Abstract
This study aimed to evaluate the imaging and pathological findings in axillary lymph nodes in patients with breast cancer who received concurrent ipsilateral coronavirus disease 2019 (COVID-19) vaccination. Of the 19 women with breast cancer who received concurrent COVID-19 vaccination shot in the arm ipsilateral to breast cancer, axillary lymphadenopathy was observed in 84.2% (16 of 19) of patients on ultrasound (US) and 71.4% (10 of 14) of patients on magnetic resonance imaging (MRI), and 21.0% (4 of 19) of patients were diagnosed with metastasis. Abnormal US and MRI findings of cortical thickening, effacement of the fatty hilum, round shape, and asymmetry in the number or size relative to the contralateral side were noted in more than half of the non-metastatic and metastatic lymph nodes; however, statistical significance was not noted. Axillary lymphadenopathy is commonly observed in patients with breast cancer who receive concurrent ipsilateral COVID-19 vaccination without specific differential imaging features. Thus, understanding the limitations of axillary imaging and cautious interpretation is necessary to avoid overestimation or underestimation of the axillary disease burden.
Keywords: Breast Neoplasms, COVID-19, Lymphadenopathy, Vaccination
The coronavirus disease 2019 (COVID-19) pandemic has significantly impacted morbidity and mortality worldwide. The first COVID-19 vaccine dose was administered on December 14, 2020, under Emergency Use Authorization from the U.S. Food and Drug Administration. Since then, Moderna, Pfizer/BioNTech, Oxford-AstraZeneca, and Janssen Pharmaceuticals vaccines have been used for preventing COVID-19 infection in Korea. Local and systemic reactions have been observed in relation to the administration of COVID-19 vaccines, with the most commonly reported adverse event being unilateral “axillary swelling or tenderness” in women receiving Moderna (occurring in 11.6% and 16.0% of recipients following the first and second doses, respectively) and Pfizer-BioNTech vaccines [1,2]. A recent paper [3] reported ipsilateral axillary nodal reactivity on fluorine-18 2-deoxy-2-fluoro-D-glucose positron emission tomography/computed tomography, occurring in as high as 57.0% (4 of 7) of patients after the second dose of the Moderna vaccine and 15.0% (3 of 20) of patients after the second dose of the Pfizer-BioNTech vaccine; these proportions are higher than those reported in clinical trials. Unilateral lymphadenopathy develops in the ipsilateral axilla and supraclavicular regions because the COVID-19 vaccine is usually injected into the deltoid muscle [4,5,6]. The Moderna vaccine induces clinically detected lymphadenopathy within 2–4 days after vaccination and lasts for 1–2 days on an average [2]. The Pfizer/BioNTech vaccine also induces clinically detected lymphadenopathy within 2–4 days after vaccination and lasts for an average of 10 days [1].
COVID-19 vaccine-induced unilateral axillary lymphadenopathy is particularly relevant in women with breast cancer, with a predilection for metastasis to the axillary lymph nodes. Clinicians, radiologists, and patients may be concerned about COVID-19 vaccine-induced unilateral axillary lymphadenopathy because it could be a sign of metastasis from breast cancer, leading to a diagnostic conundrum regarding whether to recommend a biopsy or short-term follow-up. A radiology scientific panel recommended an “expectant management strategy without default follow-up imaging” for patients in whom lymphadenopathy is more likely due to the COVID-19 vaccination rather than malignancy. However, in higher-risk situations, either short-term imaging follow-up or tissue biopsy is to be considered [7]. The Journal of the American College of Radiology summarized practical management plans regarding COVID-19 vaccine-induced unilateral axillary lymphadenopathy and recommended that for patients with a recent cancer diagnosis or those in the pretreatment or peritreatment setting, prompt imaging regardless of the vaccination status and vaccination in the contralateral arm or the site most distant from the cancer should be encouraged [8].
In Korea, the Oxford-AstraZeneca vaccination was initiated in people over 65 years in March 2021; this was soon followed by Moderna, Pfizer/BioNTech, and Janssen Pharmaceuticals vaccines for people aged ≥ 18 years. With widespread vaccination throughout the country, the number of patients with newly diagnosed breast cancer who received concurrent ipsilateral COVID-19 vaccination and showed lymph node enlargement during diagnostic breast ultrasound (US) or magnetic resonance imaging (MRI) examinations increased. To our knowledge, no prior studies have investigated the differentiation between metastatic and non-metastatic lymph nodes in patients with breast cancer who received concurrent ipsilateral COVID-19 vaccination using axillary imaging. Thus, our study aimed to evaluate the imaging and pathological findings of axillary lymph nodes in patients with newly diagnosed breast cancer who received concurrent ipsilateral COVID-19 vaccination using histological examination as a reference standard.
