Highlights
-
•
Dosimetric benefit of proton over x-ray treatment for thoracic esophageal cancer.
-
•
Reduction of pulmonary and cardiac toxicity by proton therapy.
-
•
Intensity modulated proton therapy beam configurations designed by tumor location.
Keywords: Proton therapy, Esophageal cancer, Beam configuration
Abbreviations: RT, radiotherapy; IMXT, intensity-modulated x-ray therapy; PBT, proton beam therapy; OARs, organs-at-risk; PSPT, passive scattering proton therapy; IMPT, intensity-modulated proton therapy; ESCC, esophageal squamous cell carcinoma; CT, computed tomography; GTV, gross target volume; CTV-HR, high-risk clinical target volume; CTV-LR, low-risk clinical target volume; PTV, planning target volume; IQR, interquartile range; RBE, relative biological effectiveness; NTCP, normal tissue complication probability; MLD, mean lung dose; BMI, body mass index; AUC, area under the receiver operating characteristics; MHD, mean heart dose
Abstract
Background and purpose
Specific proton-beam configurations are needed to spare organs at risk (OARs), including lungs, heart, and spinal cord, when treating esophageal squamous cell carcinoma (ESCC) in the thoracic region. This study aimed to propose new intensity-modulated proton therapy (IMPT) beam configurations and to demonstrate the benefit of IMPT compared with intensity-modulated x-ray therapy (IMXT) for treating ESCC.
Material and methods
IMPT plans with three different beam angle configurations were generated on CT datasets of 25 ESCC patients that were treated with IMXT. The IMPT beam designs were two commonly-used beam configurations (anteroposterior and posterior oblique) and a recently proposed beam configuration (anterosuperior with posteroinferior). The target doses were 50–54 Gy(RBE) and 60–64 Gy(RBE) to the low-risk and high-risk target volumes, respectively. Robust optimization was applied for the IMPT plans. The differences in the dose-volume parameters between the IMXT and IMPT plans were compared.
Results
With target coverage comparable to standard IMXT, IMPT had significantly lower mean doses to the OARs. IMPT with an anteroposterior opposing beam generated the lowest lung dose (mean = 7.1 Gy(RBE), V20 = 14.1%) and the anterosuperior with posteroinferior beam resulted in the lowest heart dose (mean = 12.8 Gy(RBE), V30 = 15.7%) and liver dose (mean = 3.9 Gy(RBE), V30 = 5.9%). For the subgroup of patients with an inferior tumor location (PTVs overlapping a part of the contoured heart), the novel beam demonstrated the optimal OARs sparing.
Conclusion
Compared with IMXT, the IMPT plans significantly reduced the radiation dose to the surrounding organs when treating ESCC. IMPT beam configuration selection depends on the tumor location relative to the heart.
1. Introduction
Esophageal carcinoma is the sixth leading cause of cancer death (544,000 deaths) and the seventh most common cancer worldwide (604,000 new cases) [1]. Radiotherapy (RT) is one of the mainstays of the neoadjuvant treatment of resectable cases [2], [3] and the definitive treatment for unresectable or inoperable cases [4]. However, local failure within the radiation treatment volume remains a significant problem and triggers a dose-escalation strategy to enhance local control [5]. High-dose RT improves the local tumor response in esophageal cancer patients and potentially improves overall survival; however, this can result in treatment-related toxicity, despite modern techniques with intensity-modulated x-ray therapy (IMXT) [6], [7], [8], [9].
Several dosimetric and clinical studies demonstrated that proton beam therapy (PBT) generated lower doses to the surrounding organs-at-risk (OARs) and the reduced toxicity risk, with comparable disease control with standard IMXT [10], [11], [12], [13], [14], [15], [16], [17], [18]. For trimodality treatment, PBT also reduced postoperative complications and mortality rates [15]. The first phase IIB randomized controlled trial comparing PBT with IMXT in 107 esophageal cancer patients demonstrated similar progression-free survival and overall survival at three years with a lower rate of total toxicity burden in the PBT group [19].
Previous studies mainly reported PBT outcomes using the conventional technique, passive scattering proton therapy (PSPT), which spreads the beam over the depth of the tumor, which leads to an increased entrance dose. A more highly conformal technique, pencil beam scanning or intensity-modulated proton therapy (IMPT), has been developed, which delivers dose conformity to the proximal and distal ends of the target volume. However, there are many challenges when using IMPT in esophageal cancer, such as density heterogeneity at the tumor-lung interface [20], interplay effects due to breathing and cardiac motion [21], and anatomical changes due to weight loss or tumor progression [22]. Until now, there have been few reports on the clinical outcomes of IMPT in esophageal cancer [14], [16].
