Abstract
Loop-mediated isothermal amplification (LAMP) is a promising diagnostic tool for genetic amplification, which is known for its rapid process, simple operation, high amplification efficiency, and excellent sensitivity. However, most of the existing heating methods are external for completion of molecular amplification with possibility of contamination of specimens. The present research provided an internal heating method for LAMP using magnetic nanoparticles (MNPs), which is called nano-LAMP. Near-infrared light with an excitation wavelength of 808 nm was employed as the heating source; hydroxy naphthol blue (HNB) was used as an indicator to conduct methodological research. We demonstrate that the best temperature was controlled at a working power of 2 W and 4.8 µg/µL concentration of nanoparticles. The lowest limit for the detection of HPV by the nano-LAMP method is 102 copies/mL, which was confirmed by a gel electrophoresis assay. In the feasibility investigation of validated clinical samples, all 10 positive HPV-6 specimens amplified by nano-LAMP were consistent with conventional LAMP methods. Therefore, the nano-LAMP detection method using internal heating of MNPs may bring a new vision to the exploration of thermostatic detection in the future.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00604-022-05283-9.
Keywords: Loop-mediated isothermal amplification, Genetic amplification, Magnetic nanoparticles, Human papillomavirus, Nano-LAMP
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted viruses worldwide. In the past 40 years, more than 100 types of HPV have been identified including high risk that can cause cancer and low-risk types that develop condyloma acuminatum (CA) [1, 2]. Over 90% of the cause of condyloma acuminatum (CA) is attributed to low-risk HPV type 6 or 11 that has been identified by molecular biology [3, 4], and it has become one of the most frequent sexually transmitted diseases in China [5]. It is also a benign tumor, with genitalia and anus as the main site of disease [6]. Surgery, cryotherapy, laser therapy, and other treatments are used for clinically obvious lesions that are nonspecific and painful for patient, rather than addressing the cause of the disease. First episode patients should be sexually transmitted disease (STD) screened. Therefore, an effective and reliable test for the identification of HPV genotype is urgently needed.
Currently, DNA or RNA molecular testing of exfoliated or biopsied tissue specimens is applied to test for HPV infection. The conventional methods for the identification of HPV genotype include polymerase chain reaction (PCR), immunohistochemistry, and Luminex suspension array technology, which have some shortcomings, such as requiring precise equipment, complex operation, low sensitivity, and being time-consuming [7–9]. In the past 20 years, loop-mediated isothermal amplification (LAMP) has been used to detect HPV disease [10]. It is a nucleic acid amplification method, first invented and developed by Notomi and co-workers, to amplify a specific DNA region through two elongation reactions completed by chain replacement DNA polymerase working under isothermal conditions [11–13]. Its unique advantage of simple technology and high efficiency initiated a new platform in the diagnostic field, including screening of mutant virus, bacterial strains, analysis of fungicide resistance mutation, drug identification and gene analysis and detection [14–17]. Our previous study used LAMP HPV-type-specific DNA amplification to detect and distinguish six HPV subtypes (HPV-6, 11, 16, 42, 43, 44) [18]. LAMP has been implemented in a variety of fields as an alternative to circumvent the disadvantages of PCR.
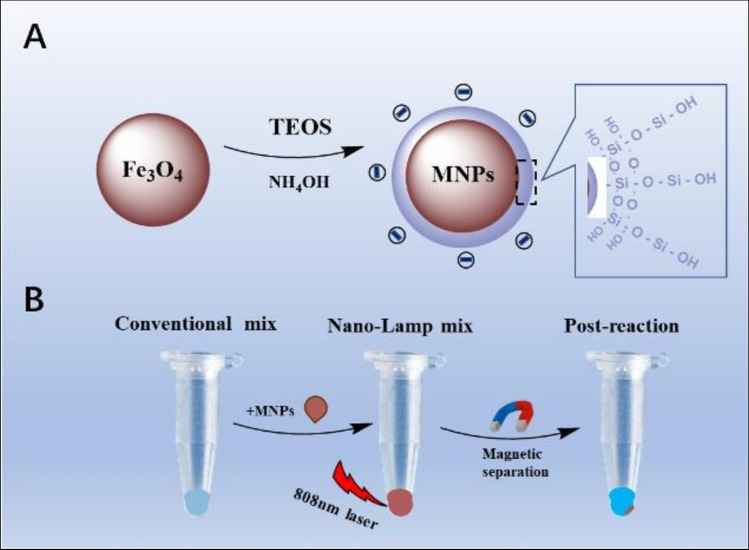
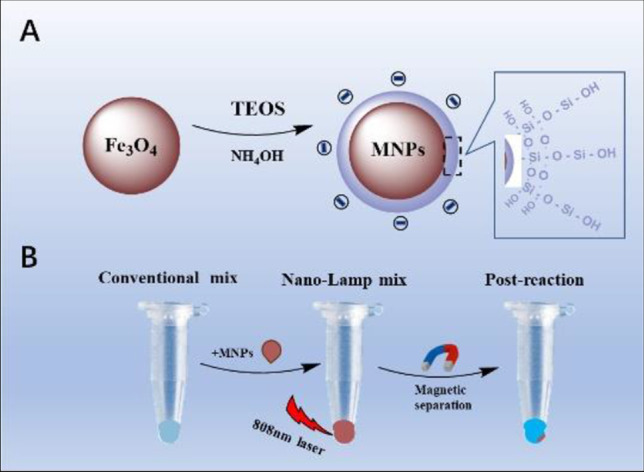
