (±5.1) | 71.6 (±4.6) | 0.68 |
Data are expressed as n (%) unless otherwise specified.
In those who recurred
CT, chemotherapy; IVRT, intravaginal radiotherapy; EBRT, pelvic external beam radiation; LND, lymph node dissection; RT, radiotherapy; SLN, sentinel lymph node.
The median follow-up time was 33 months (range, 1–205) for the SLN cohort and 55.3 months (range, 1–269) for the LND cohort (P=0.001). Recurrence was observed in 88 (44%) patients, and recurrence rates were similar between the two cohorts (P=0.26). The median time to recurrence in those who recurred was 11 months (range 1–60) for SLN and 12 months (range 1–99) for LND. Patterns of disease recurrence are detailed in Table 2. An isolated nodal recurrence occurred in two (2%) SLN and four (4%) LND (P=0.26). The three-year PFS rates were 62.9% (SE ±5.2) for SLN and 52.3 (SE ±5.3) for LND (P=0.13) (Figure 1). On univariate analysis, patients with advanced stage disease, ≥50% myometrial invasion, and positive washings had significantly shorter three-year PFS (Table 3). At the time of data analysis, 85 (43%) of 199 patients were deceased. The three-year OS rates were 72.1 (SE ±5.1) for SLN and 71.6 (SE ±4.6) for LND (Figure 2). On univariate analysis, patients with advanced stage disease, ≥50% myometrial invasion, and positive washings had significantly shorter OS (Table 4). There remained no difference in PFS or OS between the SLN and LND cohorts when adjusted for the type of postoperative therapy administered (chemotherapy