Abstract
Background
The association between body mass index (BMI) and mortality is controversial. Thus, the purpose of our research was to survey the association between BMI and postoperative 30-day mortality in brain tumor patients undergoing craniotomy.
Methods
This study analyzed data collected in a multicenter, cross-sectional study that consecutively and nonselectively collected data from a total of 18,642 patients undergoing craniotomy for tumors in the ACS NSQIP from 2012 to 2015. We constructed three linear and non-linear binomial logistic models (the inflection point was set at 18.5) to evaluate the association between BMI and postoperative 30-day mortality, respectively. We also conducted subgroup analyses. Additionally, we compared non-linear models with vs. without interaction with sex.
Results
A total of 17,713 patients were included in this analysis. Of these, 47.38% were male. The postoperative 30-day mortality of the included cases was 2.39% (423/17,713), and the mean BMI was 28.41 ± 6.05 kg/m2. The linear logistic models suggested that after adjusting for the covariates, BMI was not associated with postoperative 30-day mortality (OR=0.999; 95% CI: 0.981, 1.017). The non-linear binomial logistic models suggested a nonlinear relationship between BMI and postoperative 30-day mortality. When BMI was < 18.5, we observed a stronger negative association between them after adjusting for covariates; the OR and 95% CI were 0.719, 0.576-0.896. When BMI was > 18.5, the relationship between them was not significant. We also found that a one-unit decrease in BMI for male patients with BMI < 18.5 kg/m2 was related to a 34.6% increase in the risk of postoperative 30-day mortality (OR=0.654, 95% CI (0.472, 0.907). There was no significant association between them in male patients with BMI > 18.5 kg/m2 or female patients.
Conclusions
This study demonstrates a non-linear relationship between BMI and the risk of postoperative death. Preoperative underweight (BMI < 18.5 kg/m2) would increase the risk of postoperative death in male patients (> 18 years old) undergoing craniotomy for brain tumors. Appropriate nutritional management prior to craniotomy for brain tumors may reduce the risk of postoperative 30-day mortality in underweight men.
Keywords: body mass index, brain tumor, craniotomy, underweight, mortality risk, risk overestimation
Background
Craniotomies are the cornerstone of brain tumor treatment. However, craniotomies for intracranial tumors present significant risks of morbidity and mortality (1). The 30-day mortality, an important indicator of perioperative mortality, provides an effective evaluation of the safety of operations and their risk of postoperative complications (2). The 30-day mortality risk of a diagnostic neurosurgical procedure (e.g., resection or tissue biopsy) for a primary pediatric intracranial tumor is between 1.16% and 1.72%, consistent with contemporary data from European populations (3). With the improvement of living standards and the increase in unhealthy diets, the prevalence of obesity has reached pandemic levels in the last 50 years. Obesity represents a major health challenge because it significantly increases the risk of many diseases (such as type 2 diabetes mellitus, fatty liver disease, and hypertension) as well as several cancers (4, 5). Body mass index (BMI) is widely used to define the desirable weight index and is calculated as weight in kilograms divided by height in meters squared. A previous study found that high BMI was linked to 4 million deaths worldwide (6), and a BMI of 35 or greater was associated with significantly higher all-cause mortality (7).
J-Shaped associations between BMI and most specific causes of death and overall mortality were found in a population-based cohort study of 3.6 million adults in the UK; lower BMI was associated with an increased risk of mortality from neurological causes (8). BMI is associated with mortality from many types of cancers. In recent years, increasing studies have investigated the relationship between BMI and prognosis for various cancers. The predictive value of BMI for survival in brain tumor patients is debatable. Underweight BMIs are associated with worse outcomes following craniotomy for brain metastasis (9).
No association was found between overall survival and BMI for patients with normal weights or those with a BMI > 25 with brain metastases due to breast cancer (10). Higher BMIs were associated with longer overall and progression-free survival in adult glioblastoma multiforme patients who underwent surgery and chemoradiotherapy (11). However, another study suggested that BMI was not associated with survival in newly diagnosed and previously untreated patients with GBM (12). To date, neither studies have performed subgroup analyses nor explored the nonlinear relationship between BMI and postoperative 30-day mortality. In addition, the study was limited by the small sample size. The link between BMI and postoperative 30-day mortality has yet to be explored in patients undergoing craniotomies for brain tumors. Thus, the present study was designed to examine the relationship between BMI and postoperative 30-day mortality in cross-sectional study data from a large U.S. brain tumor population. In addition, this study may provide guidance for clinical practice by clarifying the quantitative relationship between BMI and postoperative 30-day mortality.
Participants and Methods
Study Design
The present cross-sectional study utilized data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, from records between 2012 and 2015. Our independent variable was preoperative BMI. The dependent variable was postoperative 30-day mortality.
Data Source
The data studied obtained from the ACS NSQIP database originally uploaded by Jingwen Zhang et al. (13) (data from “Sepsis and septic shock after craniotomy: Predicting a significant patient safety and quality outcome measure”; DOI: 10.1371/journal.pone.0235273). The original study was an open-access article distributed under a Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Therefore, these data could be used for secondary analysis without infringement on the authors’ rights.
Participants
A total of 18,642 adults with brain tumors were included in the original study. After excluding patients with missing values for weight and/or height (N=730) and outliers (defined as values more than ± 3 standard deviations from the mean) (14) (N=199), 17,713 cases were included in our analysis (as shown in Figure 1). Consent forms from participants were not required because our study was based on a secondary analysis of previously collected data and the original personal information was anonymous.