Most previous studies about LAMP used external heating methods to complete molecular amplification. Nevertheless, the development of nanotechnology has opened up a new horizon due to the size [19] and performance [20] advantages of nanoparticles (NPs) for clinical research. Although nanotechnology improves the sensitivity of detection, there are complex nanoprobes and tedious detection steps [21–24]. The latest studies showed that NPs were used for rapid detection of novel coronavirus [25, 26] in which Au–Fe NPs acted as enhancers for internal heating reactions called nanomaterial-assisted PCR (nano-PCR) [27–29]. However, nano-PCR needs external conditions to control constant temperature changes, and it seems easier to control a single temperature variable. Magnetic NPs (MNPs) are practical materials that have been proved to have high photothermal conversion efficiency, simple synthesis, stability, and low price [30–32]. Here, we report a reliable LAMP method for HPV diagnostics using MNPs, which is called nano-LAMP. As shown in Scheme 1, we designed negatively charged MNPs coated with a thin layer of silicon to minimize the entanglement and improve amplification efficiency, because nucleic acids are negatively charged. MNPs play the role of photothermal converter to rapidly convert light into heat energy for LAMP reaction under the irradiation of an 808-nm laser. The applicability of nano-LAMP was verified by DNA gradient and clinical sample detection, and the results were consistent with those of conventional LAMP. A similar approach can be applied to other HPV types as they share the principle of magneto-thermal effect of uniform negatively charged nano-particles. Our results indicated a new and reliable method suitable for isothermal amplification.
Scheme 1.
A depicts the synthesis process of magnetic nanoparticles (MNPs) with negative charges. B illustrates of the nano-LAMP experimental process
Experimental
Materials
Iron (III) chloride hydrate (FeCl3•6H2O), ethylene glycol, sodium acetate, ammonium hydroxide, primers of HPV-6 and agarose were purchased from Sangon Biotech. (Shanghai); tetraethyl orthosilicate (TEOS) and (3-aminopropyl) triethoxysilane (APTES) were obtained from Sigma-Aldrich (USA); and Loopamp DNA amplification kit was obtained from Rongyan Biochemistry Company. DNA extraction kit was purchased from Tellgen Life Science (Shanghai, China). Clinical samples of HPV are collected from Shanghai Tenth People’s Hospital. Deionized water with 18 MΩ· cm was used for the whole experiment. The primers of HPV-6 for LAMP amplification were designed on account of the HPV-6 sequences benefit from Primer Explorer V4 Software (Fujitsu, Tokyo, Japan). Nucleic Acid genotyping Kit for Human Papillomavirus was obtained from Topview Life Technology (Shanghai, China).
Apparatus and characterization
The following are the equipment used in our experiment: The sizes and ζ potential of IO-seeds, MNPs were measured by dynamic light scattering (JEM zetasizer Nano-ZS90, Malvern). The transmission electron microscopy (TEM) images of IO-seeds, IO@Si were obtained from a JEM-2100F electron microscope (JEOL, Japan). An 808-nm near-infrared (NIR) laser (ADR-1860, Shanghai, China) was applied for nano-LAMP experiment; meanwhile, the real-time temperature and thermal images were monitored by the infrared thermal camera (Fotric 225 s, Fotric Technology Company, Shanghai, China). A PCR System (ProFlex, Life Technology company Shanghai, China) was employed for conventional LAMP experiment.
Nucleic acid acquisition of clinical samples
A panel of 25 cervical scrape samples, including HPV-6 (n = 10), HPV-16 (n = 3), HPV-42 (n = 3), HPV-43 (n = 3), HPV-44 (n = 3), and negative samples (n = 3), were collected from Shanghai Tenth People’s Hospital and Shanghai Skin Disease Hospital. Samples of genital warts were taken from all participants using a sampling test paper in accordance with standard sampling procedures at recruitment sites. DNA for genital warts was taken from all participants in accordance with standard sampling procedures at recruitment sites. DNA extraction swabs were placed in tubes using a QIA prep Spin Miniprep Kit (Qiagen Translational Medicine Co.). The extracted DNA is dispersed in water and its quality inspection and concentration quantified by spectrophotometer.
Perform the assay with an unknown clinical specimen
The specimens were obtained from the genital warts using a swab following a strict standard procedure. Swabs were then stored in 3 mL transmission tube for DNA extraction by using a QIA prep Spin Miniprep Kit (Qiagen Translational Medicine Co.). The obtained DNA sample was dissolved in 50 μL of water. The quality and concentration of DNA were detected by U-V spectrophotometer. DNA had characteristic absorption peak at 260 nm, and the measured OD 260/280 radio between 1.8-2.0 was considered can be used for subsequent experiments. Finally, the DNA was stored at − 20 °C.
