Abstract
GT160-246, a high-molecular-weight soluble anionic polymer, was tested in vitro and in vivo for neutralization of Clostridium difficile toxin A and B activities. Five milligrams of GT160-246 per ml neutralized toxin-mediated inhibition of protein synthesis in Vero cells induced by 5 ng of toxin A per ml or 1.25 ng of toxin B per ml. In ligated rat ileal loops, 1 mg of GT160-246 neutralized fluid accumulation caused by 5 μg of toxin A. At doses as high as 80 mg/loop, cholestyramine provided incomplete neutralization of fluid accumulation caused by 5 μg of toxin A. GT160-246 protected 80% of the hamsters from mortality caused by infection with C. difficile, whereas cholestyramine protected only 10% of animals. Treatment of C. difficile-infected hamsters with metronidazole initially protected 100% of the hamsters from mortality, but upon removal of treatment, 80% of the hamsters had relapses and died. In contrast, removal of GT160-246 treatment did not result in disease relapse in the hamsters. GT160-246 showed no antimicrobial activity in tests with a panel of 16 aerobic bacteria and yeast and 22 anaerobic bacteria and did not interfere with the in vitro activities of most antibiotics. GT160-246 offers a novel, nonantimicrobial treatment of C. difficile disease in humans.
Clostridium difficile is the most commonly identified cause of infectious nosocomial diarrhea. C. difficile spores are prevalent in hospitals and health care facilities and colonize the lower intestinal tract following hospitalization. The normal intestinal flora ordinarily inhibits the growth of C. difficile. Following disruption of the normal colonic flora by antibiotics, C. difficile proliferates and produces toxins that lead to diarrhea and colitis. C. difficile-infected patients develop symptoms of profuse diarrhea, abdominal pain, and occasionally, fever and leukocytosis (8, 20). In severe cases, patients develop pseudomembranous colitis or toxic megacolon and may require surgery (25). Infection with C. difficile can prolong a patient's hospital stay by as much as 10 to 14 days and can add significant costs to overall patient care (5, 24, 28).
C. difficile-associated diarrheal disease is frequently treated with one of two antibiotics, metronidazole or vancomycin. Antibiotic therapy for C. difficile infection, while initially effective, is not an ideal approach to management of this disease. The use of oral vancomycin is restricted because of the growing rates of resistance among gut enterococci (1). Metronidazole has side effects including nausea, abdominal pain, and an unpleasant metallic taste, and it is also a potential teratogen. Most importantly, because antibiotics continue to suppress recovery of the normal intestinal flora, treatment with antibiotics results in disease recurrence in about 20% of patients.
C. difficile produces two high-molecular-weight toxins, toxins A and B, which are responsible for the development of diarrhea and colitis. Toxin A, a potent enterotoxin, is believed to play the primary role in C. difficile diarrhea; however, toxin B may also contribute to disease in humans (12, 14, 21). Because these toxins are responsible for the disease symptoms and colonization results from disruption of the normal microbial flora by antibiotics, a nonantibiotic toxin binding compound represents an attractive alternative therapy for this disease. Cholestyramine, a cationic resin that binds to C. difficile toxins, has been used as a treatment for C. difficile colitis in some patients. This resin has shown only modest activity and is not recommended for use in patients with severe cases of C. difficile colitis (2, 6, 27). Cholestyramine also binds to vancomycin and must be dosed separately if it is used in combination with this antibiotic (26).
The objective of the present study was to characterize the activity of a polymeric toxin binding compound for the treatment of C. difficile colitis. Here we describe the activity of GT160-246, a high-molecular-weight anionic polymer that binds to C. difficile toxins A and B, is nonantimicrobial, and offers a novel approach to the treatment of C. difficile colitis in humans.
MATERIALS AND METHODS
Polymers.
GT160-246 is a synthetic soluble polyanion with a molecular weight of >400,000. Cholestyramine was obtained from Sigma Chemical Co. (St. Louis, Mo.).
Protein synthesis inhibition assay.
Monolayers of Vero cells (ATCC CCL-81) were cultured to confluency in 96-well microtiter trays. Purified C. difficile toxins A and B were purchased from TechLab (Blacksburg, Va.). The cells were incubated overnight with 1.25 to 5 ng of purified toxin A or purified toxin B per ml in medium in the presence or absence of 5 mg of GT160-246 per ml. Following overnight exposure, the cells were washed and then pulsed with 1 μCi of [3H]leucine per ml and incubated for 1 h at 37°C. Following incubation, the cells were washed with phosphate-buffered saline (PBS), solubilized in 100 μl of 0.2 M KOH, precipitated with 10% trichloroacetic acid, filtered, and analyzed for incorporation of 3H by liquid scintillation counting.
Animal models.
All animal procedures were conducted under protocols approved by a local institutional animal care and use committee and according to the standards of the National Institutes of Health.
Rat ileal loop assay.
Rat ileal loop assays were performed by previously published methods (3, 19). Briefly, male Wistar rats (weight, 200 to 250 g) were fasted 18 to 22 h prior to experimentation. Water was available ad libitum. Animals were anesthetized with a mixture of 60 mg of ketamine per kg of body weight and 5 mg of xylazine per kg administered intraperitoneally, and two 5-cm ileal loops were prepared. The renal pedicles were ligated to prevent the excretion of mannitol, and 10 μCi of [3H]mannitol was injected intravenously. Each ileal loop received 0.5 ml of test agent in PBS. Five micrograms of toxin A with or without test compounds was mixed and immediately injected into the ligated loops. Following injection of the test articles the abdomen was closed and the animals were placed in shoebox cages. Four hours after the test substances were administered, the rats were killed and the ileal loops were harvested. The length-to-weight ratio was determined for each loop as a measure of fluid accumulation, and the level of [3H]mannitol accumulation in each loop was determined as a measure of loop permeability for each treatment. Treatments were replicated within an experiment, and means and standard errors were determined. Statistical analyses were performed on logarithmically transformed data to reduce or eliminate heterogeneity of variance. A one-way analysis of variance and Dunnett's posttest were performed with Graphpad Prism software.
