Highlights
-
•
Mitigating cross-signal contribution in LC-MS/MS.
-
•
Using stable isotope labelled internal standard isotopes of lesser abundance to counteract cross-signal contribution.
-
•
Novel approach to correct for non-linearity from cross-signal contribution.
Abbreviations: FLX, flucloxacillin; 13C4-FLX, 13C4 flucloxacillin; K3-EDTA, potassium ethylenediaminetetraacetic acid; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LLOQ, lower limit of quantification; MRM, multiple reaction monitoring; MS1, quadrupole 1; MS2, quadrupole 2; SIL-IS, stable isotope labelled internal standard; Q1, first quadrupole; Q3, third quadrupole; QC, quality control; ULOQ, upper limit of quantification
Keywords: Isotopic distribution, Cross-signal contribution, Stable isotope labelled internal standard, LC-MS/MS
Abstract
Background
Utilising stable isotope labelled internal standards (SIL-IS) in quantitative LC-MS/MS drug analysis is the most widely used approach to normalise for variability during sample quantification processes. However, compounds containing atoms such as Sulphur, Chlorine or Bromine, could potentially cause cross-signal contribution to the SIL-IS from the naturally occurring isotopes, resulting in non-linear calibration curves. A simple, novel method of mitigating the effect is presented here. It entails monitoring of a less abundant SIL-IS isotope, as the precursor ion, of a mass that has no/minimal isotopic contribution from the analyte isotopes.
Methods
Experiments were conducted on two LC-MS/MS analysers: Waters Xevo TQ-S and Shimadzu 8050. Flucloxacillin (FLX) was used as an example. Two transitions were selected for FLX (m/z 454 → 160 → 295) and one for each of the SIL-IS isotopes (m/z 458 → 160 for the isotope 457 g/mol and m/z 460 → 160 for the isotope 459 g/mol). Assay biases were assessed at three SIL-IS concentrations: 0.7, 7 and 14 mg/L for each isotope.
Results
When using the SIL-IS isotope m/z 458 → 160 at a concentration of 0.7 mg/L, biases were up to 36.9 % on both instruments. Increasing the SIL-IS concentration to 14 mg/L, reduced the bias to 5.8 %. Using the less abundant isotope, m/z 460 → 160, resulted in biases of 13.9 % at an SIL-IS concentration of 0.7 mg/L.
Conclusions
Applying this method will mitigate cross-signal contribution from the analyte isotopes to the corresponding SIL-IS, minimise the use of SIL-IS, and, thereby, reduce overall cost.
Introduction
For almost 30 years, liquid chromatography tandem mass spectrometry (LC-MS/MS) has proven to be a sensitive and specific analytical technique used in a range of applications. It has proven particularly useful in the quantitative analysis of drugs in biological fluids. When using LC-MS/MS for quantitative assays, the use of a stable isotope labelled internal standard (SIL-IS), of each analyte, is recognised as the most suitable approach to normalise for a range of phenomena that occur during sample preparation and quantification [1], especially suppression or enhancement of ionisation in the ion source.
SIL-ISs are analyte analogues where one or more of the atoms in the molecule are substituted with stable isotopes, such as 13C, 2H or 15N, allowing for detection based on mass differential, rather than chromatographic separation. They are generally considered “ideal” for normalising sample-to-sample analyte ionisation efficiency, as they are regarded to possess the same physico-chemical properties as the analyte. However, they have proven not to be without drawbacks [2], [3], [4], [5].
Cross-signal contribution is one such drawback. If one of the analyte isotopes has the same molecular mass as the selected SIL-IS (due to natural isotopic distribution), and even if natural abundance of this isotope is relatively low, in assays with a wide concentration range, the contribution from the analyte to the internal standard, especially at the upper limit of concentration (ULOQ) of the assay where the analyte concentration is highest, could be substantial. The increase in the analyte concentration causes an increase in the SIL-IS response, resulting in a decrease in the apparent analyte/SIL-IS ratio and, consequently, a non-linear calibration curve.
Several approaches, described previously [6], [7], [8], [9], have been designed to mitigate or correct for cross-signal contribution in LC-MS/MS assays. However, these are either less practical [8] to use in routine clinical laboratories, more expensive [6], [7] or both. One such example is narrowing the assay range. This, however, only minimises the error and reduces the assay’s analytical range. Another approach, previously presented, uses a product ion with lower cross-signal contribution from the analyte isotopes [6]. However, if the selected product ion has a low response, an increased concentration of the SIL-IS is required, which has cost implications. Rule et al., [8] have described a method of subtracting the percentage of the analyte contribution to SIL-IS, where a ‘response contribution factor’ is experimentally determined for each analyte and SIL-IS pair [8]. Although, this approach is probably the most valid of those presented to date, it is complex in its application. Another method presented by Tan et al. [7] uses an increased concentration of SIL-IS in the extraction [7]. However, this can be costly if the percentage of interference is relatively high, requiring that the concentration of the SIL-IS be as high as the ULOQ [7]. Furthermore, ionisation suppression of the analyte of interest can be caused by high SIL-IS concentration [10]. Conversely, if the SIL-IS is not isotopically pure, increasing its concentration will increase the interference in the analyte, thus resulting in a positive intercept of the calibration curve.
