Abstract
Cervical spinal cord injury (cSCI) severs bulbospinal projections to respiratory motor neurons, paralyzing respiratory muscles below the injury. C2 spinal hemisection (C2Hx) is a model of cSCI often used to study spontaneous and induced plasticity and breathing recovery post-injury. One key assumption is that C2Hx dennervates motor neurons below the injury, but does not affect their survival. However, a recent study reported substantial bilateral motor neuron death caudal to C2Hx. Since phrenic motor neuron (PMN) death following C2Hx would have profound implications for therapeutic strategies designed to target spared neural circuits, we tested the hypothesis that C2Hx minimally impacts PMN survival. Using improved retrograde tracing methods, we observed no loss of PMNs at 2- or 8-weeks post-C2Hx. We also observed no injury-related differences in ChAT or NeuN immunolabeling within labelled PMNs. Although we found no evidence of PMN loss following C2Hx, we cannot rule out neuronal loss in other motor pools. These findings address an essential prerequisite for studies that utilize C2Hx as a model to explore strategies for inducing plasticity and/or regeneration within the phrenic motor system, as they provide important insights into the viability of phrenic motor neurons as therapeutic targets after high cervical injury.
Keywords: phrenic motor neurons, cervical spinal injury, C2Hx, hemisection, spinal injury, Cholera toxin B, CtB, motor neurons, ChAT, NeuN
1. Introduction
Cervical spinal cord injury (cSCI) disrupts descending bulbospinal pathways to spinal respiratory motor neurons, causing respiratory muscle paralysis, diminished breathing capacity and ventilatory failure (Baussart et al., 2006; Brown et al., 2006; Goshgarian et al., 1986). Restoration of breathing capacity is an important goal since respiratory failure is a leading cause of morbidity and mortality in individuals with cSCI (NSCISC, 2018; Winslow and Rozovsky, 2003).
Considerable research has been devoted to identifying potential strategies to improve breathing ability in people with cSCI. Such efforts have generally focused on either regeneration of severed neural pathways (Alilain et al., 2011; Silver and Miller, 2004) or strengthening of spared neural pathways (Courtine et al., 2009; Fuller et al., 2003; Gonzalez-Rothi et al., 2015a; Goshgarian, 2003; Ling et al., 1994; Lovett-Barr et al., 2012). Cervical spinal hemisection at C2 (C2Hx) is an experimental model frequently used to study breathing recovery and plasticity within respiratory motor circuits after cSCI (Alilain and Goshgarian, 2007; Dougherty et al., 2017; Fuller et al., 2008; Gonzalez-Rothi et al., 2017; Goshgarian, 2009; Navarrete-Opazo et al., 2015). In these studies, one fundamental assumption is that C2Hx dennervates spinal respiratory motor neurons caudal to injury without causing significant neuronal pathology or cell death, thus sparing the necessary substrates for functional recovery of breathing ability. However, a recent study reported substantial presumptive motor neuron death caudal to C2Hx (Satkunendrarajah et al., 2016), using stereological analysis of choline acetyltransferase (ChAT) positive cells to assess motor neuron survival. Since there is considerable evidence for neurochemical plasticity in motor neurons post-injury (Fuller et al., 2005; Ganzer et al., 2018; Grossman et al., 2000; Mantilla et al., 2012; Murray et al., 2010), it is possible that C2Hx may alter motor neuron ChAT expression without actual loss/death of motor neurons themselves. Given the fundamental importance of distinguishing between motor neuron loss versus injury-related changes in the neurochemical profile of surviving neurons, it is essential to accurately assess the actual number of cells within the motor pool of interest. Indeed, the report of Satkunendrarajah and colleagues (2016) did not probe the fate of motor neurons in specific motor pools (e.g., phrenic vs. neck vs. forelimb), but performed regional quantification of ChAT expressing cells (e.g. all motor neurons in the cervical spinal cord). This is an important consideration, as there is some historical evidence of caudal motor neuron sparing after C2 spinal cord hemisection(Gonzalez-Rothi et al., 2015b; Lane et al., 2008a), although this was not the primary outcome for either of these studies.
To accurately quantify motor neuron survival caudal to injury, precise identification of the motor neurons of interest is essential. One method frequently used to identify specific motor neuron pools is retrograde tracing of a target muscle or nerve. For example, intrapleural injections of Cholera toxin B fragment (CtB) yield robust retrograde labelling of phrenic motor neurons without non-specific labeling in nearby cells (Mantilla et al., 2009). We utilized a refined version of this technique to test the hypothesis that phrenic motor neuron survival is minimally affected by C2Hx at sub-acute (2 wk) and chronic (8 wk) post-injury time points. We also assessed the impact of chronic spinal injury on expression of commonly used markers of neurons (NeuN-Neuronal Nuclei) and motor neurons (ChAT) in retrogradely labelled phrenic motor neurons to explore whether injury affected expression of these markers in the absence of frank cell death. We report no effect of C2Hx on phrenic motor neuron survival at 2- or 8-weeks post-injury, nor did we observe altered NeuN or ChAT expression in CtB-labelled phrenic motor neurons. These findings have important implications for studies using the C2Hx model to explore spontaneous and/or induced plasticity within the phrenic motor system, since these neurons are an essential substrate for recovery of breathing function post-injury. Thus, confirmation of their overall survival at chronic post-injury time-points would suggest a robust population of neurons available as targets for therapeutic intervention. Conversely, significant loss of phrenic motor neurons would limit the potential for strategies designed to induce plasticity in surviving neuron populations to effect real and meaningful functional recovery without sufficient viable substrate to target.
2. Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committees of the University of Florida and the University of Wisconsin-Madison, and complied with all standards set forth in the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. Rats were double-housed and maintained on a 12-hour light/dark cycle with access to food and water ad libitum.
2.1. Experimental groups
Our primary goal was to assess the impact of chronic C2Hx on phrenic motor neuron survival post-injury. Thus, intrapleural injections of the monosynaptic retrograde tracer CtB were performed in adult male Sprague-Dawley rats (~300 grams, Envigo, Indianapolis IN, Colony 208A, University of Florida) to enable the precise identification of phrenic motor neurons in the cervical spinal cord (Figure 1A). Fourteen days after tracer injections, rats were randomized to one of four groups: 1) spinal intact (no surgery/injury) 2-week time controls (n=7); 2) spinal intact 8-week time controls (n=7); 3) 2-weeks post-C2Hx (n=8); and 4) 8-weeks post-C2Hx (n=9).
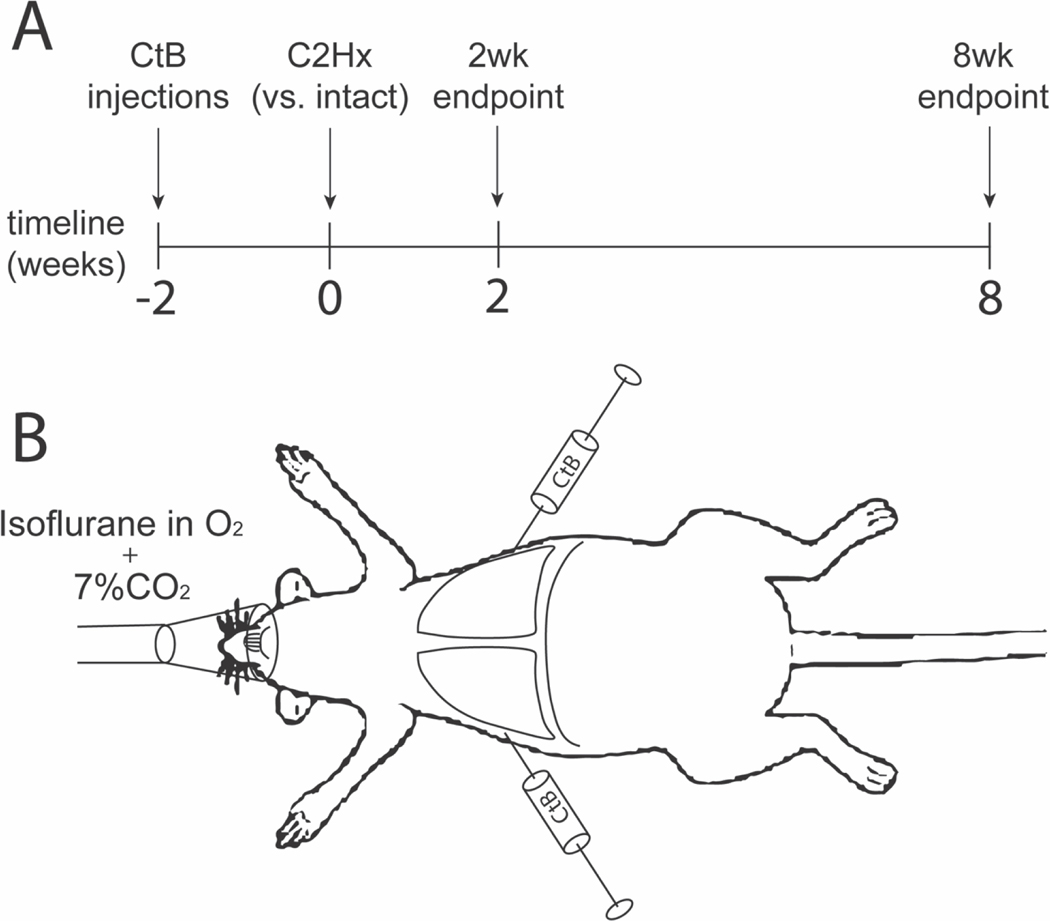
Figure 1: Experimental Model.