Hamster studies.
Hamsters were housed in groups of five animals each in microisolator cages on sterile Alpha-Chip bedding with free access to autoclaved chow (Purina 5000) and autoclaved tap water. They were infected on day −1 by oral gavage with 106 CFU of an overnight broth culture of C. difficile (strain HUC 2-4 was provided by A. Onderdonk, Boston, Mass.). They were injected subcutaneously with 10 mg of clindamycin phosphate per kg on day 0 to induce disease. The hamsters (n = 10/group) were treated with saline or 500 to 1,500 mg of test articles per kg per day administered in three daily doses by oral gavage. The animals were observed daily for morbidity and mortality and the presence or absence of diarrhea for 7 to 15 days. The animals judged to be in extremis were euthanatized by asphyxiation with CO2. For histological studies, the hamsters were killed on day 2 following clindamycin treatment, and the ceca were removed. The ceca were fixed in formalin, paraffin embedded, sectioned, and stained with hematoxylin-eosin for histological analysis. Sections from each animal were coded and randomized by an observer unaware of the treatments.
Microbial studies.
The antimicrobial activity of GT160-246 was evaluated by a microtiter broth dilution MIC assay and compared with the activities of relevant antibiotics according to standard NCCLS methods (16, 17). Test strains were American Type Culture Collection (ATCC) control or type strains or clinical isolates. The activity of GT160-246 against a panel of 13 aerobic bacteria and 3 species of Candida was compared to the activities of vancomycin and gentamicin. Briefly, serial twofold dilutions of GT160-246 from 5,000 to 150 μg/ml and of vancomycin or gentamicin from 256 to 0.12 μg/ml were prepared in cation-supplemented Mueller-Hinton broth (CSMHB) and aliquoted into 96-well microtiter plates. The plates were inoculated with overnight cultures of bacteria or Candida spp. at concentrations of approximately 5 × 105 CFU/ml. The plates were incubated at 35°C. Growth to turbidity was scored at 18 to 24 h of incubation, and the MICs were determined. The activity of GT160-246 against a panel of bacterial strains representative of the normal anaerobic flora was compared with the activities of metronidazole and imipenem. GT160-246 was tested at twofold dilutions from 5,130 to 2.5 μg/ml in anaerobic brucella broth. Metronidazole was tested at twofold dilutions from 128 to 0.06 μg/ml, and imipenem was tested from 32 to 0.015 μg/ml. All dilutions were tested in duplicate against a panel of 22 species (i.e., two strains each of 12 species and a single strain each of 10 species). Bacteria were cultured on brucella agar, and suspensions of cells were prepared in brucella broth immediately prior to inoculation of microtiter trays. The wells were inoculated with 1.5 × 106 CFU/ml, and the trays were incubated for 2 days in an anaerobic chamber. Growth to turbidity was scored, and the MICs were determined.
Antibiotic interference.
Microtiter trays containing duplicate serial dilutions of antibiotics were prepared in CSMHB or supplemented brucella broth (supplemented with laked horse blood, vitamin K1, and hemin, according to NCCLS guidelines). GT160-246 was prepared in either CSMHB for aerobic cultures or supplemented brucella broth for anaerobic cultures. The final test concentration of GT160-246 was 2,500 μg/ml. Solutions of antibiotics were prepared in CSMHB or supplemented brucella broth. Trimethoprim-sulfonamide was prepared as two separate solutions that were mixed at a ratio of 1:20. Twofold serial dilutions of each antibiotic were prepared and dispensed into 96-well microtiter trays (50 μl/well), in duplicate.
ATCC control strains of Staphylococcus aureus (ATCC 29213), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) were transferred onto blood agar plates for two passages to ensure purity and growth. A 0.5 McFarland suspension was prepared in saline, and 0.2 ml was diluted in 15 ml of CSMHB or CSMHB containing 5 mg of GT160-246 per ml. Microtiter trays containing antibiotic were inoculated with 50 μl of the microorganism suspensions, in duplicate. The trays were incubated for 20 h at 36.4°C prior to being read for determination of the MIC (the lowest concentration of antibiotic with no growth). All manipulations and incubations of anaerobic organisms were performed in an anaerobic chamber. ATCC control strains of Bacteroides fragilis (ATCC 25285), Bacteroides thetaiotaomicron (ATCC 29741), Eubacterium lentum (ATCC 43055), and Clostridium perfringens (ATCC 13124) were transferred to supplemented brucella agar for two passages to ensure purity and good growth. A 0.5 McFarland suspension of each organism was prepared in standard brucella broth. This suspension was diluted by adding 0.5 to 15 ml of brucella broth or brucella broth containing 5,000 μg of GT160-246 per ml. Microtiter trays containing antibiotic were inoculated with 50 μl of the microorganism suspensions, in duplicate, and were incubated in an anaerobic chamber at 36.4°C for 48 h. The MICs of each antibiotic were recorded.
RESULTS
Effect of GT160-246 on toxin-mediated inhibition of protein synthesis.
