Abstract
Parasitic ascaridoid nematodes occur in a wide range of marine organisms across the globe. Some species of the anisakid family (Ascaridoidea: Anisakidae) can cause gastrointestinal disease in humans (i. e. anisakidosis). Despite their importance as potentially hazardous parasites, the occurrence and infection characteristics of ascaridoids are still poorly known from many host species and geographical areas. This study investigated the diversity and infection levels of ascaridoid parasites in various commercial fish and squid host species off Bangladesh. Fish and squid specimens were visually inspected for nematodes using the UV-press method. Nematodes were assigned to genus level based on morphology and identified by sequence analyses of the entire ITS region and partial 28S rDNA and mtDNA cox2 genes. Third-stage larvae (L3) of Anisakis typica occurred at low prevalence (P = 10% and 8%, respectively) in the viscera of Selar crumenophthalmus and Trichiurus lepturus, while Hysterothylacium amoyense occurred in the viscera of Sardinella fimbriata (P = 1%) and the viscera and muscle of Harpadon nehereus (P = 32%) and T. lepturus (P = 76%). Lappetascaris sp. Type A L3 occurred in the mantle of the squid Uroteuthis duvaucelii (P = 11%). Anisakis and Lappetascaris species, and H. amoyense were firstly identified in the Bay of Bengal. The potentially zoonotic A. typica was only found in fish viscera. Hysterothylacium amoyense and Lappetascaris sp., both generally regarded as non-zoonotic, occurred at low prevalence in the muscle or mantle of fish or squid, respectively. Since consumption of raw or lightly processed seafood seems to be rare in Bangladesh, the risk of acquiring anisakidosis from consuming fishery products from off Bangladesh appears to be low. Due to its reddish appearance, the visual presence of H. amoyense larvae in fish flesh may represent a food quality issue.
Keywords: Anisakis, Hysterothylacium, Lappetascaris, Fish parasite, Anisakiasis, Bay of Bengal
Highlights
-
•
Anisakis sp. (A. typica) firstly identified in Bay of Bengal fishes.
-
•
Hysterothylacium amoyense firstly identified in Bay of Bengal fishes.
-
•
Lappetascaris sp. type A larva firstly identified in Bay of Bengal squid.
-
•
Potentially zoonotic A. typica only present in low prevalence in fish viscera.
-
•
The risk of fish-borne zoonosis caused by ascaridoids appears low in Bangladesh.
1. Introduction
Anisakiasis is an underestimated emerging fish-borne zoonotic disease of global concern (Bao et al., 2019). This gastrointestinal disease is caused by fish parasitic nematodes of the genus Anisakis (Nematoda: Ascaridoidea: Anisakidae) in the third larval stage (L3) (Audicana and Kennedy, 2008). The term anisakidosis is also used when the disease is caused by any member of the family Anisakidae (e. g. the genera Anisakis, Pseudoterranova and Contracaecum) (Audicana and Kennedy, 2008). The aetiological agent may be accidentally transmitted to humans through consumption of parasitized raw, marinated or undercooked fishery products which contain a viable parasite (Audicana and Kennedy, 2008; Bao et al., 2019).
The disease can be classified into different clinical forms depending on the location of the larva and symptoms. The parasite may try to invade the gastrointestinal tract provoking acute/chronic gastric, intestinal or extra-gastrointestinal anisakiasis with mild to severe symptoms (e.g. abdominal pain, vomiting, intestinal obstruction, granuloma formation, etc.) (Nieuwenhuizen, 2016). Transient form of disease occur where the parasite larvae do not invade the human digestive tract, and after hours to weeks abandons the body, usually alive (Shamsi and Andrew, 2011; Smith, 1999). Asymptomatic or symptomless cases can also occur and are usually not diagnosed (Carrascosa et al., 2015; Takasaki et al., 2020). Gastro-allergic anisakiasis may occur when allergic symptoms (ranging from urticaria, angioedema to life-threatening anaphylaxis) predominate (Daschner et al., 2000). In addition, allergy to Anisakis may occur when the larval allergens that may be present in a fishery product (with no necessity of live parasite) trigger an allergic response in sensitized individuals (Adroher-Auroux and Benítez-Rodríguez, 2020; Audicana and Kennedy, 2008; Bao et al., 2019; EFSA-BIOHAZ, 2010). Occupational allergy has been also described in fishmongers (Añíbarro and Seoane, 1998) and fish-processing workers (Nieuwenhuizen et al., 2006).
It is estimated that the total number of worldwide anisakidosis (almost all anisakiasis) cases up to December 2017 may be over 76,000, mainly from developed countries such as Japan, South Korea, Spain and Italy (Bao et al., 2019). It appears that there is an increasing trend in annual hospitalizations in Spain (Herrador et al., 2019), South Korea (Kim et al., 2019) and Japan (Murata et al., 2021; Watahiki et al., 2020), which was not observed in Italy (Cavallero et al., 2018). A retrospective survey carried out over the period 2010 to 2014 in France showed a decrease in clinical cases (Yera et al., 2018). In addition, recent studies suggest that the estimated annual cases could be between 8000 and 21,000 in Spain (Bao et al., 2017; Herrador et al., 2019) and over 7000 in Japan (Murata et al., 2021).
To the best of our knowledge, the disease has been reported in littoral countries of the Bay of Bengal such as Malaysia (Amir et al., 2016) and Thailand (Hemsrichart, 1993) but not yet in Bangladesh, India, Indonesia, Myanmar or Sri Lanka (Bao et al., 2019; FAO/WHO, 2014; Wiwanitkit and Wiwanitkit, 2016). Recently, Wiwanitkit and Wiwanitkit (2016) reviewed the situation of anisakiasis in Southeast Asia and concluded that the disease is possible and should become a new focused interest in tropical coastal medicine.
The Bay of Bengal is one of the world's 64 Large Marine Ecosystems (BOBLME, 2013). Over 400 million people of its littoral countries harvest the coastal and marine fishery resources to support their livelihood (BOBLME, 2013). The Bay of Bengal has a distinct tropical marine ecosystem with profuse drainage from upstream rivers into the northern part and abundant swamp and mangroves that further supports diverse marine fish assemblages (about 511 marine fish species) including coral reefs, tropical dolphins and sharks (Murshed-e-Jahan et al., 2014). Bangladesh has one of the biggest delta areas, contributing to very rich fresh and brackish water fisheries and aquaculture industries (3,084,399 metric tons/year), in addition to marine fisheries (599,846 metric tons/year) (Shamsuzzaman et al., 2017). More than 17 million people depend on the fishery sector for their livelihood, and fish supplements about 60% of the people's daily animal protein intake (Shamsuzzaman et al., 2017).
The study of fish parasites in Bangladesh has a relatively short history, dating back to a few scattered records contained in the works of Thomas Southwell and colleagues at the first quarter of the 20th Century (Arthur and Ahmed, 2002). Arthur and Ahmed (2002) provided the first parasite-host list, highlighting that some or all ascaridoid records may involve misidentification. Later, Chandra (2006) recorded 290 species of fish parasites in Bangladesh; however, most of these were from freshwater. Anisakids and raphidascaridids such as Hysterothylacium spp. (Nematoda: Ascaridoidea: Raphidascarididae) are very common parasites of marine fishes and squids of all oceans and may occur in freshwater too (Klimpel and Rückert, 2005; Li et al., 2016a; Mattiucci et al., 2018; Shamsi et al., 2013). However, many of the commercially important Bangladeshi marine fish species have not been examined for ascaridoid parasites yet.
The aim of the present study was to investigate various marine fish and squid species caught within the exclusive economic zone of Bangladesh, for the presence and spatial distribution of potentially zoonotic ascaridoid parasites.
2. Material and methods
2.1. Fish and squid sampling
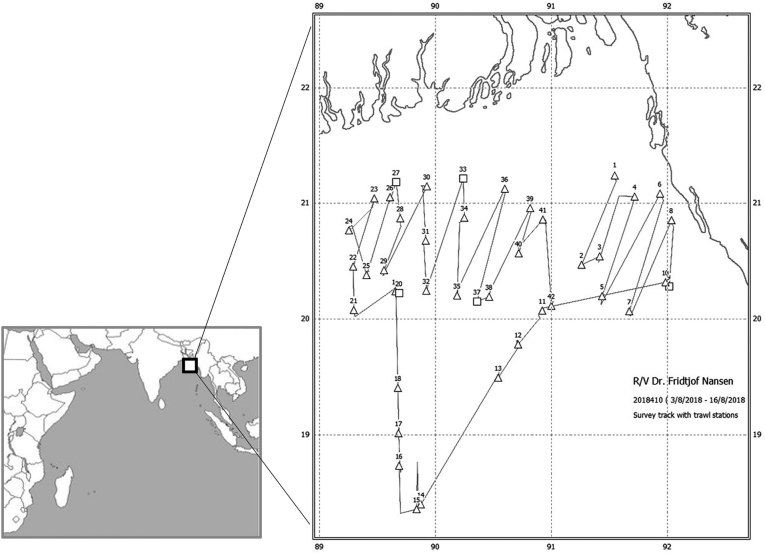
A total of 14 teleost fish (N = 464) and 2 squid (N = 31) species were caught in August 2018 during a research cruise onboard the research vessel R/V “Dr. Fridtjof Nansen” (NORAD-FAO project GCP/INT/730/NOR; survey number 2018410) using pelagic and bottom trawl on the continental shelf off Bangladesh, and extending offshore to deeper waters for mesopelagic trawling transects (Fig. 1; Table 1). Fishes and squids from each catch were identified to species level by local taxonomists. Total length (mantle length for squids), total weight and sex were recorded for each specimen (Table 1).
Fig. 1.
Course track and trawl stations made by the research vessel R/V “Dr. Fridtjof Nansen” in the sampling area.