Preparation of nano-LAMP mixture
Loopamp DNA amplification kit was obtained from Rongyan Biochemistry Company that was employed in LAMP experiment. Specifically, LAMP requires five primers (B3, F3, BIP, LB, and FIP) to recognize HPV-6 DNA sequences. Conventional LAMP reactions were containing Tris–HCL 40 mM, KCL 20 Mm, MgSO4 16 mM, (NH4)2SO4 20 Mm, Tween20 0.2%, Betaine 1.6 M, HPV-6 specific primers (FIP 40 pmol, BIP 40 pmol, Loop-F*6 20 pmol, Loop-B*6 40 pmol), Bst DNA polymerase 1 μL, deionized water 3.5 μL, HNB 120 μmol/L, DNA determinand 2 μL.
Nano-LAMP mix containing Tris–HCL 40 mM, KCL 20 Mm, MgSO4 16 mM, (NH4)2SO4 20 mM, Tween20 0.2%, Betaine 1.6 M, HPV-6 specific primers (FIP 40 pmol, BIP 40 pmol, Loop-F*6 20 pmol, Loop-B*6 40 pmol), Bst DNA polymerase 1 μL, deionized water 0.5 μL, HNB 0.5 μL, sample determinand 2 μL, 3 μL 40 μg/mL MNPs magnetic nanoparticles, and DNA determinand 2 μL.
The HPV-6 specific target sequences are respectively F3 (5′-AAGTGCAAATGCCTCCAC -3′), B3 (5′- TCAAAGTGTCTATATTGGTTGAT -3′), FIP (5′-TGCATTCTTGCAAAACACACAATTA-GACCAGTTGTGCAAGACG -3′), BIP (′5- AACAGCTAAAGGTCCTGTTTCG-CCATGAAATTCTAGGCAGCA -3′), LB (5′- AGGCGGCTATCCATATGCAG -3′) (accession numbers: HG793938.1 (HPV-6).
Measurement of aqueous MNP photothermal effect
To measure the photothermal effect (PTT) in experiment, a NIR laser (808 nm) was used in aqueous MNP photothermal effect and the following clinical DNA specimen detection experiment. The total reaction volume is 25 μL containing Nano-LAMP mix and MNPs without DNA template. The mixture solution was placed in a 0.2 mL EP tube at stepwise increased concentrations (0 mg/mL; 2.4 mg/mL; 3.6 mg/mL; 4.8 mg/mL) and set different exciting laser (1.5 W, 2 W, 3 W) powers for 10 min respectively. The temperature was real-time monitored in 10 min and recorded every 60 s’ temperature change by a photothermal imaging instrument.
Verification of sensitivity and specific of nano-LAMP
The HPV DNA was diluted in a gradient and the concentrations respectively were 107 copies/mL–101 copies/mL in turn. Negative control (template DNA is replaced by water) was included in each experiment to avoid the contamination. The conventional reactions placed in a polymerase chain reaction (PCR) instrument at 64 °C constant temperature mode for 60 min. The nano-LAMP reactions were treatment with a NIR laser (808 nm) keeping the temperature at 64–65 °C for 60 min.
To determine the specificity of nano-LAMP, we randomly select five single infection subtypes (HPV-6, HPV-16, HPV-42, HPV-43, HPV-44) clinical DNA templates. All the primers added in the reaction are HPV-6 primers. The conventional reactions placed in a PCR instrument at 64 °C constant temperature mode for 60 min. The nano-LAMP reactions were treated with a NIR laser (808 nm) keeping the temperature at 64 °C for 60 min.
Statistical analysis
All statistics were presented as the mean ± standard deviation of three separate experiments in each group.
Results and discussion
Synthesis and characterizations
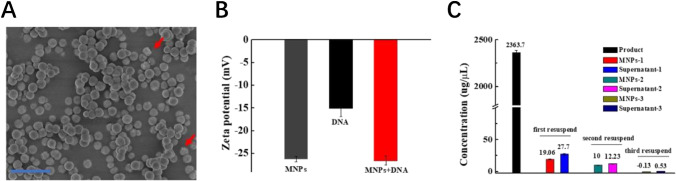
The scanning electron microscope (SEM) (Fig. 1A) shows the resultant MNPs are uniform in size and consistent with the average hydrodynamic diameter of MNPs in the range of 400 nm (Figure S1C) with a core–shell structure. By comparing ζ potential, we verified that MNPs had little effect on nucleic acid. The ζ potential of MNPs was about − 26 mV, which could be attributed to exposure of the negative charge of silicon-linked hydroxyl groups (Fig. 1B). The ζ potential of HPV DNA was about − 15 mV, while the ζ potential of the mixture of MNPs and DNA was similar to the potential of MNPs. To explore the adsorption of the analyte onto the MNP surface, we measured the concentration of the product (about 2363 μg/μL) by the NanoDrop-2000. In addition, MNPs were re-suspended and dispersed with ultra-pure water and magnetically separated again for 3 times. It can be clearly seen from Fig. 1C that there are a little amount of product (19.6 μg/μL) adsorbed on the surface of MNPs non-specifically after the first magnetic separation. However, the product left on the surface of the MNPs is nearly completely removed after three magnetic separations that confirmed low adhesion of MNPs.
Fig. 1.