Institutional review board of Seoul National University Hospital (IRB) approved this study (IRB. No. H-2111-058-1271) and waived the requirement for informed consent. From April 2021 to September 2021, we retrospectively searched our institution’s electronic medical records for patients who were newly diagnosed with breast cancer, received concurrent COVID-19 vaccination in the ipsilateral arm, and underwent biopsy or surgery for axillary lymph nodes. The exclusion criterion was the unavailability of axillary lymph node pathology data.
At our institution’s breast cancer center, patients diagnosed with breast cancer are examined using mammography, US, and MRI for preoperative staging. For breast US, we used a 14–16 MHz linear transducer with 3 machines: Arietta 850 (Fujifilm Healthcare Corporation, Tokyo, Japan), Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France), and Aplio i800 system (Canon Medical Systems, Tustin, CA, USA). Breast radiologists or breast fellows (with 1–22 years of experience in breast imaging) performed axillary US after scanning the entire breast, with knowledge of the patient’s clinical information. The patient was placed in the supine oblique position with the arm raised above the head. The entire axilla, including the axillary tail of the breast, was scanned in an orthogonal direction. Breast MRI was performed using a 3.0-T MRI unit with a dedicated 8-channel breast coil. Axillary lymph nodes were evaluated using a standard MRI sequence of T1-weighted, contrast-enhanced (after a dynamic breast sequence), and fat-saturated Dixon imaging. The field-of-view was optimized to cover the bilateral axillae (levels I–III), supraclavicular fossa, and inferior portion of the neck (levels IV–VII). Information regarding COVID-19 vaccination was documented during the US examination, which made our analysis feasible.
Two breast radiologists (Chang JM and Ha SM, with 14 and 6 years of breast imaging experience, respectively) reviewed all imaging examinations, including US, and, if available, MRI, and were blinded to the medical records and final axillary lymph node status. Axillary lymph nodes were considered abnormal on US based on cortical abnormalities, including focal or diffuse cortical thickening ≥ 3 mm, round shape, or complete or near-complete effacement of the fatty hilum [9]. On MRI, axillary lymph nodes with a round shape, absence of fatty hilum, irregular margin, eccentric cortical thickening, or asymmetry in the number or size relative to the contralateral side on axial or sagittal scans were considered suspicious [10,11,12,13].
We identified 21 women with newly diagnosed breast cancer who received concurrent ipsilateral COVID-19 vaccinations. Of the 21 women, we excluded one patient who received a second dose of Oxford-AstraZeneca during the mid-cycle of neoadjuvant chemotherapy and one patient who did not have pathology results from either biopsy or surgery. In total, 19 women (median age ± standard deviation [SD], 56 ± 11.4; range, 39–78 years) constituted the study population (Supplementary Table 1). The median interval between the most recent vaccination and imaging assessment was 26 days (range, 4–49 days). Most women underwent imaging examinations within 4 weeks (63.2%, 12 of 19) after the COVID-19 vaccination. There were 4 types of COVID-19 vaccines: Pfizer/BioNTech (n = 11, 57.9%), Oxford-AstraZeneca (n = 5, 26.3%), Moderna (n = 2, 10.5%), and Janssen Pharmaceuticals (n = 1, 5.2%). Among the 19 women, 17 underwent upfront surgery (89.5%); of those patients who underwent upfront surgery, 9 (52.9%) underwent breast-conserving surgery, and 8 (47.1%) underwent total mastectomy. There were stage 0 (n = 1), stage I (n = 8), and stage II (n = 8) breast cancer cases. The histological types of breast cancer in these 17 patients were invasive ductal carcinoma in 13 (76.5%), invasive lobular carcinoma in 2 (11.8%), ductal carcinoma in situ in one (5.9%), and malignant phyllodes tumor in one (5.9%).