Using SIL-IS with additional labelling/labelled at different positions, may potentially overcome the issue, but these options are often not available or involve substantial additional cost.
An alternative approach, presented here, relies on selecting a SIL-IS isotope of lower abundance, as a precursor ion, but of a mass that has no/minimal contribution from the analyte isotopes.
Others have used less abundant natural isotopes of the analytes, in LC-MS/MS, to expand the linear dynamic range [11] and to allow for simultaneous quantification of abundant with less abundant analytes [12]. Recently, Yuan et al. [9] published a strategy to overcome interferences in LC-MS/MS using a less abundant SIL-IS isotope for the application in microdose bioavailability studies. Here, we present a similar approach using a less abundant isotope of a SIL-IS to mitigate cross-signal contribution from the analyte isotopes applicable to therapeutic drug monitoring. In addition, we compared, on two LC-MS/MS systems, the theoretical cross-signal contribution with that observed. Flucloxacillin, incorporated in a simultaneous LC-MS/MS method for quantification of ten antibiotics, is used as an example. The presented strategy has also been applied to cefotaxime, cefepime and cefazolin, which were part of the developed method, but for which the data is not included here.
Materials and Methods
Chemicals and Reagents
Flucloxacillin sodium (FLX, 95 % purity) and 13C4-FLX sodium (SIL-IS, 99.4 % isotopic purity) were purchased from Toronto Research Chemicals (PM Separations, Australia). Acetonitrile (LC-MS grade) and water were purchased from Fisher Scientific (Scoresby, VIC, Australia). Water was further purified using a Simplicity UV purification system from Millipore Australia (North Ryde, NSW, Australia). Formic acid (LC-MS grade) was purchased from Fluka (Sigma- Aldrich, Australia). Disodium hydrogen phosphate was obtained from Chem-Supply (Gillman, SA, Australia). Expired human plasma (K3-EDTA) was obtained from the blood bank (SydPath, St Vincent’s Hospital, Sydney).
LC-MS/MS equipment and conditions
Two LC-MS/MS systems were used. These were Waters Acquity UPLC system coupled to a triple quadrupole mass spectrometer, Xevo TQ-S (Waters Australia, Rydalmere, NSW, Australia), and Shimadzu UFLC system with triple quadrupole mass spectrometer, Shimadzu-8050 (Shimadzu Oceania, Rydalmere, NSW, Australia). The Waters system consisted of a binary solvent LC pump, a degasser, an autosampler and a column compartment. The Shimadzu system consisted of a solvent delivery system (Nexera X2 LC-30AD), an autosampler (Nexera X2 SIL-30AC), a vacuum degasser (DGU-20A5R), a column oven (Prominence CTO-20A) and a system controller (CBM-20A). Autosampler temperature was set to 8 °C and the column to 40 °C on both instruments. Compounds were chromatographically separated on a Waters Acquity BEH C18 (2.1 × 50 mm, 1.7 µm) column, with gradient elution using water and acetonitrile, each containing 0.1 % formic acid, as mobile phases A and B, respectively. The gradient starting at 0 % B was linearly increased to 100 % B in 3.5 min and held for a minute, before it was returned to initial conditions and equilibrated for a further minute. The flow rate was 0.4 mL/min and the total run time was 5.5 min.
