Abstract
Increasing number of Janus kinase (JAK) inhibitors have been approved for chronic haematopoietic neoplasms and inflammatory/autoimmune diseases. We aimed to assess safety of the first three approved JAK inhibitors: ruxolitinib, tofacitinib and baricitinib. In this retrospective observational study, pharmacovigilance data were extracted from the World Health Organization database. Adverse events are classified according to Medical Dictionary for Regulatory Activities hierarchy. Until February 28, 2021, all Individual Case Safety Reports [ICSRs] with the suspected drug ruxolitinib, tofacitinib or baricitinib were included. Disproportionality analysis was performed and the information component (IC) was estimated. Adverse events were considered a significant signal if the lower end of the 95% credibility interval of the IC (IC025) was positive. We identified 126,815 ICSRs involving JAK inhibitors. Ruxolitinib, tofacitinib and baricitinib were associated with infectious adverse events (IC025 1.7, especially with viral [herpes and influenza], fungal, and mycobacterial infectious disorders); musculoskeletal and connective tissue disorders (IC025 1.1); embolism and thrombosis (IC025 0.4); and neoplasms (IC025 0.8, especially malignant skin neoplasms). Tofacitinib was associated with gastrointestinal perforation events (IC025 1.5). We did not find a significant increase in the reporting of major cardiovascular events. We identified significant association between adverse events and ruxolitinib, tofacinitib and baricitinib in international pharmacovigilance database.
Subject terms: Epidemiology, Adverse effects, Biological therapy, Immunological disorders
Introduction
For a decade, growing interest in clinical immunology and rheumatology regarding targeted therapies to block cytokines and their signaling have led to the development and use of Janus kinase (JAK) inhibitors. Janus kinases are cytokine transmembrane receptors: JAK1, JAK2, JAK3 and TYK2. JAK-STAT (signal transducer and activator of transcription) pathway plays roles in orchestrating of immune system, cell proliferation and haematopoiesis1. JAK-STAT pathway is implicated in the pathogenesis of inflammatory and autoimmune diseases including rheumatoid arthritis, psoriasis, and inflammatory bowel disease as well as malignancies1.
Three of the JAK inhibitors have been approved for a few years by the US Food and Drug Administration/European Medicines Agency (FDA/EMA). Tofacitinib, a selective JAK1 and JAK 3 inhibitor, has been approved for treating rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. Ruxolitinib, a selective JAK1 and JAK2 inhibitor, has been approved for treating myelofibrosis and polycythemia vera. Baricitinib selectively inhibits JAK 1 and JAK 2 and has been approved for treating rheumatoid arthritis and atopic dermatitis. The success of JAK inhibitors in the treatment of inflammatory diseases or malignancies demonstrates that intracellular signaling pathways can be targeted to treat inflammatory and autoimmune diseases. Perspectives for the use of these three JAK inhibitors are now wider, for other inflammatory/autoimmune diseases2,3. Moreover, increasing number of JAK inhibitors have been recently approved or assessed in clinical trials, including research into cancer treatment4–7. In this context of the intensive development of these JAK inhibitors, safety data are crucial.
The first three approved JAK inhibitors (ruxolitinib anti-JAK1,2, tofacitinib anti-JAK1,3, and baricitinib anti-JAK1,2,) can offer sufficient perspectives for safety studies, for patients who participated into clinical trials or those receiving treatment with the approval of these treatments in the United States, Asia and Europe.
As for other biologic agents, risk of serious infections and opportunistic infections has been reported, mostly among patients participating in clinical trials8–14. As compared with patients using biologics (anti-TNF, abatacept, rituximab and tocilizumab), among those receiving tofacitinib, the rate of herpes zoster infection doubled in a real-world American study15. Apart from infection risk, studies to evaluate the risk of serious heart-related events and cancer were planned at the time tofacitinib was approved. Recent concerns about ruxolitinib involved occurrence of non-melanoma skin-cancer and second malignancies16 and concerns about tofacitinib and baricitinib involved embolism and thrombotic events17–22, intestinal perforations10,23–26 and malignancies13,25,27. Thus, the EMA Committee for Medicinal Products for Human Use and the FDA added thrombosis to the bariticinib and tofacitinib warnings and precautions28 as well as intestinal perforations29. Post-marketing reporting constitutes an important source to identify safety signals. In this study, we assessed the safety of the first three approved JAK inhibitors—ruxolitinib, tofacitinib and baricitinib—by using the World Health Organization (WHO) international pharmacovigilance database, VigiBase, which contains more than 24 million individual case safety reports (ICSRs) and classifies adverse events according to the Medical Dictionary for Regulatory Activities (MedDRA). To identify safety concern, we used disproportionality analysis.