Table 1.
Fish and squid biometrics and trawl stations from where they were caught.
| Fish and squid^ species (Family) | Common name | N | Station | TL | TW | Sex (f, m, u) |
|---|---|---|---|---|---|---|
| Bregmaceros mcclellandi (Bregmacerotidae) | Unicorn cod | 100 | 28 | 5* | 0.91** | NS |
| Cubiceps pauciradiatus (Nomeidae) | Bigeye cigarfish | 60 | 13 | 11.1 ± 0.7 (10–12.5) | 13.2 ± 2.4 (9–19) | 32/24/4 |
| Dussumieria elopsoides (Dussumieriidae) | Slender rainbow sardine | 30 | 23 | 20.8 ± 0.4 (20.0–21.5) | 79.1 ± 4.2 (71–89) | 29,1,0 |
| Eupleurogrammus muticus (Trichiuridae) | Smallhead hairtail | 3 | 31 | 62.2 ± 1.3 (61.0–63.5) | 100.5 ± 3.5 (98–103) | 2/1/0 |
| Harpadon nehereus (Synodontidae) | Bombay-duck | 50 | 27, 33 | 25.9 ± 2.8 (12.5–31.5) | 113.7 ± 35.5 (62–288) | 3/0/47 |
| Megalaspis cordyla (Carangidae) | Torpedo scad | 15 | 31 | 25.3 ± 2.2 (22.5–29.0) | 140.9 ± 32.6 (103–190) | 7/5/3 |
| Mene maculata (Menidae) | Moonfish | 30 | 23 | 25.1 ± 1.4 (19.0–25.0) | 186.8 ± 23.7 (112−212) | 5/25/0 |
| Sardinella fimbriata (Clupeidae) | Fringescale sardinella | 100 | 6 | 16.4 ± 0.5 (15.5–17.5) | 39.9 ± 3.6 (31.0–51.0) | 59/41/0 |
| Selar crumenophthalmus (Carangidae) | Bigeye scad | 30 | 24 | 24.3 ± 1.0 (22.5–26.5) | 196.5 ± 32.3 (134–273) | 19/11/0 |
| Trichiurus lepturus (Trichiuridae) | Largehead hairtail | 38 | 4, 9, 31 | 57.8 ± 10.4 (42.5–82.0) | 133.1 ± 115.0 (29–497) | 26/9/3 |
| Ommastrephes bartramii^ (Ommastrephidae) | Neon flying squid | 22 | 17 | 8.1 ± 1.1 (6.5–10.5) | 17.0 ± 6.8 (9–32) | NS |
| Uroteuthis duvaucelii^ (Loliginidae) | Indian squid | 9 | 10 | 8.2 ± 0.9 (7.0–9.5) | 24.8 ± 5.8 (17–32) | NS |
| Chirocentrus dorab (Chirocentridae) | Dorab wolf-herring | 2 | 36 | 37.8 ± 1.8 (36.5–39.0) | 154.5 ± 33.2 (131–178) | 0/1/1 |
| Scomberomorus guttatus (Scombridae) | Indo-Pacific king mackerel | 4 | 30, 36 | 39.9 ± 10.9 (32.0–56.0) | 426.8 ± 358.0 (192–960) | 1/0/3 |
| Scomberomorus commerson (Scombridae) | Narrow-barred Spanish mackerel | 1 | 30 | 55 | 890 | 0/0/1 |
| Scomberoides tol (Carangidae) | Needlescaled queenfish | 1 | 36 | 34.5 | 226 | 1/0/0 |
N = number of fishes sampled; Station = trawl station from where fish/squid were caught; TL = total length (mantle length for squids) in cm (mean ± SD = standard deviation (minimum and maximum range values)); TW = total weight in g (mean ± SD = standard deviation (minimum and maximum range values)); f = female, m = male, u = unknown; NS = not sexed. * Due to small body size, only 1 fish per sample was measured for TL. ** average TW per fish (TW of sample = 91 g/100 fish).
2.2. Parasite inspection
Fish and squid specimens were dissected, and the body cavity and internal organs initially inspected for ascaridoid nematodes by the naked eye under good light conditions. Thereafter, the internal organs and fillets were placed in individual transparent plastic bags and inspected using the UV-press method (Karl and Leinemann, 1993; Levsen and Lunestad, 2010). The method uses the fluorescence of dead by freezing anisakid nematodes (Pippy, 1970). The pressed samples were stored frozen at −20 °C for at least 24 h before thawing and examining each bag under 366 nm UV-light in a dark room (Karl and Leinemann, 1993; Levsen and Lunestad, 2010). Ascaridoid parasites may be tentatively assigned to genus level (e.g. Anisakis, Hysterothylacium), depending on differences in shape and size/thickness, shade as well as intensity and brightness of fluorescence (Bao et al., 2021) (see also “morphological identification” subsection below).
The nematodes recorded were stored frozen at −20 °C for further morphological and molecular study.
2.3. Morphological identification
A subsample of 114 out of 270 ascaridoid nematodes, randomly selected depending on their macroscopic appearance (see Results section), from the viscera and flesh or mantle of infected fish or squid hosts, were subjected to morphological identification using light microscopy. The ascaridoids were assigned to genus and their developmental stage determined according to morphological characters such as: presence/absence of lips or boring tooth depending on developmental stage (fourth larval stage (L4)/adult or third larval stage (L3)); presence/absence and appearance of ventricle, intestinal caecum, ventricular appendix and terminal mucron, as well as the position of the excretory pore relative to boring tooth (if present) and nerve ring (Berland, 1989; Li et al., 2016b; Li et al., 2012; Li et al., 2008; Nagasawa and Moravec, 2002).
2.4. Molecular identification
A subsample of 30 ascaridoid nematodes, randomly selected among those initially identified to genus level, were specifically identified by sequence analyses of the mitochondrial cytochrome c oxidase II (mtDNA cox2) gene, entire internal transcribed spacers (ITS rDNA) region and/or 28S rDNA gene (see Table 2 and Table A.1).
Table 2.
Morphological identity and developmental stage of the parasites found per host species, and sequence similarity scores obtained by BLAST (Basic Local Alignment Search Tool) for the present ascaridoid ITS, cox2 and 28S gene sequences, with the conclusion for parasite species identity.
| Host species | Morphology | Gene | BLAST (N, bp) | Acc. nr. | Parasite species |
|---|---|---|---|---|---|
| E. muticus | 1 L3 Anisakis sp. larval type I | NA | |||
| 1 L3 H. amoyense/H. zhoushanense | NA | ||||
| H. nehereus | 16 L3 H. amoyense/H. zhoushanense | ITS (n = 13) | 100% H. amoyense (381 to 804 bp) | KP252131 | H. amoyense |
| cox2 (n = 4) | 97.01–97.97% H. amoyense (356 to 583 bp) | MF120253 | |||
| 28S (n = 3) | 100% H. amoyense (724 to 725 bp) | MF094276 | |||
| S. fimbriata | 1 L3 H. amoyense/H. zhoushanense | ITS (n = 1) | 100% H. amoyense (292 bp) | KP252131 | H. amoyense |
| cox2 (n = 1) | 97.35% H. amoyense (432 bp) | MF120253 | |||
| 28S (n = 1) | 100% H. amoyense (724 bp) | MF094276 | |||
| S. crumenophthalmus | 3 L3 Anisakis sp. larval type I | ITS (n = 1) | 100% A. typica (897 bp) | JQ912690 | A. typica |
| cox2 (n = 2) | 97.74–99.30% A. typica (574 bp) | JQ859931 and JQ859923 | |||
| 28S (n = 2) | 100% A. typica (731 and 751 bp) | KX098562 | |||
| T. lepturus | 4 L3 Anisakis sp. larval type I | ITS (n = 2) | 100% A. typica (720 to 814 bp) | JQ912690 | A. typica |
| cox2 (n = 2) | 98.74–99.48% A. typica (578 bp) | KC928267 | |||
| 87 L3 + 2 L4 H. amoyense/H. zhoushanense | ITS (n = 4) | 99.87–100% H. amoyense (743 to 809 bp) | KP252131 | H. amoyense | |
| cox2 (n = 6) | 97.01–97.60% H. amoyense (462 to 587 bp) | MF120253 | |||
| 28S (n = 5) | 100% H. amoyense (711 to 725 bp) | MF094276 | |||
| U. duvaucelii | 1 L3 Lappetascaris sp. type A | ITS (n = 1) | 100% Hysterothylacium sp. or Lappetascaris sp. (874 bp) | MT365537 or MW750364 | Lappetascaris sp. |
| cox2 (n = 1) | 98.78% Lappetascaris sp. (575 bp) | MW775335 | |||
| 28S (n = 1) | Matches <96.5% |
Morphology: number of ascaridoids identified to species or larval type and developmental stage (i.e. L3 = third larval stage, L4 = fourth larval stage); Gene: ribosomal (ITS or 28S) or mitochondrial (cox2) DNA sequences; BLAST: blast results (percent identity (%) and species identity), bp: number of base pairs; Acc. Nr.: Accession number of the highest match from published deposited samples; Parasite species = conclusion for species identity. NA: not available.
Genomic DNA was extracted using the DNeasy® Blood & Tissue Kit (QIAGEN® GmbH, Hilden, Germany) according to the manufacturer's instructions.
The mtDNA cox2 gene of 16 ascaridoids was amplified using the primers 211F (5′-TTTTCTAGTTATATAGATTGRTTTYAT-3′) and 210R (5′-CACCAACTCTTAAAATTATC-3′) (Nadler and Hudspeth, 2000), following procedures of Mattiucci et al. (2014), modified by Bao et al. (2021).