US-guided biopsy was recommended and performed in 9 of the 19 (47.3%) women before surgery. Two women diagnosed with invasive ductal carcinoma received neoadjuvant chemotherapy and remaining 17 women underwent upfront surgery with axillary staging. Sentinel lymph node biopsy was performed in 15 (88.2%) women, and axillary lymph node dissection was performed in 2 (11.8%) women. One patient diagnosed with ductal carcinoma in situ underwent total mastectomy with sentinel lymph node biopsy because of the possibility of upstaging to invasive cancer. Another patient with a final diagnosis of malignant phyllodes tumor underwent total mastectomy with axillary lymph node dissection because of a preoperative core-needle biopsy result of breast sarcoma.
Of the 19 women, 16 (84.2%) showed axillary lymphadenopathy on US, and 4 (21.0%) were diagnosed with axillary lymph node metastasis on biopsy (n = 1, Pfizer/BioNTech) and sentinel lymph node biopsy (n = 3; one Oxford-AstraZeneca, 2 Pfizer/BioNTech). Two women with clinically suspicious lymphadenopathy underwent axillary lymph node dissection; however, the final pathological examination revealed no metastasis. Regarding the US findings, in the 15 women without axillary lymph node metastasis, focal or diffuse cortical thickening was the most frequent finding (53.3%), and complete or near-complete effacement of the fatty hilum was less frequent (26.7%); 2 of the 4 (50.0%) women who had lymph node metastasis showed complete or near-complete effacement of the fatty hilum (Table 1).
Table 1. Imaging findings of the axillary lymph nodes in women with breast cancer who received ipsilateral coronavirus disease 2019 vaccination.
| Imaging findings | No. (%) | No metastasis (n = 15) | Metastasis (n = 4) | p-value | |
|---|---|---|---|---|---|
| US finding | 0.605 | ||||
| Normal | 3 (15.8) | 3 (20.0) | 0 | ||
| Focal or diffuse cortical thickening only | 10 (52.6) | 8 (53.3) | 2 (50.0) | ||
| Complete or near complete effacement of the fatty hilum | 6 (31.6) | 4 (26.7) | 2 (50.0) | ||
| Cortical thickness on US (mm) | 0.567 | ||||
| Mean ± standard deviation | 4.2 ± 1.6 | 4.3 ± 1.6 | 3.5 ± 1.4 | ||
| Abnormal lymph node noted | 1.000 | ||||
| No | 3 (15.8) | 3 (20.0) | 0 | ||
| Level I only | 12 (63.2) | 9 (60.0) | 3 (75.0) | ||
| Level I–II | 3 (15.8) | 2 (13.3) | 1 (25.0) | ||
| Level I–III | 1 (5.3) | 1 (6.7) | 0 | ||
| MRI finding (n = 14)* | 1.000 | ||||
| Normal | 4 (28.6) | 3 (27.3) | 1 (33.3) | ||
| Focal or diffuse cortical thickening only | 4 (28.6) | 3 (27.3) | 1 (33.3) | ||
| Suspicious finding | 6 (42.8) | 5 (45.4) | 1 (33.3) | ||
Data in parenthesis are percentages.
MRI = magnetic resonance imaging; US = ultrasound.
*Suspicious findings on MRI included lymph nodes with more than one of following features: round shape, absence of fatty hilum, eccentric cortical thickening, or asymmetry in number or size relative to the contralateral side.
The cortical thickness of the largest node in women with and without metastasis was 6.3 mm and 7.9 mm, respectively. The mean cortical thickness of the lymph nodes on US was 4.3 mm (SD, 1.6 mm) and 3.5 mm (SD, 1.4 mm) for women with and without metastasis, respectively. The mean cortical thickness in 9 women recommended for US-guided lymph node biopsy was 4.2 mm (range, 2.6–7.2 mm). One patient, who received the Pfizer/BioNTech vaccination 44 days before US examination and had suspicious lymph nodes with a thickened cortex (4.8 mm) at level I, showed negative results on preoperative lymph node biopsy; however, the final pathology report of sentinel lymph node biopsy revealed metastasis (Table 2). Among the 15 women without lymph node metastasis, 3 (20.0%) showed normal lymph nodes and 12 (80.0%) showed axillary lymphadenopathy at level I. Of the 12 patients whose cases were considered as level I, 9 (75.0%) were exclusively at level I, 2 were at levels I and II (16.7%), and one was at levels I, II, and III (8.3%) (Figure 1). In comparison, axillary lymphadenopathy was detected at level I in 3 of 4 (75.0%) patients and at levels I and II in one of 4 (25.0%) patients with metastasis (Figure 2).