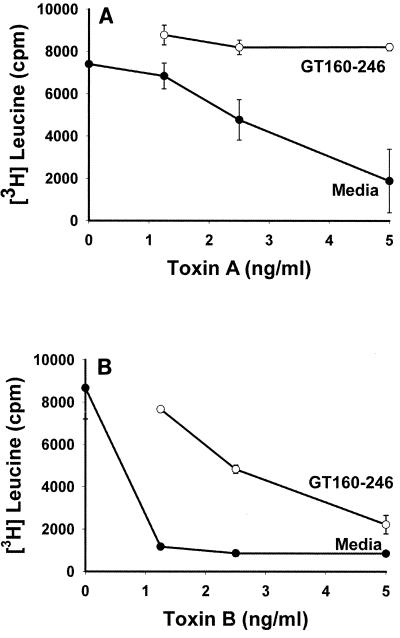
The ability of GT160-246 to modulate the effects of C. difficile toxins A and B on Vero cell protein synthesis was determined. Protection of cells from inhibition of protein synthesis by toxins A and B is shown in Fig. 1. Toxins A and B induced a dose-dependent inhibition of [3H]leucine incorporation in Vero cells. Toxin B was at least fivefold more potent than toxin A at inhibiting Vero cell protein synthesis, with complete inhibition occurring at 1.25 ng/ml. GT160-246 completely blocked inhibition of protein synthesis mediated by 5 ng of toxin A per ml. GT160-246 was able to block 90% of the activity of 1.25 ng of toxin B per ml. GT160-246 also completely blocked toxin A- and toxin B-mediated Vero cell rounding in vitro (data not shown).
FIG. 1.
Effects of GT160-246 on [3H]leucine incorporation by Vero cells in culture. Serial dilutions of C. difficile toxin A or toxin B were incubated with medium or GT160-246 (5 mg/ml) for 1 h at room temperature and then added to Vero cell monolayers. The cells were pulsed with [3H]leucine, and the level of incorporation was measured after 1 h of incubation. (A) Effect of toxin A with either medium alone or GT160-246. (B) Effect of toxin B in the presence of medium alone or GT160-246. Data represent the means for triplicate wells at each concentration. Error bars represent the standard errors of the means.
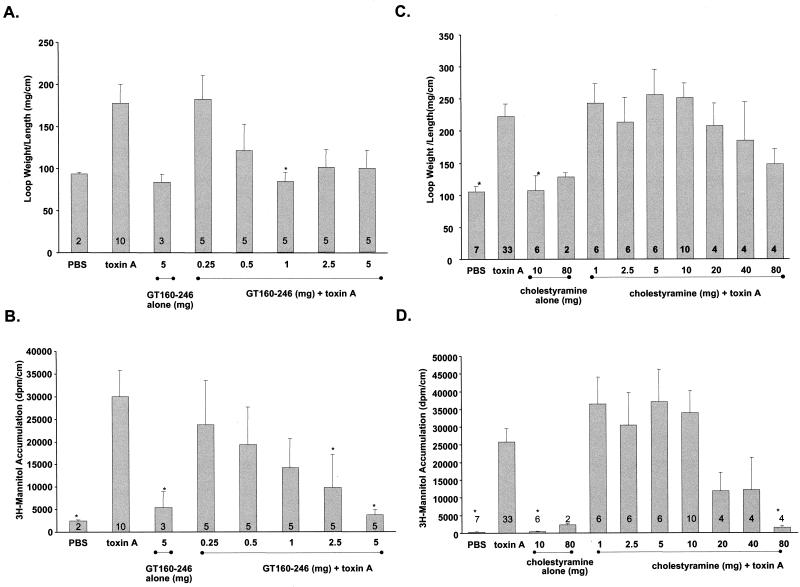
GT160-246 blocks the enterotoxic activity of toxin A in rat ileal loops.
The ability of GT160-246 or cholestryamine to neutralize the enterotoxic activity of toxin A was evaluated in a rat ileal loop model. GT160-246 or cholestyramine was mixed with toxin A and injected into rat ileal loops. Fluid accumulation and permeability to [3H]mannitol were measured over a 4-h period. A dose of 1 mg of GT160-246 caused a significant inhibition (P < 0.05) of fluid accumulation mediated by 5 μg of toxin A (Fig. 2A). A similar trend was seen at higher doses of GT160-246. Treatment of each loop with 2.5 to 5 mg of GT160-246 also caused a significant inhibition (P < 0.05) of toxin A-mediated increases in intestinal permeability (Fig. 2B). In contrast, up to 80 mg of cholestyramine per loop incompletely inhibited toxin A-mediated fluid accumulation, and 80 mg of cholestyramine per loop was required to completely block intestinal permeability (Fig. 2C and D). Taken together, these data show that GT160-246 can block the enterotoxic activities of toxin A in rat ileum and that GT160-246 is significantly more potent than cholestyramine.
FIG. 2.
Effect of GT160-246 or cholestyramine on the enterotoxic activity of toxin A in rat ileal loops. (A) Fluid accumulation induced by 5 μg of toxin A was measured in the presence of 0.25, 0.5, 1, 2.5, or 5 mg of GT160-246. Fluid accumulation was also measured in loops receiving PBS alone (control) or 5 mg of GT160-246 alone. (B) Inhibition of toxin A-mediated increases in intestinal permeability in rat intestinal loops by GT160-246. The permeability of ligated ileal loops to [3H]mannitol following treatment with 5 μg of toxin A was measured in the presence of 0.25, 0.5, 1, 2.5, or 5 mg of GT160-246. The permeability to mannitol was also measured in loops receiving PBS alone (control) or 5 mg of GT160–246 alone. (C) Fluid accumulation induced by 5 μg of toxin A was measured in the presence of 1.0, 2.5, 5.0, 10, 20, 40, or 80 mg of cholestyramine. Fluid accumulation was also measured in loops receiving PBS alone (control), 10 mg of cholestyramine alone, or 80 mg of cholestyramine alone. (D) Permeability of ligated ileal loops to [3H]mannitol following treatment with 5 μg of toxin A was measured in the presence of 1.0, 2.5, 5.0, 10, 20, 40, or 80 mg of cholestyramine. Permeability to mannitol was also measured in loops receiving PBS alone (control), 10 mg of cholestyramine alone, or 80 mg of cholestyramine alone. The number of loops in each treatment group is shown on each column. Error bars represent the standard errors of the means. Data were analyzed by analysis of variance and Dunnett's posttest to compare treatments to toxin control. ∗, P < 0.05.