The entire ITS rDNA region (ITS1, 5.8S rDNA gene and ITS2) of 22 ascaridoids was amplified using the primers NC5 (5′-GTAGGTGAACCTGCGGAAGGATCATT-3′) and NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3′) following Zhu et al. (2000) with modifications, i. e. the annealing temperature was set up at 54 °C instead of 55 °C.
In addition, the partial 28S rDNA gene (28S) of 12 ascaridoids was amplified using the primers 28SF (5′-AGCGGAGGAAAAGAAACTAA-3′) and 28SR (5′-ATCCGTGTTTCAAGACGGG-3′) (Nadler and Hudspeth, 1998), following procedures of Li et al. (2018).
Purification and sequencing of PCR products of correct size were carried out by Eurofins (Cologne, Germany) using the primers 210R, NC5 and 28SF for the amplification of the cox2, ITS and 28S genes, respectively.
The obtained sequences were searched for similarity by BLAST (Basic Local Alignment Search Tool), using Blastn query with the nucleotide collection (nr/nt) database and the megablast program selection, through web servers of the National Center for Biotechnology Information (USA) (Altschul et al., 1990).
2.5. Parasite infection analyses
Parasite infection descriptors such as prevalence, mean abundance and mean intensity were calculated as described in Bush et al. (1997). Any correlations between parasite abundance and fish size (total length and weight) were calculated for the most parasitized host species using Spearman rank test. The significance level was set at 0.05 and all calculations and tests were run in Statistica v 13.4.0.14.
3. Results
3.1. Necropsy and gross parasite identification
A total of 269 parasitic nematodes were detected in the fish examined. At first, n = 261 nematodes were grossly classified as ascaridoid sp. A and 8 as ascaridoid sp. B (see infection level section below for further details). Ascaridoid sp. A had a reddish appearance. These larvae were encapsulated or moving freely around the viscera (Fig. A.1.), as well as the fish flesh (Fig. A.2). The parasites were not fluorescent under UV-light. Ascaridoid sp. B had a whitish appearance. The larvae were found in or around the viscera and showed brightly bluish fluorescence under UV-light. In addition, one single larva, initially classified as ascaridoid sp. C, was found in the mantle of the squid U. duvaucelii, and appeared bluish upon UV light exposure.
3.2. Parasite species identification
A total of 105 ascaridoid sp. A, 8 ascaridoid sp. B and 1 ascaridoid sp. C specimens were morphologically assigned to Hysterothylacium amoyense or Hysterothylacium zhoushanense, Anisakis type I and Lappetascaris sp. type A (Fig. A.3) larvae, respectively (Table 2). Out of 30 specimens molecularly identified, according to the obtained mtDNA cox2, rDNA ITS and/or 28S gene sequences, 25H. amoyense/H. zhoushanense, 4 Anisakis sp. larval type I and 1 Lappetascaris sp. morphotypes were assigned to the species H. amoyense, A. typica and Lappetascaris sp., respectively (Table 2). Sequences of the former were deposited in GenBank with the accession numbers (ITS: ON065558–60, ON098267–85), (cox2: ON109753–67, ON109536) and (28S: ON098258–66, ON117607–09) (further details at Table A.1).
The ITS sequences of the H. amoyense specimens in the present study were identical except for 1 larva from Trichiurus lepturus that had 1 nucleotide different from the others at alignment position 377 (G instead of A). They matched 99.87% to 100% with the H. amoyense sequence deposited in GenBank (KP252131) (Table 2). The 28S sequences were identical among them and to H. amoyense GenBank sequence MF094276 (Table 2). The cox2 sequences matched at 97.01%–97.97% with the H. amoyense sequence deposited in GenBank (MF120253) (Table 2). Further details at the discussion section.
3.3. Parasite infection levels
The majority of ascaridoids, i. e. 261H. amoyense and 8 A. typica, were found in just four fish species, i.e. Harpadon nehereus, Sardinella fimbriata, Selar crumenophthalmus and T. lepturus (Table 3). A single Lappetascaris sp. type A larva occurred in the mantle of the squid Uroteuthis duvaucelii. The most infected fish species was T. lepturus showing 76% overall ascaridoid prevalence and 6.0 (7.8) mean abundance (standard deviation). Hysterothylacium amoyense was found in the viscera and sometimes in the muscle of H. nehereus and T. lepturus, whilst A. typica only occurred in the viscera of S. crumenophthalmus and T. lepturus (Table 3). Anisakis type I (N = 1, likely A. typica) and H. amoyense or H. zhoushanense (N = 1, likely H. amoyense) larvae were found in the viscera of Eupleurogrammus muticus. No ascaridoids were found in Bregmaceros mcclellandi, Chirocentrus dorab, Cubiceps pauciradiatus, Dussumieria elopsoides, Megalaspis cordyla, Mene maculata, Ommastrephes bartramii, Scomberomorus commerson, Scomberomorus guttatus and Scomberoides tol (Table 3).
Table 3.
Infection levels of the ascaridoid parasites found in fish and squid species collected off Bangladesh in the Bay of Bengal.
| Host | Parasite | Muscle |
Viscera |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | mA | mI | P | mA | mI | P | mA | MI | ||
| B. mcclellandi (N = 100) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| C. pauciradiatus (N = 60) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| D. elopsoides (N = 30) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| E. muticus (N = 3) | Ascaridoid spp. (N = 2) | 0% | – | – | 67% | 0.7 (0.6) | 1.0 (0.0) {1–1} | 67% | 0.7 (0.6) | 1.0 (0.0) {1–1} |
| Anisakis sp. (N = 1) | 0% | – | – | 33% | 0.3 (0.6) | 1 | 33% | 0.3 (0.6) | 1 | |
| H. amoyense or H. zhoushanense (N = 1) | 0% | – | – | 33% | 0.3 (0.6) | 1 | 33% | 0.3 (0.6) | 1 | |
| H. nehereus (N = 50) | H. amoyense (N = 31) | 12% | 0.1 (0) | 1.0 (0) {1–1} | 30% | 0.5 (0.9) | 1.7 (1.1) {1–4} | 32% | 0.6 (1.1) | 1.9 (1.1) {1–4} |
| M. cordyla (N = 15) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| M. maculata (N = 30) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| S. fimbriata (N = 100) | H. amoyense (N = 1) | 0% | – | – | 1% | 0.0 (0.1) | 1 | 1% | 0.0 (0.1) | 1 |
| S. crumenophthalmus (N = 30) | A. typica (N = 3) | 0% | – | – | 10% | 0.1 (0.3) | 1.0 (0.0) {1–1} | 10% | 0.1 (0.3) | 1.0 (0.0) {1–1} |
| T. lepturus (N = 38) | A. typica (N = 4) | 0% | – | – | 8% | 0.1 (0.4) | 1.3 (0.6) {1–2} | 8% | 0.1 (0.4) | 1.3 (0.6) {1–2} |
| H. amoyense (N = 228) | 5% | 0.1 (0.2) | 1.0 (0.0) {1–1} | 76% | 5.9 (7.7) | 7.8 (7.9) {1−33} | 76% | 6.0 (7.8) | 7.9 (8.0) {1–34} | |
| Ascaridoid spp. (N = 232) | 5% | 0.1 (0.2) | 1.0 (0.0) {1–1} | 76% | 6.1 (7.8) | 7.9 (8.1) {1–34} | 76% | 6.1 (8.0) | 8.0 (8.2) {1–35} | |
| O. bartramii (N = 22) | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| U. duvaucelii (N = 9) | Lappetascaris sp. (N = 1) | 11% | 0.1 (0.3) | 1 | 0% | – | – | 11% | 0.1 (0.3) | 1 |
| C. dorab (N = 2) * | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| S. guttatus (N = 4) * | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| S. commerson (N = 1) * | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
| S. tol (N = 1) * | Not infected | 0% | – | – | 0% | – | – | 0% | – | – |
Host = host species sampled, number (N) of individuals sampled in parenthesis; Parasite = Ascaridoid species identified, number (N) of parasites found in parenthesis; Muscle = infection data for the muscle; Viscera = infection data for the viscera; Total = infection data in viscera and muscle; P = prevalence of infection as percentage; mA = mean abundance, standard deviation in parenthesis; mI = mean intensity, standard deviation in parenthesis and range (minimum – maximum number of parasites in the sample) with curly brackets. * Just the belly flaps (i. e. anterior-ventral portion of the fillets) were examined in these fishes.
Mean abundance of ascaridoid nematodes in T. lepturus (N = 38) was weakly but significantly positively related to fish host length (Spearman R = 0.48, p = 0.002) and body weight (Spearman R = 0.45, p = 0.005).
4. Discussion
The parasitic nematode fauna of marine fish species from Bangladesh waters is poorly known, and some earlier records were possibly misidentified (Arthur and Ahmed, 2002; Chandra, 2006). To the best of our knowledge, herein we present the first report of molecularly identified ascaridoid nematodes of the genera Anisakis and Lappetascaris and of H. amoyense found in fishes and squids from off Bangladesh (and Bay of Bengal).
Anisakis spp. have heteroxenous life cycles in the marine environment, in which marine mammals (mainly cetaceans) act as definitive hosts, small crustaceans as intermediate hosts, and fish and squids as second intermediate or paratenic hosts (Mattiucci et al., 2018). The anisakid species A. typica was reported from the viscera of S. crumenophthalmus and T. lepturus with low prevalence of infection; 10% and 8%, respectively. As far as we know, this is the first molecular report of an Anisakis species in the Bay of Bengal. E. muticus appears to be new fish paratenic or intermediate host record of Anisakis sp. (possibly A. typica).