The electrospray ionisation source interface was operated in positive ion mode and used for multiple reaction monitoring (MRM) analysis on both instruments. For the Waters system, the ionisation source temperature was maintained at 130 °C, and the de-solvation temperature at 380 °C while de-solvation and cone gas flow rates were set to 800 and 50 L/h, respectively. For the Shimadzu system, interface, heating block and de-solvation temperatures were set to 200, 300 and 150 °C, respectively. Nitrogen was used as the nebulising gas, set to 2.8 L/min, and also used as the heating and drying gas at flow rates of 9 L/min. Argon, set to 270 kPa, was used as the collision gas, on both instruments. Capillary probe voltages were set to 1 kV, on both instruments. Optimisation of voltages and energies were performed by infusion of FLX and SIL-IS solutions. Two MRM transitions were selected for the analyte (m/z 454 → 160, 454 → 295) and one for each of the SIL-IS isotopes (most abundant isotope (457 g/mol), transition monitored m/z 458 → 160; less abundant isotope (459 g/mol), transition monitored m/z 460 → 160) and parameters optimised for both instruments. Data acquisition and processing used Waters MassLynx software version 4.1 and Shimadzu LabSolution software version 5.96. Linear regression analysis, weighted 1/x2, using the peak area ratio of the analyte/SIL-IS versus the concentration, were used to determine the analyte concentration. For the Waters system, low and high mass resolutions were set to 3 and 15 (arbitrary values) for MS1 and MS2, respectively. The Shimadzu system, mass spectrometer resolution for first and third quadrupoles (Q1 and Q3) were set to “unit” with a full-width-half-mass of 0.51–0.80 Daltons. Inter-channel delay and dwell times were 5 and 45 ms (ms) for Waters system and 3 and 10 ms for Shimadzu, respectively.
Calibrator, control and sample preparation
A stock solution of FLX (10 g/L) was prepared in isotonic buffer (0.1 M di-sodium hydrogen phosphate, pH = 7.4, (adjusted with concentrated ortho-phosphoric acid) containing 0.3 % sodium chloride). This, in turn was used to prepare working calibrators by appropriate dilution in analyte free plasma. Calibrators were prepared at the following concentrations: 2.0, 5.0, 50, 100 and 250 mg/L. Quality control (QC) sample concentrations were 2.5, 20 and 200 mg/L prepared from an identical, but independently prepared, stock solution. SIL-IS stock solution (1 g/L) was prepared in water. Working SIL-IS solutions at concentrations of 0.1, 1.0 and 2.0 mg/L (corresponding to 0.7, 7 and 14 mg/L SIL-IS concentration in the extraction) were prepared in acetonitrile. Sample preparation was by protein precipitation. Fifty microliters of water were added to 25 µL of the samples, calibrators and QC followed by a working SIL-IS solution, in acetonitrile of 175 µL. Samples were vortex mixed (2 min), centrifuged (14,000 × g, 10 min) and diluted with water (1:5) before injection into the LC-MS/MS (5 µL on the Waters system and 1 µL on the Shimadzu system).
Theoretical and experimental cross-signal contribution
-
1)
The theoretical isotopic distribution of FLX, SIL-IS and the potential of FLX cross-signal contribution to SIL-IS, based on the precursor ion, for the most abundant and a lesser abundant SIL-IS isotope, was estimated using online software, E-mass [13].The theoretical estimation based on MRM, (m/z 458 → 160), was determined using the online software IsoPatrn [14]. The observed isotopic distribution of FLX and its corresponding SIL-IS was obtained by performing a mass spectrometer 1 scan of a pure solution of each compound.
-
2)
The “apparent” signal, from the naturally occurring analyte isotopes, was assessed by extracting a set of calibrators in a single batch, and substituting acetonitrile for the usual working stock of SIL-IS in acetonitrile. These extracts were then analysed on both LC/MS-MS systems using transitions for the two SIL-IS isotopes, 457 g/mol (m/z 458 → 160) and 459 g/mol (m/z 460 → 160). Similarly, blank plasma was extracted using SIL-IS working stock at a concentration of 7 mg/L to obtain the true signal from the SIL-IS. From these data, the percentage cross-signal contribution from the analyte to SIL-IS was calculated at each calibrator concentration.
-
3)
Calibration curve linearity was assessed for three of the SIL-IS MRMs: m/z 458 → 160, m/z 458 → 299 and m/z 460 → 160, at SIL-IS concentration of 7 mg/L. For isotope 457 g/mol, two MRMs were tested (m/z 458 → 160, m/z 458 → 299) to determine if, potentially, a different fragment ion might be less affected by the cross-signal contribution from the analyte.
-
4)
Assay accuracy was assessed by extracting calibrators and QC in a single batch, on four separate occasions, with working stocks of SIL-IS at concentrations of 0.7, 7 and 14 mg/L. They were analysed on both instruments and assay accuracy, against weighed-in concentrations, was determined based on each of the two monitored SIL-IS MRMs.
-
5)
Analysis of patient samples with the proposed method, using SIL-IS transition m/z 460 → 160, was performed on both instruments. Twenty-one patient samples, requested for routine monitoring of FLX, were selected to cover the assay analytical range. Samples described in the study were collected for measurement of FLX concentrations for clinical purposes. The use of these samples for research purposes is consistent with the Australian National Statement on Research Ethics. (https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018#toc__725).