Figure 1.
Flowchart of study participant selection.
Variables
BMI
Weight and height were recorded as continuous variables. The definition of BMI was as follows: BMI = weight in kilograms divided by height in meters squared (kg/m2). The data were collected under standardized conditions and treated following uniform procedures.
Postoperative 30-Day Mortality
The 30-day mortality was defined as mortality after discharge for the first 30 postoperative days (13).
Covariates
In our study, covariates were selected according to our clinical experience and the previous literature. Thus, the following variables were treated as covariates: (1) continuous variables: preoperative blood test indicators (serum sodium, blood urea nitrogen, white blood cell count, hematocrit, platelet count), BMI and duration of the operation; and (2) categorical variables: sex (female or male), race (Asian, White, African American or Unknown), age ranges (18-40, 41-60, 60-80, >80 years old), smoking status, ventilator dependence, steroid use, preoperation transfusions, and emergency case status, and a history of diabetes [No, Yes (Noninsulin-dependent) or Yes (Insulin-dependent)], severe chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), hypertension, renal failure, disseminated cancer, and open wound infection. More specific details are presented in the original study (13). In addition, WBC counts less than or equal to 10 × 109/L were considered low risk, and those greater than 10 × 109/L were considered high risk (15). Thus, the continuous variable WBC count was divided into dichotomous variables (presence of low or high risk) for subgroup analyses.
Statistical Analysis
We subgroup analyses the participants by quartiles of BMI. The mean ± standard deviation (SD) (normally distributed variables) or median (interquartile range) (non-normally distributed variables) was reported for continuous variables, and frequencies and percentages were presented for categorical variables. We used χ2 tests (categorical variables), one-way ANOVAs (normally distributed variables), or Kruskal–Wallis H tests (non-normally distributed data) to test for significant differences among different BMI groups. To examine the exact link between BMI and postoperative 30-day mortality, three distinct covariate unadjusted and multivariable non-linear and linear binomial logistic regression models were constructed according to the STROBE statement guidelines, including a nonadjusted model (no covariates were adjusted), a minimally adjusted model (adjusting for sex, race, age range, smoking status, severe COPD, CHF, and renal failure), and a fully adjusted model (adjusting for sex, race, age range, smoking status, preoperation transfusions, ventilator dependence, severe COPD, congestive heart failure, renal failure, steroid use, serum sodium, blood urea nitrogen, white blood cell count, hematocrit and platelet count). The coefficient of association (OR) and their 95% confidence intervals were recorded. We adjusted the coefficient of association when covariances were added to the model, and the odds ratio was changed by 10% or more (16). BMI < 18.5 kg/m2 was defined as underweight according to the World Health Organization. The log-likelihood ratio test was employed to determine the most suitable model for describing the association between BMI and postoperative 30-day mortality. When a nonlinear relationship was found, we set the inflection point of BMI at 18.5, then built a two-piece binary logistic regression model on either side of the inflection point. We also compared non-linear models with vs. without interaction with sex. Tests for interaction were performed with the likelihood ratio test to explore the difference of non-linear relationship between BMI and 30-day mortality in male and female patients.
Subgroup analyses were performed using a binary logistic regression model for the various subgroups: sex, age range, race, diabetes, smoking status, severe COPD, hypertension, disseminated cancer, steroid use, emergency case, and white blood cell count. First, we converted the continuous white blood cell count to a categorical variable based on the clinical threshold mentioned above (<10× 109/L, ≥10 × 109/L) (15). Second, in addition to the subgroup factor itself, we adjusted each subgroup for all factors (race, age range, diabetes, smoking status, ventilator dependence, severe COPD, congestive heart failure, hypertension, renal failure, disseminated cancer, open wound infection, steroid use, preoperative transfusions, emergency case, serum sodium, blood urea nitrogen, white blood cell count, hematocrit, platelet count, and duration of operation). Last, we tested for interaction using the likelihood ratio test for models with and without interaction terms (17, 18).
We also explored the potential for unknown confounds on the relationship between BMI and postoperative 30-day mortality by calculating E-values (19). All results were reported according to the STROBE statement guidelines (16, 20).
The number of participants with missing values for Na, BUN, WBC, HCT, PLT, and duration of operation was 775 (4.37%), 1,415 (7.99%), 575 (3.25%), 428 (2.41%), 565 (3.19%), and 2 (0.01%), respectively. The missing values were replaced by the mean value for statistical analysis.
Modeling was performed with EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the statistical software package R (http://www.R-project.org, The R Foundation). P values < 0.05 (two-sided) were considered statistically significant.
Results
Characteristics of Participants
Table 1 provides the demographic and clinical characteristics of the participants included in the study. A total of 17,713 patients were included in our study, of whom 47.38% were male. The age distribution proportions were 16.4% (18-40), 41.53% (41-60), 38.8% (61-80) and 3.27% (>81). The mean BMI was 28.41 ± 6.05 kg/m2. The postoperative 30-day mortality of the included cases was 2.39% (423/17,713). We assigned participants into subgroups using BMI quartiles: Q1 (11.21-24.10 kg/m2), Q2 (24.10-27.58 kg/m2), Q3 (27.58-31.91 kg/m2), and Q4 (31.91-48.90 kg/m2). No significant differences were observed among the different BMI groups regarding the covariates of ventilator dependence, renal failure, open wound infection, steroid use, or preoperative transfusions (all P values > 0.05). Compared with those of participants with lower BMI (11.21-24.10 kg/m2), the highest BMI (31.91-48.90 kg/m2) significantly positively correlated with sex, race, age range, preoperative blood test indicators (serum sodium, blood urea nitrogen, white blood cell count, hematocrit, platelet count), severe COPD, smoking status, congestive heart failure (CHF), hypertension, diabetes, disseminated cancer, emergency case and duration of operation (all P values < 0.05). Although these baseline indicators (such as serum sodium) were statistically significant due to the large sample size, they were not clinically significant.