A SEM of MNPs. (B) ζ potential of MNPs, HPV DNA and MNPs + DNA. (C) The concentration of the product and concentrations of the supernatant and the surface of MNPs was measured after three washes and re-suspension was measured by the NanoDrop-2000
Photothermal properties of MNPs
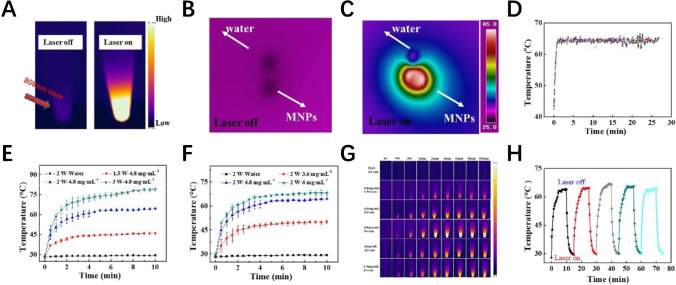
We evaluated the photothermal performance of MNPs before nano-LAMP experiment. Figure 2A shows the frontal thermal imaging of MNP aqueous solution under 808-nm laser. When the switch was turned on, the thermal image of the solution changed significantly, indicating absorption of near-infrared light by the MNPs. According to the microscopic droplet diagrams in Fig. 2B and C, the temperature change of the droplets was more obvious than that of water droplets in the presence of MNPs. At a certain power and a fixed concentration of MNPs, the final temperature tended to be steady-state.
Fig. 2.
A The NIR thermal images of nano-LAMP mixture recorded by infrared camera during a 808-nm laser irradiation. (B) NIR thermal images of droplets containing MNPs at 40 μg/μL, and ultrapure water droplet with laser off (B) and laser on (C). (D) Thermal imager recording of 25 min of temperature change. (E) Photothermal conversion curves of different concentrations (n = 3) and different powers (F) (n = 3). (G) Thermal images of different samples recorded by infrared camera during 808-nm laser irradiation. (H) Photothermal transformation curve of five cycles
The optimal reaction conditions for the experiment were controlled between 60 and 65 °C in our study (Fig. 2D). Constant temperature was achieved by adjusting power and concentration. To verify the photothermal effect of MNPs, dispersive MNP solutions with different concentrations (0, 3.6, 4.8, and 6 mg/mL) were studied by near-infrared light laser (808 nm, 2 W/cm2) irradiation for 10 min. With the increase of sample concentration, the temperature elevation rate increased significantly (Fig. 2E). In particular, the final temperature reached 67.5 °C after being irradiated by near-infrared laser for 10 min at a concentration of 6 mg/mL, indicating good photothermal conversion ability of MNPs. In addition to sample concentration-dependent manner, MNP-mediated temperature could be adjusted by altering the output power of the NIR laser. The conversion heat was positively correlated with the NIR power of illumination (Fig. 2F). For a clear view of the local photothermal generation, the infrared camera real-time recorded the thermal images of MNPs with different concentrations under different NIR irradiation. The results of infrared thermal imaging were matched with those of the digital thermometer (Fig. 2F). MNPs still showed excellent photothermal conversion capability after five cycles of NIR laser irradiation, indicating its high photothermal stability (Fig. 2H). These consequences indicated that MNPs had excellent photothermal conversion performance and were a prerequisite for nano-LAMP experiments.
Sensitivity of nano-LAMP for HPV-6 detection
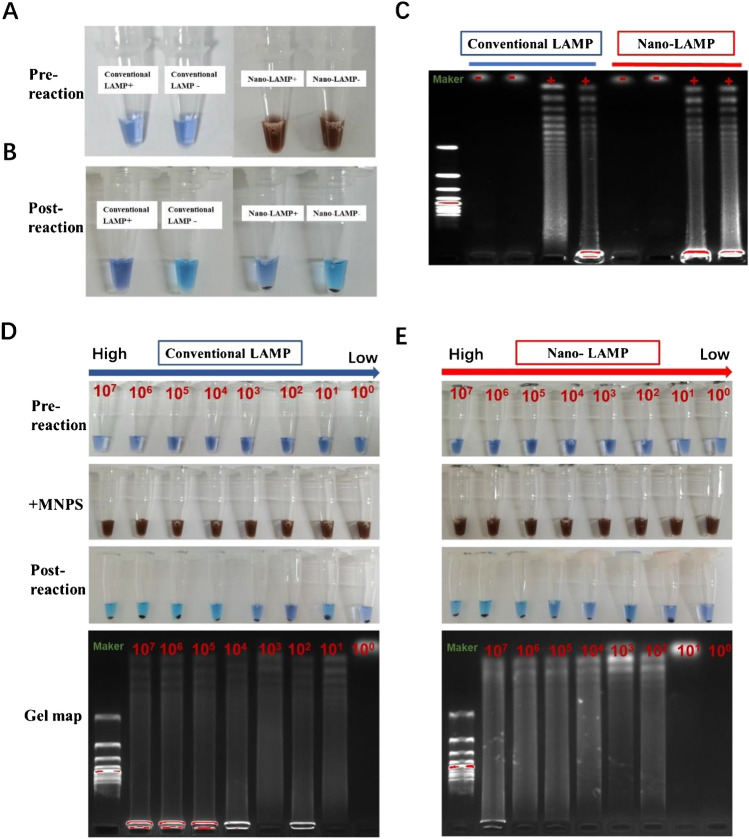
To evaluate the feasibility of nano-LAMP for HPV-6 detection, a comparative experiment was conducted between nano-LAMP and conventional LAMP. Hydroxynaphthol blue (HNB) was used as color indicator in situ by combining with Mg2+ ions and the initial color was blue. When the reaction proceeded, Mg2+ ions reacted with pyrophosphate to produce magnesium pyrophosphate precipitation. When HNB lost Mg2+, the color became sky blue, while the unreacted solution was still violet blue. The amplified products were recognized by 1.5% agarose gel electrophoresis at 120 V for 35 min. The electrophoresis results of positive samples produced a ladder pattern containing repeat structures and a long trailing structure, while the negative sample did not.
