Abstract
Irritable bowel syndrome (IBS) is a common disorder that affects the large intestine. Oxidative stress and inflammation play a major role in IBS. Considering the antioxidant properties of ellagic acid (EA), this study was designed to evaluate the effect of EA on oxidative stress index, inflammatory markers, and quality of life in patients with IBS. This research was conducted as a randomized, double-blind, placebo-controlled clinical trial; 44 patients with IBS were recruited. Patients who met the inclusion criteria were randomly allocated to consume a capsule containing 180 mg of EA per day (n = 22) or a placebo (n = 22) for 8 weeks. Serum levels of total antioxidant capacity (TAC), malondialdehyde (MDA), C-reactive protein (CRP), and interleukin-6 (IL-6) were measured at the beginning and the end of the study. Also, quality of life was assessed using a self-report questionnaire for IBS patients (IBS-QOL). At the end of the study, we saw a significant decrease and increase in the MDA and TAC in the intervention group, respectively (p < 0.05). Also, EA consumption reduced CRP and IL-6 levels, and these changes were significant in comparison with placebo group changes (p < 0.05). The overall score of IBS-QOL significantly decreased, and quality of life was increased (p < 0.05), but there were no significant changes in the placebo group. According to these findings, receiving polyphenols, such as EA, may help maintain intestinal health by modulating inflammation and oxidative stress and ultimately improving the quality of life in IBS patients.
Trial Registration
Iranian Registry of Clinical Trials Identifier: IRCT20141025019669N11
Keywords: Ellagic acid, Oxidative stress, Quality of life, Irritable bowel syndrome
INTRODUCTION
Abdominal pain is one of the few clinical symptoms commonly referred to as irritable bowel syndrome (IBS) disease, which ultimately leads to a change in the function of the digestive system [1]. The prevalence rates of IBS range from 12% to 30% of the world’s population [2]. Based on the investigation results conducted on IBS patients, they had a low quality of life, an average of 73 days a year. Problems such as depression, incomplete daily activities, and dietary restrictions have been reported in these patients [3]. The cost to society in terms of direct and indirect costs ranges from $15–30 billion annually [4].
Although the underlying cause of IBS is still unknown, scientific evidence suggests that it’s related to increased sensitivity of the gut by factors such as food, bile acids, antibiotics, infections, sexual relations, and social and psychological incidents [5]. These changes increase the permeability of the intestine by activating local and brain immunity. The neuroendocrine responses and changes in the microbiome of the intestine ultimately leading to exacerbation of intestinal abnormality and the activation of the intestinal motion sensor, which is associated with the duration and severity of symptoms [5]. Significant changes in the inflammatory cytokines profile in these patients have been observed in scientific studies compared with healthy subjects. According to the reports of these investigations, increases in proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) occur in people with IBS [6]. In addition, anti-inflammatory factors reductions such as IL-10 has been reported in these patients [7]. Various scientific studies have confirmed the relationship between oxidative stress and inflammation. For example, the production of reactive oxygen species by resident cells such as vascular smooth muscle and endothelial cells occurs in the activity of leukocytes [8]. These oxidants are inactivated by the antioxidant defense system [9]. Polyphenols, active ingredients in fruits and vegetables, are classified among 50 antioxidant substances. Based on the results of scientific research, the protective and therapeutic properties of polyphenols have been proven in the management and remedy of IBS.
It should be noted that these health effects contribute to the treatment of the disease by preventing the inflammatory pathways' function, reducing inflammation, and improving the antioxidant status [10]. One of the strong antioxidants from the polyphenols family found in fruits and vegetables is the ellagic acid (EA) [11]. This polyphenol with a strong hydrogen bonding network can participate in many reactions [12]. EA plays an important role in reducing lipid peroxidation and inflammatory symptoms of type 1 diabetes. EA also shows anti-inflammatory and anti-proliferative effects in various types of cancer and prevents oxidative stress [13].
To date, scientific and interesting results have been reported on the effects of EA sources on reducing inflammation in the intestine. Rosillo et al. [14] investigated the EA-enriched pomegranate extract (PE) effects on the chronic Crohn’s disease model in mice. Trinitrobenzenesulfonic acid was used for colonic injury through intracolonic instillation. Mice were subjected to different diets for 30 days. At the end of the study, significant decreases in myeloperoxidase activity and TNF-α levels were reported in mice fed an EA-rich diet. Also, based on the study results, a significant decrease in the expression of genes related to oxidative stress factors was observed [14]. Also, the results of Marín et al. [15] indicated that in the acute ulcerative colitis (UC) model, EA improved disease severity as well as reduced inflammatory factors (IL-6 and TNF-α). Also, in the chronic model of the disease, EA reduced intestinal inflammation and prevented the progression of the UC.