GT160-246 treatment protects hamsters from mortality due to C. difficile colitis.
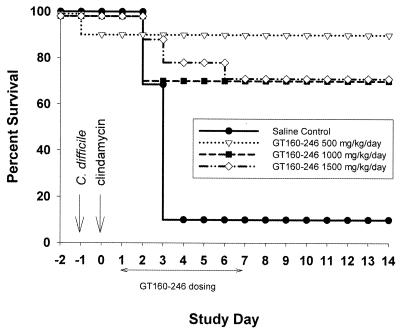
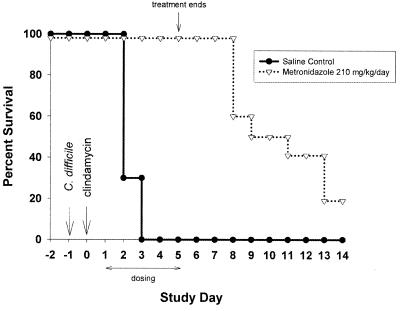
We used the hamster model of C. difficile colitis to evaluate the in vivo toxin-neutralizing activity of GT160-246. C. difficile colitis was induced by inoculation of 106 CFU of C. difficile and treatment with 10 mg of clindamycin phosphate per kg to deplete the normal intestinal flora. GT160-246 does not bind to clindamycin (data not shown) and does not interfere with its activity in vitro (see Table 3). Hamsters were treated with GT160-246 or saline orally on days 1 through 7 following clindamycin treatment; and observations for diarrhea, morbidity, and mortality were made through day 14. All of the hamsters developed clinical symptoms of infection including diarrhea (wet tail), lethargy, and/or ruffled coat. Ninety percent of the animals that received saline developed disease and died by day 4. Treatment with 500, 1,000, or 1,500 mg of GT160-246 per kg per day led to survival of 90, 70, and 70% of the animals, respectively, through day 14 (Fig. 3). Ninety percent of the hamsters treated with GT160-246 (500 mg/kg/day) had no diarrhea by day 14, and 100% of the hamsters receiving 1,000 or 1,500 mg/kg/day had no diarrhea by day 8, with no recurrence of symptoms through the end of the study (day 14). In a separate study, hamsters were treated with metronidazole (210 mg/kg/day) for 5 days. This dose was selected as the lowest dose of metronidazole that completely protected the hamsters from mortality and disease symptoms during active antibiotic treatment (data not shown). Hamsters were completely protected from mortality while receiving antibiotic; however, within 72 h of removal of antibiotic, the hamsters began to develop disease and die, with only 20% survival by day 14 (Fig. 4).
TABLE 3.
Interference by GT160-246 with the antimicrobial activities of antibiotics
| Antibiotic | Concn range (μg/ml) | Interferencea with activity against the following bacteria (ATCC no.):
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus (29213) | B. fragilis (25285) | E. faecalis (29212) | E. coli (25922) | P. aeruginosa (27853) | B. thetaiotaomicron (29741) | E. lentum (43055) | C. perfringens (13124) | ||
| Metronidazole | 0.125–16 | NS | − | NS | NS | NS | − | − | − |
| Vancomycin | 0.25–32 | − | − | − | NS | NS | NS | − | − |
| Penicillin | 0.125–128 | − | − | − | − | NS | − | − | − |
| Ampicillin | 0.125–128 | − | − | − | NS | − | − | − | − |
| Oxacillin | 0.06–64 | − | − | − | NS | NS | − | − | − |
| Amoxacillin w/clavulanic acid | 0.06–64 | − | − | − | − | NS | − | − | − |
| Clindamycin | 0.03–64 | − | − | − | NS | NS | − | − | − |
| Cephalexin | 0.06–128 | − | − | − | − | NS | − | − | − |
| Cefuroxime | 0.25–64 | − | NS | NS | − | NS | NS | − | − |
| Azithromycin | 0.25–64 | + | − | + | + | − | − | − | − |
| Erthromycin | 0.125–64 | − | − | − | − | − | − | − | − |
| Clarithromycin | 0.06–32 | − | − | − | NS | NS | − | − | − |
| Ciprofloxacin | 0.002–16 | − | − | − | − | − | NS | − | − |
| Gatifloxacin | 0.004–8 | − | − | − | − | − | − | − | − |
| Levofloxacin | 0.004–32 | − | − | − | − | − | − | − | − |
| Tetracycline | 0.06–128 | − | − | − | − | − | − | − | − |
| Trimethoprim-sulfonamide | 0.125/2.13–128/2461 | − | − | − | − | NS | − | − | − |
Interference was considered positive (+) if the MIC was raised by >2 dilutions in two replicate wells. −, no interference; NS, not susceptible to the indicated antibiotic (alone) at the highest concentration tested.
FIG. 3.
GT160-246 treatment of hamsters with C. difficile colitis. Hamsters were infected with C. difficile on day −1 and received 10 mg of clindamycin subcutaneously on day 0. Hamsters received saline alone (n = 10) or GT160-246 at 500 mg/kg/day (n = 10), 1,000 mg/kg/day (n = 10), or 1,500 mg/kg/day (n = 10) orally on days 1 through 7. Hamsters were observed for clinical signs of disease and mortality through day 14.
FIG. 4.
Metronidazole treatment of hamsters with C. difficile colitis. Hamsters were infected with C. difficile on day −1 and received 10 mg of clindamycin per kg subcutaneously on day 0. Hamsters received 210 mg of metronidazole per kg per day orally (n = 5) on days 1 through 5. Hamsters were observed for morbidity and mortality through day 14.
GT160-246 prophylaxis prevents mortality from C. difficile colitis in hamsters.