Anisakis typica has been reported in fishes (e. g. S. crumenophthalmus and T. lepturus) from Indonesian (Kuhn et al., 2013; Palm et al., 2017; Palm et al., 2008), South and East China Sea (Guo et al., 2020; Kong et al., 2015), Papua New Guinea (Koinari et al., 2013) and northern Taiwan waters (Sonko et al., 2020). It has been also reported in fishes from Japanese and northern Australian waters (Jabbar et al., 2012; Takano et al., 2021). A new taxon closely related to A. typica, and temporarily indicated as Anisakis sp. 1, was reported as L3 from the fish Nemipterus sp. from western Malaysian waters (Mattiucci et al., 2018). It appears that this provisional taxon (also reported as A. typica var. indonesiensis, A. typica sp. T or A. typica) is frequent in fishes (e.g. M. cordyla, S. crumenophthalmus, S. tol and T. lepturus) from Indonesian (Anshary et al., 2014; Kuhn et al., 2013; Mattiucci et al., 2018; Palm et al., 2017; Palm et al., 2008), Thailand (Eamsobhana et al., 2018; Tunya et al., 2020), Vietnamese (Van Hien et al., 2021) and South China Sea (Guo et al., 2020) waters but has not been found in the present study. Interestingly, one L3 from S. crumenophthalmus (acc. Num. ON109756) showed a highest match of 97.7% with an A. typica sequence deposited in GenBank (acc. Num. JQ859931), lower than the other three specimens here identified, matching ≥98.3% with the same specimen, and being therefore identified as A. typica. Once aligned with these specimens, this sequence showed several differences, suggesting that it could likely represent an undescribed A. typica genotype.
Palm et al. (2017) reported A. typica/ A. typica var. indonesiensis and A. typica prevalence of 81,1% and 2,9% for S. crumenophthalmus and T. lepturus from Indonesia, respectively. Anisakis spp. prevalence raised up to 30% in the viscera of T. lepturus from the Gulf of Thailand (Purivirojkul, 2009). In terms of definitive host, it appears that A. typica is a common parasite of various dolphin and kogiid species in warmer and tropical waters (Kuhn et al., 2016; Mattiucci et al., 2018). It has been reported from the pygmy sperm whale (Kogia breviceps) and dwarf sperm whale (Kogia sima) from Philippines' waters (Quiazon, 2016; Quiazon et al., 2013). In addition, A. typica (possibly Anisakis sp. 1) has been reported from the Indo-Pacific bottlenose dolphin (Tursiops aduncus) in the Northern Red Sea (Kleinertz et al., 2014).
Anisakis spp. are very common parasites of marine fishes from all oceanic waters (Mattiucci et al., 2018). The low infection level of Anisakis sp. found in the present study might be explained by an absence/scarcity of suitable cetacean definitive hosts around sampling areas of Bangladeshi waters. Other unknown biological/physical factors (e. g. presence of suitable crustacean intermediate hosts, influence of river exports, etc.) might be affecting parasite recruitment and transmission dynamics in the area as well. Interestingly, a complete disappearance of Anisakis spp. larvae in recent years in fishes from New South Wales (Australia) waters was recently reported despite a common and increasing population of marine mammals in the region (reviewed by Shamsi (2021)). Shamsi (2021) hypothesized that the remarkable finding may be explained by environmental changes or a dramatic decline in the population of crustacean intermediate hosts. Further parasitological studies in the same and other important fish species (e.g. Tenualosa ilisha) caught in different seasons, areas (e. g. outer shelf and deep sea) and commercial sizes are recommended to better understand Anisakis epidemiology of Bangladesh fishes.
Species of Hysterothylacium are very common in marine fishes worldwide (Klimpel and Rückert, 2005; Li et al., 2016a; Shamsi et al., 2013). They are parasites of cold-blooded organisms using mainly fishes as both intermediate/paratenic and/or definitive hosts, and small crustaceans as first intermediate hosts (Balbuena et al., 1998; Bao et al., 2021; Køie, 1993; Levsen et al., 2016). The raphidascaridid H. amoyense was found in the viscera of H. nehereus, S. fimbriata and T. lepturus. The parasite was also found in the muscle of H. nehereus and T. lepturus. To the best of our knowledge, this is the first molecular report of an Hysterothylacium species from off Bangladesh waters. The moray eel Muraenesox cinereus from Chinese waters was reported as definitive host of H. amoyense (originally described as Contracaecum amoyensis Hsü, 1933 from the M. cinereus type host) (Li et al., 2018; Li et al., 2008). The conger eel Conger myriaster was also reported as definitive host (Li et al., 2018). No adult nematodes were found here but two fourth-stage larvae (L4) were present in the viscera of two T. lepturus. Third-stage larvae (L3) of H. amoyense and H. zhoushanense are very similar and cannot be distinguished only based on morphological characters (Li et al., 2016b), therefore, analysis of the rDNA ITS and 28S, as well as mtDNA cox2 sequences, identified the larvae as H. amoyense.
The ITS sequences of the specimens in the present study were identical except for 1 larva from T. lepturus that had 1 nucleotide different from the others at alignment position 377 (G instead of A). They were identical to sequences of larval H. amoyense from fishes (i.e. Lophius litulon, C. myriaster, Halieutaea stellata, Nemipterus japonicus, Platycephalous indicus) of China or Iran from published works (Chen et al., 2018; Guo et al., 2020; Li et al., 2016b; Najjari et al., 2016; Zhang et al., 2018), and deposited in GenBank (accession numbers MH211518 - MH211527; MF539810, MF539811, MF539813; KT749421, KT749422; KP252130 - KP252133; MT020111 - MT020134). Larvae have been also suggested from hagfish Eptatretus burgeri from Taiwan (Luo et al., 2016) as well as T. lepturus and Scomber japonicus from China (Kong et al., 2015) by PCR-RFLP analyses of the ITS region but no sequence has been provided. There is currently no ITS sequence available from an adult H. amoyense in the GenBank, and as explained above, L3 of H. amoyense cannot be morphologically distinguished from H. zhoushanense. Thus, Shamsi et al. (2016) proposed to refer to the larval type of Li et al. (2016b) and those described by authors from fishes (i.e. Otolithes ruber, Psettodes erumei, Saurida tumbil and S. commerson) from Irani waters, and with identical ITS1 & ITS2 sequences (as to those in the present study), as Hysterothylacium larval type XV. However, there is currently a 28S sequence (accession number MF094276) from an adult H. amoyense identified from its definitive host M. cinereus in Chinese waters (Li et al., 2018). The 28S sequences of our larval specimens are identical to the former, therefore confirming H. amoyense identity. In addition, the cox2 sequence of the same parasite isolate is also available in GenBank (accession number MF120253) (Li et al., 2018). Blast analyses of the present cox2 sequences showed 97.01–97.80% similarity to the former sequence. Thus, this is the first molecular report of H. amoyense in H. nehereus, S. fimbriata and T. lepturus fish hosts, and extends the geographical distribution of the parasite to Bangladesh waters. By syllogism, those larval individuals presented above from published studies (Chen et al., 2018; Guo et al., 2020; Li et al., 2016b; Najjari et al., 2016; Zhang et al., 2018) with identical ITS sequence as those presented in here can be considered as H. amoyense.
The raphidascaridid genus Lappetascaris was erected by Rasheed (1965) to include the type species L. lutjani, recovered from the intestine of Pakistani fishes Lutjanus sp. and T. ilisha (Rasheed, 1965). The parasite was later reported in fishes from India (De, 1990) and more recently Brazil (Vicente et al., 2002). Unfortunately, no sequence of L. lustjani, or any other Lappetascaris species (i. e. Lappetascaris suraiyae and L. chandipurensis, see http://www.marinespecies.org/aphia.php?p=taxdetails&id=990484), are available in GenBank for comparison with the Lappetascaris larva sequenced here. Third-stage larvae of Lappetascaris sp. was firstly described from the cyprinid fish Paraulaubuca cf. typus from the Mekong river in Laos (Moravec and Scholz, 1991). Later, Nagasawa and Moravec (1995) described the morphology of L3 of Lappetascaris from the mantle of squid Todarodes pacificus from the Sea of Japan, also reported by Takahara and Sakurai (2010) and Gomes et al. (2020) in same host and area, and concluded that the two larval types belong to different congeneric species. Nagasawa and Moravec (2002) included the former larvae into a Lappetascaris sp. Type A larvae further described from the muscle of the squids Thysanotheuthis rhombus, O. bartramii, Onychoteuthis borealijaponica and Gonatopsis borealis from Central and Western North Pacific Ocean. Lappetascaris sp. Type B was additionally described as morphologically similar but significantly smaller and was found encapsulated in the stomach of O. bartramii (Nagasawa and Moravec, 2002). Recently, Lappetascaris sp. Type A larvae was reported from the muscle and other tissues of the squids Histioteuthis reversa and Histioteuthis bonnellii from Mediterranean Sea (Culurgioni et al., 2010; Palomba et al., 2021). The ITS sequence of Lappetascaris sp. Type A larva obtained here is identical to those of H. reversa and H. bonnellii (accession numbers MW697754 and MW697755; MW750359 to MW750364) and to those from octopus Eledone cirrhosa and Eledone moschata from Mediterranean Sea morphologically identified as Lappetascaris sp. or Hysterothylacium sp., and deposited in GenBank as Hysterothylacium sp. (accession numbers MT365529 to MT365537) (Guardone et al., 2020; Palomba et al., 2021). According to phylogenetic studies of the ITS region and cox2 gene locus, Lappetascaris sp. appears to be closely related to other ascaridoids of the genus Hysterothylacium (i.e. H. brucei, H. tetrapteri, H. kajikiae and H. corrugatum) which have predator billfishes of the family Xiphiidae and Istiophoridae as definitive hosts (Guardone et al., 2020; Palomba et al., 2021). Thus, Lappetascaris sp. Type A is tentatively identified for the first time in U. duvaucelii and extends the geographical distribution of the parasite to Bangladeshi waters. Further morphological and molecular studies on adult Lappetascaris spp. from type fish hosts (e. g. T. ilisha) as well as adult raphidascaridids from billfishes are recommended to clarify its identity and taxonomic status.