Results and Calculations
Theoretical and experimental cross-signal contribution
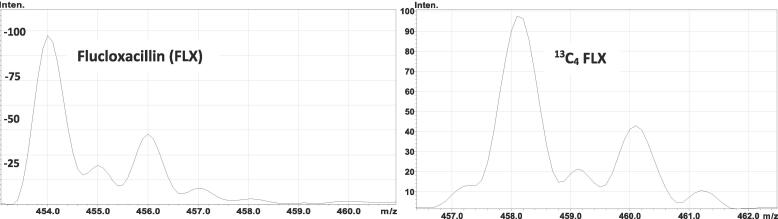
Using E-mass software, the most abundant isotope of FLX has a mass of 453 g/mol, but other isotopes, (e.g., 454, 455, 456, 457 g/mol) are present in lower abundance (Fig. 1). The maximum estimated potential of cross-signal contribution from the FLX isotope, 457 g/mol, to SIL-IS, was 2.8 % of the analyte concentration, based on the precursor ion. Theoretically estimated percent cross-signal contribution for the MRM, (m/z 458 → 160), using the IsoPatrn online calculator, was 0.54 % (data not shown). The observed isotopic distribution of FLX and SIL-IS displayed a similar pattern to the predicted (Fig. 2).
Fig. 1.
Natural relative abundance (%) of flucloxacillin (FLX) and 13C4-FLX (SIL-IS), using E-mass[13]on-line software, and the potential of cross-signal contribution. FLX isotope molecular weight 457 g/mol, with natural relative abundance of 2.8 %, has identical mass to the 13C4-FLX, used as the SIL-IS. Potential cross-signal contribution from FLX to its SIL-IS, based on the precursor ion, is up to 2.8 % of the analyte concentration.
Fig. 2.
Mass spectrometer 1 scan of flucloxacillin (FLX) and 13C4-FLX (SIL-IS). Isotopic distribution of FLX and its SIL-IS, obtained experimentally, showed a similar pattern to that theoretically predicted, when using the on-line software shown in Fig. 1.
Apparent signal
The apparent signal for the SIL-IS isotopes (m/z 458 → 160 and m/z 460 → 160) is shown in Fig. 3, expressed as the percent of the actual SIL-IS signal, when used at concentration of 7 mg/L. Absolute increase in the SIL-IS response, at the 250 mg/L (ULOQ), was on average 39.8 % and 10.9 % for the Waters and Shimadzu instruments, respectively (Fig. 3a). Minimal cross-signal contribution was observed for the SIL-IS isotope m/z 460 → 160 on both systems (0.9 % and 0.2 % at ULOQ for Waters and Shimadzu instruments, respectively (Fig. 3b)).
Fig. 3.
Measured percent (%) cross-signal contribution of flucloxacillin (FLX) isotopes 457 g/mol (m/z 458 → 160) and 459 g/mol (m/z 460 → 160) to 13C4-FLX across the assay concentration range for each of two LC-MS/MS systems. Percent cross-signal contribution, from the analyte isotopes to the SIL-IS, was calculated from the apparent SIL-IS response. Calibrators without the addition of the SIL-IS were analysed and the response compared to the response of the extracts prepared with SIL-IS working stock at a concentration of 7 mg/L. Cross-signal contribution across the assay concentration range for Waters and Shimadzu systems was 0.6 and 0.35 %, respectively. This corresponded to an absolute increase in the SIL-IS peak area, at the assay ULOQ, on average of up to 39.8 % for the Waters system and 10.9 % for the Shimadzu system (a). Negligible increase in the SIL-IS response was observed for isotope 459 g/mol (m/z 460 → 160) for both systems (0.9 % and 0.2 % at the ULOQ for Waters and Shimadzu systems, respectively) (b). Data points represent the mean and standard deviation of a triplicate analysis.
Linearity
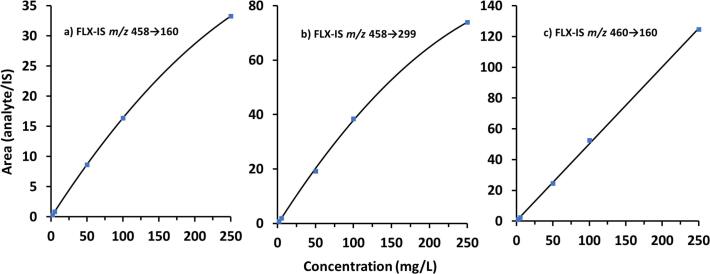
The calibration curves displayed a quadratic fit for the 457 g/mol isotope with both SIL-IS MRMs m/z 458 → 160 and m/z 458 → 299, with the latter being less affected by the cross-signal contribution from the analyte. The calibration curve for the SIL-IS 459 g/mol isotope using m/z 460 → 160 was linear (Fig. 4).