Results
Among the 24,416,850 ICSRs in VigiBase, the number involving JAK inhibitors was 126,815. Tofacitinib had the highest number of reports (supplementary Table S1). Physicians reported 12% to 29% of the ICSRs for JAK inhibitors. In 16.3% of the ICSRs for ruxolitinib, 9.6% for tofacitinib and 12.9% for baricitinib, the adverse events caused or prolonged hospitalization. In 14.0% of the ICSRs for ruxolitinib, 1.9% for tofacitinib and 1.4% for baricitinib, the adverse events caused death. The median number of Preferred Terms (PTs) declared by ICSRs was 2.0 (IQR 1.0–3.0). For patients, the median age was 70, 61 and 61 years for ruxolitinib, tofacitinib and baricitinib reports, respectively. More than 75% of the ICSRs for tofacitinib and baricitinib involved women. Rheumatoid arthritis was most frequently reported in tofacitinib and baricitinib ICSRs (55% and 79.7%, respectively), whereas myelofibrosis and polycythemia vera were reported in ruxolitinib ICSRs (43.5% and 19.3%).
Adverse events
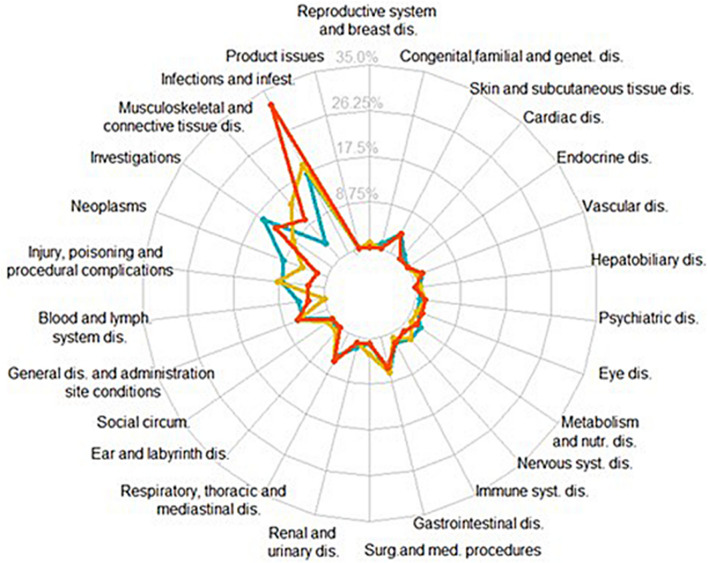
A total of 376,487 adverse events were reported in the 126,815 ICSRs (including 6179 different PTs). We identified four main System Organ Classes (SOCs) for which adverse event reporting was significantly increased for JAK inhibitors compared with the full database (Fig. 1 and supplementary Table S2): “infections and infestations” (IC025 1.7, i.e. lower limit of the 95% credibility interval of the information component estimated thanks to disproportionality analysis), “musculoskeletal and connective tissue disorders” (IC025 1.1), “investigations” (IC025 0.9), and “neoplasms benign, malignant and unspecified” (IC025 0.8). Six other SOCs (including blood and lymphatic system and respiratory, thoracic and mediastinal disorders) represented also significant increased reporting of adverse event associated with JAK inhibitors (Fig. 1 and supplementary Table S2). We did not find any association for 17 of the 27 different SOCs, including nervous system, psychiatric, vascular, cardiac, skin and subcutaneous tissue disorders (Fig. 1 and supplementary Table S2).
Figure 1.
Proportion of Preferred Terms (MedDRA) with positive IC025 related to each JAK inhibitor according to the 27 different System Organ Classes. “Pregancy, puerperium and perinatal conditions” is not represented here because of no positive IC025 observed for any of the three drugs. Yellow curve represents tofacitinib, orange baricitinib and blue ruxolitinib. MedDRA: Medical Dictionary for Regulatory Activities; IC025: lower limit of the 95% credibility interval of the information component. A positive IC025 is the statistical threshold used in VigiBase.
We further described the results regarding (1) infections and infestations, (2) musculoskeletal and connective tissue disorders and (3) neoplasms. We did not describe “investigations” SOC which includes blood test abnormalities because we focused on clinical events rather than isolated biological data. We finally focused on PTs of interest for embolism and thrombosis, gastrointestinal perforations and serious heart-related events.
