Abstract
Animal studies have shown that fungal burden correlates with survival during treatment with new antifungal therapies for histoplasmosis. The purpose of this report is to compare the clearance of fungal burden in patients with histoplasmosis treated with liposomal amphotericin B versus itraconazole. In two separate closed clinical trials that evaluated the efficacy of liposomal amphotericin B and itraconazole treatment of disseminated histoplasmosis in patients with AIDS, blood was cultured for fungus and blood and urine were tested for Histoplasma antigen. The clinical response rates were similar; 86% with liposomal amphotericin B (n = 51) versus 85% with itraconazole (n = 59). Of the patients with positive blood cultures at enrollment, after 2 weeks of therapy cultures were negative in over 85% of the liposomal amphotericin B group versus 53% of the itraconazole group (P = 0.0008). Furthermore, after 2 weeks, median antigen levels in serum fell by 1.6 U in the liposomal amphotericin B group versus 0.1 U in the itraconazole group (P = 0.02), and those in urine fell by 2.1 U in the liposomal amphotericin B group and 0.2 U in the itraconazole group (P = 0.0005). The more rapid clearance of fungemia supports the use of liposomal amphotericin B rather than itraconazole for initial treatment of moderately severe or severe histoplasmosis.
Human immunodeficiency virus (HIV)-infected patients with disseminated histoplasmosis are treated with a 12-week course of induction treatment to reverse the clinical illness and suppress the fungal burden in the tissues (4, 5). Recent studies show that in patients with moderately severe or severe manifestations, liposomal amphotericin B is more effective than the deoxycholate formulation of amphotericin B. A clinical response after 2 weeks of treatment occurred in 88% of patients receiving liposomal amphotericin B versus 64% of patients receiving the standard deoxycholate preparation (P. Johnson, L. J. Wheat, G. Cloud, C. Thomas, W. Dismukes, M. Goldman, R. Hafner, and W. Powderly, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 232, p. 15). Survival during the first 2 weeks of therapy also was improved in the liposomal amphotericin B group compared to the deoxycholate amphotericin B group (98 versus 88%, respectively). Despite the better clinical response with liposomal amphotericin B the rates of clearance of fungemia and H. capsulatum antigen from the serum and urine were similar with the two treatments. After 2 weeks of induction therapy, treatment was changed to itraconazole for another 10 weeks of consolidation therapy.
In an earlier study, targeted for patients with less severe disease manifestations, itraconazole given for 12 weeks also was highly effective, inducing remission in 85% of patients, based upon resolution of signs and symptoms of histoplasmosis and clearance of positive cultures (4). Itraconazole also cleared the organisms from the blood and antigen from the serum and urine of patients who responded to therapy. A randomized trial comparing liposomal amphotericin B to itraconazole which is required to clearly prove the relative effectiveness of these two regimens, is not feasible because of the large sample size and the declining incidence of histoplasmosis in patients with AIDS in the era of potent antiretroviral therapy. Although the eligibility criteria of the studies differed, excluding severe disease in the itraconazole study and mild disease in the liposomal amphotericin B study, these databases provided the opportunity to compare the antifungal effects of these two regimens on clearance of fungemia and Histoplasma capsulatum antigenemia and antigenuria in these AIDS patients with disseminated histoplasmosis.
MATERIALS AND METHODS
Patient groups.
Enrollment into the itraconazole study occurred in 1991 and 1992 (4), followed by enrollment in the liposomal amphotericin B study between August 1995 and April 1999 (Johnson et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect.). Patients were more than 13 years old and had serologically documented HIV infection. Study entry required diagnosis of a first episode of disseminated histoplasmosis based on compatible clinical findings and laboratory evidence, consisting of the demonstration of organisms resembling H. capsulatum var. capsulatum by stain of tissues or body fluids, positive cultures, or detection of antigen in serum or urine. Patients were excluded from the itraconazole study if they had severe disease, namely, systolic blood pressure of <90 mm Hg, hypoxia with pO2 of <60 torr, or central nervous system histoplasmosis (4), while such patients were specifically targeted for the liposomal amphotericin B study. Patients were excluded from the itraconazole study if they were unable to take oral medications, had Karnofsky scores of below 30, hemoglobin levels of <7 g/dl, neutrophil counts of <600/mm3, platelet counts of <50,000/mm3, hepatic enzymes of ≥5 times the upper limit of normal, or bilirubin levels of ≥2.5 times the upper limit of normal (4). Such patients could, however, participate in the liposomal amphotericin B study (Johnson et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect.). Patients with renal impairment were excluded from both studies (for itraconazole, creatinine levels of >3 times the upper limit of normal; for liposomal amphotericin B, creatinine levels of >2 times the upper limit of normal).