A. Schema depicting the experimental timeline. All rats received bilateral intrapleural injections of CtB. Two weeks after injection, rats were randomized to one of four groups: 2-wk intact, 8-wk intact, 2-wk C2Hx, or 8-wk C2Hx. Rats were sacrificed at the pre-determined end-point and cervical spinal tissues processed for CtB, NeuN, and ChAT immunohistochemistry. B. Schema depicting intrapleural tracer injection methods. Rats were anesthetized with isoflurane (2.5%) while under hypercapnic conditions (FICO2=7.1%, FIO2=20.8%, balance N2). Bilateral intrapleural injections of CtB were administered, isoflurane was withdrawn, and animals were allowed to recover from anesthesia while remaining under hypercapnic normoxia.
2.2. Intrapleural CtB injections
The basic methods for intrapleural CtB injections were adapted from previously published reports (Allen et al., 2018; Mantilla et al., 2009; Nichols et al., 2015b) as described in Figure 1B. Briefly, anesthesia was induced with 2.5% isoflurane (in 100% O2) and maintained at 2% via nose cone. Based on findings from an initial study (Fig. 2), intrapleural tracer injections were performed under hypercapnic conditions (FICO2=7.1%, FIO2=20.8%, balance N2) to increase breathing, and maximize retrograde labeling of the phrenic motor pool (increased motor neuron activity and/or greater CtB mixing in the pleural space). Upon transfer to the nose cone (Figure 1B), all rats were exposed to hypercapnic normoxia (FICO2=7.1%, FIO2=20.8%, balance N2) during and after intrapleural injections to maximize retrograde labelling of phrenic motor neurons with CtB. 12.5 μL of CtB (0.2% w/v CtB; dissolved in sterile H2O; Calbiochem, Billerica, MA) was loaded into a 25 μL Hamilton syringe attached to a 9.52 mm sterile needle (Hamilton Company, Reno, NV; syringe: 7636-01, needle: 7804-04). Injections were administered bilaterally (2 × 12.5 μL = 25μL total per animal) at the 5th intercostal space at a depth of approximately 6 mm. Following injections, isoflurane was discontinued, and rats were placed in Plexiglas chambers to recover from anesthesia while under the same hypercapnic normoxic conditions for 15 minutes. Rats were monitored closely for any signs of respiratory distress; however no such signs were observed.
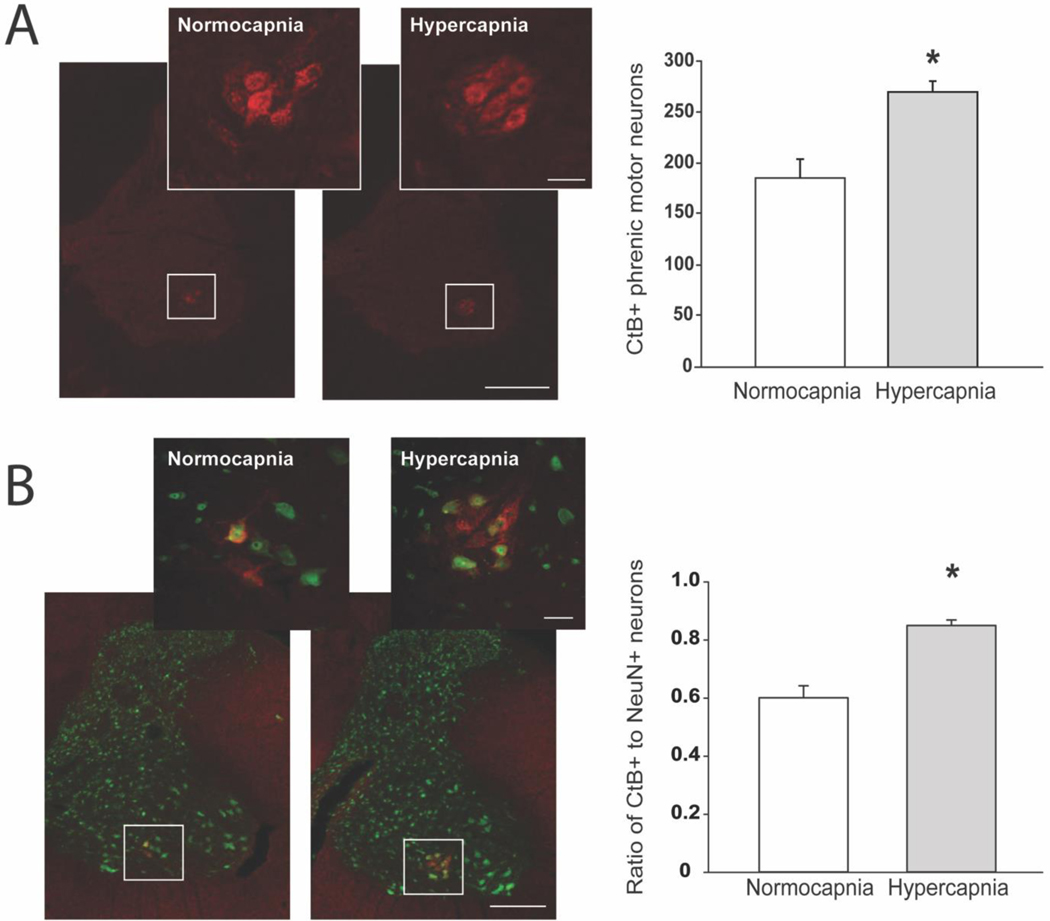
Figure 2. Validation of Modified Intrapleural Injection Methods to Optimize Retrograde Labeling of Phrenic Motor Neurons with Cholera toxin B.
In preliminary experiments, we validated improved intrapleural CtB tracing methods in spinal intact rats by comparing phrenic motor neuron labelling after injections delivered under isoflurane anesthesia with either poikilocapnic (n=7; FICO2=0%, FIO2=100%; white bars) or hypercapnic conditions (n=7; FICO2=7.1%, FIO2=20.8%, balanced N2; gray bars) using previously published methods (Nichols et al., 2013; Nichols et al., 2017; Nichols et al., 2015a). Briefly, transverse sections from the C4 spinal cord were dual labeled for CtB and NeuN. Tissues were incubated in primary antibodies (goat anti-CtB (1:2000); mouse anti-NeuN (1:500)) overnight at 4°C, followed by secondary antibodies (Alexa Fluor® 594 (donkey anti-goat 594; 1:1000); 488 (donkey anti-mouse 488; 1:1000) for 2 hours at room temperature. The total number of phrenic motor neurons within the C4 region was extrapolated from manual phrenic motor neuron cell counts from six C4 sections per animal (left and right phrenic motor neuron counts were not different from each other, and thus were averaged together for each section for extrapolation); briefly, we divided the total C4 segment length (2000 μm) by the thickness of each section (40 μm), and multiplied by the average number of phrenic motor neurons per animal. A. Representative images depict CtB labeling in the cervical spinal ventral horn from isoflurane anesthetized normocapnia and hypercapnia treated rats. (4x with a 20x inset of the phrenic motor pool; Scale bar at 4x = 400μm, 20x = 50μm. We observed increased CtB-labelling with injections performed under hypercapnia vs. normocapnia (*p<0.001). B. Tissue sections from these preliminary studies were also dual labeled with NeuN and manually counted by a blinded observer. The number of large, NeuN-labelled neurons was not different between groups (normocapnia: 307±13, hypercapnia: 320±18; data not shown). Thus, the ratio of CtB-labelled to NeuN-labelled cells was increased by hypercapnia (normocapnia: 0.60±0.04; hypercapnia 0.85±0.02; p<0.001), suggesting improved CtB labelling efficiency of phrenic motor neurons. Data were analyzed using Student’s t test. Differences were considered significant if p< 0.05. Data are displayed as mean±SEM.
2.3. Surgical preparation
Anesthesia methods and surgical preparation have been previously described (Dougherty et al., 2012; Gonzalez-Rothi et al., 2015b). Briefly, anesthesia was induced with isoflurane (2.5% in 100% O2) and maintained via nose cone for the duration of the surgical procedure (2–2.5% isoflurane in 100% O2). Adequate anesthesia was confirmed by the absence of toe pinch and palpebral responses. Body temperature was maintained at 36.5–37.5°C with a heating pad. Artificial tears were applied, nails clipped, and the surgical site shaved and prepped.
2.4. Cervical hemisection
C2Hx methods have been described previously (Dougherty et al., 2012; Gonzalez-Rothi et al., 2015b). Briefly, after anesthetic induction and pre-operative preparation, a dorsal cervical incision was made from the base of the skull to C3, and the skin and dorsal paraspinal muscles were separated to expose the C2 spinous process. The spinal cord was exposed at C2 via dorsal laminectomy. A lateralized hemisection was performed on the left side of the spinal cord just caudal to the dorsal C2 spinal nerve roots using a microscalpel followed by gentle aspiration. After confirming completeness of the C2Hx injury, the dura and overlying muscles were sutured (9–0 and 4–0 sutures, respectively), and the skin closed with stainless steel wound clips. After surgery, rats were administered buprenorphine (0.03 mg/kg, Hospira, IL), meloxicam (2 mg/kg, Portland, ME) and lactated Ringer’s (5mL) subcutaneously for pain management and fluid replacement. Intact rats in control groups did not undergo any surgical procedures.