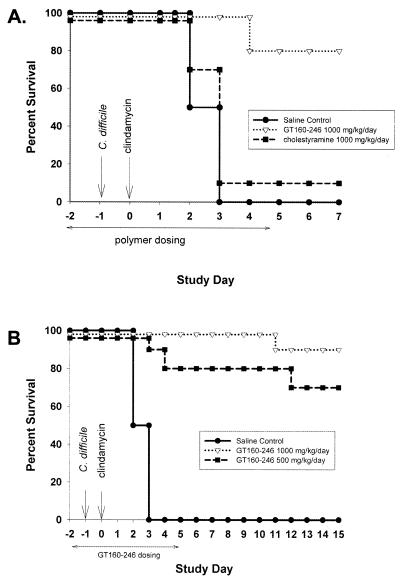
To determine whether a toxin binding compound could prevent C. difficile colitis, hamsters were treated with saline, GT160-246, or cholestyramine. Hamsters were infected with C. difficile on day −1, received clindamycin on day 0, and were treated with compounds for days −2 through day 4 (Fig. 5). All hamsters treated with saline died by day 3. Treatment with 1,000 mg of GT160-246 per kg per day resulted in protection of 80% of hamsters from mortality through day 7, while treatment with 1,000 mg of cholestyramine per kg per day protected only 10% of the hamsters through day 7 (Fig. 5A). In an extended prevention study, GT160-246 was administered at two doses, 500 and 1,000 mg/kg/day, for days −2 through day 4. Hamsters were observed through day 15. All of the animals treated with saline died by day 3. A dose of 500 mg of GT160-246 per kg per day protected 70% of hamsters from death, and a dose of 1,000 mg/kg/day protected 90% of hamsters from death through day 15 (Fig. 5B). By day 15, 57% (four of the seven remaining hamsters) of the hamsters treated with GT160-246 at 500 mg/kg/day and 78% (seven of the nine remaining hamsters) of the hamsters treated with GT160-246 at 1,000 mg/kg/day were also free of diarrhea.
FIG. 5.
GT160-246 pretreatment protects hamsters from mortality from C. difficile colitis. (A) Hamsters were infected with C. difficile on day −1 and received 10 mg of clindamycin per kg subcutaneously on day 0. Hamsters were treated with saline (n = 10), 1,000 mg of GT160-246 per kg per day (n = 10), or 1,000 mg of cholestyramine per kg per day (n = 10) orally on days −2 through day 4. They were observed for morbidity and mortality through day 7. (B) Hamsters were treated with saline (n = 10) or GT160-246 at 500 mg/kg/day (n = 10) or 1,000 mg/kg/day (n = 10) orally from day −2 through day 4. They were observed for development of morbidity and mortality through day 15.
GT160-246 blocks intestinal inflammation resulting from C. difficile infection.
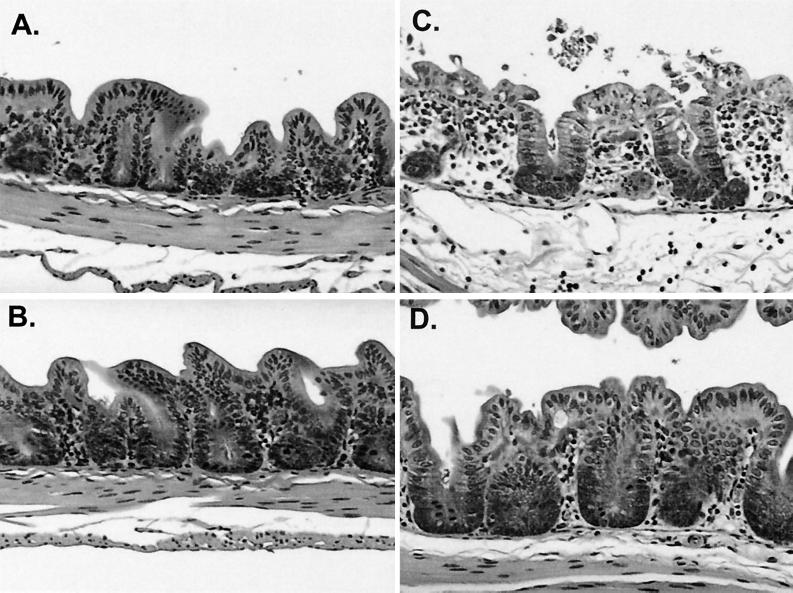
C. difficile toxins cause severe inflammation in the colon, sometimes resulting in pseudomembranous colitis in humans. Hamsters also develop a severe cecitis in response to the toxins; however, pseudomembranes are usually absent. GT160-246 may reduce cecal inflammation caused by the toxins through binding and blocking of their interaction with intestinal receptors. To evaluate the impact of GT160-246 treatment on cecal inflammation, hamster ceca were examined on day 2 for evidence of cecal inflammation resulting from C. difficile infection. Representative sections of cecal tissue are shown in Fig. 6. Ceca from hamsters infected with C. difficile and treated with saline exhibited a pronounced inflammatory infiltrate, edema, and necrosis of villi (Fig. 6C). Ceca from uninfected controls (Fig. 6A) and GT160-246-treated ceca (Fig. 6B) appeared normal, with no significant inflammation, necrosis, or edema. Ceca from C. difficile-infected, GT160-246-treated hamsters (Fig. 6D) showed minimal inflammation and no significant edema or necrosis of the villi. These observations suggest that GT160-246 had a significant effect on toxin-mediated tissue damage and subsequent inflammation at day 2 postinfection.
FIG. 6.
GT160-246 treatment prevents histological damage caused by C. difficile. Representative histological sections from ceca of each treatment group are shown. (A) Noninfected hamster. (B) Noninfected hamster administered GT160-246. (C) C. difficile-infected hamster. (D) C. difficile-infected hamster treated prophylactically with 1,000 mg of GT160–246 per kg per day orally.