The obvious color changes of reactions were consistent with those before reaction (Fig. 3A) and after reaction (Fig. 2B). With the addition of matched DNA template, the positive reaction appeared blue-green, indicating that the nucleic acid amplified steadily free from MNP interference. Simultaneously, the low level of interference by MNPs on LAMP was verified by gel electrophoresis (Fig. 3C). There were bands in the positive reaction and no bands in the negative reaction, which was consistent with the color reaction, suggesting that MNPs had little impact on the conventional LAMP reaction. Non-interference by MNPs was the prerequisite for our nano-LAMP experiment. On this basis, the sensitivity of nano-LAMP was formulated by gradient experiment through the internal heating means. As shown in Fig. 3D and E, the electrophoresis experiment results represent the reaction product of different concentrations of HPV nucleic acid through nano-LAMP and conventional LAMP experiments. In the conventional LAMP test, all positive samples had specific trailing bands, while negative samples did not generate reaction products. However, at a concentration of 10 copies/mL, the results using nano-LAMP were identified as negative, while conventional LAMP experiment results were positive, indicating that the sensitivity of nano-LAMP for HPV-6 was 100 copies/mL, close to that of conventional LAMP. These outcomes clearly demonstrated the feasibility of nano-LAMP for the detection of HPV-6. At the higher concentrations, the results of two methods were consistent at high concentrations and the results were similar at low concentrations. The specificity of nano-LAMP for HPV-6 is sufficient to detect HPV clinical specimens.
Fig. 3.
A Images of pre-reactions for conventional LAMP (conventional LAMP + and conventional LAMP −) and reactions added MNPs. (B) Post-reaction images for nano-LAMP (nano-LAMP + and nano-LAMP −) after magnetic separation. (C) Gel electrophoresis images of LAMP products in A and B. (D) Color contrast pictures and electrophoretic contrast of HPV DNA diluted to different concentrations (107 copies/mL–101 copies /mL) of HPV-6 DNA were involved in conventional LAMP reactions and in nano-LAMP reactions (E)
The first consideration was to design MNPs with simple synthesis, low cost, and high photothermal efficiency. The second consideration was to minimize adsorption between MNPs and nucleic acid, thus reducing interference. MNPs met these requirements, with strong photothermal efficiency and little interference with nucleic acid. We reviewed some recent literature on nanomaterial-based optical methods for the determination of HPV (Table 1). To compare the performance of this nanomaterial-based optical methods for the determination of HPV, the nano-LAMP method can detect HPV directly by observing the results of color changes, and the internal heating method can reduce aerosol pollution. Importantly, the materials we use are laber free, that is, no antibodies, enzymes or polymers are used to modify the surface of the material and it takes into account high sensitivity and time saving in the experiment.
Table 1.
An overview on recently reported nanomaterial-based optical methods for the determination of HPV
| Analyte | Nanomaterials | Transducer | Biomarker | Limit of detection | Determination | Reference |
|---|---|---|---|---|---|---|
| HPV-16 | PtS2 nanosheets | PEG-PtS2 fluorescence biosensor | HPV-16e6 gene fragment | ~ 0.44 nM | Fluorescence (0.5 h) | [20] |
| HPV-16 | AgNCs | DNA-templated AgNCs | DNAzyme | ~ 5.7 pM | Fluorescence (> 7 h) | [21] |
| HPV-16/18 | Nanodiamonds | Spermine-modified nanodiamonds | Electrostatic interaction | ~ 5 nM | Mass spectrometry (2 h) | [19] |
| HPV | Gold nanoparticles | A silicon substrate | Silane chemistry | ~ 1 pM | Fluorescence (2 h) | [23] |
| HPV | Gold nanoparticles | AuNPs | Positively charged avidin | ~ 1 nM | Bare eyes (1.5 h) | [22] |