Following surgery, rats were housed in a heated incubator overnight, then returned to their home cages the following day. Subsequent post-operative care and monitoring was provided twice per day (AM/PM) until rats were fully upright, gaining weight, and displaying no overt signs of pain or distress. Pain was managed by administering buprenorphine (0.03 mg/kg, 2x/day, Hospira, IL) and meloxicam (2 mg/kg, Portland, ME) subcutaneously for 72 hours post-injury. Rats received subcutaneous injections of lactated Ringers (5 mL/day) and were manually fed a nutritional supplement (Diet Gel Boost; Clear H2O; Westbrook, ME) until volitional drinking and eating resumed. Nutritional supplement and chow were provided at the bottom of home cages to ensure food was easily accessible to injured rats.
2.5. Tissue Preparation
Rats were sacrificed at either 2- or 8-weeks post-C2Hx (or the equivalent time point in spinal intact controls). Rats were anesthetized and perfused intracardially with cold 0.1M phosphate buffered saline (PBS), followed by paraformaldehyde (4% w/v in 0.1M PBS, pH 7.4). The spinal cord was removed from the vertebral column and post-fixed in paraformaldehyde overnight. Spinal cords were cryoprotected in 20% sucrose solution in 0.1M PBS for 3 days, followed by 30% sucrose solution in 0.1M PBS for 3 days. The cervical spinal cord (C3–7) was sectioned longitudinally (40 μm) using a freezing microtome (Leica SM2000R, Buffalo Grove, IL) and individual tissues placed sequentially into cell culture wells. Sections were stored in antifreeze solution at −20°C until processed (30% glycerol, 30% ethylene glycol, 40% 0.1 M PBS, pH 7.4.).
2.6. Immunolabeling
Immunofluorescence was used to identify CTB-positive phrenic motor neurons in the cervical spinal cord of spinal intact and C2Hx rats. Manual counts of CtB-positive phrenic motor neurons were conducted in all tissue sections. All tissue sections were processed for visualization of CtB, with a subset of these tissues co-labeled with antibodies to NeuN (1 out of every 4 sections) and the remaining co-labeled with antibodies to ChAT (3 out of 4 sections). The antibody used against CtB (goat, Millipore Cat# 227040, RRID:AB_211712) recognizes the B subunit of Cholera toxin. Goat anti-CtB does not bind to any protein if CtB has not been introduced to the animal (Seven et al., 2018). The antibody used for ChAT (rabbit, Millipore Cat# AB143, RRID:AB_2079760) has been validated in conditional ChAT knockout mice (Young et al., 2008). The antibody for NeuN clone A60 (rabbit, Millipore Cat# ABN78, RRID:AB_10807945) has been used extensively and targets most neurons (Mullen et al., 1992; Wolf et al., 1996).
Tissue sections were washed 3 × 5 minutes with 0.1M PBS and then incubated in a blocking solution (10% NDS in 0.1M TBS-Triton (0.1%), pH 7.4; SD32–0500, Equitech-Bio, Inc, Kerrville, TX) for 1 hour at room temperature. Tissues were then incubated in primary antibody solution (3% NDS in 0.1M TBS-Triton (0.1%), pH 7.4) with goat anti-CtB (1:2500), and either rabbit anti-ChAT (1:100), or rabbit anti-NeuN (1:500) overnight at 4°C. The following day, tissues were washed 3 × 5 minutes with 0.1M TBS-Triton (0.1%) and then incubated in secondary antibody solution (3% NDS in 0.1M TBS-Triton (0.1%), pH 7.4) with secondary antibodies conjugated to Alexa Fluor® 488 (A-21206, 1:1000; Thermo Fisher Scientific, Waltham, MA) and 594 (A212207, 1:500; Thermo Fisher Scientific, Waltham, MA) for 2 hours at room temperature to label CtB and ChAT or NeuN, respectively. Tissues were then washed 3 × 5 minutes with 0.1M TBS and mounted on positively charged slides using VectaShield Hardset mounting medium (Vector Laboratories, UK) and then coverslipped. Slides were stored at 4°C until staining was assessed. Control experiments included incubating tissues without primary or secondary antibodies. Tissues were processed slightly differently in the initial, pilot study to test the impact of hypercapnia on phrenic motor neuron labeling (see figure legend 2).
2.7. Image acquisition
All longitudinal tissue sections were imaged using an epifluorescent microscope with 10x magnification (Keyence BZ-X700, Keyence Corporation of America, Itasca, IL). For tissues dual labelled with CtB and ChAT, a GFP filter was used for CtB (OP-87763; 0.43 seconds) and a Texas Red filter was used for ChAT (OP-87765; 0.10 seconds). For tissues dual labelled with CtB and NeuN, a GFP filter was used for CtB (OP-87763; 0.59 seconds) and a Texas Red filter for NeuN (OP-87765; 0.33 seconds). All image sets (CtB+ChAT and CtB+NeuN) were collected with the same exposure time setting and no binning was performed. Separate investigators imaged and analyzed data, to ensure injury and time-point remained blinded until analyses completion.
2.8. Image analysis
CtB-positive phrenic motor neurons were manually counted from single plane 10x images in all longitudinal cervical tissue sections. During tissue blocking, the right side of the cervical spinal cord was marked to ensure accurate determination of “side” during imaging and analyses. Only CtB-labelled neurons with a visible nucleus were counted in order to minimize double counting of labelled neurons. Data were expressed as number of cells.
In addition to manual counts of all CtB labelled neurons, we assessed expression of two proteins commonly used to identify neurons (NeuN) and more specifically motor neurons (ChAT) within CtB-labelled phrenic motor neurons. Optical density of ChAT or NeuN expression was measured within CtB-labelled phrenic motor neurons using semi-automatic quantification software (Keyence Hybrid Cell Count, Keyence Corporation of America, Itasca, IL). This algorithm identified CtB-positive phrenic motor neuron soma and quantified the optical density of ChAT or NeuN immunoreactivity within each soma. Data were expressed as arbitrary units (a.u.).
Group data were averaged and expressed as mean ± standard error of the mean. data were analyzed using three-way Mixed Model ANOVA with repeated measures (independent variables: injury, time-point and side relative to injury as the repeated measure). Statistical analyses were performed using SAS JMP (Cary, NC). Differences were considered significant if p<0.05.
3. Results
3.1. Phrenic motor neurons survive caudal to cervical hemisection
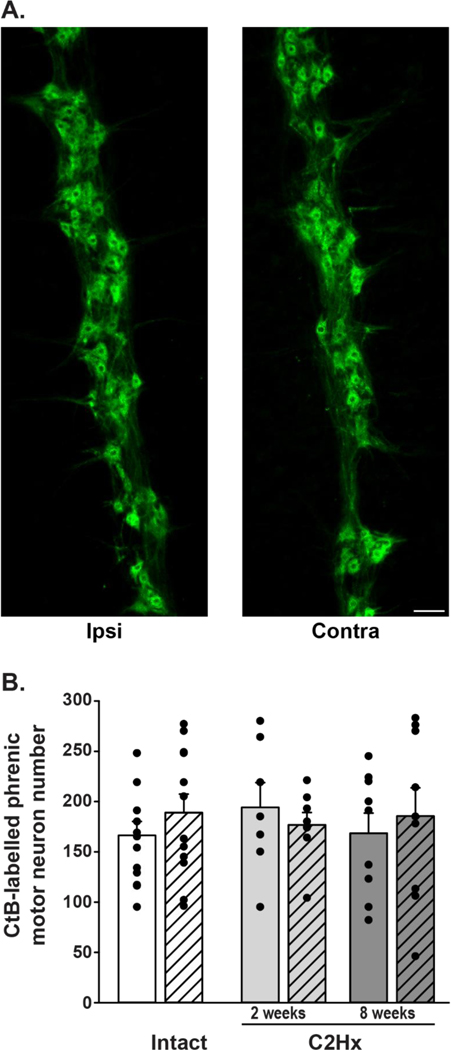
Phrenic motor neurons were retrogradely labelled with CtB via intrapleural injections in both intact and C2Hx rats. CtB-positive cells in the C3-C6 ventral cervical spinal cord were identified via immunoreactivity (Figure 2), and were organized in a rostro-caudal column of clustered cells, consistent with prior reports (Goshgarian and Rafols, 1984; Kinkead et al., 1998; Lane et al., 2008b; Mantilla et al., 2009). Labelled cells with a visible nucleus were manually counted from all longitudinal tissue sections. We observed no differences in phrenic motor neuron number regardless of group (intact vs. injured; p=0.89), side (left/ipsilateral vs. right/contralateral; p=0.11) or time (2 vs. 8 weeks; p=0.49). Comparison of the 2- and 8-week intact groups revealed no differences in phrenic motor neuron number (intact, time: p=0.47; side: p=0.09), so these data were combined into a single intact control group for comparison with injured rat groups. There was no measurable loss of phrenic motor neurons at either 2- or 8-weeks post-C2Hx on either side of the spinal cord versus intact rats (intact ipsilateral: 159±15; intact contralateral: 197±19; C2Hx, 2 weeks, ipsilateral: 194±25; C2Hx, 2 weeks, contralateral: 177±12; C2Hx, 8 weeks, ipsilateral: 169±20; C2Hx, 8 weeks, contralateral: 186±28; group: p=0.93; side: p=0.33; Figure 2).