Parasitic ascaridoids in commercial fish are important from food safety and quality perspectives (Bao et al., 2021; Bao et al., 2019; Bao et al., 2018). Anisakiasis is an emerging fish-borne zoonosis of worldwide concern caused by species of the genus Anisakis (Bao et al., 2017). Heating (e. g. to >60 °C at the core of the product for at least 1 min) and freezing (to not more than −20 °C at the core of the product for not less than 24 h) of fish fillets are the recommended methods to devitalize the parasite and prevent anisakiasis (EFSA-BIOHAZ, 2010). Out of the 9 fully described Anisakis species, A. simplex (s. s.) and A. pegreffii have been molecularly confirmed as causative agents so far (Kołodziejczyk et al., 2020; Mattiucci et al., 2013). Recently, A. typica was identified as cause of anisakiasis in Japan (The 86th Annual Meeting of the Japanese Society of Parasitology (2017) cited in Suzuki et al. (2021)). The potentially zoonotic A. typica was found only in the viscera of T. lepturus and S. crumenophthalmus (and possibly E. muticus) in low numbers (i. e. prevalence ≤10% for the two former fish species). However, a few specimens of A. typica (s. l.) were reported in the muscle of Auxis rochei rochei and S. crumenophthalmus from Indonesia (Palm et al., 2017; Palm et al., 2008). Anshary et al. (2014) observed an Anisakis (likely A. typica/Anisakis sp. 1) migrating into the fish musculature.
To date, anisakiasis has not been reported in Bangladesh, but it was reported in other littoral countries of the Bay of Bengal such as Malaysia and Thailand (Amir et al., 2016; Hemsrichart, 1993). Uga et al. (1996) reported seroprevalence to Anisakis spp. of 11% (n = 26 out of 244 individuals attending the hospital for routine examinations or presenting diarrhoea) tested by ELISA in Indonesia, even though anisakiasis has neither been reported there yet. As far as we know, there is currently no tradition of consumption of raw, marinated, cold-smocked or generally lightly cooked fish in Bangladesh. In addition, the parasite was present with low prevalence in fishes (i. e. S. crumenophthalmus, T. lepturus and probably E. muticus) but was not found in the muscle. Thus, the risk of anisakiasis appears to be low but cannot be completely discarded (see also further comments below).
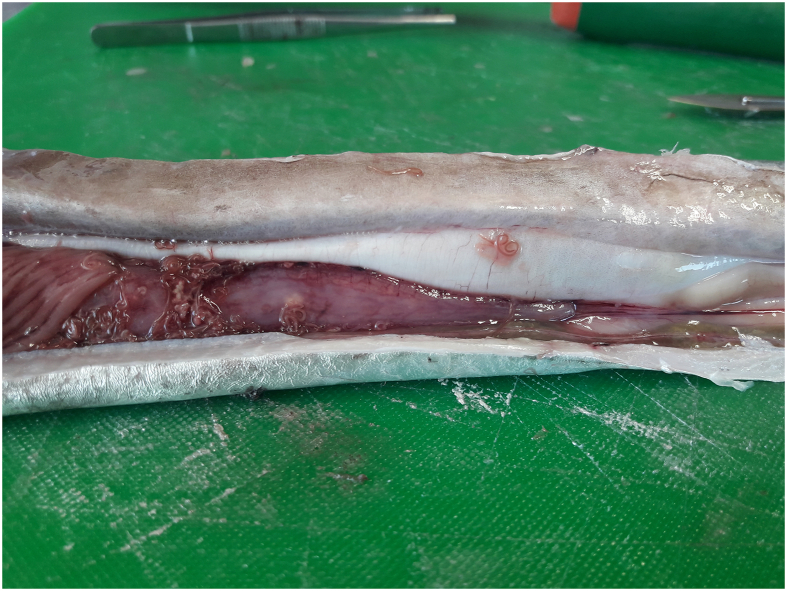
The genus Hysterothylacium is generally considered non-zoonotic, however, this aspect is yet to be thoroughly elucidated and therefore remains somehow controversial (Bao et al., 2021; Shamsi et al., 2018). Hysterothylacium amoyense was observed to probably cause pathological effects (i.e. tumour-like lesions and lymphocytic infiltration in stomach) in mice model (Najjari et al., 2016). The parasite was found in the muscle of N. nehereus and T. lepturus with a prevalence of 12% and 5%, respectively. However, it is generally accepted that the pathogenic potential of anisakids (e. g. Anisakis) surpasses those of raphidascaridids (e. g. Hysterothylacium and Lappetascaris), specially comparing hundreds of well reported clinical anisakidosis cases to few and dubious raphidascarididosis. Thus, the risk of fish-borne zoonosis caused by ascaridoids from consumption of the Bangladeshi fish/squid species studied here appears to be low. However, prevention methods (i. e. heating or freezing) should prevail if fishery products are meant to be consumed as raw or lightly cooked. The reddish colour of H. amoyense larvae, making them easily visible in fillets, may represent a food quality issue (see Fig. A.1, Fig. A.2). Prompt evisceration and removal of visible parasites from flesh are recommended.
5. Conclusions
Nematodes of the genera Anisakis (i. e. A. typica), Lappetascaris (i. e. Lappetascaris sp. Type A) and Hysterothylacium (i. e. H. amoyense) were firstly identified in waters of the Bay of Bengal (i. e. Bangladesh). The raphidascaridid nematode H. amoyense was the most abundant parasite species and is generally regarded as non-zoonotic. The potentially zoonotic A. typica was only present in fish viscera in low abundances, suggesting that its life cycle is hampered by unknown ecological and/or physical factors. Moreover, there is currently no tradition of consumption of raw or lightly processed fishery products in Bangladesh. Thus, the risk of fish-borne zoonosis caused by ascaridoids appears to be low. Prevention methods (heating or freezing) to kill any possible worm in the fish before consumption should prevail.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Bineesh K. K. (Zoological Survey of India) for the taxonomic identification of the present fish species. We thank Natalia Drivenes for the excellent technical support and help with the molecular analyses.
This paper uses data collected through the scientific surveys with the R/V Fridtjof Nansen as part of the collaboration between the EAF Nansen Programme and Bangladesh, where the survey took place. The EAF-Nansen Programme is a partnership between the Food and Agriculture Organization of the United Nations (FAO), the Norwegian Agency for Development Cooperation (NORAD), and the Institute of Marine Research (IMR), Norway, for sustainable management of the fisheries of partner countries. This research was partially funded by the NORAD.
Appendix
Fig. A.1.
Hysterothylacium amoyense third stage larvae in situ in the viscera of Trichiurus lepturus.
Fig. A.2.
Hysterothylacium amoyense third stage larva in situ in the muscle of Harpadon nehereus.
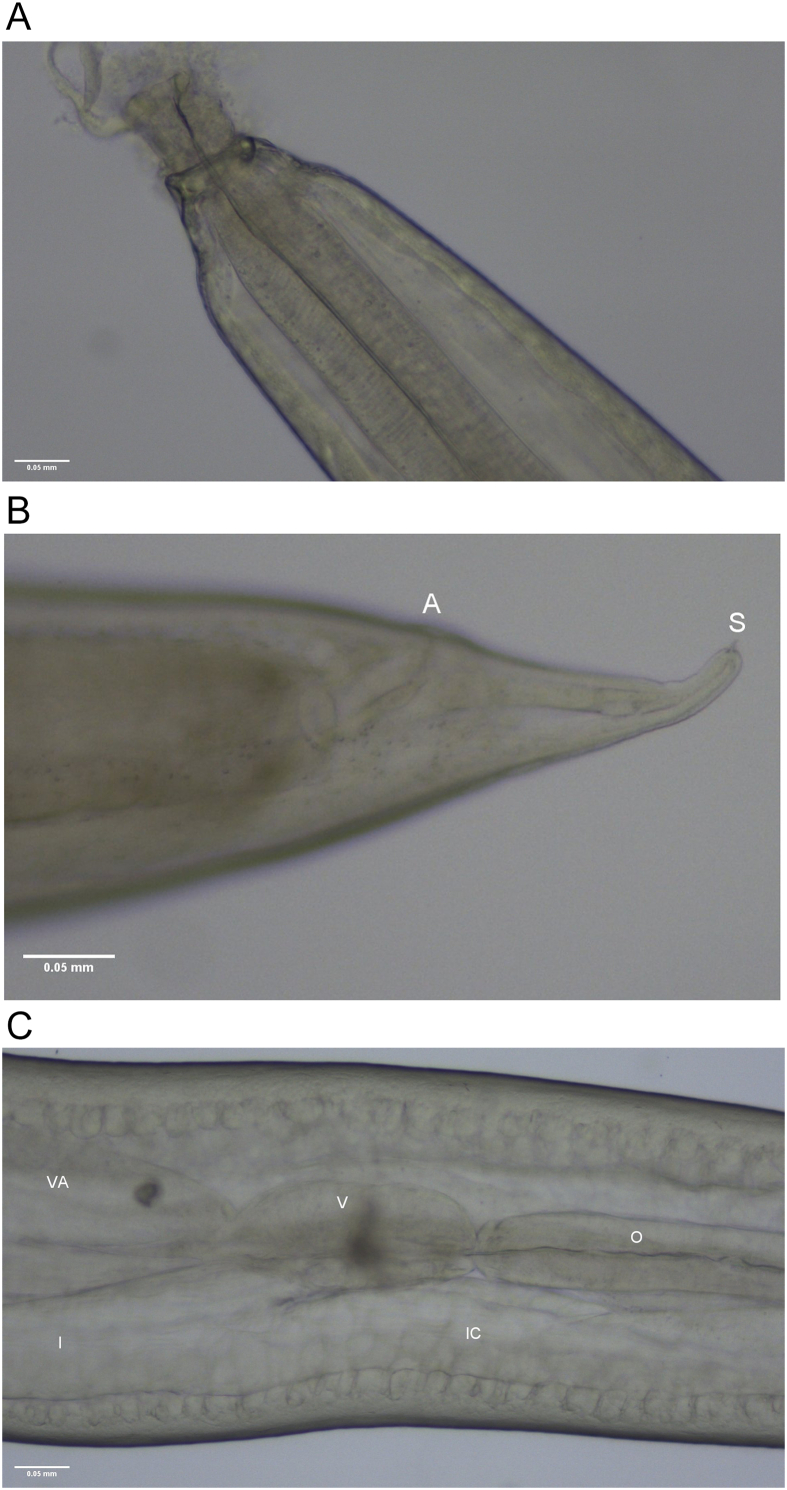
Fig. A.3.