These scientific reports indicate the beneficial effects of polyphenols on intestinal performance and their better function in health. Thus considering the beneficial effects of phenolic compounds such as EA on human health and its beneficial effects on inflammatory indicators, this study designed for the first time to evaluate the effects of EA supplementation on the index of oxidative stress, inflammatory factors, and quality of life in patients with IBS.
MATERIALS AND METHODS
Patients
This randomized, double-blinded, placebo-controlled clinical trial was done on 44 subjects aged 19–60 years old. This research was conducted at Velayat Hospital of Qazvin University of Medical Sciences, Qazvin, Iran, from January 2019 to September 2019. A total of 44 IBS patients including both genders, with normal body mass index (BMI; 19–25 kg/m2) after a clinical examination and gastroenterologist confirmation were enrolled in the study. Patient’ selection in this study was based on Rome III’s diagnostic criteria for functional digestive disorders [16]. Patients with a history of abdominal surgery, gastrointestinal diseases such as celiac disease, and pregnant and lactating women, and those who have been taking supplements in the last 3 months were not included in the study. Also, having underlying illnesses like diabetes, severe psychiatric and behavioral disorders, and aspirin, warfarin, heparin, and anti-inflammatory drugs (including non-steroids, steroids, antihistamines, and mast cell stabilizers) have been other exclusion criteria. After approving approval with the ethics committee of Qazvin University of Medical Sciences, Qazvin, Iran (grant number: IR.QUMS.REC.1397.201), the protocol of the study was registered in the Iranian Registry of Clinical Trials website by the IRCT20141025019669N11 code (https://en.irct.ir/trial/35574).
Study design
All patients who met the inclusion criteria were randomly allocated to consume a capsule containing 180 mg of EA per day (n = 22) or a placebo (n = 22) for 8 weeks. Placebo capsules contained 180 mg starch that their shape, color, and size were similar to the supplement.
The supplement was purchased from Supplement Spot (Kokomo, IN, USA), and the placebo was made by the School of Pharmacy, Tabriz University of Medical Sciences (Tabriz, Iran). It should be noted that the effective dose for EA supplementation was taken from Falsaperla et al. [17]. Since oral supplementation with EA has been shown to reduce inflammation (one of the main goals of this research project), this dose was chosen as the dose of choice in this study.
The pharmacological remedy was similar in the 2 groups. All patients were advised not to alter their diet and physical activity habits during the study. The allocation sequence was blinded by using a table of random numbers. The patients were divided into 2 groups by randomized block allocation according to BMI. In this study, the patient, researcher, and specialist physician were blind to supplements and placebo. The capsules were packaged by a third party outside the study and grouped with codes A and B so that the researcher was unaware of the contents of the capsule. After allocation, the participants were referred to the lab for blood sample testing the next day. Anthropometric measurements, clinical history, and demographic data of each individual were evaluated. Quality of life was assessed using a self-report questionnaire for IBS patients (IBS-QOL). IBS-QOL has 34 items about physical and psychosocial function due to IBS. This questionnaire includes a 5-point Likert response scale: not at all, slightly, moderately, quite a lot, and extremely. All questions of this questionnaire are divided into 8 subscales, including dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual, and relationships. The minimum score for this test is 34 and a maximum of 170. The higher the individual’s score, the lower the quality of life [18]. Height and weight were measured at the beginning and the end of the study using digital scales and stadiometers. BMI was calculated by dividing the weight in kilograms by height in meters squared. Three-day food recalls were used to assess dietary intake, and Nutritionist IV program (First Databank Inc., San Bruno, CA, USA) modified for Iranian food composition was used to estimate participants’ dietary intake. Also, to evaluate the physical activity, we used the International Physical Activity Questionnaire (IPAQ). Data from the IPAQ were converted to metabolic equivalent minutes/week using existing guidelines [19].
Laboratory methods
After 10–12 hours of overnight fasting, blood samples were collected from patients. Each sample contains 10 mL of blood. A temperature of −20°C was used to freeze the serums, and then samples were stored at −80°C for future laboratory evaluations. Serum levels of total antioxidant capacity (TAC) were measured by a spectrophotometric method using Randox TAS (Randox Laboratories, Crumlin, UK) by an autoanalyzer (Model Alcyon 300; Abbott, Abbott Park, IL, USA). Serum malondialdehyde (MDA) levels were measured by thiobarbituric acid method. Inflammatory factors, including IL-6 and C-reactive protein (CRP) concentration, were measured by ELISA kit (Koma Biotech Inc., Seoul, Korea) and immune turbid metric assay (Pars Azmoon kit; Pars Azmoon Inc., Tehran, Iran), respectively.