Table 1.
Baseline characteristics of participants.
| BMI (quartile) | Q1 (11.21-24.10) | Q2 (24.10-27.58) | Q3 (27.58-31.90) | Q4 (31.91-48.90) | P value |
|---|---|---|---|---|---|
| N | 4410 | 4441 | 4429 | 4433 | |
| Sex N (%) | <0.001 | ||||
| Male | 1714 (38.9%) | 2359 (53.1%) | 2397 (54.1%) | 1923 (43.4%) | |
| Female | 2696 (61.1%) | 2082 (46.9%) | 2032 (45.9%) | 2510 (56.6%) | |
| Race, N (%) | <0.001 | ||||
| White | 3094 (70.2%) | 3209 (72.3%) | 3347 (75.6%) | 3354 (75.7%) | |
| Asian | 243 (5.5%) | 174 (3.9%) | 77 (1.7%) | 42 (0.9%) | |
| African American | 256 (5.8%) | 246 (5.5%) | 293 (6.6%) | 408 (9.2%) | |
| Unknown | 817 (18.5%) | 812 (18.3%) | 712 (16.1%) | 629 (14.2%) | |
| Age range, N (%) | <0.001 | ||||
| 18-40 | 894 (20.3%) | 716 (16.1%) | 615 (13.9%) | 713 (16.1%) | |
| 41-60 | 1722 (39.0%) | 1785 (40.2%) | 1807 (40.8%) | 2042 (46.1%) | |
| 61-80 | 1633 (37.0%) | 1747 (39.3%) | 1849 (41.7%) | 1613 (36.4%) | |
| > 81 | 161 (3.7%) | 193 (4.3%) | 158 (3.6%) | 65 (1.5%) | |
| Serum sodium (Mean ± SD) | 138.4 ± 3.3 | 138.6 ± 3.1 | 138.7 ± 3.1 | 138.9 ± 3.0 | <0.001 |
| Blood urea nitrogen (Mean ± SD) | 16.8 ± 7.8 | 17.5 ± 7.8 | 17.8 ± 7.7 | 17.6 ± 8.5 | <0.001 |
| White blood cell count (Mean ± SD) | 9.2 ± 4.4 | 9.4 ± 4.4 | 9.6 ± 4.4 | 9.7 ± 4.4 | <0.001 |
| Hematocrit (Mean ± SD) | 39.3 ± 4.8 | 40.6 ± 4.7 | 40.9 ± 4.6 | 40.6 ± 4.6 | <0.001 |
| Platelet count (Mean ± SD) | 250.7 ± 81.6 | 239.8 ± 74.0 | 238.7 ± 70.4 | 245.7 ± 73.5 | <0.001 |
| Duration of operation (Mean ± SD) | 202.2 ± 124.7 | 209.2 ± 134.0 | 214.6 ± 131.6 | 227.6 ± 136.1 | <0.001 |
| Diabetes, N (%) | <0.001 | ||||
| No | 4163 (94.4%) | 4058 (91.4%) | 3897 (88.0%) | 3547 (80.0%) | |
| Yes (Noninsulin-dependent) | 162 (3.7%) | 247 (5.6%) | 336 (7.6%) | 546 (12.3%) | |
| Yes (Insulin-dependent) | 85 (1.9%) | 136 (3.1%) | 196 (4.4%) | 340 (7.7%) | |
| Smoking status, N (%) | <0.001 | ||||
| No | 3261 (73.9%) | 3624 (81.6%) | 3661 (82.7%) | 3734 (84.2%) | |
| Yes | 1149 (26.1%) | 817 (18.4%) | 768 (17.3%) | 699 (15.8%) | |
| Ventilator dependence, N (%) | 0.067 | ||||
| No | 4362 (98.9%) | 4396 (99.0%) | 4372 (98.7%) | 4401 (99.3%) | |
| Yes | 48 (1.1%) | 45 (1.0%) | 57 (1.3%) | 32 (0.7%) | |
| Severe COPD, N (%) | <0.001 | ||||
| No | 4120 (93.4%) | 4285 (96.5%) | 4251 (96.0%) | 4264 (96.2%) | |
| Yes | 290 (6.6%) | 156 (3.5%) | 178 (4.0%) | 169 (3.8%) | |
| Congestive heart failure, N (%) | 0.023 | ||||
| No | 4396 (99.7%) | 4432 (99.8%) | 4420 (99.8%) | 4410 (99.5%) | |
| Yes | 14 (0.3%) | 9 (0.2%) | 9 (0.2%) | 23 (0.5%) | |
| Hypertension, N (%) | <0.001 | ||||
| No | 3330 (75.5%) | 2934 (66.1%) | 2535 (57.2%) | 2177 (49.1%) | |
| Yes | 1080 (24.5%) | 1507 (33.9%) | 1894 (42.8%) | 2256 (50.9%) | |
| Renal failure, N (%) | 0.057 | ||||
| No | 4409 (100.0%) | 4441 (100.0%) | 4424 (99.9%) | 4428 (99.9%) | |
| Yes | 1 (0.0%) | 0 (0.0%) | 5 (0.1%) | 5 (0.1%) | |
| Disseminated cancer, N (%) | <0.001 | ||||
| No | 3146 (71.3%) | 3542 (79.8%) | 3572 (80.7%) | 3673 (82.9%) | |
| Yes | 1264 (28.7%) | 899 (20.2%) | 857 (19.3%) | 760 (17.1%) | |
| Open wound infection, N (%) | 0.221 | ||||
| No | 4369 (99.1%) | 4408 (99.3%) | 4400 (99.3%) | 4388 (99.0%) | |
| Yes | 41 (0.9%) | 33 (0.7%) | 29 (0.7%) | 45 (1.0%) | |
| Steroid use, N (%) | 0.058 | ||||
| No | 3741 (84.8%) | 3797 (85.5%) | 3708 (83.7%) | 3792 (85.5%) | |
| Yes | 669 (15.2%) | 644 (14.5%) | 721 (16.3%) | 641 (14.5%) | |
| Preoperation transfusions, N (%) | 0.600 | ||||
| No | 4391 (99.6%) | 4427 (99.7%) | 4417 (99.7%) | 4419 (99.7%) | |