Table 2. Summary of 19 breast cancer women with ipsilateral coronavirus disease 2019 vaccination.
| Case No. | Age | Vaccine type | Vaccine dose | Symptom at axillary region | US finding | Cortical thickness on initial US (mm) | MRI finding | Biopsy | Result on biopsy | Type of axillary surgery | Result on lymph node surgery | Histology of breast cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | Pfizer | 2 | No | Fatty hilum effacement and cortical thickening | 3.1 | Diffuse cortical thickening | No | N/A | SLNB | Negative | DCIS |
| 2 | 57 | Pfizer | 1 | No | None | 2.0 | None | No | N/A | SLNB | Negative | IDC |
| 3 | 67 | Az | 2 | No | Cortical thickening | 4.1 | Asymmetry, eccentric cortical thickening | Yes | Negative | SLNB | Negative | IDC |
| 4 | 74 | Pfizer | 2 | No | Fatty hilum effacement and cortical thickening | 7.9 | Asymmetry, round shape | No | N/A | ALND | Negative | IDC |
| 5 | 61 | Az | 2 | No | Cortical thickening | 3.0 | None | No | N/A | SLNB | Negative | IDC |
| 6 | 50 | Pfizer | 1 | No | Cortical thickening | 4.5 | N/A | No | N/A | SLNB | Negative | IDC |
| 7 | 56 | Moderna | 1 | No | Cortical thickening | 3.8 | Asymmetry, eccentric cortical thickening | Yes | Negative | SLNB | Negative | ILC |
| 8 | 46 | Pfizer | 2 | No | None | 1.9 | Asymmetry, Eccentric cortical thickening | No | N/A | SLNB | Negative | ILC |
| 9 | 48 | Janssen | 1 | No | Cortical thickening | 3.5 | Diffuse cortical thickening | Yes | Negative | SLNB | Negative | IDC |
| 10 | 59 | Pfizer | 1 | No | Fatty hilum effacement and cortical thickening | 4.3 | N/A | Yes | Negative | SLNB | Negative | IDC |
| 11 | 56 | Pfizer | 1 | No | Cortical thickening | 2.6 | N/A | Yes | Negative | SLNB | Negative | IDC |
| 12 | 49 | Pfizer | 1 | No | Cortical thickening | 4.6 | N/A | Yes | Negative | SLNB | Negative | IDC |
| 13 | 51 | Moderna | 1 | No | Cortical thickening | 3.5 | None | No | N/A | SLNB | Negative | IDC |
| 14 | 64 | Az | 1 | No | None | 1.5 | Diffuse cortical thickening | No | N/A | ALND | Negative | Malignant phyllodes tumor |
| 15* | 39 | Az | 2 | No | Fatty hilum effacement and cortical thickening | 7.2 | Asymmetry, eccentric cortical thickening, fatty hilum loss | Yes | Negative | N/A | N/A | IDC |
| 16* | 78 | Pfizer | 2 | No | Fatty hilum effacement and round shape | 3.3 | Asymmetry, irregular margin | Yes | Positive | N/A | N/A | IDC |
| 17 | 56 | Az | 2 | No | Cortical thickening | 3.6 | None | No | N/A | SLNB | 2 lymph nodes Positive | IDC |
| 18 | 77 | Pfizer | 2 | No | Cortical thickening | 4.8 | Diffuse cortical thickening | Yes | Negative | SLNB | 1 lymph node Positive | IDC |
| 19 | 48 | Pfizer | 1 | No | Fatty hilum effacement and cortical thickening | 6.3 | N/A | No | N/A | SLNB | 1 lymph node Positive | IDC |
ALND = axillary lymph node dissection; Az = Oxford-AstraZeneca; DCIS = ductal carcinoma in situ; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; MRI = magnetic resonance imaging; SLNB = sentinel lymph node biopsy; US = ultrasound; N/A = not applicable.
*Two women receiving neoadjuvant chemotherapy without upfront surgery.