Infections and infestations (Table 1, Fig. 2 and supplementary Tables S3 and S4)
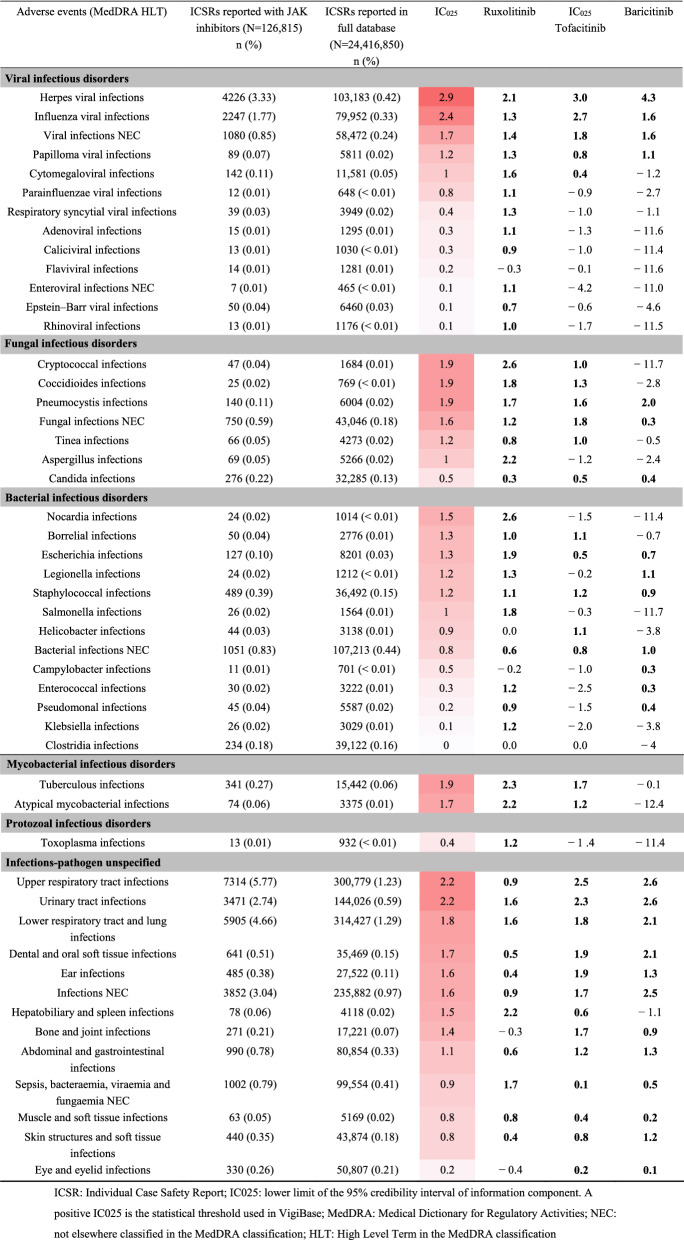
Table 1.
Infectious adverse events related to Janus kinase (JAK) inhibitors.
Significant values are given in bold.
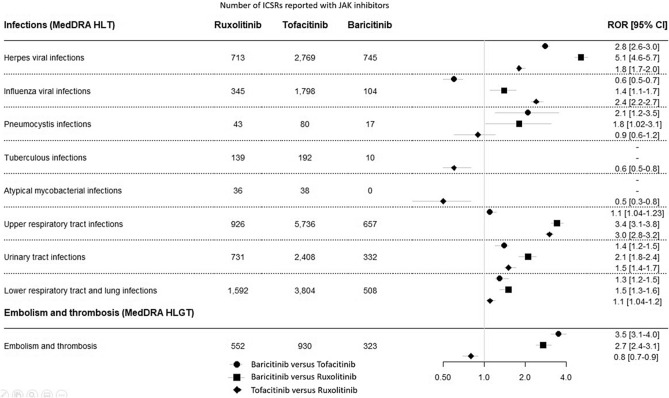
Figure 2.
Comparison between the three JAK inhibitors for selected adverse events. ICSR Individual Case Safety Report; ROR [95% CI] reporting odds ratio and 95% confidence interval. MedDRA Medical Dictionary for Regulatory Activities; HLT High Level Term in the MedDRA classification; HLGT High Level Group Term in the MedDRA classification; PT Preferred Term in the MedDRA classification.
The main significant increased reporting of adverse events for viral infections were herpes infections, including herpes viral infections (IC025 2.9), and influenza viral infections (IC025 2.4) (Table 1). We found a differential reporting between the three JAK inhibitors (Fig. 2). Over-reported herpes viral infections were ranked from the highest for baricitinib, then tofacitinib, then ruxolitinib, whereas over-reported influenza viral infections were ranked from the highest for tofacitinib, then baricitinib, then ruxolitinib. High dose of baricitinib was significantly associated with increased reporting of herpes viral infections and influenza viral infections compared with low dose (supplementary Tables S3 and S4). Regarding fungal infectious disorders, we identified pneumocystis infections and cryptococcal and coccidioides infections as significant higher reporting (IC025 1.9 for all three). We found a significant increased reporting concerning pneumocystis infections for each JAK inhibitor, with an over-reporting for baricitinib versus tofacitinib and ruxolitinib (Fig. 2). Similarly, tuberculous and atypical mycobacterial infections had IC025 values close to 2 (1.9 and 1.7, respectively). Tuberculous infections were over-reported for ruxolitinib versus tofacitinib, with no signal observed for baricitinib. Finally, we observed a significant increased reporting of infections according to organ localization: upper respiratory tract infections (IC025 1.9), urinary tract infections (IC025 1.9), and lower respiratory tract and lung infections (IC025 1.9). Over-reported respiratory and urinary tract infections were ranked from the highest for baricitinib, then tofacitinib, then ruxolitinib. High dose of baricitinib was significantly associated with increased reporting of upper respiratory tract infections compared with low dose whereas high dose of tofacitinib was significantly associated with decreased reporting of lower respiratory tract and lung infections and urinary tract infections compared with low dose. No other differences in an over reporting of infections were associated to the dose of either baricitinib or tofacitinib (supplementary Tables S3 and S4).
Musculoskeletal and connective tissue disorders (supplementary Table S5)
The adverse events “synovial and bursal disorders”, “musculoskeletal and connective tissue deformities” and “joint disorders” were the main significant adverse events reported (IC025 3.4, 2.1 and 1.9, respectively).