Fig. 4.
Calibration curves of flucloxacillin (FLX) using three different stable isotope labelled internal standards (SIL-IS) MRMs. A quadratic fit model was observed with SIL-IS m/z 458 → 160 and 458 → 299 due to cross-signal contribution from the analyte. Calibration curve was linear with SIL-IS m/z 460 → 160. The concentration of the SIL-IS was 7 mg/L.
Assay accuracy
Interpolated QC values deviated from the weighed-in values by up to 36.9 % using the SIL-IS isotope m/z 458 → 160 (Table 1). Assay accuracy was acceptable only after the SIL-IS concentration was increased by 20-fold to 14 mg/L. Good accuracy was obtained, on both instruments, when using the isotope m/z 460 → 160 at SIL-IS concentration of 0.7 mg/L (Table 1). At SIL-IS concentration of 7 mg/L, for the isotope m/z 458 → 160, bias for the high QC (200 mg/L) was as much as −15.4 % and −19.1 % at the ULOQ (250 mg/L; data not shown).
Table 1.
Impact of SIL-IS concentration and isotope selection on assay accuracy. Accuracy (expressed as % average difference from the weighed-in concentration) of flucloxacillin controls with both SIL-IS isotopes, (m/z 458 → 160 and 460 → 160), at 0.7, 7 and 14 mg/L concentrations, analysed on Waters and Shimadzu systems, (n = 4).
| SIL-IS = 0.7 mg/L | SIL-IS = 14 mg/L | SIL-IS = 7 mg/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Waters Instrument | Shimadzu Instrument | Waters Instrument | Shimadzu Instrument | Waters Instrument | ||||||
| QC (mg/L) | Mean ± SD | % Diff | Mean ± SD | % Diff | Mean ± SD | % Diff | Mean ± SD | % Diff | Mean ± SD | % Diff |
| (m/z 458 → 160) | ||||||||||
| 2.5 | 3.11 ± 0.24 | 24.4 | 2.87 ± 0.26 | 14.7 | 2.65 ± 0.03 | 5.8 | 2.65 ± 0.04 | 6.0 | 2.55 ± 0.07 | 2.0 |
| 20 | 27.37 ± 0.83 | 36.9 | 26.70 ± 1.18 | 33.5 | 21.1 ± 0.48 | 5.4 | 20.8 ± 0.25 | 4.1 | 20.15 ± 0.57 | 0.8 |
| 200 | 141.57 ± 2.46 | −29.2 | 138.68 ± 4.79 | −30.7 | 194.7 ± 3.24 | −2.6 | 197.6 ± 2.82 | −1.2 | 169.3 ± 3.75 | −15.4 |
| (m/z 460 → 160) | ||||||||||
| 2.5 | 2.85 ± 0.22 | 13.9 | 2.74 ± 0.15 | 9.4 | 2.61 ± 0.07 | 4.3 | 2.67 ± 0.08 | 6.7 | 2.61 ± 0.07 | 4.3 |
| 20 | 19.90 ± 0.99 | −0.5 | 20.61 ± 1.50 | 3.0 | 20.3 ± 0.30 | 1.3 | 20.1 ± 0.18 | 0.3 | 19.53 ± 0.45 | −2.4 |
| 200 | 192.28 ± 9.44 | −3.9 | 197.79 ± 12.77 | −1.1 | 206.9 ± 6.57 | 3.4 | 206.2 ± 2.20 | 2.6 | 194.4 ± 5.10 | −2.8 |
Application of the method to routine clinical samples
Patient samples for routine therapeutic drug monitoring of FLX were analysed on both instruments. The results are shown in Fig. 5 as a Bland-Altman plot of the average between the instruments plotted against the percent difference (Waters – Shimadzu instruments). These data show good agreement with < 12% difference between instruments, except for one point at low concentration (i.e.,17 %, Fig. 5). The mean difference between the instruments was −1.7 %.
Fig. 5.
Bland-Altman plot demonstrating the difference between the two instruments for flucloxacillin patient results. Patient samples (n = 21) were analysed on Waters and Shimadzu systems. The SIL-IS isotope used for quantification of the results was m/z 460 → 160 at concentrations of 7 mg/L (Waters system) and 0.7 mg/L (Shimadzu system). Good agreement was observed between the two methods with < 12% difference, except for one point at low concentration (17%).
Discussion
Instrument parameters, such as resolution, may affect the extent of cross-signal contribution, hence two instruments were included in the evaluation, as well as the theoretical estimation of the potential cross-signal contribution, using online software packages [13], [14].