Lappetascaris sp. type A third larval stage from U. duvaucelii. A) cephalic extremity with ventral part protruding more anteriorly than dorsal one. B) ventricle (V) part connected to the end of the muscular oesophagus (O), ventricular appendix (VA) and intestine (I), and intestinal caecum (IC) extending anteriorly alongside the ventricle and oesophagus. C) caudal extremity showing anus (A) and terminal spike (S).
Table A.1.
GenBank accession numbers (i. e. ITS number, cox2 number, 28S number) of ascaridoid parasite isolates from fish and squid host species of Bangladesh.
| Ascaridoid species | Isolate | Host species | ITS number | cox2 number | 28S number |
|---|---|---|---|---|---|
| H. amoyense | HARP12_H | Harpadon nehereus | NA | ON109760 | NA |
| HNMUS23H | H. nehereus | ON098267 | ON109762 | ON098262 | |
| HNVIS1H | H. nehereus | ON098269 | ON109767 | ON098265 | |
| HNMUS43H | H. nehereus | ON098268 | ON109763 | ON098263 | |
| HARPOON15ST27vis1 | H. nehereus | ON098270 | NA | NA | |
| HARPOON34ST33vis1 | H. nehereus | ON098271 | NA | NA | |
| BOMBAY DUCK18ST27–2 | H. nehereus | ON098272 | NA | NA | |
| BOMBAY DUCK18ST27–1 | H. nehereus | ON098273 | NA | NA | |
| HARPOON35ST33mus1 | H. nehereus | ON098274 | NA | NA | |
| HARPODON22_ST27_MUS1 | H. nehereus | ON098275 | NA | NA | |
| HARPODON4_ST29_VIS1 | H. nehereus | ON098276 | NA | NA | |
| HARPODON34_ST33_VIS2 | H. nehereus | ON098277 | NA | NA | |
| HARPODON34_ST33_VIS3 | H. nehereus | ON098278 | NA | NA | |
| HARPODON4_ST29_VIS3 | H. nehereus | ON098279 | NA | NA | |
| SFVIS24H | S. fimbriata | ON098280 | ON109761 | ON098261 | |
| Hystero1 T.Lepturus 28 | Trichiurus lepturus | NA | ON109757 | ON098258 | |
| Hystero2 T.Lepturus 28 | T. lepturus | NA | ON109758 | ON098259 | |
| Hystero1 T.Lepturus 17 | T. lepturus | NA | ON109759 | ON098260 | |
| TLVIS20H | T. lepturus | ON098281 | ON109764 | ON098264 | |
| TLVIS18H | T. lepturus | NA | ON109765 | NA | |
| TLVIS14H | T. lepturus | NA | ON109766 | NA | |
| TL17VISH | T. lepturus | ON098282 | NA | ON098266 | |
| TLEPT34ST9mus1 | T. lepturus | ON098283 | NA | NA | |
| TLEPT29ST9mus1 | T. lepturus | ON098284 | NA | NA | |
| A. typica | SCVIS15A | S. crumenophthalmus | NA | ON109756 | ON117607 |
| SCVIS20N | S. crumenophthalmus | ON065558 | ON109753 | ON117608 | |
| TLEPT29ST9vis30 | T. lepturus | ON065559 | ON109754 | NA | |
| TLEPT31ST9vis1 | T. lepturus | ON065560 | ON109755 | NA | |
| Lappetascaris sp. | UDMAN6A2 | U. duvaucelii | ON098285 | ON109536 | ON117609 |
References
- Adroher-Auroux F.J., Benítez-Rodríguez R. Anisakiasis and Anisakis: an underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020 doi: 10.1016/j.rvsc.2020.08.003. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amir A., Ngui R., Ismail W.H.W., Wong K.T., Ong J.S.K., Lim Y.A.L., Lau Y.-L., Mahmud R. Anisakiasis causing acute dysentery in Malaysia. Am. J. Trop. Med. Hyg. 2016;95:410–412. doi: 10.4269/ajtmh.16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Añíbarro B., Seoane F.J. Occupational conjunctivitis caused by sensitization to Anisakis simplex. J. Allergy Clin. Immunol. 1998;102:331–332. doi: 10.1016/S0091-6749(98)70108-3. [DOI] [PubMed] [Google Scholar]
- Anshary H., Sriwulan Freeman M.A., Ogawa K. Occurrence and molecular identification of Anisakis Dujardin, 1845 from marine fish in southern Makassar Strait, Indonesia. Korean J. Parasitol. 2014;52:9–19. doi: 10.3347/kjp.2014.52.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J.R., Ahmed A.T.A. 2002. Checklist of the Parasites of Fishes of Bangladesh, FAO Fisheries Technical Paper 369/1. Rome. [Google Scholar]
- Audicana M.T., Kennedy M.W. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008;21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbuena J.A., Karlsbakk E., Saksvik M., Kvenseth A.M., Nylund A. New data on the early development of Hysterothylacium aduncum (Nematoda, Anisakidae) J. Parasitol. 1998;84:615. doi: 10.2307/3284732. [DOI] [PubMed] [Google Scholar]
- Bao M., Pierce G.J., Pascual S., González-Muñoz M., Mattiucci S., Mladineo I., Cipriani P., Bušelić I., Strachan N.J.C. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci. Rep. 2017;7:43699. doi: 10.1038/srep43699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M., Pierce G.J., Strachan N.J.C., Martínez C., Fernández R., Theodossiou I. Consumers’ attitudes and willingness to pay for Anisakis-free fish in Spain. Fish. Res. 2018;202:149–160. doi: 10.1016/j.fishres.2017.06.018. [DOI] [Google Scholar]
- Bao M., Pierce G.J., Strachan N.J.C., Pascual S., González-Muñoz M., Levsen A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: challenges in risk assessment. Trends Food Sci. Technol. 2019;86:298–310. doi: 10.1016/J.TIFS.2019.02.013. [DOI] [Google Scholar]
- Bao M., Cipriani P., Giulietti L., Drivenes N., Levsen A. Quality issues related to the presence of the fish parasitic nematode Hysterothylacium aduncum in export shipments of fresh Northeast Arctic cod (Gadus morhua) Food Control. 2021;121 doi: 10.1016/j.foodcont.2020.107724. [DOI] [Google Scholar]
- Berland B. In: Nematode Problems in North Atlantic Fish. Report from a Workshop in Kiel, 3-4 April 1989. Möller H., editor. 1989. Identification of fish larval nematodes from fish; pp. 16–22. [Google Scholar]
- BOBLME . 2013. Marine Protected Areas in Bangladesh - a Framework for Establishment and Management BOBLME-2013-Ecology-04. [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83:575. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Carrascosa M.F., Mones J.C., Salcines-Caviedes J.R., Román J.G. A man with unsuspected marine eosinophilic gastritis. Lancet Infect. Dis. 2015;15:248. doi: 10.1016/S1473-3099(14)70892-8. [DOI] [PubMed] [Google Scholar]
- Cavallero S., Martini A., Migliara G., De Vito C., Iavicoli S., D’Amelio S. Anisakiasis in Italy: analysis of hospital discharge records in the years 2005-2015. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra K. Fish parasitological studies in Bangladesh: a review. J. Agric. Rural Dev. 2006;4:9–18. doi: 10.3329/jard.v4i1.762. [DOI] [Google Scholar]
- Chen H.-X., Zhang L.-P., Gibson D.I., Lü L., Xu Z., Li H.-T., Ju H.-D., Li L. Detection of ascaridoid nematode parasites in the important marine food-fish Conger myriaster (Brevoort) (Anguilliformes: Congridae) from the Zhoushan fishery. China. Parasit. Vectors. 2018;11:274. doi: 10.1186/s13071-018-2850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culurgioni J., Cuccu D., Mereu M., Figus V. Larval anisakid nematodes of Histioteuthis reversa (Verril, 1880) and H. bonnellii (Férussac, 1835) (Cephalopoda: Teuthoidea) from Sardinian Channel (western Mediterranean) Bull. Eur. Assoc. Fish Pathol. 2010;30:220–228. [Google Scholar]
- Daschner Á., Alonso-Gómez A., Cabañas R., Suárez de Parga J.M., López-Serrano M.C. Gastroallergic anisakiasis: borderline between food allergy and parasitic disease-clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J. Allergy Clin. Immunol. 2000;105:176–181. doi: 10.1016/S0091-6749(00)90194-5. [DOI] [PubMed] [Google Scholar]
- De N.C. Some new data on the morphology of the nematode Lappetascaris lutjani Rasheed, 1965 (Nematoda: Anisakidae) Folia Parasitol. 1990;37(4):295–299. [PubMed] [Google Scholar]
- Eamsobhana P., Yong H.S., Song S.L., Tungtrongchitr A., Roongruangchai K. Genetic differentiation of Anisakis species (Nematoda: Anisakidae) in marine fish Priacanthus tayenus from Gulf of Thailand. Trop. Biomed. 2018;35:669–677. [PubMed] [Google Scholar]
- EFSA-BIOHAZ Scientific opinion on risk assessment of parasites in fishery products. EFSA J. 2010;8:1543. doi: 10.2903/j.efsa.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . Food and Agriculture Organization of the United Nations/World Health Organization; 2014. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites, Microbiological Risk Assessment Series. [Google Scholar]
- Gomes T.L., Quiazon K.M.A., Kotake M., Itoh N., Yoshinaga T. Anisakis spp. in fishery products from Japanese waters: updated insights on host prevalence and human infection risk factors. Parasitol. Int. 2020;78 doi: 10.1016/j.parint.2020.102137. [DOI] [PubMed] [Google Scholar]