Neoplasms (Table 2 and supplementary Tables S4 and S6)
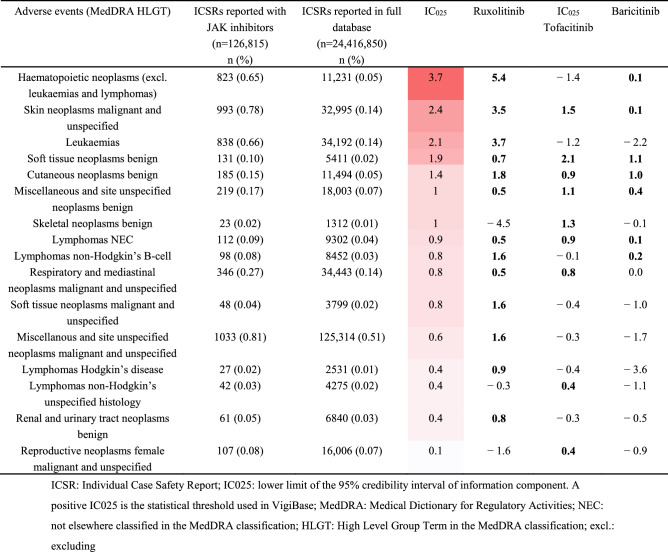
Table 2.
Neoplasm adverse events related to JAK inhibitors.
Significant values are given in bold.
We identified malignant neoplasms for which adverse event reporting was significanty increased: “hematopoietic neoplasms (excluding leukaemias and lymphomas)” (IC025 3.7), “skin neoplasms malignant and unspecified” (IC025 2.4), “leukaemias” (IC025 2.1) and “soft tissue neoplasms benign” (IC025 1.9). “Respiratory and mediastinal neoplasms malignant” also presented a significant increase in reports (IC025 0.8). No differences in an over reporting of neoplams were associated to the dose of either baricitinib or tofacitinib (Supplementary Tables S4 and S6).
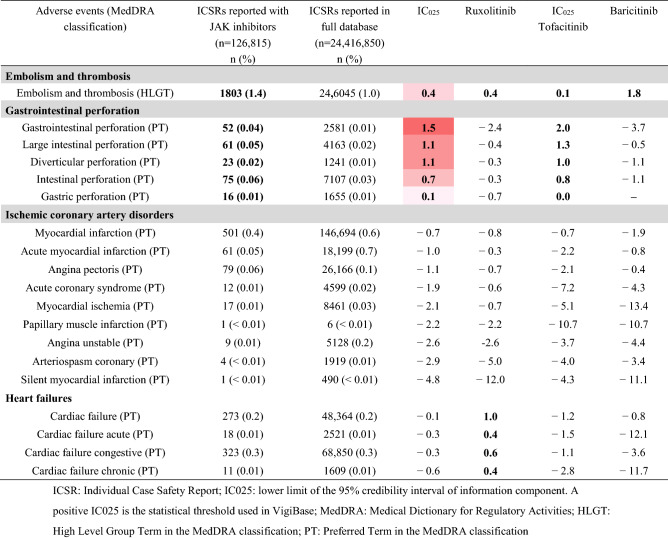
Embolism and thrombosis (Table 3 and Fig. 2 and supplementary Tables S4 and S7)
Table 3.
Embolism and thromboembolic, gastrointestinal perforation and major cardiovascular adverse events related to JAK inhibitors.
Significant values are given in bold.
Among the 126,815 ICSRs, 1803 (1.4%) described an embolism and thrombosis adverse event (IC025 0.4). Over-reported embolism and thrombosis adverse events were ranked from the highest for baricitinib, then ruxolitinib, then tofacitinib (Fig. 2). No differences in an over reporting of embolism and thrombosis events were associated to the dose of either baricitinib or tofacitinib (supplementary Tables S4 and S7).
Gastrointestinal perforation (Table 3 and Fig. 2 and supplementary Table S7)
The JAK inhibitors were associated with higher reporting of “gastrointestinal perforation”, “large intestinal perforation”, “diverticular perforation”, “intestinal perforation” and “gastric perforation”. At the drug level, only tofacitinib had a significant increase of adverse event reporting. Of note, no increase for baricitinib and ruxolitinib did not mean no event: from 3 to 16 events were described for ruxolitinib and from 1 to 4 events for baricitinib.
Major cardiovascular events (Table 3 and supplementary Table S7)
No major cardiovascular adverse event were associated with higher reporting for JAK inhibitors. Similarly, no cerebrovascular events were reported with JAK inhibitors. At the drug level, only ruxolitinib had a significant increase of reporting for adverse events “cardiac failure”, “cardiac failure acute”, “cardiac failure congestive” and “cardiac failure chronic” compared with the full database.
Discussion
In this pharmacovigilance study, JAK inhibitors were most commonly associated with infectious adverse events, embolism and thrombosis, neoplasms and gastrointestinal perforation events. We also identified significant increase in adverse event reporting regarding musculoskeletal and connective tissue disorders. Finally, we found no association with major cardiovascular events.