Cross-signal contribution from naturally occurring analyte isotopes to its corresponding SIL-IS, in LC-MS/MS, is difficult to counteract/avoid. Selecting a SIL-IS with a mass difference of at least 3 amu (atomic mass units) from the analyte will generally minimize the effect. However, for compounds containing Sulfur (S), Chlorine (Cl) or Bromine (Br) atoms, such as FLX, a larger difference in the mass units is required, due to their wide isotopic distribution.
Cross-signal contribution depends on several factors, namely: the isotopic distribution of the precursor ion; the product ion selected [6], [15]; concentration of the SIL-IS used in the assay [7], [16], and; the type of regression employed for the calibration curve [7]. Likewise, structurally related compounds [17], such as isomers, isobars, and drugs from the same chemical class, can all interfere with one another when co-eluting. Other fragments created in the collision cell, by-product ions, can also interfere. For this reason, assessment of the isotopic interference from analyte to the SIL-IS and vice versa is required during method development [1]. Theoretical estimation of likely interferences can be achieved by using online calculators [13], [14], [15]; however, experimental assessment should be performed, regardless, and chromatographic modification undertaken to resolve the interfering compounds whenever possible.
The predicted cross-signal contribution from FLX isotopes to the SIL-IS, using E-mass software [13], was 2.8 %. This takes into consideration all the FLX isotopes that have a mass of 457 g/mol (i.e., the precursor ion), based on natural isotope distribution of the atoms constituting the molecule. When SIM (selected ion monitoring) is used for quantification, the percent cross-signal contribution is in agreement with the theoretical estimation, as all the molecules with the m/z 458 would pass through Q1 (first quadrupole) and be detected. However, when using MRM, the location of the stable isotopes in the molecule determines the m/z of the fragment ion, hence, the percent of the cross-signal contribution depends on the product ion selected, which is always less than the isotopic distribution of a precursor ion. Theoretically estimated percent cross-signal contribution for the MRM, m/z 458 → 160, using the IsoPatrn online calculator [14], was 0.54 %, which agreed with the obtained experimental data (0.65 and 0.35% for Waters and Shimadzu systems, respectively). An example of the two possible FLX isotopes, m/z 458, is shown in Fig. 7c and 7d. Both molecules will pass through Q1, but only the isotope in 7c, with the stable isotopes located in the left-hand side of the molecule from the point of cleaving, will produce a fragment ion of m/z 160 and pass through Q3. With this in mind, the effect of analyte cross-signal contribution on a SIL-IS ion fragment, m/z 458 → 299 (Fig. 7b), available for this analyte, was also assessed to determine whether it could be used as an alternative MRM. However, given the calibration curve remained quadratic with this MRM (Fig. 4), it was not a suitable alternative in this case.
Fig. 7.
Chemical structures and fragmentation patterns of flucloxacillin (FLX), 13C4 FLX (SIL-IS) and two examples of FLX isotopes of the same mass as SIL-IS, as a possible cause of cross-signal contribution. A) FLX, MW = 453 g/mol (m/z 454), with the fragmentation pattern, b) 13C4 FLX, MW = 457 g/mol (m/z 458), with the fragmentation pattern, c) and d) FLX isotopes, MW = 457 g/mol, containing various stable isotopes distributed across the molecules. FLX isotope in 7c, although it is constituted of different stable isotopes (13C,15N and 37Cl) than 13C4 FLX in 7b, it has the same molecular weight as 13C4 FLX and produces the same ion fragment (m/z 160) when the ring cleaves off. This will, therefore, contribute to the signal of 13C4 FLX used as a SIL-IS. The FLX isotope in 7d, containing 34S and 37Cl, also has the same molecular weight as 13C4 FLX, but the stable isotopes are located on each side of the molecule where the bond cleavage will occur, producing a fragment ion of m/z 162 and, therefore, will not contribute to the 13C4 FLX signal. The cross-signal contribution from naturally occurring isotopes of the analyte to the SIL-IS is, therefore, dependent on the product ion selected, which is always less than the isotopic distribution of a precursor ion.
As there are no clear guidelines regarding SIL-IS concentration for LC-MS/MS analysis, our practice has been to employ the approach used in high pressure liquid chromatography analysis. This entails using a concentration of the SIL-IS that will produce a response, approximately equivalent, to that of the analyte at the mid-point of the calibration curve. However, in LC-MS/MS where suppression of ionisation is of concern, and where response is not proportional to the analyte concentration, unnecessarily using a high concentration of SIL-IS may only result in suppression of ionisation in the ion source [10].