The commonly-used proton beam configurations for treating esophageal cancer include anteroposterior opposing beams, anterior with right and left posterior oblique beams, and left lateral with posterior beams [12], [13], [17], [23], [24]. For IMPT, the posterior oblique beam arrangement provided optimal sparing of all OARs, whereas the anteroposterior opposing beams and the posterior oblique beams resulted in the lowest lung and cardiac doses, respectively [17], [23]. However, Feng et al. reported that the superior-inferior posterior oblique beams had better liver, heart, and lung sparing doses, compared with posterior obliques beams, when considering uncertainties and the interplay effect [25]. Notably, these previous studies consisted of patients with cancers of the distal esophagus or esophagogastric junction (EGJ) where posterior-beam IMPT resulted in heart sparing. In contrast, esophageal squamous cell carcinoma (ESCC) is a more common tumor histology in the Asian population with evidence of a dose–response relationship [1], [8], [26]. This cancer predominantly locates in the thoracic region where surgery is less feasible. Thus, definitive high dose radiation might be offered for these patients. However, the best IMPT beam configurations that achieve dose escalation with optimal normal tissue sparing for thoracic ESCC tumors are currently unknown. Therefore, the aim of this study was to investigate the IMPT beam angle arrangements to achieve dose escalation with optimal normal tissue sparing for thoracic ESCC patients.
2. Material and methods
We retrospectively reviewed the medical records of 25 patients with squamous cell carcinoma of the thoracic esophagus that were treated with IMXT in our institution between 2011 and 2019. The study was approved by the Institutional Review Board (no. 038/62).
The patients’ median age was 62 years-old (range 44–77 years-old), and most were male (92%). The patients received concurrent chemotherapy for neoadjuvant (20%) and definite setting (80%). Nineteen patients (76%) had upper or mid thoracic esophageal cancer and the supraclavicular region was treated in 16 patients (64%). The median tumor length was 7.5 cm (range, 2.4–14.0 cm).
2.1. Treatment planning
The computed tomography (CT) simulation images and structures from the IMXT treatment plan were used to generate dummy IMPT plans with three different beam configurations as described below. The target delineation and dose prescription were previously described [8]. Briefly, the gross target volume (GTV) consisted of a gross primary tumor with the whole circumferential wall of the involved esophagus (GTV-P), and pathologic nodes (GTV-N). A high-risk clinical target volume (CTV-HR) included the GTV-P with a 0.5-cm radial and a 2-cm craniocaudal margin along the esophagus, and GTV-N. A low-risk CTV (CTV-LR) encompassed GTV-P with a 0.5-cm radial and a 4-cm craniocaudal margin, GTV-N, and elective nodal region. A 1-cm isotropic margin was added to the CTVs to create planning target volumes (PTVs). The median PTV-LR and PTV-HR volumes were 514.9 cm3 (interquartile range (IQR), 414.4–663.5) and 340.6 cm3 (IQR, 243.4–432.2), respectively. The median prescription doses were 50 Gy for PTV-LR and 60 Gy for PTV-HR.
The prescription doses were 50–54 Gy relative biological effectiveness (RBE) followed by a cone-down boost to 60–64 Gy(RBE), at 2 Gy(RBE) per fraction, for the low-risk and high-risk PTV (PTV-LR and PTV-HR), respectively. The supraclavicular region was electively treated when a tumor involved the upper thoracic esophagus or upper mediastinal lymph nodes metastasis. The lungs, heart, liver, and spinal cord were contoured for all patients; however, the kidneys were delineated for those with PTVs beyond the EGJ. The heart delineation started at the caudal edge of the left pulmonary artery, following the pericardium until it blended with the diaphragm.
2.2. Beam arrangement
For the IMXT plan, 6 MV photon beams were used. The typical seven beam angles of the IMXT plan were separated into five beams for co-planar (240°, 330°, 45°, 135°, and 60°) and two beams for non-coplanar anterior fields (10° and 350° with 270° couch rotation). However, the beam angles were adjusted for each patient depending on the tumor location.