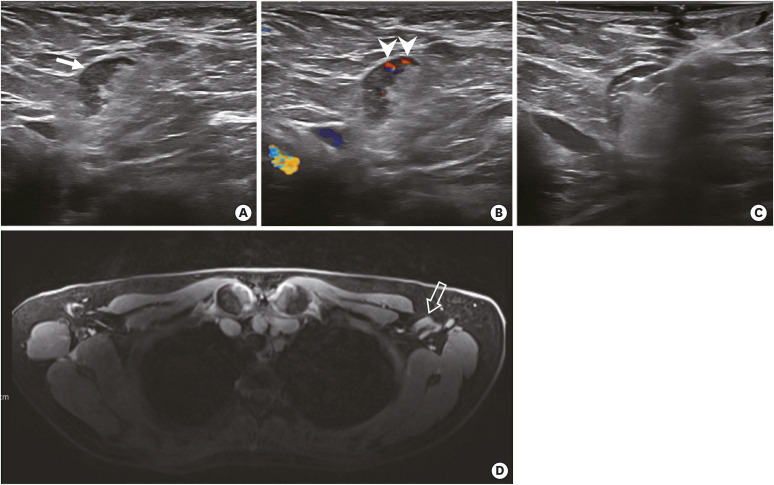
Figure 1. A 56-year-old woman with invasive lobular carcinoma of the left breast. The woman received the first dose of the Moderna coronavirus 2019 vaccine in the left deltoid muscle 22 days before the preoperative axillary US. (A, B) Axillary US demonstrates lymphadenopathy with cortical thickening (arrow) and increased nonhilar blood flow (arrowheads). (C) US guided-biopsy was performed and was negative for carcinoma. (D) T1-weighted fat-saturated MRI demonstrates corresponding lymphadenopathy at level I (transparent arrow). There was no lymph node metastasis on the final sentinel lymph node biopsy.
US = ultrasound; MRI = magnetic resonance imaging.
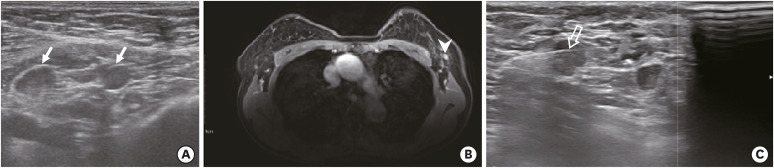
Figure 2. A 78-year-old woman with invasive ductal carcinoma of the left breast, which was hormone receptor negative, human epidermal growth factor receptor 2 positive. The woman received the second dose of the Pfizer/BioNTech coronavirus 2019 vaccine in the left deltoid muscle 27 days before the preoperative axillary US. (A) Axillary US demonstrates lymphadenopathy with cortical thickening and effacement of the fatty hilum at level I (arrows). (B) T1-weighted fat-saturated MRI demonstrates corresponding lymphadenopathy (arrowhead). (C) Subsequent US guided-biopsy was performed and was positive for carcinoma (transparent arrow). The patient is receiving neoadjuvant chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab.
US = ultrasound; MRI = magnetic resonance imaging.
Fourteen of the 19 women in the study cohort underwent a preoperative MRI. In the women without lymph node metastasis (n = 11), MRI revealed negative findings (27.3%, 3 of 11), focal or diffuse cortical thickening only (27.3%, 3 of 11), and suspicious findings (45.4%, 5 of 11). In the women with lymph node metastasis (n = 3), MRI revealed negative findings (33.3%, 1 of 3), focal or diffuse cortical thickening only (33.3%, 1 of 3), and suspicious findings (33.3%, 1 of 3). No significant findings could be used to differentiate non-metastatic and metastatic lymph nodes according to the US findings (p = 0.605), cortical thickness (p = 0.567), location of lymphadenopathy (p = 1.000), or MRI findings (p = 1.000) (Table 1).
In patients with breast cancer, accurate assessment of axillary lymphadenopathy is essential for staging and deciding appropriate treatment because the nodal status determines the need for systemic therapy, the extent of surgery, reconstruction options, and need for radiation therapy [14,15]. Axillary US is the primary imaging tool for assessing axillary lymph nodes with a sensitivity of 26.0%–97.0% and specificity of 55.0%–98.0% [16,17], respectively, and breast MRI or chest computed tomography imaging can provide additional information for extensive nodal disease.
With the COVID-19 vaccination, preoperative imaging assessment and prediction of the axillary lymph node status can be less accurate and cause overtreatment in women with newly diagnosed breast cancer. According to our results, there was no specific imaging clue for differentiating metastatic lymph nodes from reactive changes due to vaccination. Differentiation between reactive and metastatic lymph nodes was challenging because even in women with axillary metastasis, the findings were non-specific, with associated axillary lymphadenopathy due to vaccination.