Treatment regimens and study assessments.
In the itraconazole study, the loading dose was 300 mg by mouth twice daily for 3 days and then 200 mg twice daily for 12 weeks. Patients were evaluated for clinical response weekly for the first 4 weeks, biweekly for the next 4 weeks, and then at the completion of induction at 12 weeks. Fungal blood cultures were done at the local institution at weeks 0, 1, 2, 4, 8, and 12.
In the liposomal amphotericin B study, patients were randomized 2:1 to receive liposomal amphotericin B at 3 mg/kg/day or deoxycholate amphotericin B at 0.7 mg/kg/day for 2 weeks, followed by itraconazole at 200 mg twice daily for another 10 weeks. For this report, since clearance of positive cultures and antigen were similar in the liposomal amphotericin B and deoxycholate amphotericin B arms but fewer patients were available in the deoxycholate amphotericin B group, only the liposomal amphotericin B group is compared to itraconazole group. Fungal blood cultures using the lysis-centrifugation method at a central laboratory were done at weeks 0, 1, 2, 4, 8, and 12 (Johnson et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect.).
Histoplasma antigen assay.
The test used to detect antigen in serum and urine is an enzyme-linked immunoassay that has been previously described (2). Results are expressed as enzyme immunoassay units, and results of 1 U or higher are regarded as positive. For the calculation of median antigen values, the actual units were used, including values between 0.5 and 0.9 U in those with negative results. Urine and blood specimens at baseline, week 2, and week 12 were compared. If baseline specimens were unavailable or inadequate for testing, week 1 specimens were substituted. Similarly, if specimens at week 12 were not available, the date closest to week 12, between weeks 10 and 20, was used, as in prior studies. Patients with negative antigen results at baseline or without follow-up specimens at week 2 or 12 were excluded.
Statistical methods.
Continuous baseline characteristics and outcomes were compared using the Kruskal-Wallis test for ordered measurements, and the Fisher exact test was used to compare categorical characteristics. The Cox proportional hazard model was used to assess the effect of multiple factors that appeared to affect the time to culture negativity (1). The Kaplan-Meier method was used in blood culture conversion analysis, and the treatment groups were compared by the log rank test (3). A significance level of alpha = 0.05 (two sided) was used to test all hypotheses.
RESULTS
Comparison of baseline characteristics.
Selected baseline characteristics are compared in Table 1. CD4 counts and Karnofsky scores were similar between the two groups. Amphotericin B treatment during the 3 days before enrollment (P = 0.03) and prior antiretroviral treatment (P = 0.04) were greater in the liposomal amphotericin B group. Blood culture positivity and quantitative Histoplasma antigenemia and antigenuria results were not statistically different between the two groups. Blood cultures were positive in 79% of patients in the liposomal amphotericin B group versus 80% in the itraconazole group (P = 0.55). Antigenemia was present in 83% of patients in the liposomal amphotericin B group versus 80% in the itraconazole group (P = 0.47), and antigenuria was present in 87% of the liposomal amphotericin B group versus 98% of the itraconazole group (P = 0.06). Median antigen levels also were similar in the two groups.
TABLE 1.