GT160-246 antimicrobial activity.
GT160-246 showed little antimicrobial activity at concentrations of up to 5,000 μg/ml against 16 aerobic organisms (Table 1). The MICs of vancomycin and gentamicin for reference strains S. aureus (ATCC 29213), P. aeruginosa (ATCC 27853), and E. coli (ATCC 25922) were within acceptable quality control ranges, as defined by NCCLS. GT160-246 also showed little antimicrobial activity against a diverse panel of anaerobic organisms including Clostridium spp. (Table 2). B. thetaiotaomicron (ATCC 29741) and E. lentum (ATCC 43055) were included as reference control strains for these susceptibility assays. MICs of imipenem for reference strains of B. thetaiotaomicron and E. lentum were within acceptable quality control ranges, as defined by NCCLS. No NCCLS control values have been established for microtiter anaerobic broth dilution assays with metronidazole. These data indicate that GT160-246 is nonantimicrobial in its activity and is unlikely to disrupt the normal intestinal flora in vivo.
TABLE 1.
MICs of GT160-246 for aerobic organisms compared with those of vancomycin and gentamicin
| Microorganism (ATCC no.) | MIC (μg/ml)
|
||
|---|---|---|---|
| GT160-246 | Vancomycin | Gentamicin | |
| Candida albicans (18804) | >5,000 | >256 | >256 |
| Candida tropicalis (1369) | >5,000 | >256 | 256 |
| Candida glabrata (90030) | >5,000 | >256 | >256 |
| Staphylococcus aureus (29213) | >5,000 | 0.5 | <0.12 |
| Streptococcus pyogenes (12344) | >5,000 | 0.25 | 4 |
| Enterococcus faecium (19434) | >5,000 | 0.5 | 8 |
| Campylobacter jejuni (33560) | >5,000 | NTa | NT |
| Burkholderia cepacia (25416) | >5,000 | >256 | 128 |
| Pseudomonas aeruginosa (27853) | >5,000 | >256 | 1 |
| Escherichia coli (25922) | >5,000 | 256 | 0.25 |
| Salmonella enteritidis (4931) | >5,000 | NT | NT |
| Serratia marcescens (13880) | >5,000 | >256 | 0.5 |
| Klebsiella pneumoniae (13883) | >5,000 | NT | NT |
| Yersinia enterocolitica (9610) | >5,000 | NT | NT |
| Proteus mirabilis (29906) | >5,000 | NT | NT |
| Aeromonas hydrophila (35654) | >5,000 | NT | NT |
NT, not tested.
TABLE 2.
MICs of GT160-246 for anaerobic organisms compared with those of metronidazole and imipenem
| Microorganism (ATCC no.) | MIC (μg/ml)
|
||
|---|---|---|---|
| GT160-24 | Metronidazole | Imipenem | |
| Bacteroides fragilis (10619) | >5,120 | 1.0 | 0.125 |
| Bacteroides fragilis (25285) | >5,120 | 0.5 | 0.125 |
| Bacteroides distasonis (10618) | >5,120 | 0.5 | 0.5 |
| Bacteroides distasonis (10566) | >5,120 | 1 | 2 |
| Bacteroides thetaiotaomicron (10624) | >5,120 | 2 | 0.25 |
| Bacteroides thetaiotaomicron (29741) | >5,120 | 1 | 0.25 |
| Bacteroides ovatus (10610) | >5,120 | 1 | 0.125 |
| Bacteroides ovatus (10600) | >5,120 | 2 | 0.25 |
| Fusobacterium mortiferum (10494) | >5,120 | 0.25 | 0.5 |
| Fusobacterium mortiferum (10009) | >5,120 | 0.5 | 0.5 |
| Fusobacterium varium (10479) | >5,120 | 0.5 | 1 |
| Fusobacterium varium (10039) | >5,120 | 0.5 | 0.5 |
| Acidaminococcus fermentum (10351) | >5,120 | 0.25 | 0.5 |
| Acidaminococcus fermentum (9904) | >5,120 | 0.5 | 0.25 |
| Clostridium perfringens (10436) | >5,120 | 2 | 0.06 |
| Clostridium perfringens (10385) | >5,120 | 2 | 0.125 |
| Clostridium innocuum (10617) | 5,120 | 1 | 2 |
| Clostridium innocuum (10537) | 5,120 | 0.125 | 0.015a |
| Clostridium clostridiofonne (10616) | >5,120 | 0.25 | 2 |
| Clostridium clostridiofonne (10588) | >5,120 | ≤0.06 | 1 |
| Clostridium ranosum (10421) | >5,120 | 8 | 0.5 |
| Clostridium ranosum (10329) | >5,120 | 2 | 0.25 |
| Clostridium difficile (9311) | >5,120 | 0.25 | 4 |
| Clostridium difficile (4973) | >5,120 | 0.25 | 4 |
| Bifidobacterium adolescentis (539) | >5,120 | 0.5 | 0.06 |
| Bifidobacterium bifidum (544) | >5,120 | 8 | ≤0.015 |
| Lactobacillus acidophilous (9804) | >5,120 | >128 | 8 |
| Lactobacillus casei (9866) | >5,120 | >128 | 8 |
| Eubacterium limosum (536) | 5,120 | 4 | 0.25 |
| Eubacterium contortum (537) | 5,120 | 4 | 0.03 |
| Eubacterium lentum (43055) | 5,120 | 0.125 | 0.5 |
| Peptostreptococcus magnus (357) | 2,560 | 0.5 | 0.03 |
| Peptostreptococcus ashccharolyricus (368) | 5,120 | 0.25 | ≤0.015 |
| Peptostreptococcus producrus (7207) | 5,120 | 0.25 | 1 |
GT160-246 does not interfere with the activities of most antibiotics in vitro.