3.2. Neuronal nuclei immunoreactivity in Phrenic Motor Neurons is not affected by C2Hx
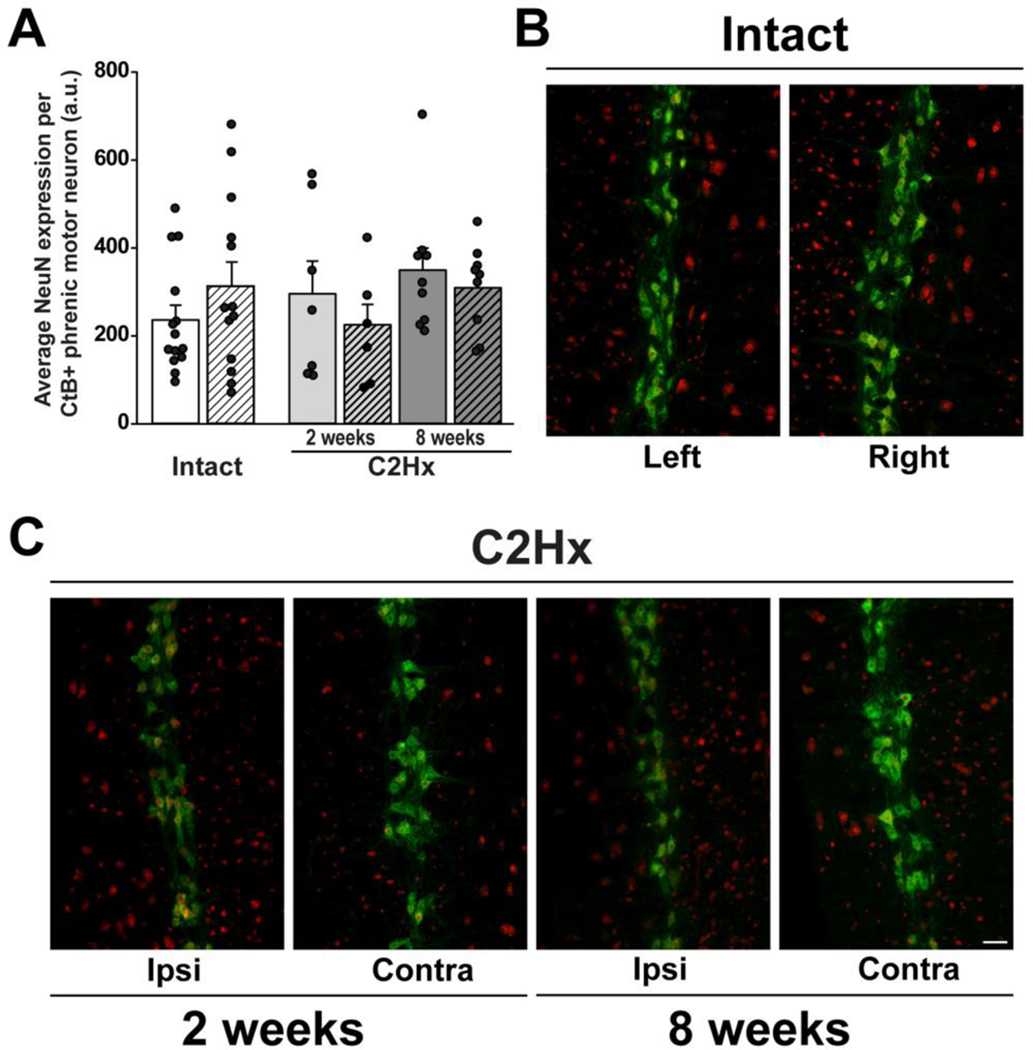
We also explored the impact of C2Hx on expression of NeuN immunoreactivity in large cells in the ventral horn that were co-labelled with CtB (Figure 3), since NeuN is a commonly used neuronal marker in studies assessing cell survival after injury (Gusel’nikova, 2015). NeuN immunoreacitivty was observed in CtB-labelled cells with a distribution consistent with literature reports (Mullen et al., 1992). We observed no injury, side or time-related differences in NeuN immunoreactivity within CtB-labelled phrenic motor neurons (injury: p=0.59; time: p=0.08; side: p=0.79). Similar to our findings with CtB counts, no differences in NeuN immunoreactivity were detected within CtB-labelled phrenic motor neurons among intact groups (intact, time: p=0.25; side: p=0.23), therefore these data were combined into a single control group for comparison with 2- and 8-week injured groups (Figure 3A). We found no effect of C2Hx on NeuN immunoreactivity in CtB-labelled phrenic motor neurons at either time versus spinal intact rats, regardless of side (intact, ipsilateral: 236.2±32.4; intact, contralateral: 313.0±51.3; C2Hx, 2 weeks ipsilateral: 295.8±64.7; C2Hx, 2 weeks, contralateral: 225.3±40.0; C2Hx, 8 weeks, ipsilateral: 349.7±47.2; C2Hx, 8 weeks, contralateral: 309.4±31.2; group: p=0.40, side: p=0.93, Figure 3).
Figure 3: CtB-labelling of phrenic motor neurons in spinal intact and C2Hx rats, 2- and 8-weeks post-injury.
A: Representative images from spinal intact rats illustrating CtB-labelled phrenic motor neurons in longitudinal sections from the cervical spinal cord (40μm). B: Phrenic motor neuron counts in spinal intact (white bars) and C2Hx rats, 2- and 8-weeks post-injury (light grey and dark grey bars, respectively). Left (ipsilateral) and right (contralateral) sides of the cervical spinal cord are indicated by solid and hashed bars, respectively. Data were initially analyzed with repeated measures ANOVA with injury (intact vs. C2Hx) and time-point (2 weeks vs. 8 weeks) as between subject variables, and side (left/ipsi vs. right/contra) as the within subject repeated measure. Since we observed no differences in motor neuron number between 2 and 8 week intact groups (time: p=0.47, side: p=0.09), these data were combined to form a single control group for comparison with the 2 and 8 week C2Hx groups (panel B). Similar to our findings with separate control groups, we found no effect of injury, nor did we observe differences in motor neuron counts between sides (group: p=0.93, side: p=0.33). Scale bar: 100μm. Differences were considered significant if p<0.05. Data are displayed as mean ± SEM.
3.3. ChAT immunoreactivity in Phrenic Motor Neurons not affected by C2Hx
ChAT immunoreactivity was also assessed within CtB-labelled phrenic motor neurons (Figure 4) since it is a frequently used marker of motor neurons. The distribution of ChAT immunoreactivity in CtB-labelled phrenic motor neurons was consistent with literature reports (Barber et al., 1984). C2Hx had minimal impact on ChAT immunoreactivity on either side of the spinal cord at either 2- or 8-weeks post-injury versus intact rats (injury: p=0.18, time: p=0.96, side: p=0.12). Since we did not observe differences in ChAT immunoreactivity in CtB-labelled phrenic motor neurons between intact groups, these data were combined into a single comparison group for 2- and 8-week C2Hx groups (Figure 4A; time: p=0.25, side: p=0.85). When comparing intact versus C2Hx at 2- and 8-weeks post-injury, no injury effect was observed (group: p=0.39), however there was an effect of side (p=0.04; intact, ipsilateral: 872.2±67.1; intact, contralateral: 864.9±84.1; C2Hx, 2 weeks, ipsilateral: 1132.1±64.7; C2Hx, 2 weeks, contralateral: 928.5±110.4; C2Hx, 8 weeks, ipsilateral: 1233.2±224.8; C2Hx, 8 weeks, contralateral: 899.8±190.6; Figure 4). Specifically, average ChAT expression is elevated on the side contralateral to injury, although these differences did not reach statistical significance in post-hoc analyses.
Figure 4: NeuN expression in CtB-labelled phrenic motor neurons in spinal intact and C2Hx rats, 2- and 8-weeks post-injury.
A. Quantification of NeuN expression in CtB-labelled phrenic motor neurons in spinal intact (2- and 8- week groups combined; white bars) and C2Hx rats at 2- and 8-weeks post-injury (light and dark grey bars, respectively). Left/ipsilateral and right/contralateral sides of the cervical spinal cord are indicated by solid and hashed bars, respectively.Data were analyzed with repeated measures ANOVA with “group” (intact vs. 2wk C2Hx vs. 8wk C2Hx) as the between subjects variable and “side” (left/ipsilateral vs. right/contralateral) as the within subject repeated measure. For this analysis, intact groups (2 vs. 8 weeks) were combined to form a single control group, as there were no differences between the two time points in intact rats (time: p=0.25, side: p=0.23). NeuN expression in CtB-labelled phrenic motor neurons was not affected by C2Hx compared to spinal intact rats at 2- or 8-weeks post-injury (group: p=0.40) on either the left/ipsilateral or right/contralateral sides of the spinal cord (side: p=0.93). B-D: Representative images illustrating NeuN expression (red) in CtB-labelled phrenic motor neurons (green) in spinal intact (B) and C2Hx rats at 2- (C) and 8-week (D) time-points (10x). Scale bar: 100μm. A.U.: arbitrary units. Differences were considered significant if p<0.05. Data are displayed as mean ± SEM.