This work demonstrates that the concentration of a SIL-IS can be kept to a minimum, if the signal response is adequate, for the instrument, and there is no/negligible cross-signal contribution from the analyte isotopes. In the method described herein, the concentration of the SIL-IS that produced an adequate signal response for the instrument was 7 mg/L. This response was, in fact, only approximately 50 % higher than that produced by the lowest calibrator of the analyte, but sufficient to produce accurate results (Fig. 6). The robustness of the proposed method was tested at 10-fold above and below the concentration of the SIL-IS used for routine analysis (0.7 and 14 mg/L, respectively). Assay biases when using the SIL-IS isotope m/z 460 → 160, for quantification, at concentration of 0.7 mg/L, were found to be acceptable. On the contrary, when using the SIL-IS isotope m/z 458 → 160, to quantify the results, the method was only acceptable when SIL-IS concentration was 14 mg/L. It is however worth noting that using such a low SIL-IS concentration, as in the experiments above, is not always possible. For example, compounds with low ionisation efficiency may require higher SIL-IS concentration to produce adequate signal and accurate results if a less abundant isotope ion is to be used. A SIL-IS at such a low concentration was only used to demonstrate the cross-signal contribution effect.
Fig. 6.
Chromatogram peaks of flucloxacillin at LLOQ (2 mg/L) (a) and SIL-IS (m/z 460 → 160) at concentration of 7 mg/L (b). Average peak intensity of the SIL-IS was approximately 50% higher than the LLOQ (Mean ± SD, 69939 ± 6451 cps (counts per second) (FLX); 104595 ± 8396 cps (SIL-IS), (n = 3)).
Another factor to consider when utilising this approach is the variation in the isotope abundance of either the analyte or the SIL-IS with changing of a supplier. If the starting material and/or the route of synthesis are different, the response can be affected. Similarly, isotopic and/or chemical (im)purity, of either the analyte or the SIL-IS, may also affect the signal response slightly, as the percent of cross-signal contribution might vary. It is, therefore, advisable to always test new products and/or batches of products before introducing them into routine use and determine the maximum allowable limit of the isotopic contribution at the SIL-IS concentration intended for assay use. Further, if a less abundant isotope of the analyte is used for calibration, quantification error may occur as the unknowns will differ in their isotopic content relative to the calibrators. However, none of the above-mentioned factors will impact the accuracy of the results of the approach proposed here, since the same amount of the SIL-IS is added to the calibrators, QC, and unknowns.
No difference in the results was observed between the two systems used when analysing patient samples. Measured percent cross-signal contribution from the analyte isotopes to the SIL-IS was marginally lower on the Shimadzu LC-MS/MS system (0.35 vs. 0.6 % across the concentration range). The differences observed may be attributed to the instrument capacity to resolve cross talk in the collision cell, based on the instrument specifications. The Shimadzu 8050 LC-MS/MS was a newer model and had a lower maximum inter-channel cross talk between two MRMs (<0.003 % at its minimum inter-channel delay and dwell time (pause time = 1 ms, dwell time = 0.8 ms). The Waters Xevo TQ inter-channel cross talk at its minimum pause and dwell time (3 ms and 1 ms, respectively) was < 0.01 %.
The application presented here can be used to correct for the interference observed between the analyte and SIL-IS in any area of research. However, given the work was for the purpose of mitigating the effect in the assay used for therapeutic drug monitoring, the application is useful for the detection of toxicity. Specifically, a significant risk of neurotoxicity with FLX trough concentration > 125 mg/L has been suggested [18].
Conclusion
This work demonstrates that by selecting a SIL-IS precursor ion of lower relative abundance, but of a mass that has minimal potential for cross-signal contribution from the analyte isotopes, assay error due to cross-signal contribution can be avoided, analytical range expanded, and the concentration of the SIL-IS kept to a minimum, reducing the cost per analysis.
The method was successfully applied to quantitative analysis of patient samples requested for routine monitoring of FLX.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The provision of a Shimadzu 8050 LC-MS/MS by Shimadzu Australia to undertake this work is greatly appreciated.
The work in the manuscript has been presented at the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) 2018 Congress, September 16-19, Brisbane, Australia, in a poster format.