| Yes | 19 (0.4%) | 14 (0.3%) | 12 (0.3%) | 14 (0.3%) | |
| Emergency case, N (%) | <0.001 | ||||
| No | 4131 (93.7%) | 4218 (95.0%) | 4201 (94.9%) | 4274 (96.4%) | |
| Yes | 279 (6.3%) | 223 (5.0%) | 228 (5.1%) | 159 (3.6%) | |
| 30 day mortality events, N (%) | 117 (2.65%) | 91 (2.05%) | 115 (2.60%) | 100 (2.26%) | 0.021 |
Covariate Unadjusted Analyses Using a Binary Logistic Regression Model
The covariate unadjusted analysis indicated that patients that were female, 41–60 years old, 61–80 years old, >81 years old, had diabetes (Noninsulin-dependent), diabetes (Insulin-dependent), ventilator dependence, severe COPD, congestive heart failure, hypertension, renal failure, disseminated cancer, open wound infections, steroid use, preoperation transfusions, emergency cases, levels of Na, BUN, WBCs, HCT, PLTs and durations of operation were positively associated with postoperative 30-day mortality. In contrast, patients that were Asian, African American, of unknown race and those that smoked were negatively associated with postoperative 30-day mortality (see Supplementary Table 1 for details).
Multivariable Analyses Using the Non-Linear and Linear Binomial Logistic Models
To investigate the exact association between BMI and postoperative 30-day mortality, we constructed three models using non-linear and linear binary logistic regression models, respectively. From the linear binary logistic regression models, we found no significant association between BMI and postoperative 30-day mortality rates in Model 1, Model 2, or Model 3 (Table 2). The trend of ORs and 95% CIs were robust irrespective of the covariates adjusted for in the model (Model 1: OR=0.987; 95% CI: (0.971, 1.003), Model 2: OR=0.993; 95% CI: (0.975, 1.010), Model 3: OR=0.999; 95% CI: (0.981, 1.017). All p-values for the three standard linear binomial logistic models (Model 1, Model 2, or Model 3) were more than 0.05 (Table 2).
Table 2.
The results of the standard linear and two-piecewise linear regression model.
| Postoperative 30-day mortality (OR, 95% CI, P value) | |
|---|---|
| Model 1 Fitting model by standard linear regression | 0.987 (0.971, 1.003) 0.1061 |
| Fitting model by two-piecewise linear regression | |
| Inflection point of BMI, kg/m2 | 18.5 |
| ≤18.5 | 0.681 (0.553, 0.838) 0.0003 |
| >18.5 | 0.993 (0.976, 1.009) 0.3917 |
| P for log-likelihood ratio test | 0.003 |
| Model 2 Fitting model by standard linear regression | 0.993 (0.975, 1.010) 0.3999 |
| Fitting model by two-piecewise linear regression | |
| Inflection point of BMI, kg/m2 | 18.5 |
| ≤18.5 | 0.716 (0.576, 0.889) 0.0026 |
| >18.5 | 0.998 (0.981, 1.016) 0.8669 |
| P for log-likelihood ratio test | 0.009 |
| Model 3 Fitting model by standard linear regression | 0.999 (0.981, 1.017) 0.8998 |
| Fitting model by two-piecewise linear regression | |
| Inflection point of BMI, kg/m2 | 18.5 |
| ≤18.5 | 0.719 (0.576, 0.896) 0.0033 |
| >18.5 | 1.005 (0.987, 1.023) 0.5905 |
| P for log-likelihood ratio test | 0.010 |
OR, odds ratios; CI, confidence,
Model 1 (nonadjusted model): not adjusted for any covariates.
Model 2 (minimally adjusted model): adjusted sex, race, age range, smoking status, severe COPD, congestive heart failure, and renal failure.
Model 3 (fully adjusted model): adjusted sex, race, age range, smoking status, ventilator dependence, severe COPD, congestive heart failure, renal failure, steroid use, preoperative transfusions, serum sodium, blood urea nitrogen, white blood cell count, hematocrit, and platelet count.