Our study has several limitations. Most notably, our study has a retrospective design, and the assessment was limited to static images. In addition, owing to the small number of cases in our study, no discrepancy between the clinical assessment and blinded reviews by the radiologists was noted, although interobserver variability might exist. In our study, a direct correlation of suspicious lymph nodes on imaging and pathological results was not made because not all imaging-based suspicious lymph nodes were biopsied preoperatively. In patients who met the American College of Surgeons Oncology Group Z0011 criteria, sentinel lymph node biopsy was performed regardless of the imaging findings, and lymph node biopsy was performed in patients who would benefit from neoadjuvant chemotherapy. Indeed, there is still controversy regarding the need for preoperative axillary imaging [18,19,20,21]. Metastatic sentinel lymph nodes can show false-negative findings even after axillary US examination [22], and negative US-guided biopsy results cannot rule out lymph node metastasis [23], as we have seen disagreement between suspicious lymph nodes on US and metastatic sentinel lymph nodes.
The decision-making process requires multidisciplinary communication and collaboration among radiologists, surgeons, medical oncologists, and radiation oncologists [18,19,20,21]. Thus, to avoid confusion, vaccination in the contralateral arm or the site most distant from the index cancer should be encouraged; however, in patients who have already received COVID-19 vaccination shot in the arm ipsilateral to the diagnosed breast cancer site, management should be cautiously determined considering index tumor information and risk assessment with the engagement of a multidisciplinary team. In patients who receive upfront surgical treatment without neoadjuvant chemotherapy, interval changes in the cortical thickness from the vaccination date [24] and the location of axillary lymphadenopathy on imaging [25,26] may reflect COVID-19 vaccine-induced unilateral axillary lymphadenopathy rather than metastasis. However, this is not always the case; thus, tissue biopsy should be considered. At the same time, surgeons must be more precise and try to avoid unnecessary axillary lymph node dissection, in lieu of sentinel lymph node biopsy, due to the diagnostic challenges caused by COVID-19 vaccination. In particular, in the presence of extensive nodal involvement at levels II and III, caution in the decision to perform axillary lymph node dissection is needed, as shown in 2 of our cases. Currently, there is insufficient data regarding the duration of radiologically evident lymphadenopathy or appropriate follow-up intervals [1]. Further research is needed to provide optimal management of COVID-19 vaccine-induced lymphadenopathy in patients with breast cancer.
COVID-19 vaccination induces ipsilateral axillary lymphadenopathy that may lead to unnecessary lymph node biopsy with suspicion of metastasis from breast cancer, which may be falsely considered to be caused by reactive changes due to recent vaccination rather than cancer, based either on imaging or biopsy results, delaying cancer diagnosis and thus potentially having a detrimental effect on patient management [3]. For patients with a high suspicion of breast cancer, it is important to educate the clinician and patients to receive COVID-19 vaccination in the contralateral arm or the site most distant from the cancer. Recognizing COVID-19 vaccine-induced unilateral axillary lymphadenopathy as a potential differential diagnosis and making efforts to investigate the patient’s vaccination history are crucial to avoid overestimation of the axillary disease burden and make appropriate therapeutic recommendations.
Footnotes
Funding: This study was supported by JW Medical Corporation’s research grant, Seoul National University Hospital (grant No. 06-2021-1410).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Ha SM, Chang JM.
- Data curation: Ha SM, Cheun JH, Chang JM.
- Formal analysis: Ha SM, Chang JM.
- Resources: Lee SH, Kim SY, Park AR, Kim YS, Yoen H, Lee Y, Cho N, Moon WK.
- Supervision: Chang JM.
- Writing - original draft: Ha SM.
- Writing - review & editing: Ha SM, Cheun JH, Lee SH, Kim SY, Park AR, Kim YS, Yoen H, Lee Y, Cho N, Moon WK, Chang JM.
SUPPLEMENTARY MATERIAL
Information related to ipsilateral coronavirus disease 2019 vaccination in women with newly diagnosed breast cancer
References
- 1.Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events. 2021. [Accessed February 22, 2022]. cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html .
- 2.Centers for Disease Control and Prevention. The Moderna COVID-19 Vaccine’s Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events. 2021. [Accessed February 22, 2022]. cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html .