- Guardone L., Bilska-Zając E., Giusti A., Malandra R., Cencek T., Armani A. Larval ascaridoid nematodes in horned and musky octopus (Eledone cirrhosa and E. moschata) and longfin inshore squid (Doryteuthis pealeii): safety and quality implications for cephalopod products sold as fresh on the Italian market. Int. J. Food Microbiol. 2020;333 doi: 10.1016/j.ijfoodmicro.2020.108812. [DOI] [PubMed] [Google Scholar]
- Guo N., Chen H.X., Zhang L.P., Zhang J.Y., Yang L.Y., Li L. Infection and molecular identification of ascaridoid nematodes from the important marine food fish Japanese threadfin bream Nemipterus japonicus (Bloch) (Perciformes: Nemipteridae) in China. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104562. [DOI] [PubMed] [Google Scholar]
- Hemsrichart V. Intestinal anisakiasis: first reported case in Thailand. J. Med. Assoc. Thail. 1993;76:117–121. [PubMed] [Google Scholar]
- Herrador Z., Daschner Á., Perteguer M.J., Benito A. Epidemiological scenario of anisakidosis in Spain based on associated hospitalizations: the tip of the iceberg. Clin. Infect. Dis. 2019;69:69–76. doi: 10.1093/cid/ciy853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar A., Asnoussi A., Norbury L.J., Eisenbarth A., Shamsi S., Gasser R.B., Lopata A.L., Beveridge I. Larval anisakid nematodes in teleost fishes from Lizard Island, northern great barrier reef, Australia. Mar. Freshw. Res. 2012;63:1283. doi: 10.1071/MF12211. [DOI] [Google Scholar]
- Karl H., Leinemann M. A fast and quantitative detection method for nematodes in fish fillets and fishery products. Arch. Leb. 1993;44:105–128. [Google Scholar]
- Kim J.Y., Yi M., Yong T.-S. Parasitic infections and medical expenses according to health insurance review assessment claims data in South Korea, 2011–2018. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living indo-Pacific bottlenose dolphins (Tursiops aduncus) in the northern Red Sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- Klimpel S., Rückert S. Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol. Res. 2005;97:141–149. doi: 10.1007/s00436-005-1407-6. [DOI] [PubMed] [Google Scholar]
- Køie M. Aspects of the life cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae) Can. J. Zool. 1993;71:1289–1296. doi: 10.1139/z93-178. [DOI] [Google Scholar]
- Koinari M., Karl S., Elliot A., Ryan U., Lymbery A.J. Identification of Anisakis species (Nematoda: Anisakidae) in marine fish hosts from Papua New Guinea. Vet. Parasitol. 2013;193:126–133. doi: 10.1016/j.vetpar.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Kołodziejczyk L., Szostakowska B., Sobecka E., Szczucki K., Stankiewicz K. First case of human anisakiasis in Poland. Parasitol. Int. 2020;76 doi: 10.1016/j.parint.2020.102073. [DOI] [PubMed] [Google Scholar]
- Kong Q., Fan L., Zhang J., Akao N., Dong K., Lou D., Ding J., Tong Q., Zheng B., Chen R., Ohta N., Lu S. Molecular identification of Anisakis and Hysterothylacium larvae in marine fishes from the East China Sea and the Pacific coast of Central Japan. Int. J. Food Microbiol. 2015;199:1–7. doi: 10.1016/j.ijfoodmicro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Kuhn T., Hailer F., Palm H.W., Klimpel S. Global assessment of molecularly identified Anisakis Dujardin, 1845 (Nematoda: Anisakidae) in their teleost intermediate hosts. Folia Parasitol. 2013;60:123–134. doi: 10.14411/fp.2013.013. [DOI] [PubMed] [Google Scholar]
- Kuhn T., Cunze S., Kochmann J., Klimpel S. Environmental variables and definitive host distribution: a habitat suitability modelling for endohelminth parasites in the marine realm. Sci. Rep. 2016;6:30246. doi: 10.1038/srep30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levsen A., Lunestad B.T. Anisakis simplex third stage larvae in Norwegian spring spawning herring (Clupea harengus L.), with emphasis on larval distribution in the flesh. Vet. Parasitol. 2010;171:247–253. doi: 10.1016/j.vetpar.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Levsen A., Paoletti M., Cipriani P., Nascetti G., Mattiucci S. Species composition and infection dynamics of ascaridoid nematodes in Barents Sea capelin (Mallotus villosus) reflecting trophic position of fish host. Parasitol. Res. 2016;115:4281–4291. doi: 10.1007/s00436-016-5209-9. [DOI] [PubMed] [Google Scholar]
- Li L., Xu Z., Zhang L. Redescription of three species of Hysterothylacium (Nematoda: Anisakidae) from marine fishes from the Yellow Sea, China, with the synonymy of Hysterothylacium muraenesoxin (Luo, 1999) Zootaxa. 2008;1878:55. doi: 10.11646/zootaxa.1878.1.2. [DOI] [Google Scholar]
- Li L., Liu Y.-Y., Zhang L.-P. Morphological and genetic characterization of Hysterothylacium zhoushanensis sp. nov. (Ascaridida: Anisakidae) from the flatfish Pseudorhombus oligodon (Bleeker) (Pleuronectiformes: Paralichthyidae) in the East China Sea. Parasitol. Res. 2012;111:2393–2401. doi: 10.1007/s00436-012-3095-3. [DOI] [PubMed] [Google Scholar]
- Li L., Gibson D., Zhang L.-P. An annotated catalogue of the ascaridoid nematode parasites of Chinese vertebrates. Syst. Parasitol. 2016;93:1–35. doi: 10.1007/s11230-015-9617-5. [DOI] [PubMed] [Google Scholar]
- Li L., Zhao W.-T., Guo Y.-N., Zhang L.-P. Nematode parasites infecting the starry batfish Halieutaea stellata (Vahl) (Lophiiformes: Ogcocephalidae) from the east and South China Sea. J. Fish Dis. 2016;39:515–529. doi: 10.1111/jfd.12374. [DOI] [PubMed] [Google Scholar]
- Li L., Lü L., Nadler S.A., Gibson D.I., Zhang L.-P., Chen H.-X., Zhao W.-T., Guo Y.-N. Molecular phylogeny and dating reveal a terrestrial origin in the early carboniferous for ascaridoid nematodes. Syst. Biol. 2018;67:888–900. doi: 10.1093/sysbio/syy018. [DOI] [PubMed] [Google Scholar]
- Luo H.-Y., Chen H.-Y., Chen H.-G., Shih H.-H. Scavenging hagfish as a transport host of Anisakid nematodes. Vet. Parasitol. 2016;218:15–21. doi: 10.1016/j.vetpar.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Fazii P., De Rosa A., Paoletti M., Megna A., Glielmo A., De Angelis M., Costa A., Meucci C., Calvaruso V., Sorrentini I., Palma G., Bruschi F., Nascetti G. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg. Infect. Dis. 2013;19:496–499. doi: 10.3201/eid1903.121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiucci S., Cipriani P., Webb S.C., Paoletti M., Marcer F., Bellisario B., Gibson D.I., Nascetti G. Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex, with a species designation as Anisakis berlandi n. sp. for A. simplex sp. C (Nematoda: Anisakidae) J. Parasitol. 2014;100:199–214. doi: 10.1645/12-120.1. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Cipriani P., Levsen A., Paoletti M., Nascetti G. Molecular epidemiology of Anisakis and anisakiasis: an ecological and evolutionary road map. Adv. Parasitol. 2018:93–263. doi: 10.1016/bs.apar.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Moravec F., Scholz T. Observations on some nematodes parasitic in fresh-water fishes in Laos. Folia Parasitol. 1991;38:163–178. [PubMed] [Google Scholar]
- Murata R., Suzuki J., Kodo Y., Kobayashi K., Sadamasu K., Takano T., Iwaki T., Waki T., Ogawa K. Probable association between Anisakis infection in the muscle of skipjack tuna (Katsuwonus pelamis) and human anisakiasis in Tokyo, Japan. Int. J. Food Microbiol. 2021;337 doi: 10.1016/j.ijfoodmicro.2020.108930. [DOI] [PubMed] [Google Scholar]
- Murshed-e-Jahan K., Belton B., Viswanathan K.K. Communication strategies for managing coastal fisheries conflicts in Bangladesh. Ocean Coast. Manag. 2014;92:65–73. doi: 10.1016/j.ocecoaman.2014.01.003. [DOI] [Google Scholar]
- Nadler S.A., Hudspeth D.S.S. Ribosomal DNA and phylogeny of the Ascaridoidea (Nemata: Secernentea): implications for morphological evolution and classification. Mol. Phylogenet. Evol. 1998;10:221–236. doi: 10.1006/mpev.1998.0514. [DOI] [PubMed] [Google Scholar]
- Nadler S.A., Hudspeth D.S.S. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology hypotheses of structural and sequence evolution. J. Parasitol. 2000;86:380–393. doi: 10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Moravec F. Larval anisakid nematodes of Japanese common squid (Todarodes pacificus) from the sea of Japan. J. Parasitol. 1995;81:69. doi: 10.2307/3284008. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Moravec F. Larval anisakid nematodes from four species of squid (Cephalopoda: Teuthoidea) from the central and western North Pacific Ocean. J. Nat. Hist. 2002;36:883–891. doi: 10.1080/00222930110051752. [DOI] [Google Scholar]