Through the non-linear binary logistic regression models, we observed that the association between BMI and postoperative 30-day mortality rates was nonlinear (Figure 2). When the infection point was set at 18.5, the P for the log-likelihood ratio test was less than 0.05 in our study (Table 2). After adjusting for confounding factors (sex, race, age range, smoking status, ventilator dependence, severe COPD, congestive heart failure, renal failure, steroid use, preoperative transfusions, serum sodium, blood urea nitrogen, white blood cell count, hematocrit, and platelet count), we observed a stronger negative association between BMI and 30-day mortality. When BMI was less than 18.5, the OR and 95% CI were 0.719, 0.576-0.896, respectively. When BMI was >18.5, the relationship between BMI and 30-day mortality was not significant, the coefficient of association and 95% CI were 1.005, 0.987-1.023, respectively (Table 2).
Figure 2.
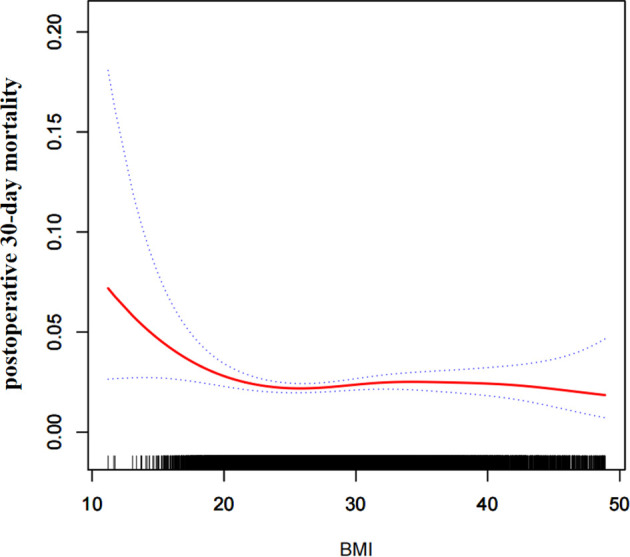
The nonlinear relationship between BMI and risk of postoperative 30-day mortality.
We also computed an E-value to assess the sensitivity to unmeasured confounders. The E-value was 1.08, which was greater than the relative risk of unmeasured confounders influencing the relationship between BMI and postoperative 30-day mortality, suggesting that unmeasured or unknown confounders had little effect on the relationship.
Subgroup Analyses
We performed subgroup analyses to consider other factors that might influence the relationship between BMI and postoperative 30-day mortality. We used sex, age range, race, diabetes, smoking status, severe COPD, hypertension, disseminated cancer, steroid use, emergency case, and white blood cell count as subgroup variables to detect the trend of coefficient of association. Table 3 shows that there was no significant differences in the relationship in different age range, race, diabetes, smoking status, severe COPD, hypertension, disseminated cancer, steroid use, emergency case or white blood cell count groups (P for interaction > 0.05); only sex modified the relationship between BMI and postoperative 30-day mortality rates (P for interaction < 0.05). A stronger association was observed in males (OR=0.975, 95% CI: 0.950-1.002), and a weaker association was found in females (OR=1.012, 95% CI: 0.988-1.037).
Table 3.
Results of interaction and subgroup analyses.
| Characteristic | OR (95% CI) | P for interaction |
|---|---|---|
| Sex | 0.004 | |
| Male | 0.975 (0.950, 1.002) | |
| Female | 1.012 (0.988, 1.037) | |
| Age range | 0.2003 | |
| 18-40 | 1.057 (0.988, 1.131) | |
| 41-60 | 0.986 (0.956, 1.017) | |
| 61-80 | 0.999 (0.974, 1.025) | |
| > 81 | 0.954 (0.888, 1.024) | |
| Race | 0.6447 | |
| White | 0.998 (0.977, 1.020) | |
| Asian | 1.023 (0.869, 1.204) | |
| African American | 0.959 (0.896, 1.026) | |
| Unknown | 0.981 (0.937, 1.027) | |
| Diabetes | 0.1382 | |
| No | 1.001 (0.981, 1.022) | |
| Yes (Noninsulin-dependent) | 0.940 (0.885, 0.998) | |
| Yes (Insulin-dependent) | 0.990 (0.935, 1.048) | |
| Smoking status | 0.4939 | |
| No | 0.992 (0.972, 1.014) | |
| Yes | 1.008 (0.970, 1.048) | |
| Severe COPD | 0.3361 | |
| No | 0.997 (0.978, 1.016) | |
| Yes | 0.968 (0.914, 1.026) | |
| Hypertension | 0.1216 | |
| No | 1.010 (0.982, 1.038) | |
| Yes | 0.981 (0.958, 1.005) | |
| Disseminated cancer | 0.7233 | |
| No | 0.991 (0.966, 1.016) | |
| Yes | 0.997 (0.971, 1.025) | |
| Steroid use | 0.0944 | |
| No | 0.984 (0.963, 1.005) | |
| Yes | 1.018 (0.984, 1.052) | |
| Emergency case | 0.4764 | |
| No | 0.989 (0.970, 1.009) | |
| Yes | 1.011 (0.957, 1.068) | |
| White blood cell count | 0.2992 | |
| WBC < 10 | 1.003 (0.977, 1.030) | |
| WBC >10 | 0.984 (0.959, 1.009) |
Sex Differences in the Nonlinear Relationship
Based on the above results, we further explored the effect of sex on the relationship between BMI and postoperative 30-day mortality. Through the non-linear binary logistic regression models, we found a nonlinear relationship between BMI and postoperative 30-day mortality in males but not in females (Figure 3). The mortality rate of male patients was higher than that of female patients (Figure 3). As shown in Table 4, When the inflection point was set at 18.5, curve fitting and threshold effect analysis revealed that for male patients with BMI < 18.5 kg/m2, a one-unit decrease in BMI was related to a 34.6% increase in the risk of postoperative 30-day mortality (OR=0.654, 95% CI (0.472, 0.907); for male patients with BMI > 18.5 kg/m2, the effect of a one-unit increase in BMI on risk of postoperative 30-day mortality was not significant. No significant association was found between BMI and postoperative 30-day mortality in female patients (Figure 3). We also compared non-linear models with vs. without interaction with sex in Table 4. The results of the interaction test were statistically significant, suggesting that the non-linear relationship between BMI and 30-day mortality was different between male and female patients.