Sample size calculation
We used the MDA factor before and after the intervention to determine the sample size used in Hosseini et al.’s study [20]. Therefore, if the mean and standard deviation of the MDA before and after the supplementation was 3/3 ± 1 and 2/1 ± 0/7, it was calculated as 18 people for each group. Considering the drop out in participants during the study, 22 people were considered for each group.
| N = [(Z1 − α/2 + Z1 − β)2 (SD12 + SD22)]/Δ2 |
Statistical analysis
Statistical analyses were conducted using SPSS version 20 (IBM Corp., Armonk, NY, USA). All data were presented as mean ± standard deviation and were checked for normality by the Kolmogorov-Smirnov test. Due to the normal distribution of variables, the paired sample t-test and the independent sample t-test were applied to analyze differences in variables within and between groups, respectively. The p < 0.05 was considered statistically significant.
RESULTS
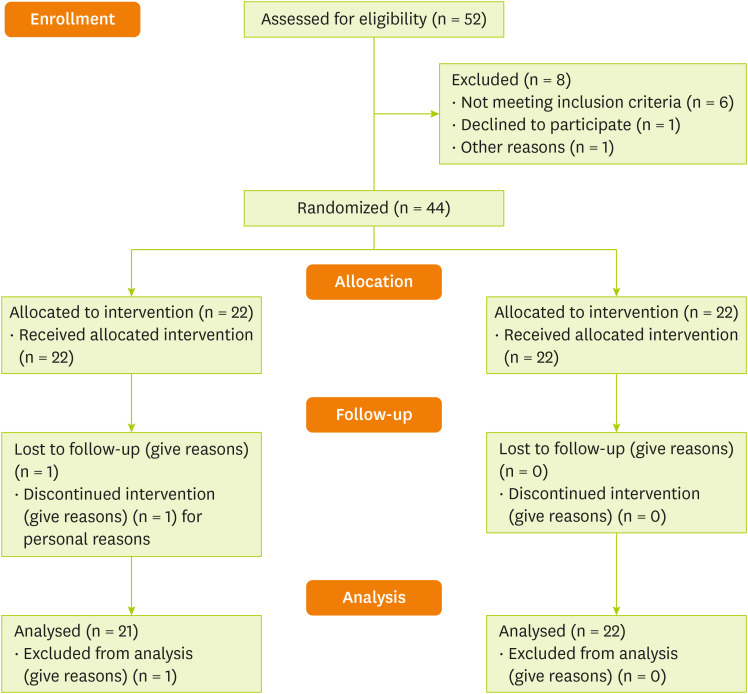
Total of 44 patients with IBS were recruited for the study. Twenty-two patients completed the study in the intervention group. One patient from the placebo group dropped out for personal reasons (Figure 1). Patient compliance in this study was 97.72%. The final analysis was done on the subjects who finished the study. The patient’s demographic and baseline characteristics are presented in Table 1. The mean age of all participants was 34.91 ± 5.34 years. There was no significant difference in age between the intervention and placebo groups (34.55 ± 4.93 vs. 35.29 ± 5.84 years). Also, there was no significant difference between the EA and placebo groups in terms of weight (63.67 ± 7.91 vs. 63.48 ± 9.14), BMI (23.81 ± 1.34 vs. 23.75 ± 1.42 kg/m2), and physical activity (37.53 ± 2.76 vs. 36.21 ± 2.91) at the beginning of the study (p > 0.05; Table 1). However, there was no significant difference in BMI, weight, and physical activity between the patients (Table 1). In this study, we did not receive any adverse effects reports from patients about EA consumption.
Figure 1. Trial profile and design.
Table 1. The comparison of baseline characteristics of the participants.
| Characteristics | Placebo (n = 21) | Ellagic acid (n = 22) | P1 | |
|---|---|---|---|---|
| Age (yr) | 35.29 ± 5.84 | 34.55 ± 4.93 | 0.611 | |
| Weight (kg) | ||||
| Baseline | 63.48 ± 9.14 | 63.67 ± 7.91 | 0.941 | |
| 2 mon change | 63.03 ± 8.45 | 63.66 ± 7.81 | 0.799 | |
| P2 | 0.843 | 0.903 | ||
| BMI (kg/m2) | ||||
| Baseline | 23.75 ± 1.42 | 23.81 ± 1.34 | 0.827 | |
| 2 mon change | 23.61 ± 1.36 | 23.81 ± 1.39 | 0.690 | |
| P2 | 0.813 | 0.890 | ||
| Physical activity (met-h/week) | ||||
| Baseline | 36.21 ± 2.91 | 37.53 ± 2.76 | 0.136 | |
| 2 mon change | 36.72 ± 3.03 | 38.20 ± 2.80 | 0.104 | |
| P2 | 0.603 | 0.189 | ||
Data are expressed as means ± standard deviation. P1: comparison of the mean of baseline characteristics between the 2 groups of ellagic acid and placebo (independent samples t-test). P2: comparison of mean of baseline characteristics in each group at baseline and end of study (paired samples t-test).