In our study, infections were frequently reported for JAK inhibitors, as was expected according to safety data from clinical trials24,30. We found a significant increase in reporting compared with the full database for some microorganisms (viral [herpes and influenza], fungal, and mycobacterial infectious disorders) and two main organ locations (respiratory and urinary tract infections).
Herpes zoster has been identified as a complication of JAK inhibitors in clinical trials25,26,31,32 and in a pharmacovigilance study of adverse events reported from the United States22. Of note, in our study, herpes viral infections (MedDRA HLT) also include herpes simplex virus. Herpes zoster induced most of the treatment discontinuation due to infections in some clinical trials25,26, but few data are available for herpes simplex infections. We observed over-reported herpes viral infections, the highest level for baricitinib, then tofacitinib, then ruxolitinib. Associated risk factors that can affect herpes zoster and herpes simplex infections for patients receiving JAK inhibitors include age, glucocorticoid exposure25, other combined therapy, and underlying immunologic dysregulation. For example, herpes zoster/simplex infections were more frequently reported in a pooled safety data analysis of baricitinib in atopic dermatitis than in rheumatoid arthritis33. Atopic dermatitis is known to be associated with herpes simplex infections, with a severe form called eczema herpeticum34. In our study, the increased risk of herpes viral infections with baricitinib versus the two other JAK inhibitors could be explained by the underlying disorder because the main indication (80%) was rheumatoid arthritis. The risk associated with ruxolitinib was difficult to assess because most patients with haematopoietic neoplasms could have received prophylactic valaciclovir.
Recent concerns about JAK inhibitors involved embolism and thrombosis17–22. Although the initial beneficial effect of ruxolitinib for risk of thrombosis was assessed in patients with polycythemia vera and myelofibrosis35, lack of evidence remains for this beneficial association. Regarding tofacitinib, in the meta-analyses including 12,410 tofacitinib-exposed patients from completed studies, the incidence rate of venous thromboembolism events was 0.25 (95% CI 0.19–0.33). In our study, we found significant disproportionality analysis results for embolism and thrombosis with the first three approved JAK inhibitors. Over-reported “embolism and thrombosis” adverse events were ranked the highest for baricitinib, then ruxolitinib, then tofacitinib.
These comparisons must be interpreted with caution. Indeed, we did not consider patient characteristics, risk factors for thromboembolism or dose and duration of treatments. In the meta-analysis of clinical trials of tofacitinib, patients with than without baseline cardiovascular risk factors were more likely to experience thromboembolic events20. Risk factors were age ≥ 50 years and with at least one criterion (current smoker, high-density lipoprotein level < 40 mg/dL, history of hypertension, diabetes, myocardial infarction or coronary heart disease). Incidence rates in patients without risk factors were very low and most patients who experienced thromboembolic events also had multiple cardiovascular risk factors at baseline. Similarly, all patients with thromboembolic events in a pooled analysis of clinical trials of baricitinib had multiple risk factors25. Therefore, the treatment must be adapted to the individual risk.
Regarding neoplasms, we found increased frequency of neoplasm reports and identified “skin neoplasms malignant and unspecified” as significant. The three JAK inhibitors were associated with increased frequency of “skin neoplasms malignant and unspecified”. This is an important finding because previous cohort studies of patients with rheumatoid arthritis did not find a difference between tofacitinib and biologic disease-modifying anti-rheumatic drugs in risk of non-melanoma skin cancer (adjusted hazard ratio 1.04 [95% CI 0.68–1.61])36. Rheumatoid arthritis is associated with increased risk of melanoma and non-melanoma skin cancer regardless of the exposure37,38. Thus, the increased frequency of “skin neoplasms malignant and unspecified” for ruxolitinib leads to a discussion of a class effect of the JAK inhibitor. “Respiratory and mediastinal neoplasms malignant” was frequently reported for all three JAK inhibitors. This finding confirmed the recent warning from Pfizer for tofacitinib39. Indeed, in this warning, the incidence rate of malignancies excluding non-melanoma skin cancer was 1.13 (95% CI 0.94–1.35), with lung cancer as the leading cancer. As for skin neoplasms, this signal concerned all three JAK inhibitors. Lastly, we found a significant increase in reporting for “leukaemias”, in particular for ruxolitinib, which is probably related to the underlying disease.
Some studies have concluded similar incidence rates of malignancies for patients receiving tofacitinib or baricitinib as for those receiving other drugs40 and for non-melanoma skin cancer41 or malignancies excluding non-melanoma cancer13,14,25. ‘Cancer immunoediting’, the process whereby the human immune system destroys cancer cells within the body, is thought to rely upon a variety of cytokines (for example, IFNγ) and cell types (such as NK cells) that could be affected by JAK inhibition42. Decrease NK cells could predispose to develop malignancies among patients treated by JAK inhibitors but this effect remains unclear30.
Exposure time within trials is relatively limited, and even if pharmacovigilance studies bring interesting data, longer follow-up is needed to further assess malignancy risk and to compare JAK inhibitors with each other.