Figure 3.
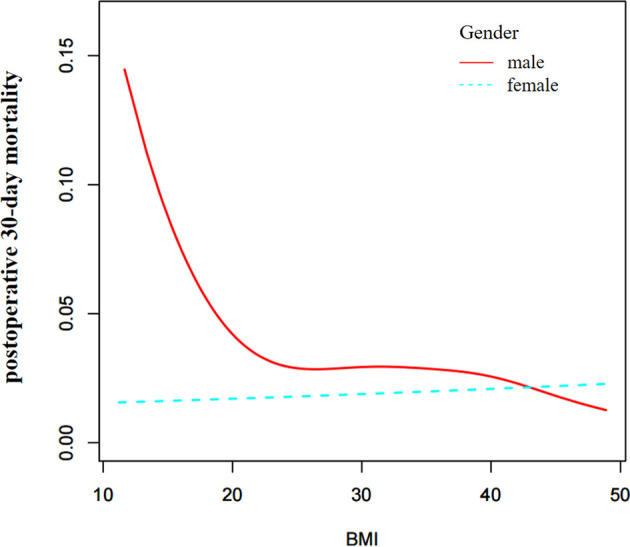
Sex differences in the effect of BMI on postoperative 30-day mortality.
Table 4.
The results of the piecewise linear regression.
| Gender | Male | Female | P for interaction | |
|---|---|---|---|---|
| 30-day mortality (OR, 95% CI, P value) | 30-day mortality (OR, 95% CI, P value) | |||
| Infection point of BMI | 18.5 | 18.5 | ||
| ≤18.5 | 0.654 (0.472, 0.907) 0.0110 | ≤18.5 | 0.741 (0.533, 1.029) 0.0738 | |
| >18.5 | 0.990 (0.964, 1.017) 0.4637 | >18.5 | 1.017 (0.992, 1.042) 0.1803 | |
| P value for log likelihood ratio test | 0.022 | 0.106 | <0.001 |
The model adjusted for race, age range, smoking status, ventilator dependence, severe COPD, congestive heart failure, renal failure, steroid use, preoperative transfusions, serum sodium, blood urea nitrogen, white blood cell count, hematocrit, and platelet count.
Discussion
Many studies have reported an increased mortality rate in cancer patients with low BMIs (21, 22). Preoperative BMI is an independent prognostic factor for non-small-cell lung cancer patients after surgical resection, with underweight patients having an unfavorable prognosis (23, 24). Additionally, being overweight did not increase the risks of complications and operative mortality compared with those of patients with a normal BMI (24). Another report suggested that underweight BMIs were associated with worse outcomes following craniotomy for brain metastasis (9). These results are similar to ours. We conclude that low BMI is associated with poor outcomes and that being overweight did not increase the risk of postoperative 30-day mortality in patients undergoing craniotomy for tumors.
The predictive value of BMI for survival in many studies is controversial. J-shaped associations between BMI and most specific causes of death and overall mortality were reported in a population-based cohort study of 3.6 million adults in the UK; for neurological causes, a lower BMI was associated with increased mortality risk (8). Based on the results of our study, we share the view that lower BMIs are associated with an increased risk of mortality due to neurological causes. However, our findings are inconsistent with previous findings because higher BMIs also increased mortality in other studies. For instance, a recent study reported that obesity was associated with greater overall mortality in cancer patients, but obese patients with melanoma, renal cell carcinoma, and lung cancer had lower risks of death than patients with the same cancers that were not obese (25). This difference may be attributed to differences in the study population and the cutoff time.
To date, clinical studies on the association between BMI and survival in patients with brain cancer are scarce, and their findings are controversial. Jones LW et al. maintained that there was no association between BMI and survival in newly diagnosed and previously untreated patients with GBM in 2010 (12). Still, Valente Aguiar, P et al. recently reported that a higher BMI was associated with a longer survival index in adult GBM patients who underwent surgery and chemoradiotherapy (11). Lareida A et al. found that underweight BMIs were associated with worse outcomes following craniotomy for brain metastasis (9), which was similar to our findings. These authors speculated that dietary factors might lead to a lower BMI in patients with brain metastasis. In addition, they argued that high BMIs were associated with better outcomes in patients with brain metastases and that improving patient nutrition may help improve their prognosis (9).