The mean energy and macronutrient intake at the study’s baseline were shown in Table 2. There were no statistically significant differences between the groups in terms of average daily intake the energy, protein, fat, saturated fatty acids, unsaturated fatty acids, and some micronutrients (p > 0.05).
Table 2. The comparison of the in dietary intake at the baseline and the end of the study in patients with irritable bowel syndrome.
| Variables | Placebo (n = 21) | Ellagic acid (n = 22) | P1 | |
|---|---|---|---|---|
| Energy (kcal) | ||||
| Baseline | 1,908.27 ± 380.67 | 1,961.31 ± 367.27 | 0.307 | |
| End | 1,890.49 ± 401.19 | 1,973.20 ± 360.09 | 0.299 | |
| P2 | 0.503 | 0.499 | ||
| Protein (g) | ||||
| Baseline | 74.34 ± 17.14 | 76.41 ± 19.02 | 0.314 | |
| End | 73.63 ± 16.97 | 76.88 ± 21.10 | 0.300 | |
| P2 | 0.407 | 0.480 | ||
| Carbohydrate (g) | ||||
| Baseline | 248.83 ± 41.93 | 254.67 ± 42.97 | 0.540 | |
| End | 245.52 ± 41.53 | 256.21 ± 43.10 | 0.492 | |
| P2 | 0.607 | 0.643 | ||
| Fat (g) | ||||
| Baseline | 71.47 ± 16.22 | 72.14 ± 20.48 | 0.716 | |
| End | 68.55 ± 37.01 | 71.31 ± 24.11 | 0.695 | |
| P2 | 0.291 | 0.382 | ||
| Saturated fatty acids (g) | ||||
| Baseline | 19.27 ± 5.00 | 19.45 ± 5.72 | 0.705 | |
| End | 18.17 ± 6.40 | 20.08 ± 3.23 | 0.612 | |
| P2 | 0.415 | 0.541 | ||
| Monounsaturated fatty acid (g) | ||||
| Baseline | 27.19 ± 7.09 | 28.02 ± 6.08 | 0.749 | |
| End | 26.11 ± 5.03 | 28.91 ± 6.11 | 0.546 | |
| P2 | 0.617 | 0.719 | ||
| Polyunsaturated fatty acid (g) | ||||
| Baseline | 22.09 ± 8.13 | 22.85 ± 7.29 | 0.780 | |
| End | 21.19 ± 6.12 | 21.01 ± 4.18 | 0.803 | |
| P2 | 0.307 | 0.243 | ||
| Fiber (g) | ||||
| Baseline | 6.39 ± 0.91 | 6.48 ± 1.04 | 0.407 | |
| End | 6.03 ± 2.24 | 6.71 ± 2.17 | 0.354 | |
| P2 | 0.207 | 0.210 | ||
| Vitamin C (mg) | ||||
| Baseline | 68.09 ± 17.09 | 69.47 ± 13.27 | 0.704 | |
| End | 66.28 ± 47.11 | 68.27 ± 9.67 | 0.501 | |
| P2 | 0.570 | 0.609 | ||
| Vitamin E (IU) | ||||
| Baseline | 6.39 ± 0.11 | 6.79 ± 0.27 | 0.405 | |
| End | 5.83 ± 0.91 | 6.80 ± 0.40 | 0.102 | |
| P2 | 0.311 | 0.821 | ||
| Selenium | ||||
| Baseline | 119.47 ± 29.12 | 120.08 ± 21.14 | 0.457 | |
| End | 118.6 ± 27.13 | 119.13 ± 23.04 | 0.263 | |
| P2 | 0.302 | 0.451 | ||
Data are expressed as means ± standard deviation.
P1: comparison of the mean of dietary intake between the 2 groups of ellagic acid and placebo (independent samples t-test). P2: comparison of mean of baseline characteristics in each group at baseline and end of study (paired samples t-test).
The effect of EA supplementation on oxidative stress and inflammatory biomarkers in IBS patients has been summarized in Table 3. EA consumption reduced CRP, and IL-6 levels in the intervention group, and this change was significant compared to placebo group changes (p < 0.05). Also, as is clear from our results, the intervention group increased TAC (3.02 ± 0.62 vs. 1.25 ± 0.35) and decreased MDA amounts in comparison to the placebo group (0.89 ± 0.17 vs. 2.06 ± 0.18), and these differences were statistically significant (p < 0.05).