| HPV | Magnetic beads | Droplet microfluidic chips | Streptavidin antibody | ~ 103 copies | Fluorescent (4.5 h) | [24] |
| HPV | Magnetic beads | MNPs | Label free | ~ 102 copies | Bare eyes (1 h) | This work |
Specificity of nano-LAMP for HPV-6 detection
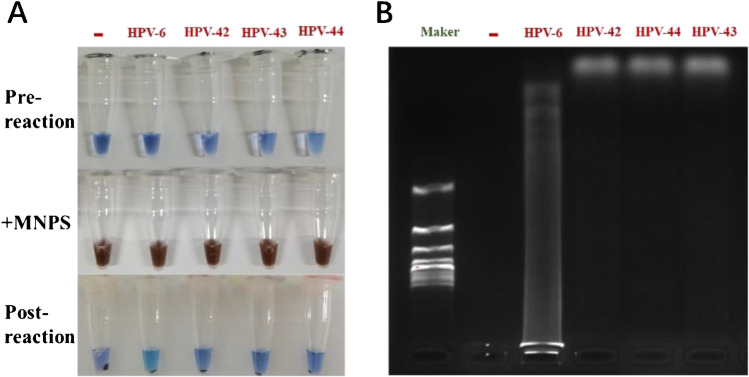
We confirmed the feasibility of nano-LAMP for HPV-6 detection in a sensitivity study. Nevertheless, the efficiency of LAMP can easily be compromised. We verified the specificity of the assay by checking its cross-reactivity, for which we selected four HPV types (HPV-6, HPV-42, HPV-43, HPV-44) and HPV-6 primers. According to color reaction (Fig. 4A) and gel electrophoresis (Fig. 4B), DNA amplification proceeded when the primers for HPV-6 were matched with target HPV-6 DNA. Besides, we have took experiments between other subtypes of primers (HPV-11, HPV-16, HPV-42, HPV-43, HPV-44) (Table S3) and clinical specimens. As shown in Figures S2, S3, S4, S5, and S6, amplification reactions could be performed only when clinical specimens’ subtypes were matched with primers, and the final color changed to sky blue and electrophoresis results showed bands representing the specificity of the reaction. This indicated that nano-LAMP could avoid cross-reaction, and that the designed primers had a high degree of genotype matching and could be classified based on genotype.
Fig. 4.
Results of cross-reaction experiment: primers of HPV-6 and different types of DNA (HPV-6, HPV-42, HPV-43, HPV-44). (A) Images of pre-reactions for conventional LAMP, reactions added MNPs and post-reaction images for nano-LAMP after magnetic separation. (B) Gel electrophoresis images of nano-LAMP products
Feasibility of nano-LAMP for clinical samples
In the simulated clinical sample group, nano-LAMP exhibited excellent non-interference with nucleic acid and significant controlled heating to maintain a constant temperature, which were expected to lead to outstanding sensitivity and specificity for the clinical specimens. To assess the clinical applicability of nano-LAMP, we evaluated clinical specimens from cohorts of patients with HPV-6 and control individuals without HPV-6. We selected HPV-6 specimens confirmed by the Clinical Diagnostic Laboratory of Shanghai Tenth People’s Hospital and independently verified.
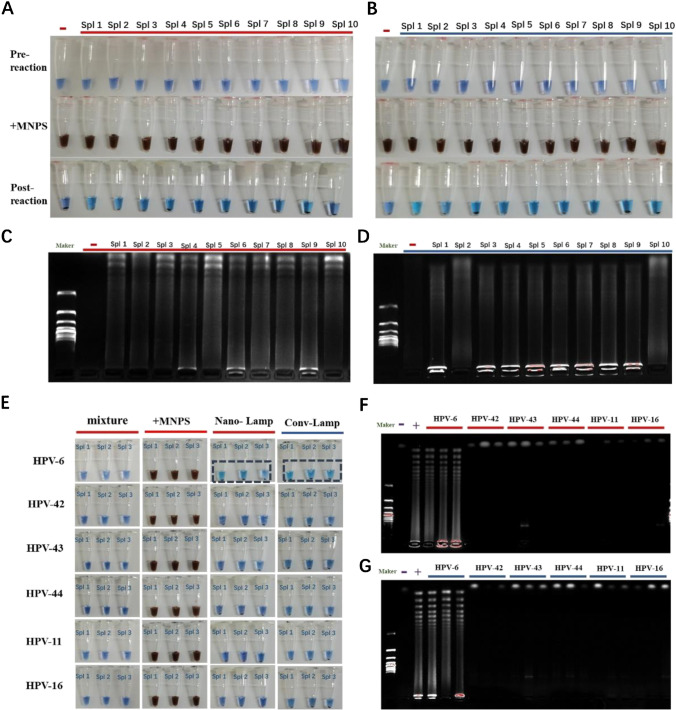
We randomly selected 10 samples of HPV-6 that had been verified in clinical trials. All 10 samples were verified by nano-LAMP and conventional LAMP methods. The reactions were completed and magnetic separation was performed (Fig. 5A, B). Color changes of the reagent and electrophoretic strips confirmed that all 10 clinical specimens were positive for HPV-6, while the negative samples had no color change and electrophoresis showed no stripe. The corresponding electrophoresis results verified the consistency between nano-LAMP and conventional LAMP. Moreover, trailing results appeared in all positive samples (Fig. 5C, D). We verified the specificity of nano-LAMP in clinical specimens by cross-reaction experiment. We selected two high-risk HPV types (HPV-11 and 16) and four low-risk HPV types (HPV-6 and 42–44), and three specimens of each type were randomly selected. We verified the uniformity between nano-LAMP and conventional LAMP. Amplification reactions could be performed only when HPV-6 clinical specimens were matched with HPV-6 primers, and the final color changed to sky blue (Fig. 5E) and electrophoresis results showed bands representing the experimental product (Fig. 5F). In contrast, the HPV-6 primer did not match the other types of samples (HPV-11, 16 and 42–44), so that products showed no color change (Fig. 5E) and no bands (Fig. 5F). Our results verified that there was no cross-reaction in nano-LAMP and conventional LAMP, and ensured the credibility of the experiment. The results showed that nano-LAMP could be used for genotyping of HPV clinical specimens.