To the best of our knowledge, our study was the first to describe a nonlinear relationship between BMI and postoperative 30-day mortality in male patients undergoing craniotomy for tumors. We used a piecewise linear regression model to clarify the nonlinear relationship between BMI and postoperative 30-day mortality. For male patients with BMI < 18.5 kg/m2, a one-unit decrease in BMI was related to a 34.6% increase in the risk of postoperative 30-day mortality (OR=0.654, 95% CI (0.472, 0.907). No significant association was found between BMI and postoperative 30-day mortality in male patients with BMI > 18.5 kg/m2 or female patients. Other baseline variables might also have influenced patients’ risk of postoperative 30-day mortality. Previously, it was widely believed that obesity, which is usually related to BMI, was associated with reduced survival in cancer patients. However, many studies have challenged this by demonstrating that obesity is associated with improved survival in cancer patients (11, 26). This finding, known as the “obesity paradox”, may be largely explained by methodological limitations, including confounds, selection bias, reverse causation, and reliance on BMI as a measure of adiposity in cancer patients (27, 28). We believe that the BMI may provide an inaccurate measurement of body composition. In general, body habitus is heterogeneous. The distribution of fat, as well as lean body mass and functional performance, may provide more precise insight regarding the association between body habitus and outcomes in cancer patients (29, 30). Moreover, there are differences in the patterns of proportion and distribution of body fat between males and females (31), which may explain the difference in the relationship between BMI and postoperative 30-day mortality between males and females in our findings. Future studies are needed to explore the prognostic significance of quantitative, alternative measures of body fat and functional performance in patients with brain tumors.
Our research has several strengths, as follows: (1) to the best of our knowledge, this is the largest study investigating the relationship between BMI and postoperative 30-day mortality rate, and this large sample size ensures sufficient statistical power; (2) strict statistical adjustments utilized to minimize residual confounds; (3) we used three distinct standard linear and non-linear binomial logistic models GAM model and smooth curve fitting (penalized spline method) to explore the relationship between BMI and postoperative 30-day mortality rate; thus, our analysis had greater clinical value, which previous studies did not explore; (4) we considered the interaction between other covariates and the relationship between BMI and postoperative 30-day mortality because other covariates have the potential to mask the actual relationship between BMI and postoperative 30-day mortality; and (5) the study of coefficient of association facilitates the use of this data in future studies.
However, we also acknowledge that our study has a few limitations, as follows: (1) because this study was a secondary analysis of published data, we cannot exclude some unmeasured and/or residual confounding factors that could influence the estimated relationship (e.g., socioeconomic factors, waist circumference, and waist-to-hip ratio). However, we calculated the E-value to quantify the potential impact of unmeasured confounders and found that they were unlikely to explain the results; and (2) we could not explore the relationship between BMI and long-term outcomes. Our study was based on data from a large and heterogeneous group of patients in a large catchment area despite these limitations. As such, the relationships and conclusions postulated remain highly plausible.
Conclusions
In patients over 18 years of age who underwent craniotomy for brain tumors, BMI (kg/m2) has a specific nonlinear relationship with postoperative 30-day mortality in male patients but not in female patients. Appropriate nutritional management prior to a craniotomy for brain tumors may reduce the risk of postoperative 30-day mortality in underweight men. Thus, our study may provide a reference for policy-makers to develop guidelines as to safer levels of BMI for male patients preparing to undergo craniotomy for brain tumors. Further research on the distribution of adiposity as well as lean body mass in relation to functional performance may provide more accurate insight into the association between body habitus and patient craniotomy outcomes.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Hechi People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Formal analysis: YL and HH. Investigation: LL and ZYL. Methodology: YL and HH. Supervision: LZ, ZLu, GH, and ZLa. Writing – original draft: YL, HH, and YH. Writing – review and editing: ZYL, ZLu, and ZLa. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors are grateful to Jingwen Zhang et al. for providing the data to be analyzed. Data were obtained from: Sepsis and septic shock after craniotomy: Predicting a significant patient safety and quality outcome measure (DOI: 10.1371/journal.pone.0235273).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.868968/full#supplementary-material
Abbreviations
BMI, body mass index; CI, Confidence Interval; ACS NSQIP, American College of Surgeons National Surgical Quality Improvement Program; SD, standard deviation; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; GAM, generalized additive model. GBM, glioblastoma multiforme.