Table 3. Changes in baseline to endpoint measures for oxidative stress and inflammatory biomarkers in 2 groups.
| Characteristics | Placebo (n = 21) | Ellagic acid (n = 22) | P1 | |
|---|---|---|---|---|
| IL-6 | ||||
| Baseline | 9.46 ± 0.99 | 9.65 ± 0.64 | 0.440 | |
| 2 mon change | 9.25 ± 1.00 | 6.66 ± 0.54 | < 0.001 | |
| P2 | 0.711 | < 0.001 | ||
| Mean change | −0.21 ± 0.01 | −2.99 ± 0.10 | 0.002 | |
| CRP (μM) | ||||
| Baseline | 12.12 ± 0.72 | 11.77 ± 0.82 | 0.140 | |
| 2 mon change | 12.00 ± 0.79 | 7.95 ± 0.33 | < 0.001 | |
| P2 | 0.270 | < 0.001 | ||
| Mean change | −0.12 ± 0.07 | −3.82 ± 0.49 | 0.001 | |
| TAC (μmol/L) | ||||
| Baseline | 1.28 ± 0.48 | 1.08 ± 0.20 | 0.080 | |
| 2 mon change | 1.25 ± 0.35 | 3.02 ± 0.62 | < 0.001 | |
| P2 | 0.555 | < 0.001 | ||
| Mean change | −0.30 ± 0.13 | 1.94 ± 0.42 | < 0.001 | |
| MDA (μmol/L) | ||||
| Baseline | 2.17 ± 0.24 | 2.55 ± 0.59 | 0.008 | |
| 2 mon change | 2.06 ± 0.18 | 0.89 ± 0.17 | < 0.001 | |
| P2 | 0.509 | < 0.001 | ||
| Mean change | −0.11 ± 0.06 | −1.66 ± 0.42 | < 0.001 | |
P1: comparison of the mean of oxidative stress biomarkers and inflammatory factors between 2 groups (independent samples t-test). P2: comparison of the mean of oxidative stress biomarkers and inflammatory factors in each group at the baseline and end of the study (paired samples t-test).
CRP, C-reactive protein; IL-6, interleukin-6; MDA, malondialdehyde; TAC, total antioxidant capacity.
The amount and changes in quality of life scores at the beginning and the end of the study are summarized in Table 4. In the intervention group, the overall score of IBS-QOL significantly decreased, and quality of life was increased (p < 0.05), but there were no significant changes in the placebo group. According to the study data in Table 4, statistically, significant changes are seen in various components of quality of life, including dysphoria, social reaction, health worries, food avoidance, social reaction, relationships, and sexual subscales in the EA group as compared to the placebo group (p < 0.05).
Table 4. Changes in baseline to endpoint measures for IBS-QOL in 2 groups.
| Characteristics | Placebo (n = 21) | Ellagic acid (n = 22) | P1 | |
|---|---|---|---|---|
| IBS-QOL overall score | ||||
| Baseline | 119.9 ± 9.02 | 123.18 ± 4.89 | 0.144 | |
| 2 mon change | 114.57 ± 8.25 | 78.31 ± 6.15 | < 0.001 | |
| P2 | 0.152 | < 0.001 | ||
| Mean change | −5.33 ± 0.77 | −44.87 ± 1.26 | < 0.001 | |
| Dysphoria | ||||
| Baseline | 33.29 ± 5.56 | 33.05 ± 3.76 | 0.870 | |
| 2 mon change | 31.43 ± 4.85 | 16.68 ± 3.65 | < 0.001 | |
| P2 | 0.617 | < 0.001 | ||
| Mean change | 0.14 ± 0.71 | −16.37 ± 0.11 | < 0.001 | |
| Social reaction | ||||
| Baseline | 13.57 ± 3.88 | 14.36 ± 1.59 | 0.382 | |
| 2 mon change | 13.00 ± 3.20 | 6.77 ± 1.44 | < 0.001 | |
| P2 | 0.804 | < 0.001 | ||
| Mean change | −0.57 ± 0.68 | −7.59 ± 0.15 | < 0.001 | |
| Health worries | ||||
| Baseline | 10.81 ± 2.50 | 11.14 ± 1.72 | 0.790 | |
| 2 mon change | 10.05 ± 2.03 | 6.82 ± 1.50 | < 0.001 | |
| P2 | 0.719 | < 0.001 | ||
| Mean change | −0.76 ± 0.47 | −4.32 ± 0.22 | 0.001 | |
| Body image | ||||
| Baseline | 12.19 ± 2.69 | 12.73 ± 1.85 | 0.454 | |
| 2 mon change | 11.34 ± 2.27 | 10.80 ± 1.25 | 0.500 | |
| P2 | 0.240 | 0.073 | ||
| Mean change | −0.85 ± 0.42 | −1.93 ± 0.60 | 0.109 | |
| Relationships | ||||
| Baseline | 10.19 ± 1.50 | 10.64 ± 1.84 | 0.408 | |
| 2 mon change | 10.38 ± 2.01 | 8.64 ± 1.43 | 0.002 | |
| P2 | 0.734 | 0.001 | ||
| Mean change | 0.19 ± 0.11 | −2.00 ± 0.41 | 0.001 | |
| Sexual | ||||
| Baseline | 6.57 ± 1.07 | 6.91 ± 1.19 | 0.336 | |
| 2 mon change | 6.76 ± 1.26 | 4.41 ± 0.59 | < 0.001 | |
| P2 | 0.802 | < 0.001 | ||
| Mean change | 0.19 ± 0.19 | −2.50 ± 0.60 | < 0.001 | |
| Food avoidance | ||||
| Baseline | 6.76 ± 1.44 | 6.73 ± 1.12 | 0.930 | |
| 2 mon change | 6.52 ± 1.63 | 4.91 ± 0.68 | < 0.001 | |
| P2 | 0.850 | < 0.001 | ||
| Mean change | −0.24 ± 0.19 | −1.82 ± 0.44 | 0.002 | |
| Interference with activity | ||||
| Baseline | 26.52 ± 5.04 | 25.64 ± 3.21 | 0.499 | |
| 2 mon change | 25.24 ± 5.15 | 19.27 ± 4.15 | 0.021 | |
| P2 | 0.063 | 0.020 | ||
| Mean change | −1.28 ± 0.11 | −6.37 ± 0.94 | 0.039 | |
P1: comparison of the mean of IBS-QoL between 2 groups (independent samples t-test). P2: comparison of the mean of IBS-QoL in each group at the baseline and end of the study (paired samples t-test).