- 3.Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA vaccine with ipsilateral axillary lymph node reactivity on imaging. JAMA Oncol. 2021;7:1241–1242. doi: 10.1001/jamaoncol.2021.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn H, Hong KJ. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin Exp Vaccine Res. 2019;8:89–93. doi: 10.7774/cevr.2019.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn RW, Mootz AR, Brewington CC, Abbara S. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3:e210008. doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology Scientific Expert Panel. Radiology. 2021;300:E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman CD, Lamb LR, D’Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol. 2021;217:584–586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 9.Abe H, Schmidt RA, Kulkarni K, Sennett CA, Mueller JS, Newstead GM. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy--clinical experience in 100 patients. Radiology. 2009;250:41–49. doi: 10.1148/radiol.2493071483. [DOI] [PubMed] [Google Scholar]
- 10.Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjøsne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000;10:1464–1471. doi: 10.1007/s003300000370. [DOI] [PubMed] [Google Scholar]
- 11.Baltzer PA, Dietzel M, Burmeister HP, Zoubi R, Gajda M, Camara O, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol. 2011;196:W641–W647. doi: 10.2214/AJR.10.4889. [DOI] [PubMed] [Google Scholar]
- 12.Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009;30:309–312. doi: 10.1002/jmri.21802. [DOI] [PubMed] [Google Scholar]
- 13.Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012;19:1825–1830. doi: 10.1245/s10434-011-2200-7. [DOI] [PubMed] [Google Scholar]
- 14.Caudle AS, Cupp JA, Kuerer HM. Management of axillary disease. Surg Oncol Clin N Am. 2014;23:473–486. doi: 10.1016/j.soc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Chang JM, Leung JW, Moy L, Ha SM, Moon WK. Axillary nodal evaluation in breast cancer: state of the art. Radiology. 2020;295:500–515. doi: 10.1148/radiol.2020192534. [DOI] [PubMed] [Google Scholar]
- 16.Houssami N, Diepstraten SC, Cody HS, 3rd, Turner RM, Sever AR. Clinical utility of ultrasound-needle biopsy for preoperative staging of the axilla in invasive breast cancer. Anticancer Res. 2014;34:1087–1097. [PubMed] [Google Scholar]
- 17.Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 18.Mango VL, Pilewskie M, Jochelson MS. To look or not to look? Axillary imaging: less may be more. J Breast Imaging. 2021;3:666–671. doi: 10.1093/jbi/wbab075. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16632729&dopt=Abstract . [DOI] [PubMed] [Google Scholar]
- 19.Saksena M, Jimenez R, Coopey S, Harris K. Axillary ultrasound evaluation in breast cancer patients: a multidisciplinary viewpoint and middle ground. J Breast Imaging. 2021;3:672–675. doi: 10.1093/jbi/wbab070. [DOI] [PubMed] [Google Scholar]
- 20.Rauch GM, Kuerer HM, Jochelson MS. To look or not to look? Yes to nodal ultrasound! J Breast Imaging. 2021;3:659–665. doi: 10.1093/jbi/wbab079. [DOI] [PubMed] [Google Scholar]
- 21.Dialani V, Dogan B, Dodelzon K, Dontchos BN, Modi N, Grimm L. Axillary imaging following a new invasive breast cancer diagnosis—A radiologist’s dilemma. J Breast Imaging. 2021;3:645–658. doi: 10.1093/jbi/wbab082. [DOI] [PubMed] [Google Scholar]
- 22.Neal CH, Daly CP, Nees AV, Helvie MA. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010;257:335–341. doi: 10.1148/radiol.10100296. [DOI] [PubMed] [Google Scholar]
- 23.Whitman GJ, Lu TJ, Adejolu M, Krishnamurthy S, Sheppard D. Lymph node sonography. Ultrasound Clinics. 2011;6:369–380. [Google Scholar]
- 24.Robinson KA, Maimone S, Gococo-Benore DA, Li Z, Advani PP, Chumsri S. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA Oncol. 2021;7:1395–1397. doi: 10.1001/jamaoncol.2021.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaza MJ, Wright J, Fernandez S. COVID-19 vaccine-related unilateral axillary lymphadenopathy: pattern on screening breast MRI allowing for a benign assessment. Clin Imaging. 2021;80:139–141. doi: 10.1016/j.clinimag.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information related to ipsilateral coronavirus disease 2019 vaccination in women with newly diagnosed breast cancer