4. Discussion
The primary finding of this study is that C2Hx had no detectable impact on phrenic motor neuron survival with either subacute (2-weeks) or chronic (8-weeks) spinal injury. Further, no effect of C2Hx on key proteins commonly used to identify neurons (NeuN) or motor neurons (ChAT) within retrogradely labelled phrenic motor neurons were observed. Our results confirm survival of phrenic motor neurons after cervical hemisection, preserving the necessary substrate for recovery of breathing function. These experiments utilize and validate a modified methodology for intrapleural tracer injections that improves retrograde labelling of phrenic motor neurons and enables more accurate determination of phrenic motor neuron survival post-injury (Figure 2).
4.1. Motor neuron survival caudal to injury C2Hx
The fate of neurons after spinal cord injury has been explored in a number of experimental models, including cervical (Nicaise et al., 2013; Nicaise et al., 2012a; Nicaise et al., 2012b), thoracic (Magnuson, 1999; Yu, 2019), thoraco-lumbar (Wen, 2015), and lumbar contusions (Magnuson, 1999), contusion with compression (Orr, 2017), cervical hemisection (Gonzalez-Rothi et al., 2015b; Lane et al., 2008a; Satkunendrarajah et al., 2016), thoracic transection (Bjugn et al., 1997; Yokota, 2019) and excitotoxic lesions (Magnuson, 1999). Wallerian degeneration is well described after SCI, whereby distal axons and dendrites of neurons directly affected by an injury degenerate over time (A.V., 1850; Becerra, 1995). Transneuronal degeneration, characterized by degeneration of neurons normally innervated by these damaged and/or degenerated axons has also been described (Bose et al., 2005; Cook, 1951; Eidelberg, 1989; Grossman et al., 2001), though there are some reports to the contrary (Kaelan, 1988; McBride and Feringa, 1992; Yokota, 2019).
There appear to be considerable differences in neuronal survival caudal to injury based on the type, level, and severity of injury, as well as other factors such as cell type, distance from the lesion epicenter (Grossman et al., 2001; Nicaise et al., 2013; Nicaise et al., 2012a; Nicaise et al., 2012b) and time post-injury (for review, see (Hassannejad et al., 2018)). For example, motor neuron loss is reported caudal to injury following moderate-severe contusion (Bose et al., 2005; Grossman et al., 2001; Nicaise et al., 2013; Nicaise et al., 2012a; Nicaise et al., 2012b). Conversely, with spinal transection, motor neuron survival has been reported (Hirakawa and Kawata, 1992; Yokota, 2019), even 52 weeks post-injury (L4-S3; (McBride and Feringa, 1992). While some of the disparities observed across studies are likely related to differences in injury type and/or severity, the methods used to evaluate motor neuron survival may also contribute. Indeed, methods used to assess neuronal survival post-injury vary considerably across studies, ranging from retrograde tracing methods (fluoro-gold, horseradish peroxidase, CtB) to immunohistochemistry/staining methods such as hematoxaline and eosin, Cresyl violet, TUNEL, ChAT, etc (Bose et al., 2005; Eidelberg, 1989; Grossman et al., 2001; McBride and Feringa, 1992; Nicaise et al., 2013; Nicaise et al., 2012a; Nicaise et al., 2012b; Satkunendrarajah et al., 2016; Yokota, 2019). Although immunohistochemical methods enable a broader view of injury-associated changes post-injury (e.g. regional/segmental assessments), they are limited by a lack of specificity to defined cell populations and by their susceptibility to injury-associated changes that do not necessarily correlate with cell loss. Conversely, although neuroanatomical tracing enables specificity of the cell population of interest, it is limited to those neurons retrogradely transporting the tracer.
The C2Hx model has been used extensively in studies exploring novel therapeutic strategies to restore breathing function, such as acute intermittent hypoxia (Dougherty et al., 2017; Golder and Mitchell, 2005; Gonzalez-Rothi et al., 2021; Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015; Vinit et al., 2009), electrical stimulation (Gonzalez-Rothi et al., 2017; Mercier et al., 2017; Sunshine et al., 2021), cellular transplantation (Alilain et al., 2011; Cheng et al., 2021; Dougherty et al., 2016; Goulao et al., 2019; Gransee et al., 2015; Houle and Reier, 1988; Sandhu et al., 2017; White et al., 2010), gene therapy (Charsar et al., 2019; Martinez-Galvez et al., 2016), and pharmacological manipulation (Basura et al., 2002; Hernandez-Torres et al., 2017; Hsu and Lee, 2015; Nantwi et al., 1996; Nantwi and Goshgarian, 1998; Sieck et al., 2021; Wollman et al., 2020; Zhou and Goshgarian, 2000; Zimmer and Goshgarian, 2006). Since the success of these interventions is largely predicated on having some degree of intact neural substrate innervating the diaphragm (e.g. phrenic motor neurons), significant injury-related neuron loss would likely have a profound functional impact. However, there have been few reports concerning the impact of C2Hx (Gonzalez-Rothi et al., 2015b; Lane et al., 2008a; Satkunendrarajah et al., 2016) on survival of neuron populations caudal to injury; and none have systematically assessed phrenic motor neuron survival specifically.
There is some evidence to suggest that phrenic motor neurons are, at least to some extent, spared after injury. For example, while exploring the effects of C2Hx on pre-phrenic interneurons, Lane et al., reported no significant reductions in ipsilateral motor neuron numbers after injury (Lane et al., 2008a), although methodological considerations related to the use of pseudorabies virus (e.g., time-dependent lysis of infected cells) limit interpretation of motor neuron counts. Functionally, restoration of ipsilateral phrenic/diaphragm output after C2Hx, whether spontaneous (Alilain and Goshgarian, 2008; Fuller et al., 2008; Nantwi et al., 1999; O’Hara and Goshgarian, 1991) or induced (Alilain et al., 2011; Gransee et al., 2013; Nantwi and Goshgarian, 1998; Sieck et al., 2021; Urban et al., 2019; Warren, 2018), requires the existance of viable phrenic motor neurons ipsilateral to the side of injury. This is certainly possible, as C2Hx does not damage these neurons directly, nor does it fully deprive them of synaptic inputs. Indeed, there is considerable evidence of crossed spinal pathways (Boulenguez et al., 2007; Dobbins and Feldman, 1994; Feldman et al., 1985; Goshgarian et al., 1991; Lipski et al., 1994; Moreno et al., 1992; Rikard-Bell et al., 1984), sensory afferent neurons (Nair et al., 2017a; Nair et al., 2017b; Road, 1990; Song et al., 1999; Speck and Revelette, 1987; Vinit et al., 2007) and a robust network of propriospinal interneurons (Cregg et al., 2017; Lane et al., 2008a; Lu et al., 2004; Satkunendrarajah et al., 2018; Streeter et al., 2017; Tian and Duffin, 1996) with known connections to phrenic motor neurons. Sparing of and plasticity within at least some of these synaptic connections after injury (Boulenguez et al., 2007; Lane et al., 2008a; Moreno et al., 1992; Streeter et al., 2020; Vinit et al., 2007) may provide sufficient neurotrophic support and synaptic activity to minimize transneuronal degeneration of phrenic motor neurons, which would be consistent with the findings of the present study. Furthermore, sprouting of neural projections may help restore neurochemical and/or synaptic innervation of phrenic motor neurons, further contributing to their survival.
4.2. NeuN and ChAT immunoreactivity following injury
In the present study, NeuN and ChAT immunoreactivity were assessed within CtB-labelled cells since they are frequently used as markers to detect neurons or, more specifically, motor neurons. Since there is considerable evidence for neurochemical plasticity within spinal motor neurons post-injury (Alilain and Goshgarian, 2008; Fuller et al., 2005; Grossman et al., 2000; Kitzman, 2006; Mantilla et al., 2012; Petrov et al., 2007; Yokota, 2019), we explored whether expression of these proteins was altered in surviving motor neurons post-injury. NeuN antibodies reliably label the nucleus and cytosol of ventral horn neurons, including phrenic motor neurons (Mullen et al., 1992), but NeuN expression is not specific to motor neurons, and may be impacted by neural injury in ways that are independent from cell survival (Gusel’nikova, 2015; Unal-Cevik, 2004). For example, hippocampal NeuN expression is reduced following ischemic injury, despite preservation of neuron numbers (Unal-Cevik, 2004; Xu et al., 2002).