IBS-QOL, quality of life was assessed using a self-report questionnaire for irritable bowel syndrome patients.
Safety and adverse events
No side effects were reported in the study. Also, there were no cointerventions in this study.
DISCUSSION
Our findings provide support for the anti-inflammatory and anti-oxidative properties of EA in patients with IBS. IBS as a gastrointestinal disease is a complex of symptoms described by the abdominal pain and changes in bowel habits, frequency, and form of stool, without any indications for other diseases to cause such symptoms [21]. This condition can considerably affect health-related quality of life [22]. Other scientific evidence showed that reactive oxygen metabolites have a possible role in the cause of almost diseases. Numerous productions of reactive oxygen metabolites have been shown to be responsible for the secretion of electrolytes and water that can result in diarrhea. Moreover, disturbance in redox balance can also change the expression of immune and inflammatory markers [23]. The results of scientific studies confirmed the role of dietary polyphenols in improving TAC and decreasing the molecular degradation caused by oxidative stress. These mechanisms result in high immune responses, especially in those patients with inflammatory diseases [24]. The anti-inflammatory properties of EA as a potent polyphenol are shown in scientific research. This property can improve oxidative stress status and reduce the tissue damage to the colon [25]. For the first time in this randomized clinical trial, oral supplementation of EA was investigated on oxidative stress index, inflammatory factors, and quality of life in IBS patients.
Results of the present study indicated that EA supplement therapy for 8 weeks decreased serum levels of the MDA, a marker of oxidative stress, and increased TAC. Various rodent models showed the anti-oxidative effect of EA by detoxification function and modifying MDA formation in the mucosa [26]. In the study of Shukla et al. [27], a model of rheumatoid arthritis was used to assess the effect of EA supplement. Based on their results, 13.6 mg/kg of EA decreased IL-6 levels in arthritic joints and reduced MDA in rats’ plasma and colon mucosa [27]. Also, findings in the study of Toklu et al. [28] are consistent with our findings. Administration of 50 mg/kg of pomegranate peel extract containing EA for 28 days in an animal model with liver fibrosis decreased MDA levels [28]. An important issue emerging from Yüce et al. [29] was the protective role of EA against cisplatin-induced oxidative stress in rats’ liver and heart tissue. EA (10 mg/kg) in the animals that received cisplatin decreased MDA levels, resulting in lower toxicity.
Inflammation has a special role in the pathogenesis of IBS that can worsen the symptoms by activating the visceral sensory system and perturbing different reflexes of the gastrointestinal [30], and the expression of pro-inflammatory mediators such as cytokines can increase pain by activating intracellular signaling and sensitizing the nervous system [31]. So it is possible to hypothesize that EA may ameliorate inflammatory symptoms due to its properties. Our study showed that oral EA supplement reduced serum levels of the inflammatory markers (IL-6, CRP) after 8 weeks. Marta et al. [15] showed that EA decreased inflammation, histological scores and disease severity through improvements in the inflammatory profile of mediators such as TNF-α, IL-6, and interferon gamma in mice with UC. Moreover, down regulation of some mediators such as cyclooxygenase-2 and inducible NO synthase were reported [32]. Also, the report of Umesalma et al. [33] on EA confirmed previous findings and contributed to our results that EA efficiently reduced the expressions of IL-6 in 1,2-dimethylhydrazine-induced rats. The results obtained by Esmaeilinezhad et al. [34] showed that receiving daily pomegranate juice for 8 weeks improved metabolic, oxidative, inflammatory, and BP outcomes in females with polycystic ovary syndrome.