In our study, we observed increased frequency of gastrointestinal perforations with the three JAK inhibitors. Few cases of gastrointestinal perforation have been reported for patients participating in clinical trials of baricitinib and tofacitinib or those covered by US Medicare/Marketscan10,23–26. These few cases were described only among patients with rheumatoid arthritis. To our knowledge, only one clinical trial of ruxolitinib for myelofibrosis reported such an event causing death in a patient in the placebo group11,43. In our study, gastrointestinal perforation was over-reported only with tofacitinib. However, cases were also reported for the other two JAK inhibitors. These adverse events would be more frequent for patients with inflammatory bowel diseases. Treatments other than JAK inhibitors such as non-steroidal anti-inflammatory drugs are associated with increased risk of gastrointestinal perforation, which is important to consider with JAK inhibitors.
Finally, the percentage of fatal cases resulted much higher for ruxolitinib than for other JAK inhibitors. We did not perform a detailed analysis and clinical review of the 7000 fatal cases. However, plausible explanation regarding the percentage for ruxolitinib relies on patient characteristics and indications.
Limitations of this study include under-reporting of events and few verifications of the clinical and laboratory tests or radiological findings leading to the diagnosis of the adverse events. Moreover, spontaneous reporting cannot be used to estimate prevalence or incidence of adverse events among patients exposed to drugs. A lack of case-causality constitutes also a main limit. Indeed, individual case safety reports are not fully reliable regarding causal association, due to lack of other potential causes described than suspected drug, and to missing data about time to onset of the adverse event. In this study, we first analyzed the more general level of the MedDRA hierarchy to retain groups of adverse events we further detailed. With this method, we missed potential signals which could be significant at deeper level of the MedDRA hierarchy. Finally, we did not perform subgroup analyses by duration or patient characteristics, which are not available for all ICSRs, but contribute to the occurrence of adverse events. Despite these limitations, pharmacovigilance analyses enable the detection of safety signals. VigiBase relies on data provided by more than 130 countries and enhances the identification of adverse events. Disproportionality analysis is a suitable method to compare spontaneous notifications of groups of drugs with other drugs while avoiding the effect of the extent of use of the product and nature of the adverse events.
Methods
Study design and data sources
In this retrospective observational study, pharmacovigilance data were extracted from VigiBase, the World Health Organization (WHO) database of adverse drug reactions reporting, which is managed by the Uppsala Monitoring Centre (UMC). It contains more than 24 million individual case safety reports (ICSRs) submitted by national pharmacovigilance centers from countries around the world since 1967. Different people can report adverse drug reactions: healthcare professionals, patients, pharmaceutical companies. For each ICSR, characteristics of the patient, general administrative information, drugs and reactions are available. A completeness score is also provided, to add a measure of ICSR quality44. The likelihood of a causal association is not the same in all reports. The information provided in this study does not represent the opinion of the WHO.
Procedures
This study included all ICSRs reported from inception to February 28, 2021, with a suspected drug among the following: tofacitinib, baricitinib and ruxolitinib. Each ICSR contains at least one adverse event, which corresponds to the most specific level of the Medical Dictionary for Regulatory Activities (MedDRA) hierarchy: Lowest Level Term (LLT). Each LLT is linked to one Preferred Term (PT), which are themselves grouped into High Level Terms (HLTs). The MedDRA hierarchy thus describes adverse events according to five levels, from the very specific (LLT) to the very general (System Organ Class [SOC]; details are available in supplementary Fig. S1). Each ICSR contains the onset date, end date, seriousness and fatal outcome of the event. A severe adverse event could be any event causing death, being life-threatening, requiring initial or prolonged hospital stay, or leading to persistent or clinically significant disability, congenital anomaly, birth defect or any other medically important condition.
Statistical analysis
To identify potential safety concern, we used disproportionality analysis, which compares the proportion of each suspected drug-induced adverse event (at different MedDRA levels) reported for a drug or a group of drugs with that for the same adverse event in the full database or for other drugs. Thus, when a proportion of an adverse event is higher for JAK inhibitors than for other drugs, this adverse event could constitute a safety concern. Two main estimations of the disproportionality analysis can be used: the information component (IC) for comparing to the full database or to other drugs and reporting odds ratios (RORs) for comparing drugs belonging to the same group of drugs. The IC was developed and validated by the UMC; it relies on a Bayesian confidence propagation neural network45 and the formula is as follows:
in which is estimated by , is the total number of reports involving the drug studied, and is the total number of reports for the adverse events, regardless of drug.
If the corresponding lower end of the 95% credibility interval (IC025) positive46, the adverse event could be considered a significant signal. This threshold has been used in the UMC and in different signal detection studies. Disproportionality analysis with the IC is illustrated in supplementary Fig. S1.
Disproportionality analysis relies on the ROR for drugs belonging to the same group. We detail the formula with the JAK inhibitors in supplementary Table S8, with corresponding 95% confidence intervals (95% CIs).