Fig. 5.
Clinical sample feasibility verification. Ten positive samples of HPV-6 were randomly selected for nano-LAMP and conventional LAMP. (A) Images of pre-reactions for conventional LAMP reactions added MNPs and post-reaction images and for nano-LAMP after magnetic separation (B). (C) Gel electrophoresis images of conventional LAMP products and gel electrophoresis images of nano-LAMP products (D). (E) Results of cross-reaction validation of clinical samples: Primers of HPV-6 were reacted with clinical samples (HPV-6, HPV-11, HPV-16, and HPV-42–44) for conventional LAMP (F) and nano-LAMP (G)
Conclusions
Here, we explored a magnetic nanoparticle–based internal heating method for HPV detection called nano-LAMP. We successfully verified the high specificity and sensitivity of clinical specimens of various types of HPV (HPV-6, 11, 16, 42, 43, 44) by nano-LAMP method. In particular, cross-pollution between specimens can be avoided and does not need a sophisticated instrument. Meanwhile, the synthesis method of nano-probe is simple and high yield, and the potential application of MNPs in temperature control is proved. Therefore, nano-LAMP has the potential feasibility and practicality of clinical HPV diagnosis may promote its application in the diagnosis and research of other diseases. Besides, there are still many challenges for nano-LAMP methods due to the amount of the MNPs unable to count to control the temperature more accurately. Therefore, the temperature control performance of MNPs needs to be improved and explored.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All clinical specimens were collected from Shanghai Tenth People’s Hospital and Shanghai Skin Disease Hospital.
Author contribution
All authors participated in the research and exploration of the experiment and worked together to complete the final manuscript.
Funding
This work was financially supported by the Outstanding Academic Leaders Plan of Shanghai (Grant No. 2018BR07), Sponsored by grant from Special Clinical Research Project of Shanghai Municipal Health Commission(202140147).
Declarations
Ethics approval
In all the tests, we use clinical specimens for the experiment and follow the rules required by the Ethical Review Board of Shanghai Tenth People’s Hospital, Tongji University (No. 20KT141).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei-Qin Chen, Email: wonderforjune@126.com.
Wei-Wei Liu, Email: huashanvivian@126.com.
References
- 1.Syrjänen S. Human papillomavirus infections and oral tumors. Med Microbiol Immunol. 2003;192(3):123–128. doi: 10.1007/s00430-002-0173-7. [DOI] [PubMed] [Google Scholar]
- 2.Dobec M, Bannwart F, Kaeppeli F, Cassinottiet P. Automation of the linear array HPV genotyping test and its application for routine typing of human papillomaviruses in cervical specimens of women without cytological abnormalities in Switzerland. J Clin Virol. 2009;45(1):23–27. doi: 10.1016/j.jcv.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Cong X, Sun R, Zhang X, Wang Y, Wang L, Yu Y. Correlation of human papillomavirus types with clinical features of patients with condyloma acuminatum in China. Int J Dermatol. 2016;55(7):775–780. doi: 10.1111/ijd.12964. [DOI] [PubMed] [Google Scholar]
- 4.Maniar KP, Ronnett BM, Vang R, Yemelyanova A. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am J Surg Pathol. 2013;37(1):53–60. doi: 10.1097/PAS.0b013e318263cda6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health. 2005;37(6-supp-S):S3–S9. doi: 10.1016/j.jadohealth.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Wu CC, Li MG, Meng HB, Liu YK, Niu WH, Zhou Y, Zhao R, Duan YM, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62(5):640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 7.Asiello PJ, Baeumner AJ. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip. 2011;11(8):1420–1430. doi: 10.1039/C0LC00666A. [DOI] [PubMed] [Google Scholar]
- 8.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RV, BURK RD, Prystowsky MB (2011) A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol 24(10):1295–1305. 10.1038/modpathol.2011.91 [DOI] [PMC free article] [PubMed]
- 9.Syrjänen K, Silvoniemi M, Salminen E, Vasankari T, Syrjänen S. Detection of human papillomavirus genotypes in bronchial cancer using sensitive multimetrix assay. Anticancer Res. 2012;32(2):625–632. doi: 10.1111/j.1471-0528.2011.02974.x. [DOI] [PubMed] [Google Scholar]
- 10.Hagiwara M, Sasaki H, Matsuo K, Honda M, Kawase M, Nakagawa H. Loop-mediated isothermal amplification method for detection of human papillomavirus type 6, 11, 16, and 18. J Med Virol. 2010;79(5):605–615. doi: 10.1002/jmv.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):E63. 10.1097/RLU.0b013e3181f49ac7 [DOI] [PMC free article] [PubMed]