References
- 1. Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative Mortality in The Netherlands: A Population-Based Analysis of Surgery-Specific Risk in Adults. Anesthesiology (2010) 112(5):1105–15. doi: 10.1097/ALN.0b013e3181d5f95c [DOI] [PubMed] [Google Scholar]
- 2. Watters DA, Hollands MJ, Gruen RL, Maoate K, Perndt H, McDougall RJ, et al. Perioperative Mortality Rate (POMR): A Global Indicator of Access to Safe Surgery and Anaesthesia. World J Surg (2015) 39(4):856–64. doi: 10.1007/s00268-014-2638-4 [DOI] [PubMed] [Google Scholar]
- 3. Hankinson TC, Dudley RW, Torok MR, Patibandla MR, Dorris K, Poonia S, et al. Short-Term Mortality Following Surgical Procedures for the Diagnosis of Pediatric Brain Tumors: Outcome Analysis in 5533 Children From SEER, 2004-2011. J Neurosurg Pediatr (2016) 17(3):289–97. doi: 10.3171/2015.7.PEDS15224 [DOI] [PubMed] [Google Scholar]
- 4. Blüher M. Obesity: Global Epidemiology and Pathogenesis. Nat Rev Endocrinol (2019) 15(5):288–98. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 5. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol (2016) 11:421–49. doi: 10.1146/annurev-pathol-012615-044359 [DOI] [PubMed] [Google Scholar]
- 6. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries Over 25 Years. N Engl J Med (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories. JAMA (2013) 309(1):71. doi: 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI With Overall and Cause-Specific Mortality: A Population-Based Cohort Study of 3·6 Million Adults in the UK. Lancet Diabetes Endocrinol (2018) 6(12):944–53. doi: 10.1016/S2213-8587(18)30288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lareida A, Terziev R, Grossenbacher B, Andratschke N, Roth P, Rohrmann S, et al. Underweight and Weight Loss Are Predictors of Poor Outcome in Patients With Brain Metastasis. J Neurooncol (2019) 145(2):339–47. doi: 10.1007/s11060-019-03300-1 [DOI] [PubMed] [Google Scholar]
- 10. Cacho-Diaz B, Spinola-Marono H, Reynoso N, Gonzalez-Aguilar A, Mohar-Betancourt A. Role of Overweight, Obesity, and Comorbidities in the Prognosis of Patients With Breast Cancer With Brain Metastases. Clin Breast Cancer (2019) 19(2):e394–8. doi: 10.1016/j.clbc.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 11. Valente AP, Carvalho B, Vaz R, Linhares P. Body Mass Index as an Independent Prognostic Factor in Glioblastoma. Cancer Cause Control (2021) 32(4):327–36. doi: 10.1007/s10552-020-01388-9 [DOI] [PubMed] [Google Scholar]
- 12. Jones LW, Ali-Osman F, Lipp E, Marcello JE, McCarthy B, McCoy L, et al. Association Between Body Mass Index and Mortality in Patients With Glioblastoma Mutliforme. Cancer Cause Control (2010) 21(12):2195–201. doi: 10.1007/s10552-010-9639-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Li YI, Pieters TA, Towner J, Li KZ, Al-Dhahir MA, et al. Sepsis and Septic Shock After Craniotomy: Predicting a Significant Patient Safety and Quality Outcome Measure. PloS One (2020) 15(9):e0235273. doi: 10.1371/journal.pone.0235273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang N, Hu X, Zhang Q, Bai P, Cai M, Zeng TS, et al. Non-High-Density Lipoprotein Cholesterol: High-Density Lipoprotein Cholesterol Ratio is an Independent Risk Factor for Diabetes Mellitus: Results From a Population-Based Cohort Study. J Diabetes (2018) 10(9):708–14. doi: 10.1111/1753-0407.12650 [DOI] [PubMed] [Google Scholar]
- 15. Sun J, Lou Y, Zhu J, Shen H, Zhou D, Zhu L, et al. Hypertriglyceridemia in Newly Diagnosed Acute Promyelocytic Leukemia. Front Oncol (2020) 10:577796. doi: 10.3389/fonc.2020.577796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Schlesselman SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Ann Intern Med (2007) 147(8):W163–94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 17. Mullee A, Romaguera D, Pearson-Stuttard J, Viallon V, Stepien M, Freisling H, et al. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern Med (2019) 179(11):1479–90. doi: 10.1001/jamainternmed.2019.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keidel D, Anto JM, Basagaña X, Bono R, Burte E, Carsin AE, et al. The Role of Socioeconomic Status in the Association of Lung Function and Air Pollution-A Pooled Analysis of Three Adult ESCAPE Cohorts. Int J Environ Res Public Health (2019) 16(11):1901. doi: 10.3390/ijerph16111901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA (2019) 321(6):602–3. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int J Surg (2014) 12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 21. Shaukat A, Dostal A, Menk J, Church TR. BMI Is a Risk Factor for Colorectal Cancer Mortality. Dig Dis Sci (2017) 62(9):2511–7. doi: 10.1007/s10620-017-4682-z [DOI] [PubMed] [Google Scholar]
- 22. Li J, Luo S, Wong MCS, Li C, Feng L, Peng J, et al. Longitudinal Associations Between BMI Change and the Risks of Colorectal Cancer Incidence, Cancer-Relate and All-Cause Mortality Among 81,388 Older Adults. BMC Cancer (2019) 19(1):1082. doi: 10.1186/s12885-019-6299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomita M, Ayabe T, Nakamura K. Low Body Mass Index Is an Independent Predictive Factor After Surgical Resection in Patients With Non-Small Cell Lung Cancer. Asian Pac J Cancer Prev (2017) 18(12):3353–6. doi: 10.22034/APJCP.2017.18.12.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Guo M, Zhang N, Wang G. Association of Body Mass Index and Outcomes Following Lobectomy for Non-Small-Cell Lung Cancer. World J Surg Oncol (2018) 16(1):90. doi: 10.1186/s12957-018-1394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open (2021) 4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association Between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidem Biomar (2017) 26(1):21–9. doi: 10.1158/1055-9965.EPI-15-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lennon H, Sperrin M, Badrick E, Renehan AG. The Obesity Paradox in Cancer: A Review. Curr Oncol Rep (2016) 18(9):56. doi: 10.1007/s11912-016-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DH, Giovannucci EL. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr Nutr Rep (2019) 8(3):175–81. doi: 10.1007/s13668-019-00280-6 [DOI] [PubMed] [Google Scholar]
- 29. Strulov SS, Williams GR. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidem Biomar (2017) 26(1):13–6. doi: 10.1158/1055-9965.EPI-16-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park Y, Peterson LL, Colditz GA. The Plausibility of Obesity Paradox in Cancer-Point. Cancer Res (2018) 78(8):1898–903. doi: 10.1158/0008-5472.CAN-17-3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bredella MA. Sex Differences in Body Composition. Adv Exp Med Biol (2017) 1043:9–27. doi: 10.1007/978-3-319-70178-3_2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.