ChAT, an enzyme necessary for acetylcholine synthesis, is normally expressed in motor neurons of the ventromedial, central and lateral columns (Barber et al., 1984), and is often used as a marker of motor neurons. Indeed, phrenic motor neurons robustly express ChAT (Barber et al., 1984; Yuan et al., 2014), although at least some spinal pre-phrenic interneurons are also ChAT positive (Lane et al., 2008b). Thus, ChAT immunoreactivity alone is not a unique identifier of motor neurons. Furthermore, ChAT immunoreactivity is variably altered by spinal injury (Hoang et al., 2003; Nakamura et al., 1996; Yokota, 2019). For example, following mild to moderate cervical compression injury, ChAT immunoreactivity at the site of injury (C6) decreased ~50% 2 days post-injury, an effect that persisted for 28 days post-injury (Nakamura et al., 1996). In this same study, the 50–60% reduction of ChAT immunoreactivity rostral (C4–5) and caudal (C7-C8) to injury had recovered by 7 days post-injury. Conversely, increased ChAT expression has been reported caudal to spinal cord transsection (Yokota, 2019), suggesting that in some chronic models, cholinergic activity is selectively maintained in distal motor neurons, despite reduced synaptic inputs and neuronal activity. Since ChAT expression is variably modulated by injury, with its regulation depending on injury type, time post-injury and distance from injury site, ChAT expression is not a reliable indicator of cell number in studies of spinal injury (Lams et al., 1988).
In a recent study, ChAT immunoreactivity was used to determine the extent of spinal motor neuron survival after C2Hx. The authors concluded that over one half of all motor neurons in the cervical spinal cord (C3-C8) die caudal to C2Hx (Satkunendrarajah et al., 2016). This conclusion is problematic since: 1) ChAT immunoreactivity may not be a reliable indicator of motor neuron survival following spinal injury (see above), and 2) ChAT expression is not necessarily limited to motor neurons (Lane et al., 2008b), or uniform across spinal cord regions (Barber et al., 1984). Furthermore, although atrophic changes in spinal regions distal to injury have been described in numerous reports (Bose et al., 2005; David et al., 2019; Gazula et al., 2004; Kitzman, 2005; Yokota, 2019), they are not necessarily the result of neuronal death, nor do they always correlate with reduced ChAT expression. Indeed, a recent report describes sparing of motor neuron size and number after complete spinal cord transection, despite significant reductions in gray matter area; in fact, ChAT expression was actually increased caudal to injury in that study (Yokota, 2019). Thus, factors other than overt cell death may contribute to spinal atrophy caudal to lesions, such as degenerative changes affecting axon tracts that course through spinal gray matter (Yokota, 2019), or reductions in motor neuron somal size or dendritic branching (Gazula et al., 2004; Hirakawa and Kawata, 1992; Kitzman, 2005).
4.3. Retrograde labeling of phrenic motor neurons in anesthetized rats
Accurate identification of phrenic motor neurons is critical to assess cell survival post-injury. Although immunolabeling techniques can be used to identify motor neuron-specific proteins, these techniques are often unreliable after injury, may not be truly reflect cell death, and do not identify specific motor pools. Although endogenous protein markers may exhibit injury-induced neurochemical plasticity not necessarily reflective of motor neuron loss, retrograde labeling techniques prior to injury provide a more definitive approach to determine motor neuron survival post-injury. Indeed, intrapleural injections of retrograde tracers, such as CtB, enable reliable identification of spinal respiratory motor neurons (Allen et al., 2018; Mantilla et al., 2009; Nichols et al., 2015b). Nevertheless, across published studies, there is a range in the reported number of phrenic motor neurons, possibly resulting from methodological differences in tracer administration (Mantilla et al., 2009; Nichols et al., 2015b).
One possible explanation for these disparities could relate to differences in neural activity and or ventilation in anesthetized versus unanesthetized rats. Reduced respiratory motor output in anesthetized rats may decrease phrenic motor neuron CtB labeling versus unanesthetized rats, possibly due to diminished CtB uptake and transport when neural activity is low and/or differences in CtB mixing within the pleural fluid. In an ex vivo diaphragm preparation, frequency-dependent CtB uptake was observed, with higher frequencies (80 Hz) increasing CtB uptake at axon terminals (Gonzalez Porras et al., 2019). Accordingly, we hypothesized that intrapleural CtB injections under hypercapnic conditions would increase phrenic motor neuron activity and, thus, CtB uptake due to increased respiratory drive. Alternately, increased breathing during hypercapnia may increase CtB mixing within intrapleural fluid, increasing access to, and uptake by, phrenic axon terminals. Regardless of the mechanism, we hypothesized that greater ventilation in unanesthetized rats leads to a relative increase in phrenic motor neuron counts using these methods (ie. CtB labeling of the phrenic motor pool) versus anesthetized rats, either due to increased neuronal activity and/or pleural mixing. In preliminary experiments exploring the impact of intrapleural injections under hypercapnic conditions (Figure 2), we found increased phrenic motor neuron labeling versus anesthetized rats kept normocapnic, supporting our working hypothesis that phrenic motor neuron CtB labeling is improved by greater breathing activity. A more detailed understanding of mechanisms whereby increased respiratory activity improves phrenic motor neuron CtB labeling is beyond the scope of this study.
Here, we manually counted all retrogradely labeled phrenic motor neurons to obtain accurate assessments of motor neuron numbers (ie. there was no need for unbiased stereology). We also evaluated NeuN and ChAT expression in CtB-labelled phrenic motor neurons to assess injury associated changes in the expression of these commonly used imarkers and observed no changes in NeuN/ChAT expression within CtB-labelled neurons. Thus, C2Hx does not affect phrenic motor neuron survival or neurochemical expression of NeuN or ChAT at the times studied post-injury. Since we focused on phrenic motor neurons, we cannot make conclusions about cell survival or neurochemical phenotype in other spinal motor nuclei.
5. Conclusions
Phrenic motor neuron survival after incomplete cSCI has profound implications for spontaneous and/or induced spinal respiratory motor plasticity and functional recovery of breathing ability. Indeed, phrenic motor neurons are often the target of experimental therapeutic strategies, predicated on the assumption that they remain viable post-injury. Although a recent report suggested profound loss of putative motor neurons caudal to C2Hx injury, we demonstrate robust phrenic motor neuron survival with both sub-acute and chronic injury. Our results utilize well-established retrograde labelling methods (prior to injury), refined to improve labelling efficiency, enabling precise identification of phrenic motor neurons. Although we confirm phrenic motor neuron survival following C2Hx, this study does not allow conclusions concerning their viability, or. Survival in other nearby motor neuron pools.
Figure 5: Choline acetyltransferase (ChAT) expression in CtB-labelled phrenic motor neurons in spinal intact and C2Hx rats, 2- and 8-weeks post-injury.
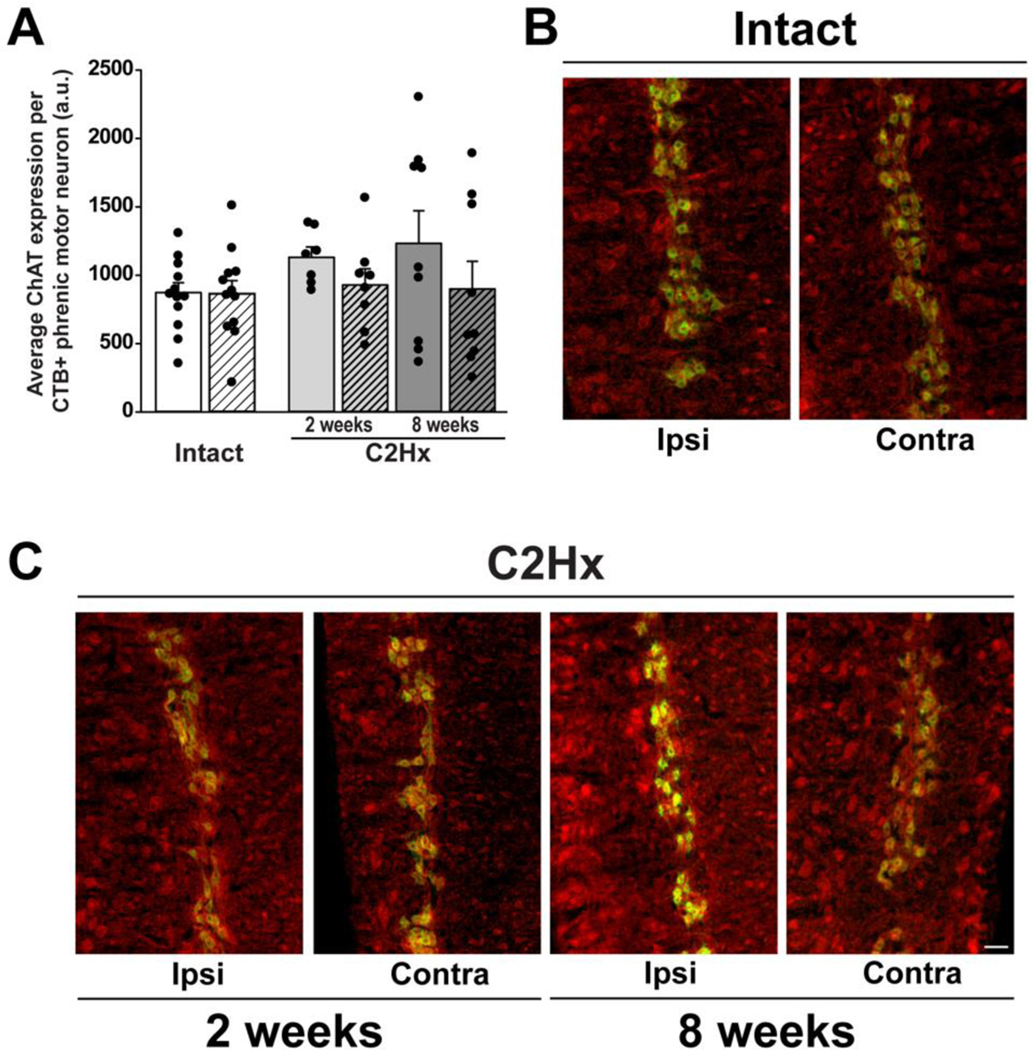
A: Quantification of ChAT expression within CtB-labelled phrenic motor neurons in spinal intact (white bars) and C2Hx rats at 2- and 8-week time-points (light and dark grey bars, respectively). Left/ipsilateral and right/contralateral sides of the cervical spinal cord are indicated by solid and hashed bars, respectively. Data were analyzed with repeated measures ANOVA with “group” (intact vs. 2wk C2Hx vs. 8wk C2Hx) as the between subjects variable and “side” (left/ipsilateral vs. right/contralateral) as the within subject repeated measure. For this analysis, intact groups (2 vs. 8 weeks) were combined to form a single control group (“intact”), as there were no differences between the two time points in intact rats (time: p=0.25, side: p=0.85). ChAT expression in CtB-labelled phrenic motor neurons was not affected by C2Hx at 2- or 8-weeks post-injury compared to spinal intact rats (group: p=0.39), however there was an effect of side (side: p=0.04). B-D: Representative images illustrating ChAT expression (red) in CtB-labelled phrenic motor neurons (green) in spinal intact (B) and C2Hx rats at 2- (C) and 8-week (D) time-points (10x). Scale bar: 100μm. A.U.: arbitrary units. Differences were considered significant if p<0.05. Data are displayed as mean ± SEM.