We first estimated the IC025 for adverse events related to JAK inhibitors at the more general level of the MedDRA hierarchy (SOC). Then, for the SOC with a positive IC025, we detailed the IC025 for adverse events at the therapeutic class level (JAK inhibitors) and for each drug at different MedDRA levels: High Level Group Terms (HLGTs) and HLTs. Finally, we focused on warnings by regulatory agencies: infections, embolism and thrombosis, serious heart-related events, gastrointestinal perforations. For selected adverse events with a positive IC025 for the three JAK inhibitors, we calculated RORs and 95% CIs. For selected adverse events with a positive IC025 for tofacitinib and baricitinib, we also estimated the IC025 of infections, embolism and thrombosis, serious heart-related events, gastrointestinal perforations according to their doses: high dose over 2 mg per day and 5 mg per day for baricitinb and tofacitinib, respectively; low-dose either. Lastly, we calculated ROR and 95% CIs for these previous adverse events using low-dose as reference. Quantitative variables are described with median (interquartile range) and categorical variables with number (percentage). Analyses involved using R 3.6.2.
Conclusion
In this international study, we identified significant increase in reporting of adverse event for the first three marketed JAK inhibitors compared with reporting of adverse eventsfor other drugs.. We confirmed some adverse effects such as infectious events and embolism and thrombosis which were already known and mentioned among cautions for use. Our results also lead to increase vigilance regarding malignancies for ruxolitinib, tofacitinib and baricitinib as well as gastrointestinal perforations for tofacitinib. We found no association with major cardiovascular events. Longer follow-up and observational studies will be helpful to improve knowledge about these risks among patients with other risk factors and treatments.
Supplementary Information
Acknowledgements
We would like to thank the UMC for providing the data. The study results and conclusions are those of the authors and not those of the UMC or WHO. We thank Laura Smales for English editing of the manuscript.
Author contributions
Conceptualization, E.S., L.H and B.L-V.; methodology, E.S. and L.H; formal analysis, L.H.; investigation, L.H; resources, B.L.-V.; data curation, L.H.; writing—original draft preparation, L.H.; writing—review and editing, L.H, B.L.-V., S.M., M.M., K.El L, L.R., A.Z., M.M., A.A., P.C., P.W., P.G., E.S.; visualization, L.H.; supervision, E.S. All authors have read and agreed to the published version of the manuscript.
Data availability
All relevant data were included in the manuscript. Data sharing not applicable.
Competing interests
Aurelien Amiot received consulting fees and travel accommodations from Pfizer. Pascal Claudepierre reported receiving personal fees from Abbvie, Pfizer, Roche-Chugai Bristol-Myers Squibb, MSD, UCB, Novartis, Janssen, Lilly, Celgene. Rest of the authors do not have any competing interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10777-w.
References
- 1.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamilloux Y, et al. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18:102390. doi: 10.1016/j.autrev.2019.102390. [DOI] [PubMed] [Google Scholar]
- 3.You H, et al. JAK inhibitors: Prospects in connective tissue diseases. Clin. Rev. Allergy Immunol. 2020;59:334–351. doi: 10.1007/s12016-020-08786-6. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas J, et al. Phase II trial of Lestaurtinib, a JAK2 inhibitor, in patients with myelofibrosis. Leuk. Lymphoma. 2019;60:1343–1345. doi: 10.1080/10428194.2018.1532509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbbVie. A Phase 2, Long-Term Extension (LTE) Study With Elsubrutinib and Upadacitinib Given Alone or in Combination (ABBV-599) in Subjects With Moderately to Severely Active Systemic Lupus Erythematosus Who Have Completed the M19-130 Phase 2 Randomized Controlled Trial (RCT). https://clinicaltrials.gov/ct2/show/NCT04451772 (2020).
- 6.Dizal Pharmaceuticals. A Phase I/II, Open-Label, Multicentre Study to Investigate the Safety, Tolerability, Pharmacokinetics and Anti-tumor Activity of AZD4205 in Patients With Peripheral T Cell Lymphoma (PTCL). https://clinicaltrials.gov/ct2/show/NCT04105010 (2020).