with or without radiotherapy vs. none/ radiotherapy alone) with an adjusted odds ratio (aOR) of 2.3 (95%CI 1.2–4.3, P=0.01) or when adjusted for age with an aOR of 1.04 (95% CI 1.01–1.07, P=0.01). Notably, PFS and OS were the same in cases that underwent a MIS approach compared to laparotomy (Table 3 and Table 4).
Figure 1:
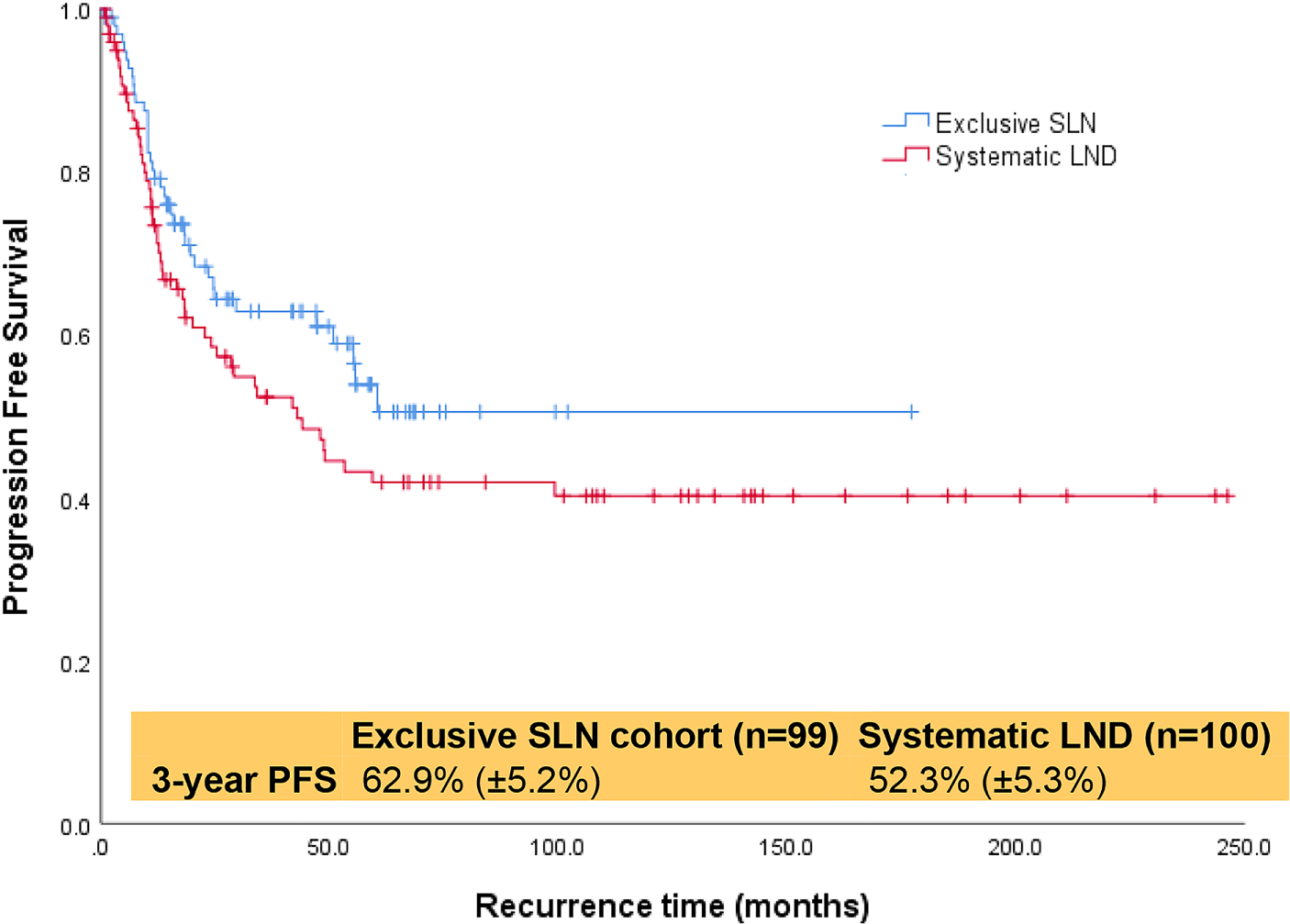
Progression-free survival of all patients with uterine carcinosarcoma.
Table 3.
Univariate analysis for PFS in patients with uterine carcinosarcoma (n=199)
| Number of patients | Number of events | Three-year PFS (±SE) rate | P-value | |
|---|---|---|---|---|
| All | 199 | 90 | — | 0.13 |
| SLN | 99 | 37 | 62.9 (±5.2) | |
| LND | 100 | 51 | 52.3 (±5.3) | |
| Procedure | 0.39 | |||
| Laparotomy | 99 | 50 | 57.0 (±5.3) | |
| MIS | 100 | 40 | 57.8 (±5.4) | |
| Stage | <0.001 | |||
| I | 116 | 40 | 66.7 (±4.7) | |
| II | 17 | 6 | 79.6 (±10.6) | |
| III | 57 | 36 | 38.5 (±7.1) | |
| IV | 9 | 8 | 16.7 (±14.2) | |
| Myometrial invasion | 0.001 | |||
| <50% | 129 | 48 | 65.9 (±4.5) | |
| ≥50% | 69 | 42 | 41.7 (±6.4) | |
| LVSI | 0.66 | |||
| Present | 91 | 41 | 53.9 (±5.7) | |
| Absent | 104 | 48 | 60.0 (±5.1) | |
| Suspicious | 1 | 0 | — | |
| Peritoneal washings | 0.01 | |||
| Positive | 43 | 28 | 64.1 (±4.4) | |
| Negative | 139 | 54 | 36.3 (±7.8) | |
| Suspicious | 3 | 1 | — |
Data are expressed as n (%) unless otherwise specified.
LVSI, lymphovascular space invasion; MIS, minimally invasive surgery; SE, standard error.