References
- 1.EMEA, European Medicines Agency. Guideline on bioanalytical method validation, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. [DOI] [PubMed]
- 2.Berg T., Strand D.H. 13C labelled internal standards–a solution to minimize ion suppression effects in liquid chromatography-tandem mass spectrometry analyses of drugs in biological samples? Journal of chromatography. A. 2011;1218:9366. doi: 10.1016/j.chroma.2011.10.081. [DOI] [PubMed] [Google Scholar]
- 3.Wang S., Cyronak M., Yang E. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J Pharm Biomed Anal. 2007;43:701–707. doi: 10.1016/j.jpba.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Lindegardh N., Annerberg A., White N.J., Day N.P. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of piperaquine in plasma stable isotope labeled internal standard does not always compensate for matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:227–236. doi: 10.1016/j.jchromb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Jemal M., Schuster A., Whigan D. Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma and urine: method validation, demonstration of using a surrogate analyte, and demonstration of unacceptable matrix effect in spite of use of a stable isotope analog internal standard. Rapid Commun. Mass Spectrom. 2003;17:1723–1734. doi: 10.1002/rcm.1112. [DOI] [PubMed] [Google Scholar]
- 6.Gu H., Wang J., Aubry A.F., Jiang H., Zeng J., Easter J., Wang J.S., Dockens R., Bifano M., Burrell R., Arnold M.E. Calculation and mitigation of isotopic interferences in liquid chromatography-mass spectrometry/mass spectrometry assays and its application in supporting microdose absolute bioavailability studies. Anal Chem. 2012;84:4844–4850. doi: 10.1021/ac300442v. [DOI] [PubMed] [Google Scholar]
- 7.Tan A., Lévesque I.A., Lévesque I.M., Viel F., Boudreau N., Lévesque A. Analyte and internal standard cross signal contributions and their impact on quantitation in LC–MS based bioanalysis. Journal of Chromatography B. 2011;879:1954–1960. doi: 10.1016/j.jchromb.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Rule G.S., Clark Z.D., Yue B., Rockwood A.L. Correction for isotopic interferences between analyte and internal standard in quantitative mass spectrometry by a nonlinear calibration function. Anal Chem. 2013;85:3879–3885. doi: 10.1021/ac303096w. [DOI] [PubMed] [Google Scholar]
- 9.Yuan L., Huang C., Liu-Kreyche P., Voronin K., Fancher R.M., Allentoff A., Zheng N., Iyer R., Zhu L., Pillutla R., Ji Q.C. A convenient strategy to overcome interference in LC-MS/MS analysis: Application in a microdose absolute bioavailability study. J Pharm Biomed Anal. 2019;165:198–206. doi: 10.1016/j.jpba.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Liang H.R., Foltz R.L., Meng M., Bennett P. Ionization enhancement in atmospheric pressure chemical ionization and suppression in electrospray ionization between target drugs and stable-isotope-labeled internal standards in quantitative liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17:2815–2821. doi: 10.1002/rcm.1268. [DOI] [PubMed] [Google Scholar]
- 11.Liu H., Lam L., Yan L., Chi B., Dasgupta P.K. Expanding the linear dynamic range for quantitative liquid chromatography-high resolution mass spectrometry utilizing natural isotopologue signals. Analytica Chimica Acta. 2014;850:65–70. doi: 10.1016/j.aca.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji M., Matsunaga H., Jinno D., Tsukamoto H., Suzuki N., Tomioka Y. A validated quantitative liquid chromatography–tandem quadrupole mass spectrometry method for monitoring isotopologues to evaluate global modified cytosine ratios in genomic DNA. Journal of Chromatography B. 2014;953–954:38–47. doi: 10.1016/j.jchromb.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 13.SISWEB, http://www.sisweb.com/mstools/isotope.htm, accessed on 13th February, 2017.
- 14.IsoPatrn, http://tarc.chemistry.dal.ca/soft_down.htm, accessed on 13th February, 2017.
- 15.Ramaley L., Herrera L.C. Software for the calculation of isotope patterns in tandem mass spectrometry. Rapid communications in mass spectrometry : RCM. 2008;22:2707–2714. doi: 10.1002/rcm.3668. [DOI] [PubMed] [Google Scholar]
- 16.Duxbury K., Owen L., Gillingwater S., Keevil B. Naturally occurring isotopes of an analyte can interfere with doubly deuterated internal standard measurement. Ann Clin Biochem. 2008;45:210–212. doi: 10.1258/acb.2007.007137. [DOI] [PubMed] [Google Scholar]
- 17.Twentyman J., Cradic K., Singh R., Grebe S. Ionic Cross Talk Can Lead to Overestimation of 3-Methoxytyramine during Quantification of Metanephrines by Mass Spectrometry. Clinical chemistry. 2012;58:1156–1158. doi: 10.1373/clinchem.2012.186601. [DOI] [PubMed] [Google Scholar]
- 18.Imani S., Buscher H., Marriott D., Gentili S., Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72:2891–2897. doi: 10.1093/jac/dkx209. [DOI] [PubMed] [Google Scholar]