- 12.Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects[J] J Microbiol. 2015;53(1):1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 13.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3(5):877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 14.Law WF, Mutalib NA, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2015;5:770. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong YP, Othman S, Lau YL, Son R, Chee HY. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol. 2009;124(3):626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becherer L, Borst N, Bakheit M, Frischmann S, Stetten FV. Loop-mediated isothermal amplification (LAMP) – review and classification of methods for sequence-specific detection[J] Anal Methods. 2020;12(6):717–746. doi: 10.1039/C9AY02246E. [DOI] [Google Scholar]
- 17.Higashimoto Y, Ihira M, Ohta A, Ohta A, Inoue S, Usui C, Asano Y. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46(8):2665–2670. doi: 10.1128/JCM.00216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Q, Li K, Chen D, Wang H, Lin Q, Liu W. Rapid detection and subtyping of human papillomaviruses in condyloma acuminatum using loop-mediated isothermal amplification with hydroxynaphthol blue dye. Br J Biomed Sci. 2018;75(3):110–115. doi: 10.1080/09674845.2017.1411864. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Yin L, Xue JJ, Wang ZH, Xet NZ. Mass spectrometry genotyping of human papillomavirus based on high-efficiency selective enrichment of nanoparticles [J] ACS Appl Mater Interfaces. 2018;48(10):41178–41184. doi: 10.1021/acsami.8b16694. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Li S, Li X, Liu M. PEG-PtS 2 nanosheet-based fluorescence biosensor for label-free human papillomavirus genotyping[J] Microchim Acta. 2020;187(7):1–7. doi: 10.1007/s00604-020-04383-8. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Ma Y, Luo L, Wang Q, Wang K, et al. Ratiometric determination of human papillomavirus-16 DNA by using fluorescent DNA-templated silver nanoclusters and hairpin-blocked DNAzyme-assisted cascade amplification[J] Microchim Acta. 2019;186(9):1–6. doi: 10.1007/s00604-019-3732-y. [DOI] [PubMed] [Google Scholar]
- 22.Mou L, Hong HH, Xu XJ, Xia Y, Jiang XY. Digital hybridization human papillomavirus assay with attomolar sensitivity without amplification. ACS Nano. 2021;15(8):13077–13084. doi: 10.1021/acsnano.1c02311. [DOI] [PubMed] [Google Scholar]
- 23.Azizah N, Hashim U, Gopinath SCB, et al. Gold nanoparticle mediated method for spatially resolved deposition of DNA on nano-gapped interdigitated electrodes, and its application to the detection of the human papillomavirus. Microchim Acta. 2016;183(12):3119–3126. doi: 10.1007/s00604-016-1954-9. [DOI] [Google Scholar]
- 24.Piao YJ, Zhou X, Wu X. Colorimetric human papillomavirus DNA assay based on the retardation of avidin-induced aggregation of gold nanoparticles. Microchim Acta. 2018;185(12):537–543. doi: 10.1007/s00604-018-3065-2. [DOI] [PubMed] [Google Scholar]
- 25.Cheong J, Yu H, Lee CY, Chang YL, Lee JU, Choi HJ, Lee JH. Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nat Biomed Eng. 2021;5(1):1–1. doi: 10.1038/s41551-020-00654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalli MA, Langmade JS, Chen X, Fronick CC, Sawyer CS, Burcea LC. Rapid and extraction-free detection of SARS-CoV-2 from saliva by colorimetric reverse-transcription loop-mediated isothermal amplification. Clin Chem. 2020;62(2):415–424. doi: 10.1093/clinchem/hvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamdy ME, Carlo MD, Hussein HA, Salah TA, Compagnone D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J Nanobiotechnol. 2018;16(1):48. doi: 10.1186/s12951-018-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun R, Liu D, Wang M. A clinical study to evaluate predictive significance of miR-513b-5p for senile cataract using nanoparticle-assisted polymerase chain reaction method[J] Mater Express. 2021;11(8):1387–1393. doi: 10.1166/mex.2021.2028. [DOI] [Google Scholar]
- 29.Wanzhe Y, Jianuan L, Peng L, Jiguo S, Ligong C, Juxiang L. Development of a nano-particle-assisted PCR assay for detection of duck tembusu virus. Lett Appl Microbiol. 2016;62(1):63–67. doi: 10.1111/lam.12509. [DOI] [PubMed] [Google Scholar]
- 30.Shi DL, Sadat ME, Dunn AW, Mast DB. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications[J] Nanoscale. 2015;7(18):8209–8232. doi: 10.1039/C5NR01538C. [DOI] [PubMed] [Google Scholar]
- 31.Xiao H, Deng Z, Zi Y, Wang Y, Zhu H, Chen B. Biomarkerless targeting and photothermal cancer cell killing by surface-electrically-charged superparamagnetic Fe3O4 composite nanoparticles. Nanoscale. 2016;9(4):1457–1465. doi: 10.1039/C6NR07161A. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Kim M, Lee KK, Chung KH, Lee CS. Effects of graphene oxide-gold nanoparticles nanocomposite on highly sensitive foot-and-mouth disease virus detection. Nanomaterials. 2020;10(10):1921. doi: 10.3390/nano10101921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.