C. difficile-associated diarrheal disease is most commonly treated with one of two antibiotics, metronidazole or vancomycin. GT160-246 was tested for inhibition of the activities of 17 antibiotics from diverse antimicrobial classes against eight representative aerobic and anaerobic bacteria (Table 3). Because we were looking for changes in absolute MICs, the activities of all 17 antibiotics were tested against all eight organisms regardless of whether the organisms would usually be considered susceptible to each antibiotic. Organisms not susceptible to the highest concentration of an individual antibiotic tested are indicated in Table 3. Interference by GT160-246 was considered positive if the MIC in the presence of GT160-246 was greater than 2 dilutions higher than the MIC of the antibiotic alone. By this criterion, GT160-246 showed no interference with the activities of most antibiotics tested (Table 3). GT160-246 did not interfere with the activities of the antibiotics used to treat human C. difficile infection, metronidazole and vancomycin. GT160-246 did raise the MIC of azithromycin by 3 to 4 dilutions for S. aureus, E. faecalis, and E. coli and was therefore considered positive for interference with these antibiotics. The MICs of two other macrolides, erythromycin and clarithromycin, were not affected by GT160-246.
DISCUSSION
C. difficile infection is an increasing problem in hospitals, with an estimated 300,000 to 500,000 cases occurring annually in the United States. The rising incidence of this disease has been associated with an increase in the number of older individuals in hospitals and the increase in the rate of use of broad-spectrum antibiotics including cephalosporins (11). While antibiotic therapy is the most common treatment and the only approved therapy for C. difficile colitis, this approach is not ideal. This disease results from disruption of the normal intestinal flora by treatment with antibiotics. Treatment with antibiotics results in disease recurrence in about 20% of patients (15). Some patients suffer from multiple episodes of disease recurrence and present a significant challenge to hospitals and health care providers. These patients may have a reduced immune response to C. difficile toxins (10).
The symptoms of C. difficile colitis result from intestinal damage caused by the action of toxins A and B. Therefore, a rational approach to therapy for this disease is to absorb the toxins in the colon, thereby blocking their ability to interact effectively with the intestinal epithelium. Various toxin binding approaches have been explored, including oral administration of toxin-neutralizing antibodies (9, 13) and receptor decoy molecules (3, 7). GT160-246 is a high-molecular-weight synthetic polymer that binds to C. difficile toxins A and B. This polymer has shown no significant antimicrobial activity in vitro and is unlikely to disrupt the normal intestinal floral in vivo. GT160-246 was also well tolerated in hamsters with severe colitis and caused no intestinal fluid accumulation, permeability changes, or inflammation in the intestinal tracts of experimental animals used in these and other studies.
C. difficile toxins A and B have potent cytotoxic activities against mammalian cells, resulting in a characteristic rounding of cells in culture (4). The C. difficile toxins also affect cellular metabolism and protein synthesis (4, 22). Incubation of toxins A and B with GT160-246 blocked toxin-mediated cell rounding and toxin-mediated inhibition of protein synthesis in Vero cells. GT160-246 appears to be more effective at binding to toxin A than to toxin B in these studies. Quantitative binding assays are in progress to determine the affinity and binding capacity of GT160-246 for these toxins.
C. difficile toxin A is a potent enterotoxin, increasing epithelial permeability and causing fluid accumulation in the intestinal lumen. GT160-246 was compared to cholestyramine for the ability to block toxin A activity in rat ileum. GT160-246 was at least 80 times more effective than cholestyramine at blocking toxin A-mediated fluid accumulation and 16 times more effective than cholestyramine at blocking intestinal permeability in rat ileal loops. Rodent intestinal epithelium lacks receptors for C. difficile toxin B; thus, the ability of GT160-246 to block toxin B activity in rat ileal loops could not be evaluated.
Hamsters are highly susceptible to colonization and infection with C. difficile (18, 23). We tested cholestyramine and GT160-246 in numerous studies of C. difficile colitis in hamsters. The death of cholestyramine-treated hamsters rarely showed more than a 1-day delay relative to the times of death of saline-treated control hamsters. In contrast, GT160-246 prevented death in 70 to 90% of hamsters when used as either a treatment or a means for the prevention of C. difficile colitis. Neither cholestyramine nor GT160-246 prevented the development of symptoms of C. difficile colitis in these hamsters. All surviving hamsters developed mild to moderate diarrhea by day 4. Most of the hamsters that received GT160-246 treatment resolved the diarrhea and were symptom free by the end of the studies. Examination of the ceca from GT160-246-treated hamsters revealed a significant decrease in intestinal inflammation relative to that for saline-treated controls. Perhaps the most exciting observation from these studies was that even after removal of GT160-246 treatment, most of the hamsters continued to resolve the disease. In contrast, metronidazole treatment of C. difficile-infected hamsters had no prolonged effect, with disease relapse and death occurring in 80% of the hamsters within 72 h of the cessation of treatment.
GT160-246 demonstrated no antimicrobial activity against a diverse panel of aerobic and anaerobic bacteria, including species that may be part of the normal human colonic flora. The nonantimicrobial nature of GT160-246 is a particularly important and desirable feature of this polymer. A nonantimicrobial approach to the treatment of C. difficile colitis should result in a reduced incidence of disease relapse compared with the incidence after treatment with antibiotics. Further studies with hamsters have shown that GT160-246 can prevent disease relapse when it is administered as monotherapy following metronidazole treatment (unpublished data).
GT160-246 blocks the biological activities of both toxins A and B in vitro and prevents mortality in a model of severe C. difficile colitis. GT160-246 is at least 16-fold more potent than cholestyramine in blocking toxin A-mediated enterotoxic activity in rat ileal loops and is significantly more effective than cholestyramine in protection of hamsters from mortality from C. difficile colitis. GT160-246 is nonantimicrobial and does not interfere with the in vitro activities of most antibiotics, including vancomycin and metronidazole. GT160-246 did elevate the MIC of azithromycin for S. aureus, E. faecalis, and E. coli. The mechanism for the interference with these three organisms is unknown.