Comparison of baseline parameters in patients with AIDS and histoplasmosis treated with itraconazole versus liposomal amphotericin B
| Parametera | Itraconazole group | Liposomal amphotericin B group | P value |
|---|---|---|---|
| Karnofsky count of <60 | 16/59 (27%) | 13/50 (26%) | 0.54 |
| Median CD4 count, 109/liter | 29 (n = 53), range, 2–346 | 18 (n = 46), range, 1–182 | 0.10 |
| Antiretroviral use | 35/59 (59%) | 39/51 (76%) | 0.04 |
| Amphotericin B in the last 72 hb | 11/58 (19%) | 19/51 (37%) | 0.03 |
| Positive blood cultures | 43/54 (80%) | 37/47 (80%) | 0.55 |
| Urine antigen positive/tested | 44/45 (98%) | 39/45 (87%) | 0.06 |
| Serum antigen positive/tested | 24/30 (80%) | 40/48 (83%) | 0.47 |
| Median urine antigenc (range) | 9.4 (0.6–12.4) | 9.6 (0.5–14.7) | 0.36 |
| Median Serum antigenc (range) | 9.0 (0.5–16.7) | 7.3 (0.5–14.4) | 0.20 |
Unless indicated otherwise, results are number positive/total number of patients, with the percentage of positive patients given in parentheses.
Patients were allowed to receive amphotericin B for a few days before enrollment in both studies, to enhance accrual by capturing patients who had received some treatment before referral or whose physician preferred one or two doses of amphotericin B if there was any delay in enrollment. The itraconazole study permitted up to 1.5 mg/kg over the prior week, and the liposomal amphotericin B study permitted up to 2.1 mg/kg over the past 3 days.
Baseline results include the entire study group, not just those evaluated for antigen clearance. For urine, there were 45 patients in both the itraconazole and the liposomal amphotericin B studies. For serum, there were 30 in the itraconazole study and 48 in the liposomal amphotericin B study. Similar findings were noted if only those patients who were evaluated for antigen clearance were included, with P values of 0.68 for urine (comparing baseline itraconazole versus liposomal amphotericin B) and 0.82 for serum, but the numbers in each group were smaller: urine and itraconazole, n = 22; urine and liposomal amphotericin B, n = 16; serum and itraconazole, n = 9, serum and liposomal amphotericin B, n = 18.
Clearance of fungemia.
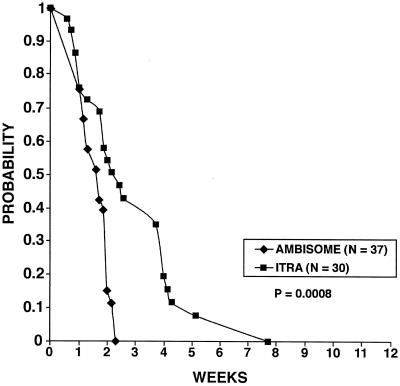
Of the patients with positive blood cultures at baseline and for whom follow-up cultures were available, fungemia cleared more rapidly with liposomal amphotericin B than with itraconazole (Fig. 1). In both studies, a total of three patients were excluded from the analysis due to death, and each died during the first week of therapy: 1 of 51 (2.0%) in the liposomal amphotericin B group and 2 of 59 (3.4%) in the itraconazole group. Kaplan-Meier estimates showed that by the end of the second week of therapy, blood cultures were negative in over 85% of the liposomal amphotericin B patients versus 53% of the itraconazole patients (P = 0.0008). If the analysis was restricted only to patients with positive antigen results in serum or urine (n = 32 in the liposomal amphotericin B group versus 25 in the itraconazole group), fungemia still cleared more quickly with liposomal amphotericin B (P = 0.004). Using the Cox proportional hazards model to assess the impact of prior amphotericin B use, prior antiretroviral therapy, and baseline CD4 count together with antifungal therapy, only therapy retained a significant correlation with the time to culture negativity (P = 0.0009). By 12 weeks of therapy, cultures were negative in all patients who were tested in both groups.
FIG. 1.
Clearance of fungemia with itraconazole (ITRA) or liposomal amphotericin B (AMBISOME). This analysis is restricted to patients with positive results at baseline for whom follow-up cultures were performed.
Clearance of antigenemia and antigenuria.