- Najjari M., Sadjjadi S.M., Derakhshanfar A., Ebrahimipour M. Hysterothylacium amoyense in Platycephalous indicus: a Persian Gulf fish and its experimental infection of mouse model. Comp. Clin. Pathol. 2016;25:1143–1149. doi: 10.1007/s00580-016-2318-x. [DOI] [Google Scholar]
- Nieuwenhuizen N. Anisakis - immunology of a food-borne parasitosis. Parasite Immunol. 2016;38:548–557. doi: 10.1111/pim.12349. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen N., Lopata A.L., Jeebhay M.F., Herbert D.R., Robins T.G., Brombacher F. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J. Allergy Clin. Immunol. 2006;117:1098–1105. doi: 10.1016/j.jaci.2005.12.1357. [DOI] [PubMed] [Google Scholar]
- Palm H.W., Damriyasa I.M., Linda Oka, I.B.M Molecular genotyping of Anisakis Dujardin, 1845 (Nematoda: Ascaridoidea: Anisakidae) larvae from marine fish of Balinese and Javanese waters, Indonesia. Helminthologia. 2008;45:3–12. doi: 10.2478/s11687-008-0001-8. [DOI] [Google Scholar]
- Palm H., Theisen S., Damriyasa I., Kusmintarsih E., Oka I., Setyowati E., Suratma N., Wibowo S., Kleinertz S. Anisakis (Nematoda: Ascaridoidea) from Indonesia. Dis. Aquat. Org. 2017;123:141–157. doi: 10.3354/dao03091. [DOI] [PubMed] [Google Scholar]
- Palomba M., Mattiucci S., Crocetta F., Osca D., Santoro M. Insights into the role of deep-sea squids of the genus Histioteuthis (Histioteuthidae) in the life cycle of ascaridoid parasites in the Central Mediterranean Sea waters. Sci. Rep. 2021;11:7135. doi: 10.1038/s41598-021-86248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippy J.H.C. Use of ultraviolet light to find parasitic nematodes in situ. J. Fish. Res. Board Can. 1970;27:963–965. doi: 10.1139/f70-107. [DOI] [Google Scholar]
- Purivirojkul W. An investigation of larval ascaridoid nematodes in some marine fish from the Gulf of Thailand. Agric. Nat. Resour. 2009;43:85–92. [Google Scholar]
- Quiazon K.M.A. Anisakis Dujardin, 1845 infection (Nematoda: Anisakidae) in pygmy sperm whale Kogia breviceps Blainville, 1838 from West Pacific region off the coast of Philippine archipelago. Parasitol. Res. 2016;115:3663–3668. doi: 10.1007/s00436-016-5169-0. [DOI] [PubMed] [Google Scholar]
- Quiazon K.M.A., Santos M.D., Yoshinaga T. Anisakis species (Nematoda: Anisakidae) of dwarf sperm whale Kogia sima (Owen, 1866) stranded off the Pacific coast of southern Philippine archipelago. Vet. Parasitol. 2013;197:221–230. doi: 10.1016/J.VETPAR.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Rasheed S. On a remarkable new nematode, Lappetascaris gen. et sp. nov. (Anisakidae: Ascaridoidea) from marine fishes of Karachi and an account of Thynnascaris inquies (Linton, 1901) n.comb. and Goezia intermedia n.sp. J. Helminthol. 1965;XXXIX:313–342. [Google Scholar]
- Shamsi S. The occurrence of Anisakis spp. in Australian waters: past, present, and future trends. Parasitol. Res. 2021;120:3007–3033. doi: 10.1007/s00436-021-07243-3. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Andrew B.R. First report of human anisakidosis in Australia. Med. J. Aust. 2011;194:199–200. doi: 10.1016/j.mcp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Gasser R., Beveridge I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013;62:320–328. doi: 10.1016/J.PARINT.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Ghadam M., Suthar J., Ebrahimzadeh Mousavi H., Soltani M., Mirzargar S. Occurrence of ascaridoid nematodes in selected edible fish from the Persian Gulf and description of Hysterothylacium larval type XV and Hysterothylacium persicum n. sp. (Nematoda: Raphidascarididae) Int. J. Food Microbiol. 2016;236:65–73. doi: 10.1016/j.ijfoodmicro.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Steller E., Chen Y. New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. Int. J. Food Microbiol. 2018;272:73–82. doi: 10.1016/J.IJFOODMICRO.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman M.M., Islam M.M., Tania N.J., Al-Mamun M.A., Barman P.P., Xu X. Fisheries resources of Bangladesh: present status and future direction. Aquac. Fish. 2017;2:145–156. doi: 10.1016/j.aaf.2017.03.006. [DOI] [Google Scholar]
- Smith J.W. Ascaridoid nematodes and pathology of the alimentary tract and its associated organs in vertebrates, including man: a literature review. Helminthol. Abstr. 1999:49–96. [Google Scholar]
- Sonko P., Chih-Cheng Chen S., Chou C.M., Huang Y.C., Hsu S.L., Barčák D., Oros M., Fan C.K. Multidisciplinary approach in study of the zoonotic Anisakis larval infection in the blue mackerel (Scomber australasicus) and the largehead hairtail (Trichiurus lepturus) in northern Taiwan. J. Microbiol. Immunol. Infect. 2020;53:1021–1029. doi: 10.1016/j.jmii.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Murata R., Kodo Y. Current status of Anisakiasis and Anisakis larvae in Tokyo, Japan. Food Saf. 2021;9:89–100. doi: 10.14252/foodsafetyfscj.d-21-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara H., Sakurai Y. Infection of the Japanese common squid, Todarodes pacificus (Cephalopoda: Ommastrephidae) by larval anisakid nematodes. Fish. Res. 2010;106:156–159. doi: 10.1016/j.fishres.2010.05.009. [DOI] [Google Scholar]
- Takano T., Iwaki T., Waki T., Murata R., Suzuki J., Kodo Y., Kobayashi K., Ogawa K. Species composition and infection levels of Anisakis (Nematoda: Anisakidae) in the skipjack tuna Katsuwonus pelamis (Linnaeus) in the Northwest Pacific. Parasitol. Res. 2021;120:1605–1615. doi: 10.1007/s00436-021-07144-5. [DOI] [PubMed] [Google Scholar]
- Takasaki T., Yamada T., Kinoshita J., Motomura Y. Asymptomatic colonic anisakiasis: is it so rare? Case Rep. Gastroenterol. 2020;14:593–597. doi: 10.1159/000508822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunya R., Wongsawad C., Wongsawad P., Chai J.Y. Morphological and molecular characteristics of Anisakis typica larvae in two species of threadfin bream, Nemipterus hexodon and N. Japonicus, from the Gulf of Thailand. Korean J. Parasitol. 2020;58:15–25. doi: 10.3347/kjp.2020.58.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga S., Ono K., Kataoka N., Hasan H. Seroepidemiology of five major zoonotic parasite infections in inhabitants of Sidoarjo, East Java, Indonesia. Southeast Asian. J. Trop. Med. Public Health. 1996;27:556–561. [PubMed] [Google Scholar]
- Van Hien H., Dung B.T., Ngo H.D., Doanh P.N. First morphological and molecular identification of third-stage larvae of Anisakis typica (Nematoda: Anisakidae) from marine fishes in Vietnamese water. J. Nematol. 2021;53 doi: 10.21307/JOFNEM-2021-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J.J., Mincarone M.M., Pinto R.M. First report of Lappetascaris lutjani Rasheed, 1965 (Nematoda, Ascaridoidea, Anisakidae) parasitizing Trachipterus arawatae (Pisces, Lampridiformes) on the Atlantic Coast of Brazil. Mem. Inst. Oswaldo Cruz. 2002;97:93–94. doi: 10.1590/S0074-02762002000100015. [DOI] [PubMed] [Google Scholar]
- Watahiki M., Uchida K., Kanatani J., Kato T., Kimata K., Isobe J., Oishi K., Tozaki K., Sekiguchi K., Horita Y., Morishima Y., Sugiyama H. Molecular identification of parasites isolated from the stomach of patients with Anisakis food poisoning in Toyama prefecture, Japan, in 2018. Jpn. J. Infect. Dis. 2020;74:85–86. doi: 10.7883/yoken.JJID.2020.289. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit S., Wiwanitkit V. Anisakiasis in Southeast Asia: a story of new tropical disease? Asian Pac. J. Trop. Med. 2016;6:382–383. doi: 10.1016/j.apjtb.2015.11.011. [DOI] [Google Scholar]
- Yera H., Fréalle É., Dutoit E., Dupouy-Camet J. A national retrospective survey of anisakidosis in France (2010-2014): decreasing incidence, female predominance, and emerging allergic potential. Parasite. 2018;25:1–6. doi: 10.1051/parasite/2018016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Xu Z., Chen H.X., Guo N., Li L. Anisakid and raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae) Int. J. Food Microbiol. 2018;284:105–111. doi: 10.1016/j.ijfoodmicro.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Zhu X., D’Amelio S., Paggi L., Gasser R.B. Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae) Parasitol. Res. 2000;86:677–683. doi: 10.1007/PL00008551. [DOI] [PubMed] [Google Scholar]