- 7.Robinson MF, et al. Efficacy and safety of PF-06651600 (Ritlecitinib), a novel JAK3/TEC Inhibitor, in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2020;72:1621–1631. doi: 10.1002/art.41316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2924–2937. doi: 10.1002/art.38779. [DOI] [PubMed] [Google Scholar]
- 9.Genovese MC, et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016;374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SB, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: Integrated analysis of data from the global clinical trials. Ann. Rheum. Dis. 2017;76:1253–1262. doi: 10.1136/annrheumdis-2016-210457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verstovsek S, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J. Hematol. Oncol. 2017;10:55. doi: 10.1186/s13045-017-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: A systematic review and meta-analysis. Am. J. Hematol. 2018;93:339–347. doi: 10.1002/ajh.24976. [DOI] [PubMed] [Google Scholar]
- 13.Wollenhaupt J, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res. Ther. 2019;21:89. doi: 10.1186/s13075-019-1866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen SB, et al. Long-term safety of tofacitinib up to 9.5 years: A comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. doi: 10.1136/rmdopen-2020-001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 2016;75:1843–1847. doi: 10.1136/annrheumdis-2016-209131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiladjian J-J, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7:e226–e237. doi: 10.1016/S2352-3026(19)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verden A, Dimbil M, Kyle R, Overstreet B, Hoffman KB. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41:357–361. doi: 10.1007/s40264-017-0622-2. [DOI] [PubMed] [Google Scholar]
- 18.Scott IC, Hider SL, Scott DL. Thromboembolism with janus kinase (JAK) inhibitors for rheumatoid arthritis: How real is the risk? Drug Saf. 2018;41:645–653. doi: 10.1007/s40264-018-0651-5. [DOI] [PubMed] [Google Scholar]
- 19.Xie W, et al. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: Systematic review and meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2019;78:1048–1054. doi: 10.1136/annrheumdis-2018-214846. [DOI] [PubMed] [Google Scholar]
- 20.Mease P, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann. Rheum. Dis. 2020;79:1400–1413. doi: 10.1136/annrheumdis-2019-216761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallejo-Yagüe E, Weiler S, Micheroli R, Burden AM. Thromboembolic safety reporting of tofacitinib and baricitinib: An analysis of the WHO VigiBase. Drug Saf. 2020;43:881–891. doi: 10.1007/s40264-020-00958-9. [DOI] [PubMed] [Google Scholar]
- 22.Peng L, Xiao K, Ottaviani S, Stebbing J, Wang Y-J. A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib. Expert Opin. Drug Saf. 2020;19:1505–1511. doi: 10.1080/14740338.2020.1799975. [DOI] [PubMed] [Google Scholar]
- 23.Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68:2612–2617. doi: 10.1002/art.39761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology. 2019;58:i34–i42. doi: 10.1093/rheumatology/key287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smolen JS, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J. Rheumatol. 2019;46:7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y-C, et al. Safety of baricitinib in East Asian patients with moderate-to-severe active rheumatoid arthritis: An integrated analysis from clinical trials. Int. J. Rheum. Dis. 2020;23:65–73. doi: 10.1111/1756-185X.13748. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein GR, et al. Tofacitinib, an oral janus kinase inhibitor: Analysis of malignancy (excluding nonmelanoma skin cancer) events across the ulcerative colitis clinical program. Inflamm. Bowel Dis. 2020 doi: 10.1093/ibd/izaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francisco, E. M. EMA confirms Xeljanz to be used with caution in patients at high risk of blood clots. European Medicines Agencyhttps://www.ema.europa.eu/en/news/ema-confirms-xeljanz-be-used-caution-patients-high-risk-blood-clots (2019).
- 29.Tofacitinib perforation—U.S. Food and Drug Administration Search Results. https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=fda1&sort_by=&query=tofacitinib+perforation.
- 30.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017;13:234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 31.Kunwar S, Collins CE, Constantinescu F. Baricitinib, a Janus kinase inhibitor, in the treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Clin. Rheumatol. 2018;37:2611–2620. doi: 10.1007/s10067-018-4199-7. [DOI] [PubMed] [Google Scholar]
- 32.Bechman K, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology. 2019;58:1755–1766. doi: 10.1093/rheumatology/kez087. [DOI] [PubMed] [Google Scholar]
- 33.Bieber T, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J. Eur. Acad. Dermatol. Venereol. 2021;35:476–485. doi: 10.1111/jdv.16948. [DOI] [PubMed] [Google Scholar]
- 34.Damour A, Garcia M, Seneschal J, Lévêque N, Bodet C. Eczema herpeticum: Clinical and pathophysiological aspects. Clin. Rev. Allergy Immunol. 2020;59:1–18. doi: 10.1007/s12016-019-08768-3. [DOI] [PubMed] [Google Scholar]
- 35.Samuelson BT, et al. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis: A meta-analysis. Blood Coagul. Fibrinolysis. 2016;27:648–652. doi: 10.1097/MBC.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 36.Comparison of malignancy and mortality rates between tofacitinib and biologic DMARDs in clinical practice: Five-year results from a US-based rheumatoid arthritis registry. ACR Meeting Abstractshttps://acrabstracts.org/abstract/comparison-of-malignancy-and-mortality-rates-between-tofacitinib-and-biologic-dmards-in-clinical-practice-five-year-results-from-a-us-based-rheumatoid-arthritis-registry/.
- 37.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res. Ther. 2008;10:R45. doi: 10.1186/ar2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Res. Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfizer Shares Co-Primary Endpoint Results from Post-Marketing Required Safety Study of XELJANZ® (tofacitinib) in Subjects with Rheumatoid Arthritis (RA)|Pfizer. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-co-primary-endpoint-results-post-marketing.
- 40.Xie W, et al. Risk of malignancy with non-TNFi biologic or tofacitinib therapy in rheumatoid arthritis: A meta-analysis of observational studies. Semin. Arthritis Rheum. 2020;50:930–937. doi: 10.1016/j.semarthrit.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Curtis JR, et al. Analysis of non-melanoma skin cancer across the tofacitinib rheumatoid arthritis clinical programme. Clin. Exp. Rheumatol. 2017;35:614–622. [PubMed] [Google Scholar]
- 42.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verstovsek S, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergvall T, Norén GN, Lindquist M. vigiGrade: A tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37:65–77. doi: 10.1007/s40264-013-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 46.Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 2013;22:57–69. doi: 10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data were included in the manuscript. Data sharing not applicable.