Median antigen concentrations in patients with paired baseline and week 2 results are shown in Fig. 2. After 2 weeks of therapy, median antigen levels in serum fell by 1.6 U in the liposomal amphotericin B group compared to 0.1 U in the itraconazole group (P = 0.02). After 2 weeks of treatment, antigen levels in serum were negative (<1.0 U) in 9 of 32 patients (28%) in the liposomal amphotericin B group versus 3 of 15 (20%) in the itraconazole group (P = 0.55).
FIG. 2.
Antigen clearance following treatment with itraconazole (Itra) or liposomal amphotericin B (Ambisome). The analysis is restricted to patients with positive results at baseline who had follow-up specimens after 2 weeks of therapy. Data are expressed as median antigen (Ag) units, the box represents the 25th and 75th percentiles, and the bars represent the 10th and 90th percentiles.
Antigen levels in urine fell by 2.1 U with liposomal amphotericin B therapy versus 0.2 U with itraconazole therapy (P = 0.0005), and at the end of the second week of treatment, antigen levels were below the detection limit in 6 of 31 patients (19%) in the liposomal amphotericin B group versus 1 of 29 (3%) in the itraconazole group (P = 0.06).
By 12 weeks of therapy, antigen levels fell further in both groups. In the liposomal amphotericin B study, treatment was changed to itraconazole after 2 weeks of liposomal amphotericin B. Antigen levels in serum were below the detection limit in 17 of 29 patients (59%) in the liposomal amphotericin B group versus 8 of 15 (53%) in the itraconazole group (P = 0.74). Antigen levels in urine were below the detection limit in 6 of 28 patients (21%) in the liposomal amphotericin B group versus 6 of 32 (19%) in the itraconazole group (P = 0.80).
DISCUSSION
This study shows more rapid clearance of fungal burden with liposomal amphotericin B than with itraconazole. Fungemia cleared by 2 weeks in over 85% of patients receiving liposomal amphotericin B compared to 53% of patients receiving itraconazole. By 12 weeks, however, blood cultures were negative in all patients in each study. Antigen concentrations also declined more rapidly with liposomal amphotericin B than with itraconazole, although less dramatically than did fungemia. For example, after 2 weeks of treatment, antigenemia cleared in 26% and antigenuria cleared in 19% of the patients receiving liposomal amphotericin B, while fungemia cleared in 85%. Antigen clearance paralleled resolution of fungemia but appeared to be less sensitive than culture for comparing the mycological response to treatment. These findings, however, do not contradict other studies that show a useful role of antigen detection for diagnosis of histoplasmosis and identification of relapse (6).
For this comparison, we chose liposomal amphotericin B rather than the deoxycholate formulation to permit a more robust statistical analysis. The comparative trial of the two formulations of amphotericin B used a 2:1 randomization schedule, and there were only 22 patients in the standard formulation arm (Johnson et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect.). Of note, however, is that fungemia, antigenemia, and antigenuria cleared at similar rates in patients treated with liposomal amphotericin B or the standard preparation, even though the clinical response to the liposomal preparation was better.
The optimal study design would have been a randomized comparison of liposomal amphotericin B to itraconazole, but such a study is not feasible. Limitations to the comparisons of these two studies include differences in eligibility criteria, imbalances in baseline characteristics of the treatment groups, and changes in treatment other than the study drugs. The liposomal amphotericin B study allowed enrollment of patients who were more severely ill than did the itraconazole study, potentially biasing the study in favor of a better response to itraconazole.
The similarity of baseline blood culture and antigen results in the two studies suggests that disease severity in the study groups may have been relatively comparable, despite the differences in eligibility criteria. Of note is that the criteria for moderately severe disease required for enrollment in the liposomal amphotericin B study greatly overlapped those for enrollment in the itraconazole study, providing a likely explanation for the similar fungal burdens in the two studies. Also, only one patient exhibited mental status changes, two patients exhibited hypotension, and none exhibited respiratory failure in the liposomal amphotericin B group, supporting an assumption that baseline severity was relatively similar in the two studies.