Highlights.
Phrenic motor neuron survival was assessed 2- and 8-weeks after C2 spinal hemisection
Improved methods were used to identify phrenic motor neurons via intrapleural CtB injections
There was no evidence of phrenic motor neuron loss after C2 hemisection
Phrenic NeuN and ChAT immunoreactivity were also unaffected by C2 hemisection
Acknowledgements.
This work was supported by NIH (HL69064). LLA was supported by T32-HD043730. JVS was supported by the McNair Scholars Program and the American Physiological Society. AEH was supported by the American Physiological Society. NLN was supported by NIH K99/R00 HL119606. EJGR was supported by K12-HD055929. The authors declare no competing financial interests.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A.V.W., 1850. Experiments on the section of the glossopharyngeal and hypoglossal nerves of a frog, and observations of the alterations produced thereby in the structure of their primitive fiber. Philos Trans R Soc Lond Biol 140, 423–429. [Google Scholar]
- Alilain WJ, Goshgarian HG, 2007. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med 30, 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG, 2008. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol 212, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J, 2011. Functional regeneration of respiratory pathways after spinal cord injury. Nature 475, 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LL, Seven YB, Baker TL, Mitchell GS, 2018. Cervical spinal contusion alters Na(+)-K(+)-2Cl- and K(+)-Cl- cation-chloride cotransporter expression in phrenic motor neurons. Respir Physiol Neurobiol 261, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE, 1984. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol 229, 329–346. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Nantwi KD, Goshgarian HG, 2002. Theophylline-induced respiratory recovery following cervical spinal cord hemisection is augmented by serotonin 2 receptor stimulation. Brain Res 956, 1–13. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadie M, Gauthier P, 2006. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis 22, 562–574. [DOI] [PubMed] [Google Scholar]
- Becerra JL, Puckett WR, Hiester ED, Quencer RM, Marcillo AE, Post JD, and Bunge RP, 1995. MR-Pathologic comparisons of wallerian degeneration in spinal cord injury Am J Neuroradiol 16, 125–133. [PMC free article] [PubMed] [Google Scholar]
- Bjugn R, Nyengaard JR, Rosland JH, 1997. Spinal cord transection--no loss of distal ventral horn neurons. Modern stereological techniques reveal no transneuronal changes in the ventral horns of the mouse lumbar spinal cord after thoracic cord transection. Exp Neurol 148, 179–186. [PubMed] [Google Scholar]
- Bose P, Parmer R, Reier PJ, Thompson FJ, 2005. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp Neurol 191, 13–23. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gauthier P, Kastner A, 2007. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res 1148, 96–104. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E, 2006. Respiratory dysfunction and management in spinal cord injury. Respir Care 51, 853–868;discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- Charsar BA, Brinton MA, Locke K, Chen AY, Ghosh B, Urban MW, Komaravolu S, Krishnamurthy K, Smit R, Pasinelli P, Wright MC, Smith GM, Lepore AC, 2019. AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury. FASEB J 33, 13775–13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sami A, Ghosh B, Urban MW, Heinsinger NM, Liang SS, Smith GM, Wright MC, Li S, Lepore AC, 2021. LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury. Neurobiol Dis 147, 105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WH, Walker JH, Barr ML, 1951. A cytological study of transneuronal atrophy in the cat and rabbit. J Comp Neurol 94, 267–291. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR, 2009. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, Chu KA, Hager LE, Maggard RSJ, Stoltz DR, Edmond M, Alilain WJ, Philippidou P, Landmesser LT, Silver J, 2017. A Latent Propriospinal Network Can Restore Diaphragm Function after High Cervical Spinal Cord Injury. Cell Rep 21, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Seif M, Huber E, Hupp M, Rosner J, Dietz V, Weiskopf N, Mohammadi S, Freund P, 2019. In vivo evidence of remote neural degeneration in the lumbar enlargement after cervical injury. Neurology 92, e1367–e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL, 1994. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347, 64–86. [DOI] [PubMed] [Google Scholar]
- Dougherty BJ, Gonzalez-Rothi EJ, Lee KZ, Ross HH, Reier PJ, Fuller DD, 2016. Respiratory outcomes after mid-cervical transplantation of embryonic medullary cells in rats with cervical spinal cord injury. Exp Neurol 278, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD, 2012. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir Physiol Neurobiol 183, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Terada J, Springborn SR, Vinit S, MacFarlane PM, Mitchell GS, 2017. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir Physiol Neurobiol 256, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg E, Nguyen LH, Polich R, Walden JG, 1989. Transsynaptic degeneration of motoneurones caudal to spinal cord lesions. Brain Res Bull 22, 39–45. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF, 1985. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5, 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS, 2005. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma 22, 203–213. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ, 2008. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Mitchell GS, 2003. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23, 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer PD, Beringer CR, Shumsky JS, Nwaobasi C, Moxon KA, 2018. Serotonin receptor and dendritic plasticity in the spinal cord mediated by chronic serotonergic pharmacotherapy combined with exercise following complete SCI in the adult rat. Exp Neurol 304, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG, 2004. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol 476, 130–145. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS, 2005. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Porras MA, Fogarty MJ, Gransee HM, Sieck GC, Mantilla CB, 2019. Frequencydependent lipid raft uptake at rat diaphragm muscle axon terminals. Muscle Nerve. [DOI] [PMC free article] [PubMed]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015a. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Rombola AM, Rousseau CA, Mercier LM, Fitzpatrick GM, Reier PJ, Fuller DD, Lane MA, 2015b. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. J Neurotrauma 32, 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Streeter KA, Hanna MH, Stamas AC, Reier PJ, Baekey DM, Fuller DD, 2017. High-frequency epidural stimulation across the respiratory cycle evokes phrenic short-term potentiation after incomplete cervical spinal cord injury. J Neurophysiol 118, 2344–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Tadjalli A, Allen LL, Ciesla MC, El Chami M, Mitchell G, 2021. Protocol-specific effects of intermittent hypoxia preconditioning on phrenic motor plasticity in rats with chronic cervical spinal cord injury. J Neurotrauma. [DOI] [PMC free article] [PubMed]
- Goshgarian HG, 2003. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol (1985) 94, 795–810. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, 2009. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 169, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL, 1991. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol 111, 135–139. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P, 1986. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol 93, 440–445. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA, 1984. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol 13, 85–109. [DOI] [PubMed] [Google Scholar]
- Goulao M, Ghosh B, Urban MW, Sahu M, Mercogliano C, Charsar BA, Komaravolu S, Block CG, Smith GM, Wright MC, Lepore AC, 2019. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 67, 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB, 2013. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8, e64755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB, 2015. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma 32, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR, 2001. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 168, 273–282. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR, 2000. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem 75, 174–184. [DOI] [PubMed] [Google Scholar]
- Gusel’nikova VV, Korzhevskiy DE, 2015. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae 7, 42–47. [PMC free article] [PubMed] [Google Scholar]
- Hassannejad Z, Zadegan SA, Shakouri-Motlagh A, Mokhatab M, Rezvan M, Sharif-Alhoseini M, Shokraneh F, Moshayedi P, Rahimi-Movaghar V, 2018. The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran J Basic Med Sci 21, 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC, 2017. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J Neurophysiol 117, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa M, Kawata M, 1992. Influence of spinal cord hemisection on the configurational changes in motor and primary afferent neurons and the chemical messenger alterations in the rat lumbar segments. J Hirnforsch 33, 419–428. [PubMed] [Google Scholar]
- Hoang TX, Nieto JH, Tillakaratne NJ, Havton LA, 2003. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J Comp Neurol 467, 477–486. [DOI] [PubMed] [Google Scholar]
- Houle JD, Reier PJ, 1988. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol 269, 535–547. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Lee KZ, 2015. Effects of serotonergic agents on respiratory recovery after cervical spinal injury. J Appl Physiol (1985) 119, 1075–1087. [DOI] [PubMed] [Google Scholar]
- Kaelan C, Jacobsen PF, Kakulas BA, 1988. An investigation of possible transsynaptic neuronal degeneration in human spinal cord injury. J Neurol Sci 86, 231–237. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS, 1998. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18, 8436–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman P, 2005. Alteration in axial motoneuronal morphology in the spinal cord injured spastic rat. Exp Neurol 192, 100–108. [DOI] [PubMed] [Google Scholar]
- Kitzman P, 2006. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in the spastic rat. Exp Neurol 197, 407–419. [DOI] [PubMed] [Google Scholar]