Figure 2:
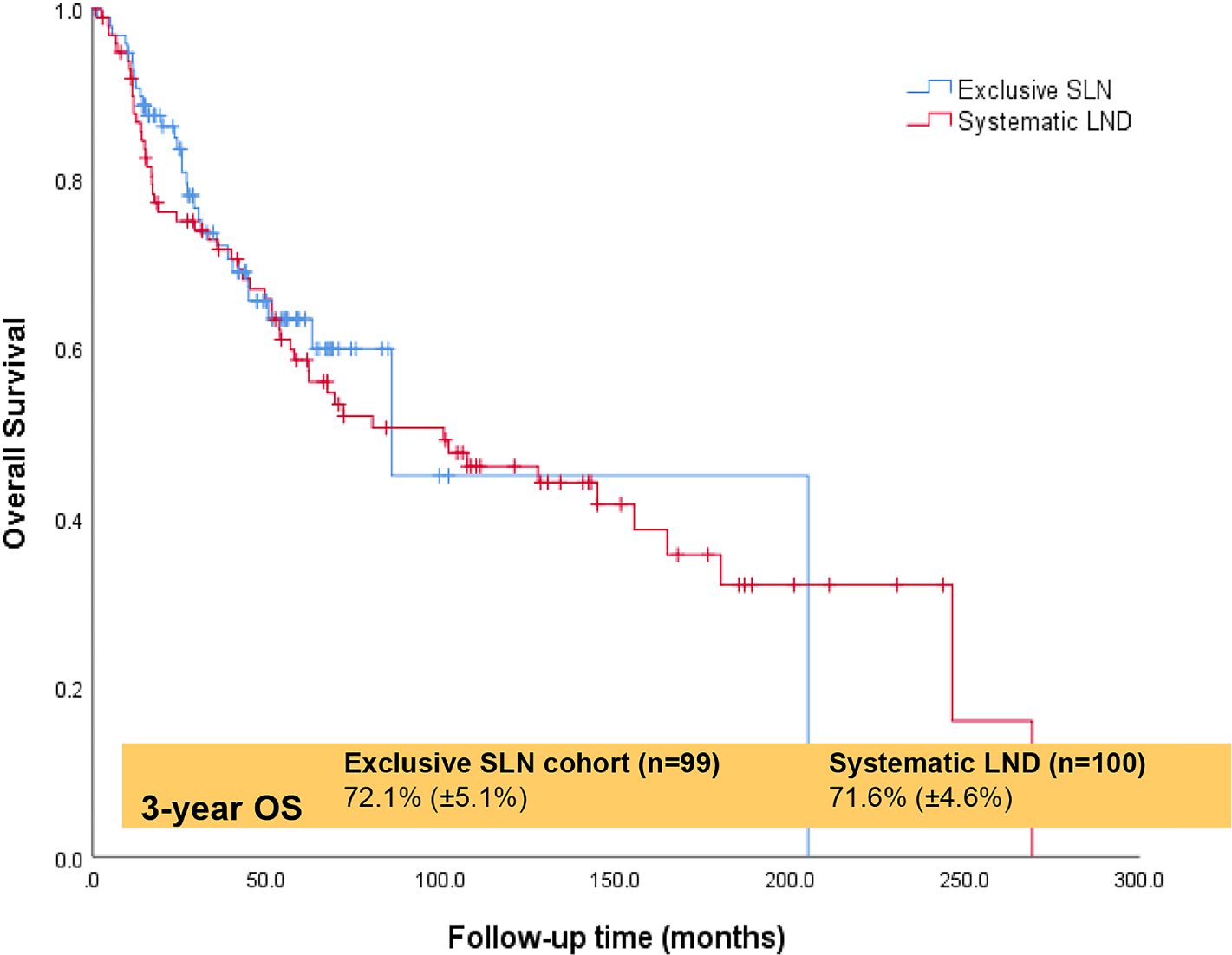
Overall survival for all patients with uterine carcinosarcoma.
Table 4.
Univariate analysis for OS in patients with uterine carcinosarcoma (n=199)
| Number of patients | Number of events | Three-year OS (±SE) rate | P-value | |
|---|---|---|---|---|
| All | 199 | 85 | — | 0.68 |
| SLN | 99 | 31 | 72.1 (±5.1) | |
| LND | 100 | 54 | 71.6 (±4.6) | |
| Procedure | 0.82 | |||
| Laparotomy | 99 | 50 | 73.9 (±4.5) | |
| MIS | 100 | 35 | 69.2 (±5.3) | |
| Stage | 0.01 | |||
| I | 116 | 43 | 74.6 (±4.3) | |
| II | 17 | 8 | 81.1 (±9.9) | |
| III | 57 | 26 | 70.6 (±6.5) | |
| IV | 9 | 8 | 37.5 (±17.1) | |
| Myometrial invasion | 0.01 | |||
| <50% | 129 | 48 | 77.9 (±3.9) | |
| ≥50% | 69 | 37 | 61 (±6.3) | |
| LVSI | 0.26 | |||
| Present | 91 | 34 | 73.6 (±5.0) | |
| Absent | 104 | 51 | 69.8 (±4.7) | |
| Suspicious | 1 | 0 | — | |
| Peritoneal washings | 0.01 | |||
| Positive | 43 | 27 | 59.6 (±7.8) | |
| Negative | 139 | 51 | 76.1 (±3.9) | |
| Suspicious | 3 | 0 | — |
Data are expressed as n (%) unless otherwise specified.