Patients in the liposomal amphotericin B group received more deoxycholate amphotericin B during the 3 days prior to enrollment, but this difference was relatively small compared to the dramatic difference seen in the clearance of fungemia. Also, the baseline culture positivity rates were similar in the two groups, suggesting that receipt of more amphotericin B before enrollment did not explain the more rapid clearance seen with liposomal amphotericin B.
If a higher proportion of patients died during the first 2 weeks of treatment in the liposomal amphotericin B group than the itraconazole treatment group, a bias in favor of more rapid clearance with liposomal amphotericin B would be indicated, as the patients who would be more likely to remain fungemic would be excluded from the analysis. Of note, however, is that only one patient in the liposomal amphotericin B study died during that interval, compared to two patients in the itraconazole study, and in no case in either study was histoplasmosis judged to be the cause of death.
The use of different blood culture methods is another limitation of this analysis. Local hospital laboratories performed the cultures in the itraconazole study using a variety of fungal culture procedures, while the lysis-centrifugation method was performed at a centralized laboratory in the liposomal amphotericin B study. To validate the accuracy of the central laboratory, during the first year of the study cultures were compared to those at the local laboratory, showing comparable results. Furthermore, it is unlikely that the culture methods used during the itraconazole study would be more sensitive than the lysis-centrifugation method. Thus, these differences probably do not explain the more rapid clearance of fungemia with liposomal amphotericin B.
Finally, the liposomal amphotericin B study followed the itraconazole study and spanned a time interval noted for major advances in antiretroviral therapy. Better control of HIV infection could affect the response to antifungal therapy, confounding the interpretation of this analysis. However, the CD4 counts were similar in the two groups and were very low, indicating that the baseline immune function was probably similar. Furthermore, analysis of the treatment records for the patients in the liposomal amphotericin B study showed that only eight patients (16%) were receiving three antiretroviral drugs, of which only four were receiving protease inhibitors, and none changed their therapy during the first 2 weeks of the study. These findings do not support a hypothesis that the use of potent antiretroviral therapy differed in the two studies, that immune function was better at baseline in the liposomal amphotericin B group, or that immune function improved due to intensification of antiretroviral therapy during the first 2 weeks of induction treatment with liposomal amphotericin B. Thus, the use of more potent antiretroviral therapy is an unlikely reason for the better response to liposomal amphotericin B than to itraconazole. However, an effect of antiretroviral therapy cannot be excluded, since the two treatments were not randomized and the analysis was retrospective.
In conclusion, the more rapid clearance of fungemia with liposomal amphotericin B supports its use, rather than itraconazole, in patients with moderate to severe histoplasmosis. Antigen testing does not add to culture methods for assessment of antifungal effect during induction therapy for histoplasmosis.
ACKNOWLEDGMENTS
This work was supported by a Merit Review Grant from the Department of Veterans Affairs, the National Institutes of Allergy and Infectious Diseases Mycoses Study Group (contract NO1-RR0255A), and the AIDS Clinical Trials Group (grant AI25859).
REFERENCES
- 1.Cox D R. Regression models and life-tables. J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 2.Durkin M M, Connolly P A, Wheat L J. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J Clin Microbiol. 1997;35:2252–2255. doi: 10.1128/jcm.35.9.2252-2255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan E L, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 4.Wheat J, Hafner R, Korzun A H, Limjoco M T, Spencer P, Larsen R A, Hecht F M, Powderly W AIDS Clinical Trial Group. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 5.Wheat J, Sarosi G, McKinsey D, Hamill R, Bradsher R, Johnson P, Loyd J, Kauffman C. Practice guidelines for the management of patients with histoplasmosis. Clin Infect Dis. 2000;30:688–695. doi: 10.1086/313752. [DOI] [PubMed] [Google Scholar]
- 6.Wheat L J, Connolly-Stringfield P, Blair R, Connolly K, Garringer T, Katz B P. Histoplasmosis relapse in patients with AIDS: detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann Intern Med. 1991;115:936–941. doi: 10.7326/0003-4819-115-12-936. [DOI] [PubMed] [Google Scholar]