- Lams BE, Isacson O, Sofroniew MV, 1988. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brainstem cholinergic neurons. Brain Res 475, 401–406. [DOI] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ, 2008a. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511, 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ, 2008b. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. Journal of Comparative Neurology 511, 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS, 1994. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res 101, 35–43. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R, 1994. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640, 171–184. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Qin C, Foreman RD, Farber JP, 2004. Chemical activation of C1-C2 spinal neurons modulates intercostal and phrenic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 286, R1069–1076. [DOI] [PubMed] [Google Scholar]
- Magnuson DSK, Trinder TC, Zhang YP, Burke D, Morassutti DJ, and Shields C, 1999. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol 156, 191–204. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC, 2012. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol 234, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC, 2009. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182, 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Galvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB, 2016. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride RL, Feringa ER, 1992. Ventral horn motoneurons 10, 20 and 52 weeks after T-9 spinal cord transection. Brain Res Bull 28, 57–60. [DOI] [PubMed] [Google Scholar]
- Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM, 2017. Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury. J Neurophysiol 117, 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG, 1992. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol 116, 219–228. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM, 1992. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K, 2010. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16, 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Bezdudnaya T, Zholudeva LV, Detloff MR, Reier PJ, Lane MA, Fuller DD, 2017a. Histological identification of phrenic afferent projections to the spinal cord. Respir Physiol Neurobiol 236, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Streeter KA, Turner SMF, Sunshine MD, Bolser DC, Fox EJ, Davenport PW, Fuller DD, 2017b. Anatomy and physiology of phrenic afferent neurons. J Neurophysiol 118, 2975–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Fujimura Y, Yato Y, Watanabe M, Yabe Y, 1996. Changes in choline acetyltransferase activity and distribution following incomplete cervical spinal cord injury in the rat. Neuroscience 75, 481494. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG, 1996. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol 140, 53–59 [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian H, 1999. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair 13, 225–234. [Google Scholar]
- Nantwi KD, Goshgarian HG, 1998. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res 789, 126–129. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS, 2015. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC, 2013. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J Neurotrauma 30, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC, 2012a. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol 235, 539–552. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC, 2012b. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma 29, 2748–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS, 2013. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med 187, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Satriotomo I, Allen LL, Grebe AM, Mitchell GS, 2017. Mechanisms of Enhanced Phrenic Long-Term Facilitation in SOD1(G93A) Rats. J Neurosci 37, 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Satriotomo I, Harrigan DJ, Mitchell GS, 2015a. Acute intermittent hypoxia induced phrenic long-term facilitation despite increased SOD1 expression in a rat model of ALS. Exp Neurol 273, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Vinit S, Bauernschmidt L, Mitchell GS, 2015b. Respiratory function after selective respiratory motor neuron death from intrapleural CTB-saporin injections. Exp Neurol 267, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCISC, 2018. The University of Alabama at Birmingham: National Spinal Cord Injury Statistical Center. [Google Scholar]
- O’Hara TE Jr., Goshgarian HG, 1991. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol 111, 244–250. [DOI] [PubMed] [Google Scholar]
- Orr MB, Simkin J, Bailey WM, Kadambi NS, McVicar AL, Veldhorst AK, and Gensel JC, 2017. Compression decreases anatomical and functional recovery and alters inflammation after contusive spinal cord injury. J Neurotrauma 34, 2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov T, Kreipke C, Alilain W, Nantwi KD, 2007. Differential expression of adenosine A1 and A2A receptors after upper cervical (C2) spinal cord hemisection in adult rats. J Spinal Cord Med 30, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS, 1984. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull 12, 469–477. [DOI] [PubMed] [Google Scholar]
- Road JD, 1990. Phrenic afferents and ventilatory control. Lung 168, 137–149. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Ross HH, Lee KZ, Ormerod BK, Reier PJ, Fuller DD, 2017. Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output after high cervical spinal cord injury. Exp Neurol 287, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG, 2018. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 562, 419–422. [DOI] [PubMed] [Google Scholar]
- Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG, 2016. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol 276, 59–71. [DOI] [PubMed] [Google Scholar]
- Seven YB, Nichols NL, Kelly MN, Hobson OR, Satriotomo I, Mitchell GS, 2018. Compensatory plasticity in diaphragm and intercostal muscle utilization in a rat model of ALS. Exp Neurol 299, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Gransee HM, Zhan WZ, Mantilla CB, 2021. Acute intrathecal BDNF enhances functional recovery after cervical spinal cord injury in rats. J Neurophysiol 125, 2158–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH, 2004. Regeneration beyond the glial scar. Nat Rev Neurosci 5, 146–156. [DOI] [PubMed] [Google Scholar]
- Song A, Tracey DJ, Ashwell KW, 1999. Development of the rat phrenic nerve and the terminal distribution of phrenic afferents in the cervical cord. Anat Embryol (Berl) 200, 625–643. [DOI] [PubMed] [Google Scholar]
- Speck DF, Revelette WR, 1987. Attenuation of phrenic motor discharge by phrenic nerve afferents. J Appl Physiol (1985) 62, 941–945. [DOI] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel SR, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD, 2020. Mid-cervical interneuron networks following high cervical spinal cord injury. Respir Physiol Neurobiol 271, 103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel SR, Liddell SS, Denholtz LE, Reier PJ, Fuller DD, Baekey DM, 2017. Coupling multielectrode array recordings with silver labeling of recording sites to study cervical spinal network connectivity. J Neurophysiol 117, 1014–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine MD, Cassara AM, Neufeld E, Grossman N, Mareci TH, Otto KJ, Boyden ES, Fuller DD, 2021. Restoration of breathing after opioid overdose and spinal cord injury using temporal interference stimulation. Commun Biol 4, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Duffin J, 1996. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 110, 196204. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T, 2004. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res 1015, 169–174. [DOI] [PubMed] [Google Scholar]
- Urban MW, Ghosh B, Block CG, Strojny LR, Charsar BA, Goulao M, Komaravolu SS, Smith GM, Wright MC, Li S, Lepore AC, 2019. Long-Distance Axon Regeneration Promotes Recovery of Diaphragmatic Respiratory Function after Spinal Cord Injury. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS, 2009. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169, 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A, 2007. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci 25, 3551–3560. [DOI] [PubMed] [Google Scholar]
- Warren PM, Steiger SC, Dick TE, MacFarlane PM, Alilain WJ, and Silver J, 2018. Rapid and robust restoration of breathing long after spinal cord injury. Nat Commun 9, 4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Sun D, Tan J, and Young W, 2015. A consistent, quantifiable, and graded rat lumbosacral spinal cord injury model. J Neurotrauma 32, 875–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, O’Steen BE, Fuller DD, Reier PJ, 2010. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol 225, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J, 2003. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82, 803–814. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I, 1996. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 44, 11671171. [DOI] [PubMed] [Google Scholar]
- Wollman LB, Streeter KA, Fusco AF, Gonzalez-Rothi EJ, Sandhu MS, Greer JJ, Fuller DD, 2020. Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp Neurol 334, 113465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GP, Dave KR, Vivero R, Schmidt-Kastner R, Sick TJ, Perez-Pinzon MA, 2002. Improvement in neuronal survival after ischemic preconditioning in hippocampal slice cultures. Brain Res 952, 153–158. [DOI] [PubMed] [Google Scholar]
- Yokota K, Kubota K, Kobayakawa K, Saito T, Hara M, Kijima K, Maeda T, Katoh H, Ohkawa Y, Nakashima Y, and Okada S, 2019. Pathological changes of distal motor neurons after complete spinal cord injury. Molecular Brain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G, 2008. Single-neuron labeling with inducible Cremediated knockout in transgenic mice. Nature neuroscience 11, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CG, Bondada V, Ghoshal S, Singh R, Pistilli CK, Dayaram K, Iqbal H, Sands M, Davis KL, Bondada S, and Geddes JW, 2019. Repositioning flubendazole for spinal cord injury. J Neurotrauma 36, 2618–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Su H, Chiu K, Lin ZX, Wu W, 2014. Assessment of the rate of spinal motor axon regeneration by choline acetyltransferase immunohistochemistry following sciatic nerve crush injury in mice. J Neurosurg 120, 502–508. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG, 2000. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol (1985) 89, 1528–1536. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG, 2006. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med 29, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]