LVSI, lymphovascular space invasion; MIS, minimally invasive surgery; SE, standard error.
Discussion:
It is becoming more apparent that systematic lymphadenectomy of grossly normal nodes at the time of surgical staging for uterine cancer does not improve survival [14]. However, assessment of whether there has been nodal spread will affect proper staging and may impact postoperative therapy decisions for some patients. An SLN algorithm is effective in detecting nodal metastases, decreases morbidity compared with lymphadenectomy, and is overall safe and well tolerated [10, 12, 15, 16]. The MSK SLN algorithm for uterine cancer demonstrates a false negative rate of 3.1% and false negative predictive value (FNPV) of 0.5% with a significant reduction in morbidity in comparison to complete lymphadenectomy [8]. The FIRES trial prospectively demonstrated that SLN biopsy is effective in patients with uterine cancer also noting a FNPV of 0.4% [17]. However, the FIRES trial only included 13 (4%) patients with CS, limiting its applicability specifically to this histology [17].
Many studies have demonstrated the diagnostic value of SLN mapping and the OS in patients with high-risk uterine cancer yet doubt still exists regarding the utility and safety of SLN alone in these high-risk cases [2, 11, 18–26]. A prospective trial of 123 patients with high-risk uterine cancer who underwent SLN biopsy with backup LND demonstrated a low FNPV of 1.4% [23]. Similarly, another prospective trial (SENTOR study) also noted a FNPV of 0.8% for SLN mapping in intermediate- and high-risk endometrial carcinomas [27]. Both prospective studies validated the use of SLN biopsy in high-risk uterine cancers but only contained a few cases of uterine CS and none assessed long-term survival. Moreover, as all patients underwent backup LND, conclusions regarding the comparative effectiveness of SLN versus LND cannot be made.
Two retrospective studies from our institution evaluated survival for patients with uterine serous carcinoma (USC) only. In our initial report, 248 patients with USC treated before and after incorporation of SLN into our practice were assessed; we found no difference in two-year PFS between the “SLN” cohort (77%, 95% CI 68–83) or the “non-SLN” group (71%, 95% CI 61–79) (P=0.3) [18]. This study was limited by including some patients in the SLN cohort who had also undergone some form of a LND – either a routine backup LND or a side-specific LND in unmapped hemipelvises. In our subsequent analysis, we excluded any SLN cases that failed mapping or had a back-up LND [11]. We noted a two-year PFS of 59% (±6.5) for the exclusive SLN group and a two-year PFS of 65% (±3.8) for the systematic LND group (P=0.48) [11]. Similar results showing no difference in PFS between cohorts have been replicated for other high-grade endometrioid histology [19, 20, 22, 28, 29].
We previously assessed the efficacy and oncologic outcomes of SLN mapping and selective LND for patients undergoing surgical staging of uterine cancer of both serous and endometrioid histologies [11, 16, 21, 30]. The largest published series on SLN mapping for patients with CS included 136 patients from our institution and noted a median PFS of 23 months (95% CI, 17.5–NE) for patients in the SLN cohort versus 23.2 months (95% CI, 15.5–41.8) for the standard lymphadenectomy cohort (P=0.7) [2]. Of note, that analysis was intended to evaluate how the incorporation of SLN mapping affected oncologic outcomes and many patients in the SLN cohort underwent either a planned backup or selective LND for failed mapping. The current study design employs stricter inclusion criteria and is intended to evaluate the oncologic outcomes of patients with CS who underwent SLN biopsy alone without additional LND. Consequently, the median total number of nodes removed was only four (range, 1–13) in the SLN cohort in the current study compared to eight (range, 1–55) in our previous study.