Contrary to these findings, a meta-analysis of 5 prospective trials did not support the possible effect of pomegranate juice on plasma CRP levels [35]. Rosillo et al. [14] studied the effect of enriched PE on colon inflammation and demonstrated the therapeutic and protective function of EA through suppressing leukocyte infiltration and proinflammatory cytokines activation. Based on Larrosa et al. [36], dietary supplementation of EA has protective function in colitis through alleviation of inflammatory response and production of proinflammatory cytokines which results in lower colonic damage. A proposed mechanism for EA, which is demonstrated among animal models of colitis, is through suppression of some signaling pathways such as nucleus factors, IKB (an enzyme complex that is involved in propagating the cellular response to inflammation), and NF-κB (a protein complex that controls cytokine production), MAPK-ERK (a chain of proteins that communicates a signal from a receptor to the DNA), and JNK (responsive to stress stimuli, such as cytokines) [37]. In this study, quality of life was increased significantly in the EA group. Since EA has some therapeutic effects against IBS disease, there might be possible correlations between the consumption of EA and the quality of life in those patients suffer from it. Nevertheless, a limited number of studies have assessed these correlations. The quality of life is strongly related to condition and severity of IBS so it requires accurate treatment and monitoring. Based on different evidences, psychological complications such as depression and anxiety, which are common signs in IBS patients, lead to impairment of quality of life [38]. The hypothalamic pituitary adrenal axis, the core endocrine stress system, can be activated by psychological stressors [39], which provides the gut immune system and the brain connection [26]. Since previous studies used several pomegranate products such as husk, seeds, or pulp, it wasn’t possible to see the exact effect of EA among other bioactive compounds. So one of the strengths of this study is that for the first time the effect of pure supplement of EA was investigated in patients with IBS. Also, the design of this study as a double-blind randomized clinical trial that had parallel groups, making the results of this study remarkable. However, the present study, like other clinical trials, had limitations such as single dose, budget deficit, and lower factor measurements. To obtain a perfect picture of EA treatment, it would be necessary to execute a randomized clinical trial with a greater number of participants and different doses.
CONCLUSIONS
Summing up the results, it can be concluded that dietary polyphenols like EA can contribute to the maintenance of gut health by the modulation of inflammation and oxidative stress. The results of our study provide evidence to support the view that EA can play an important role in enhancing the quality of life among IBS patients. Nevertheless, further studies are needed to provide additional evidence.
ACKNOWLEDGEMENTS
The authors would like to thank all of the participants who completed the study protocol.
Footnotes
Funding: This work was financially supported by a grant (number: IR.QUMS.REC.1397.201) from Vice-Chancellor for Research Affairs of Qazvin University of Medical Sciences, Qazvin, Iran.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Khadem Haghighian H, Rashidi Nooshabadi M.
- Data curation: Mirzaie Z, Bastani A and Haji-Aghamohammadi AA.
- Formal analysis: Ahadinezhad B, Khadem Haghighian H.
- Funding acquisition: Khadem Haghighian H.
- Investigation: Mirzaie Z, Khadem Haghighian H.
- Methodology: Rashidi Nooshabadi M, Bastani A.
- Project administration: Khadem Haghighian H.
- Supervision: Khadem Haghighian H.
- Validation: Haji-Aghamohammadi AA.
- Writing - original draft: Mirzaie Z, Khadem Haghighian H.
- Writing - review & editing: Rashidi Nooshabadi M, Ahadinezhad B, Bastani A.