In a recent phase 1 clinical trial, GT160-246 was demonstrated to be safe and well tolerated in healthy volunteers. On the basis of these observations, GT160-246 may offer a promising new approach to the treatment and prevention of C. difficile colitis in humans.
ACKNOWLEDGMENTS
These studies were funded entirely by GelTex Pharmaceuticals, Inc.
We thank Susan Hagen at Beth Israel Deaconess Medical Center for performing histological analyses of hamster ceca. We thank Thao Ngo for excellent technical assistance and T. Sybertz, T. Lamont, C. Pothoulakis, and A. Onderdonk for critical discussions throughout these studies.
REFERENCES
- 1.Anonymous. ASHP therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am J Health Syst Pharm. 1998;55:1407–1411. doi: 10.1093/ajhp/55.13.1407. [DOI] [PubMed] [Google Scholar]
- 2.Burbige E J, Milligan F D. Pseudomembranous colitis. Association with antibiotics and therapy with cholestyramine. JAMA. 1975;231:1157–1158. doi: 10.1001/jama.231.11.1157. [DOI] [PubMed] [Google Scholar]
- 3.Castagliuolo I, LaMont J T, Qiu B, Nikulasson S T, Pothoulakis C. A receptor decoy inhibits the enterotoxic effects of Clostridium difficile toxin A in rat ileum. Gastroenterology. 1996;111:433–438. doi: 10.1053/gast.1996.v111.pm8690209. [DOI] [PubMed] [Google Scholar]
- 4.Fiorentini C, Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29:543–567. doi: 10.1016/0041-0101(91)90050-2. [DOI] [PubMed] [Google Scholar]
- 5.Frost F, Craun G F, Calderon R L. Increasing hospitalization and death due to Clostridium difficile diarrheal disease. Emerg Infect Dis. 1998;4:619–625. doi: 10.3201/eid0404.980412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George W L, Rolfe R D, Finegold S M. Treatment and prevention of antimicrobial agent-induced colitis and diarrhea. Gastroenterology. 1980;79:366–372. [PubMed] [Google Scholar]
- 7.Heerze L D, Kelm M A, Talbot J A, Armstrong G D. Oligosaccharide sequences attached to an inert support (SYNSORB) as potential therapy for antibiotic-associated diarrhea and pseudomembranous colitis. J Infect Dis. 1994;169:1291–1296. doi: 10.1093/infdis/169.6.1291. [DOI] [PubMed] [Google Scholar]
- 8.Kelly C P, LaMont T. Clostridium difficile infection. Annu Rev Med. 1998;19:375–390. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- 9.Kink J A, Williams J A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyne L, Warny M, Qamar A, Kelly C. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 11.Levy D. Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin Ther. 2000;22:91–102. doi: 10.1016/s0149-2918(00)87980-1. [DOI] [PubMed] [Google Scholar]
- 12.Limaye A P, Turgeon D K, Cookson B T, Fritsche T R. Pseudomembranous colitis caused by a toxin A(−) B(+) strain of Clostridium difficile. J Clin Microbiol. 2000;38:1696–1697. doi: 10.1128/jcm.38.4.1696-1697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyerly D M, Bostwick E F, Binion S B, Wilkins T D. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyerly D M, Krivan H C, Wilkins T D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland L V, Surawicz C M, Rubin M, Fekety R, Elmer G W, Greenberg R N. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Method for dilution antimicrobial susceptibility testing for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS publication no. M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 4th ed. Approved standard. NCCLS publication no. M11–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 18.Onderdonk A B. Role of the hamster model of antibiotic-associated colitis in defining the etiology of the disease. In: Rolfe R, Finegold S M, editors. Clostridium difficile. Its role in intestinal disease. New York, N.Y: Academic Press, Inc.; 1988. pp. 115–125. [Google Scholar]
- 19.Pothoulakis C, Castigliuolo I, LaMont J, Jaffer A, O'Keane J, Snider R, Leeman S. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not chloera toxin. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothoulakis C, LaMont J T. Clostridium difficile colitis and diarrhea. Gastroenterol Clin N Am. 1993;22:623–637. [PubMed] [Google Scholar]
- 21.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacheri J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T, Wenzi E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman S W, Brown J E, Diecidue A, Foret D A. Differential cytotoxic effects of toxins A and B isolated from Clostridium difficile. Infect Immun. 1984;46:324–331. doi: 10.1128/iai.46.2.324-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Small J D. Fatal enterocolitis in hamsters given lincomycin hydrochloride. Lab Anim Care. 1968;18:411–420. [PubMed] [Google Scholar]
- 24.Spencer R C. Clinical impact and associated costs of Clostridium difficile-associated disease. J Antimicrob Chemother. 1998;41(Suppl. C):5–12. doi: 10.1093/jac/41.suppl_3.5. [DOI] [PubMed] [Google Scholar]
- 25.Surawicz C M, McFarland L V. Pseudomembranous colitis: causes and cures. Digestion. 1999;60:91–100. doi: 10.1159/000007633. [DOI] [PubMed] [Google Scholar]
- 26.Taylor N S, Bartlett J G. Binding of Clostridium difficile cytotoxin and vancomycin by anion-exchange resins. J Infect Dis. 1980;141:92–97. doi: 10.1093/infdis/141.1.92. [DOI] [PubMed] [Google Scholar]
- 27.Tedesco F J. Treatment of recurrent antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1982;77:220–221. [PubMed] [Google Scholar]
- 28.Wilcox M H, Cunniffe J G, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect. 1996;34:23–30. doi: 10.1016/s0195-6701(96)90122-x. [DOI] [PubMed] [Google Scholar]