Our study supports the finding of Schiavone et al. who found there is no difference in PFS between SLN mapping or LND with adjuvant therapy for CS [2]. There was no difference in three-year PFS or three-year OS between our two cohorts. However, conclusions drawn by the current study are also limited by its retrospective nature. The median follow-up time was significantly shorter for the exclusive SLN cohort at 33 months (range, 1–205) compared to 55.3 months (range, 1–269) for LND (P=0.001). This will of course have an impact on OS results. However, with the median time to recurrence being approximately 12 months in both cohorts, the difference in follow-up time is less likely to be as meaningful in the comparative analysis of PFS. This study included patients treated at our institution over a 23-year time period and reflects the evolving treatment paradigm over this time. In the more contemporary SLN cohort, 85.9% of patients received adjuvant chemotherapy ± radiotherapy compared to 71% in the systematic LND group(P=0.02). When post-surgical treatment was controlled, there was still no difference in survival outcomes between the two groups in the current study. This is unsurprising, given that the only phase III trial to assess treatment of uterine sarcomas (91 CS patients) showed no difference in OS among patients who received radiotherapy but did show improved local control with a local recurrence rate of 11/46 (24%) in the radiotherapy arm and 21/45 (47%) in the observation arm (P=0.0013) [31]. Makker et al. evaluated 49 patients with CS treated with either chemotherapy +/− radiotherapy or radiotherapy alone and their findings suggested that treatment of chemotherapy +/− radiotherapy improves both PFS and OS, although statistical significance was not reached [32]. Subsequent retrospective analyses have corroborated the notion that incorporation of chemotherapy in adjuvant treatment for CS improves PFS and OS [33–36].
This study is limited by the retrospective nature of the study design which was required given uterine carcinosarcoma is a relatively rare histotype. Although more patients in the exclusive SLN cohort received chemotherapy and more patients in the LND cohort received pelvic external beam radiation therapy, most patients in both groups were treated post-operatively and when treatment received was controlled for there remained no difference in PFS. This is the largest study published to date evaluating exclusive SLN vs LND and this cohort originates from a single institution with well annotated and consistent medical records.
Conclusions:
In our study of 199 CS patients who underwent either exclusive SLN or systematic LND there was no difference in PFS or OS. SLN biopsy alone is feasible in patients with CS, adequately detects the presence of nodal metastasis, and does not compromise oncologic outcomes. SLN biopsy should be considered part of standard surgical management of this disease. Systematic LND does not seem to offer a survival advantage for patients with CS, and routine systematic LND of non-bulky nodes is likely unnecessary.
Highlights:
Systematic LND showed no survival benefit vs. SLN biopsy alone in patients with uterine carcinosarcoma.
There is no difference in detection of nodal metastasis in SLN biopsy alone vs. systematic LND.
SLN biopsy in place of systematic LND does not compromise oncologic outcome.
Funding:
This research was funded in part by MSK’s NIH/NCI Cancer Center Support Grant, P30 CA008748.
Conflicts of interest: Outside the submitted work, Dr. Makker reports industry support and unpaid consultant fees from Astra Zeneca, Eisai, Merck, Lilly, Karyopharm, Takeda, Genentech, Clovis, Novartis, Faeth, Zymeworks, GSK, and Moreo. Dr. Makker also discloses personal fees from IBM Watson. Dr. Abu-Rustum reports institutional grants from Stryker/Novadaq and GRAIL. Dr. Leitao reports grants from KCI/Acelity, personal fees from Takeda, J&J/Ethicon, and Intuitive Surgical. All other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022, CA Cancer J Clin (2022. Jan 12), doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- [2].Schiavone MB, Zivanovic O, Zhou Q, Leitao MM Jr., Levine DA, Soslow RA, et al. Survival of Patients with Uterine Carcinosarcoma Undergoing Sentinel Lymph Node Mapping, Ann Surg Oncol 23 (1) (2016. Jan) 196–202, doi: 10.1245/s10434-015-4612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Desai NB, Kollmeier MA, Makker V, Levine DA, Abu-Rustum NR, Alektiar KM. Comparison of outcomes in early stage uterine carcinosarcoma and uterine serous carcinoma, Gynecol Oncol 135 (1) (2014. Oct) 49–53, doi: 10.1016/j.ygyno.2014.07.097 [DOI] [PubMed] [Google Scholar]