References
- 1.Laatikainen R, Koskenpato J, Hongisto SM, Loponen J, Poussa T, Hillilä M, Korpela R. Randomised clinical trial: low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:460–470. doi: 10.1111/apt.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43:541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 5.Gazouli M, Wouters MM, Kapur-Pojskić L, Bengtson MB, Friedman E, Nikčević G, Demetriou CA, Mulak A, Santos J, Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat Rev Gastroenterol Hepatol. 2016;13:77–87. doi: 10.1038/nrgastro.2015.206. [DOI] [PubMed] [Google Scholar]
- 6.Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–2243. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 7.Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43. doi: 10.1016/j.cyto.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Suresh K, Shimoda LA. Endothelial cell reactive oxygen species and Ca2+ signaling in pulmonary hypertension. Adv Exp Med Biol. 2017;967:299–314. doi: 10.1007/978-3-319-63245-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, Pawliszak W, Kedziora J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol. 2016;67:331–337. [PubMed] [Google Scholar]
- 10.Farzaei MH, Rahimi R, Abdollahi M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr Pharm Biotechnol. 2015;16:196–210. doi: 10.2174/1389201016666150118131704. [DOI] [PubMed] [Google Scholar]
- 11.Gourineni V, Shay NF, Chung S, Sandhu AK, Gu L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J Agric Food Chem. 2012;60:7674–7681. doi: 10.1021/jf3013663. [DOI] [PubMed] [Google Scholar]
- 12.Valipour M, Maghami P, Habibi-Rezaei M, Sadeghpour M, Khademian MA, Mosavi K, Ahmad F, Moosavi-Movahedi AA. Counteraction of the deleterious effects of reactive oxygen species on hemoglobin structure and function by ellagic acid. J Lumin. 2017;182:1–7. [Google Scholar]
- 13.Yu YM, Chang WC, Wu CH, Chiang SY. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem. 2005;16:675–681. doi: 10.1016/j.jnutbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Rosillo MA, Sánchez-Hidalgo M, Cárdeno A, Aparicio-Soto M, Sánchez-Fidalgo S, Villegas I, de la Lastra CA. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res. 2012;66:235–242. doi: 10.1016/j.phrs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Marín M, María Giner R, Ríos JL, Recio MC. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J Ethnopharmacol. 2013;150:925–934. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Long Y, Huang Z, Deng Y, Chu H, Zheng X, Yang J, Zhu Y, Fried M, Fox M, Dai N. Prevalence and risk factors for functional bowel disorders in South China: a population based study using the Rome III criteria. Neurogastroenterol Motil. 2017;29:e12897. doi: 10.1111/nmo.12897. [DOI] [PubMed] [Google Scholar]
- 17.Falsaperla M, Morgia G, Tartarone A, Ardito R, Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur Urol. 2005;47:449–454. doi: 10.1016/j.eururo.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Francavilla R, Piccolo M, Francavilla A, Polimeno L, Semeraro F, Cristofori F, Castellaneta S, Barone M, Indrio F, Gobbetti M, De Angelis M. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol. 2019;53:e117–e125. doi: 10.1097/MCG.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolin KY, Heil DP, Askew S, Matthews CE, Bennett GG. Validation of the international physical activity questionnaire-short among blacks. J Phys Act Health. 2008;5:746–760. doi: 10.1123/jpah.5.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini B, Saedisomeolia A, Wood LG, Yaseri M, Tavasoli S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: a randomized controlled clinical trial. Complement Ther Clin Pract. 2016;22:44–50. doi: 10.1016/j.ctcp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Stasi C, Caserta A, Nisita C, Cortopassi S, Fani B, Salvadori S, Pancetti A, Bertani L, Gambaccini D, de Bortoli N, Dell’Osso L, Blandizzi C, Marchi S, Bellini M. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: a longitudinal assessment. J Gastroenterol Hepatol. 2019;34:713–719. doi: 10.1111/jgh.14375. [DOI] [PubMed] [Google Scholar]
- 22.Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2017;15:35. doi: 10.1186/s12955-017-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, Rosaria Lauro M, Carbone C, Reis F, Pandey AK. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gessner DK, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr (Berl) 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- 26.Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, Niu R, Wei H, Xie P. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–743. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toklu HZ, Dumlu MU, Sehirli O, Ercan F, Gedik N, Gökmen V, Sener G. Pomegranate peel extract prevents liver fibrosis in biliary-obstructed rats. J Pharm Pharmacol. 2007;59:1287–1295. doi: 10.1211/jpp.59.9.0014. [DOI] [PubMed] [Google Scholar]
- 29.Yüce A, Ateşşahin A, Ceribaşi AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007;101:345–349. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 30.Alamo RZ, Quigley EM. Irritable bowel syndrome and colonic diverticular disease: overlapping symptoms and overlapping therapeutic approaches. Curr Opin Gastroenterol. 2019;35:27–33. doi: 10.1097/MOG.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, Zhang J, Chen L, Liu T, Xu G, Li C, Yuan W, Xu H, Su Z. IL-17 contributed to the neuropathic pain following peripheral nerve injury by promoting astrocyte proliferation and secretion of proinflammatory cytokines. Mol Med Rep. 2017;15:89–96. doi: 10.3892/mmr.2016.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer. 2018;70:350–375. doi: 10.1080/01635581.2018.1446091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–655. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 34.Esmaeilinezhad Z, Barati-Boldaji R, Brett NR, de Zepetnek JO, Bellissimo N, Babajafari S, Sohrabi Z. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J Endocrinol Invest. 2020;43:539–548. doi: 10.1007/s40618-019-01139-x. [DOI] [PubMed] [Google Scholar]
- 35.Sahebkar A, Gurban C, Serban A, Andrica F, Serban MC. Effects of supplementation with pomegranate juice on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;23:1095–1102. doi: 10.1016/j.phymed.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol. 2011;82:737–745. doi: 10.1016/j.bcp.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153:448–459.e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20:476–494. doi: 10.1080/10253890.2017.1369523. [DOI] [PMC free article] [PubMed] [Google Scholar]