- [4].Yamada SD, Burger RA, Brewster WR, Anton D, Kohler MF, Monk BJ. Pathologic variables and adjuvant therapy as predictors of recurrence and survival for patients with surgically evaluated carcinosarcoma of the uterus, Cancer 88 (12) (2000. Jun 15) 2782–6, doi: [DOI] [PubMed] [Google Scholar]
- [5].Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma, Curr Opin Oncol 23 (5) (2011. Sep) 531–6, doi: 10.1097/CCO.0b013e328349a45b [DOI] [PubMed] [Google Scholar]
- [6].Segarra-Vidal B, Dinoi G, Zorrilla-Vaca A, Mariani A, Student V, Garcia NA, et al. Minimally Invasive Compared With Open Hysterectomy in High-Risk Endometrial Cancer, Obstet Gynecol 138 (6) (2021. Dec 1) 828–37, doi: 10.1097/aog.0000000000004606 [DOI] [PubMed] [Google Scholar]
- [7].Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging, J Natl Compr Canc Netw 12 (2) (2014. Feb) 288–97, doi: 10.6004/jnccn.2014.0026 [DOI] [PubMed] [Google Scholar]
- [8].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes, Gynecol Oncol 125 (3) (2012. Jun) 531–5, doi: 10.1016/j.ygyno.2012.02.021 [DOI] [PubMed] [Google Scholar]
- [9].Khoury-Collado F, St Clair C, Abu-Rustum NR. Sentinel Lymph Node Mapping in Endometrial Cancer: An Update, Oncologist 21 (4) (2016. Apr) 461–6, doi: 10.1634/theoncologist.2015-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zammarrelli WA 3rd, Afonso AM, Broach V, Sonoda Y, Zivanovic O, Mueller JJ, et al. Sentinel lymph node biopsy in patients with endometrial cancer and an indocyanine green or iodinated contrast reaction - A proposed management algorithm, Gynecol Oncol 162 (2) (2021. Aug) 262–7, doi: 10.1016/j.ygyno.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Basaran D, Leitao MM Jr. UPDATE ON SENTINEL LYMPH NODE MAPPING IN ENDOMETRIAL CANCER PATIENTS WITH A HIGH RISK FOR NODAL METASTASIS, Indian J Gynecol Oncol 18 (2) (2020. Jun), doi: 10.1007/s40944-020-00386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jewell EL, Huang JJ, Abu-Rustum NR, Gardner GJ, Brown CL, Sonoda Y, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies, Gynecol Oncol 133 (2) (2014. May) 274–7, doi: 10.1016/j.ygyno.2014.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging, Int J Gynecol Cancer 23 (5) (2013. Jun) 964–70, doi: 10.1097/IGC.0b013e3182954da8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer, Cochrane Database Syst Rev 10 (10) (2017. Oct 2) Cd007585, doi: 10.1002/14651858.CD007585.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Lowery W Jeffrey, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations, Gynecol Oncol 146 (2) (2017. Aug) 405–15, doi: 10.1016/j.ygyno.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zahl Eriksson AG, Ducie J, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion, Gynecol Oncol 140 (3) (2016. Mar) 394–9, doi: 10.1016/j.ygyno.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study, Lancet Oncol 18 (3) (2017. Mar) 384–92, doi: 10.1016/s1470-2045(17)30068-2 [DOI] [PubMed] [Google Scholar]
- [18].Schiavone MB, Scelzo C, Straight C, Zhou Q, Alektiar KM, Makker V, et al. Survival of Patients with Serous Uterine Carcinoma Undergoing Sentinel Lymph Node Mapping, Ann Surg Oncol 24 (7) (2017. Jul) 1965–71, doi: 10.1245/s10434-017-5816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ducie JA, Eriksson AGZ, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease, Gynecol Oncol 147 (3) (2017. Dec) 541–8, doi: 10.1016/j.ygyno.2017.09.030 [DOI] [PubMed] [Google Scholar]
- [20].Buda A, Gasparri ML, Puppo A, Mereu L, De Ponti E, Di Martino G, et al. Lymph node evaluation in high-risk early stage endometrial cancer: A multi-institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy, Gynecol Oncol 150 (2) (2018. Aug) 261–6, doi: 10.1016/j.ygyno.2018.06.003 [DOI] [PubMed] [Google Scholar]
- [21].Schlappe BA, Weaver AL, Ducie JA, Eriksson AGZ, Dowdy SC, Cliby WA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy, Gynecol Oncol 151 (2) (2018. Nov) 235–42, doi: 10.1016/j.ygyno.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nasioudis D, Albright BB, Roy A, Ko EM, Giuntoli RL 2nd, Haggerty AF, et al. Patterns of use and outcomes of sentinel lymph node mapping for patients with high-grade endometrial cancer, Gynecol Oncol 159 (3) (2020. Dec) 732–6, doi: 10.1016/j.ygyno.2020.09.023 [DOI] [PubMed] [Google Scholar]
- [23].Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer, Gynecol Oncol 146 (2) (2017. Aug) 234–9, doi: 10.1016/j.ygyno.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhai L, Zhang X, Cui M, Wang J. Sentinel Lymph Node Mapping in Endometrial Cancer: A Comprehensive Review, Front Oncol 11 (2021. 701758, doi: 10.3389/fonc.2021.701758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schlappe BA, Weaver AL, McGree ME, Ducie J, Zahl Eriksson AG, Dowdy SC, et al. Multicenter study comparing oncologic outcomes after lymph node assessment via a sentinel lymph node algorithm versus comprehensive pelvic and paraaortic lymphadenectomy in patients with serous and clear cell endometrial carcinoma, Gynecol Oncol 156 (1) (2020. Jan) 62–9, doi: 10.1016/j.ygyno.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bogani G, Raspagliesi F, Leone Roberti Maggiore U, Mariani A. Current landscape and future perspective of sentinel node mapping in endometrial cancer, J Gynecol Oncol 29 (6) (2018. Nov) e94, doi: 10.3802/jgo.2018.29.e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cusimano MC, Vicus D, Pulman K, Maganti M, Bernardini MQ, Bouchard-Fortier G, et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging, JAMA Surg 156 (2) (2021. Feb 1) 157–64, doi: 10.1001/jamasurg.2020.5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bogani G, Papadia A, Buda A, Casarin J, Di Donato V, Gasparri ML, et al. Sentinel node mapping vs. sentinel node mapping plus back-up lymphadenectomy in high-risk endometrial cancer patients: Results from a multi-institutional study, Gynecol Oncol 161 (1) (2021. Apr) 122–9, doi: 10.1016/j.ygyno.2021.01.008 [DOI] [PubMed] [Google Scholar]
- [29].Baiocchi G, Mantoan H, Kumagai LY, Gonçalves BT, Badiglian-Filho L, de Oliveira Menezes AN, et al. The Impact of Sentinel Node-Mapping in Staging High-Risk Endometrial Cancer, Ann Surg Oncol 24 (13) (2017. Dec) 3981–7, doi: 10.1245/s10434-017-6132-8 [DOI] [PubMed] [Google Scholar]
- [30].Eriksson AG, Beavis A, Soslow RA, Zhou Q, Abu-Rustum NR, Gardner GJ, et al. A Comparison of the Detection of Sentinel Lymph Nodes Using Indocyanine Green and Near-Infrared Fluorescence Imaging Versus Blue Dye During Robotic Surgery in Uterine Cancer, Int J Gynecol Cancer 27 (4) (2017. May) 743–7, doi: 10.1097/igc.0000000000000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reed NS, Mangioni C, Malmström H, Scarfone G, Poveda A, Pecorelli S, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874), Eur J Cancer 44 (6) (2008. Apr) 808–18, doi: 10.1016/j.ejca.2008.01.019 [DOI] [PubMed] [Google Scholar]
- [32].Makker V, Abu-Rustum NR, Alektiar KM, Aghajanian CA, Zhou Q, Iasonos A, et al. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I-IV uterine carcinosarcoma, Gynecol Oncol 111 (2) (2008. Nov) 249–54, doi: 10.1016/j.ygyno.2008.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cantrell LA, Havrilesky L, Moore DT, O’Malley D, Liotta M, Secord AA, et al. A multi-institutional cohort study of adjuvant therapy in stage I-II uterine carcinosarcoma, Gynecol Oncol 127 (1) (2012. Oct) 22–6, doi: 10.1016/j.ygyno.2012.06.020 [DOI] [PubMed] [Google Scholar]
- [34].Galaal K, van der Heijden E, Godfrey K, Naik R, Kucukmetin A, Bryant A, et al. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma, Cochrane Database Syst Rev 2013 (2) (2013. Feb 28) Cd006812, doi: 10.1002/14651858.CD006812.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cha J, Kim YS, Park W, Kim HJ, Kim JY, Kim JH, et al. Clinical significance of radiotherapy in patients with primary uterine carcinosarcoma: a multicenter retrospective study (KROG 13–08), J Gynecol Oncol 27 (6) (2016. Nov) e58, doi: 10.3802/jgo.2016.27.e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vordermark D, Medenwald D, Izaguirre V, Sieker F, Marnitz S. The Role of Postoperative Radiotherapy for Carcinosarcoma of the Uterus, Cancers (Basel) 12 (12) (2020. Nov 30), doi: 10.3390/cancers12123573 [DOI] [PMC free article] [PubMed] [Google Scholar]