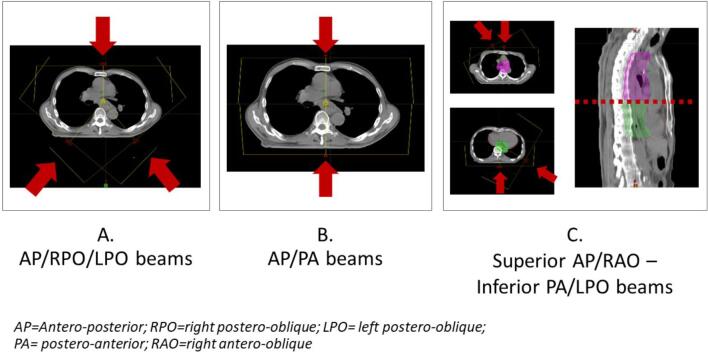
For the IMPT plan, the beam output was modulated using an RBE of 1.1. Three IMPT plans were generated using different beam angle configurations i.e., IMPT-A: anterior with posterior oblique beams (0°, 220°, and 140°), IMPT-B: anteroposterior opposing beams (0° and 180°), and IMPT-C: Superior anterior and anterior oblique (0° and 330° or 0° and 30°) -Inferior posterior and posterior oblique beams (180° and 120°) (Fig. 1S). In the IMPT-C plan, the superior and inferior beam paths were divided by the upper border of the heart. The aim of the superior beams was to treat the supraclavicular lymph node region, whereas the inferior beams avoided the heart. The superior and inferior beams were planned with a 2-cm overlapping field with a single isocenter optimization technique to avoid the uncertainties at the junction. Robust optimization (3.5% calibration curve error and 5 mm isocenter shift) was used to account for uncertainties and the interplay effect. Multifield optimization with a nonlinear universal proton optimizer was used in the IMPT technique. The criteria for dose optimization were 95% of the PTV volumes receiving the prescribed dose and maximum dose receiving lower than 107%. To evaluate the plan’s robustness, we simulated 14 error scenarios to account for possible setup and range uncertainties (3.5% for proton range uncertainties with 5 or 0 mm for setup uncertainties). In this study, the robustness evaluation criteria was that the CTV received >95% and 90% of the prescribed dose for CTV-HR and CTV-LR, respectively, in the worst-case scenario [27]. The doses in all plans were kept as low as possible to spare the lung, heart, and spinal cord.
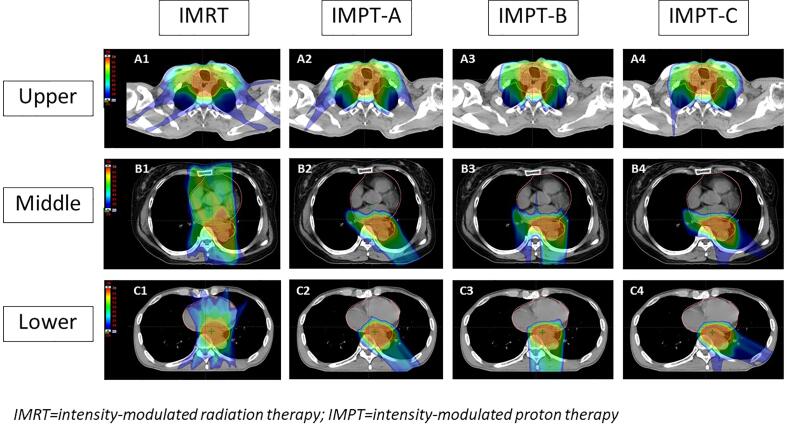
Fig. 1.
Dose distributions of the IMXT and three IMPT plans in upper (A), middle (B), lower (C) thoracic esophageal cancer.
The dose distribution of the four plans, one IMXT and three IMPT plans, based on tumor location, are depicted in Fig. 1. The treatment plans were calculated using the Eclipse™ treatment planning system (version 15.0, Varian Medical System, Palo Alto, CA).
2.3. Dose-volume analysis
The dose-volume data for the tumor and normal organs were collected from each treatment plan. The target coverage was represented by the dose covering at least 100% (D100%) of the CTVs and D95% of the PTVs. For the OARs, the mean dose and volume of the structures receiving at least × Gy (Vx) of the lungs, heart, liver, and kidneys were collected. The dose to 1 cm3 (D1 cm3) of the spinal cord was calculated. To demonstrate the effects of each plan on the heart dose, sub-group analysis was performed for tumors in the superior location (PTVs above the contoured heart) and the inferior location (PTVs overlapping a part of the contoured heart).
2.4. Normal tissue complication probability
To estimate the clinical significance of IMPT, the normal tissue complication probability (NTCP) was calculated and compared with IMXT for the heart and lung toxicity for each patient. A review of the literature revealed that there were only two NTCP models for predicting heart and lung toxicity in esophageal cancer patients [28], [29]. For postoperative lung and heart complications, the clinical and dosimetric data from 601 patients were analyzed using multivariable logistic regression analysis to define the predictive factors. The significant predictors for lung toxicity were mean lung dose (MLD), age, body mass index (BMI), and histology (squamous cell carcinoma or adenocarcinoma) with an area under the receiver operating characteristics (AUC) of 0.79. For postoperative cardiac toxicity, age was the only predictor. A published NTCP model for pericardial effusion was derived from 297 esophageal cancer patients that were treated with definitive chemoradiation of ≥50 Gy and followed for ≥6 months. Mean heart dose (MHD) was a significant predictor for any grade and symptomatic (grade ≥ 3) pericardial effusion with an AUC of 0.73–0.82 [29]. The NTCP model parameters are presented in Supplement Table 1S.
2.5. Statistical analysis
The Shapiro Wilk test was used to determine the normal distribution of the data. Dose-volume parameters of the target volumes and OARs, as well as the NTCP values were compared using the paired t-test for normally distributed data; and the Wilcoxon signed-rank test was used for non-normally distributed data. A two-sided p-value threshold of less than 0.05 was used for statistical significance. The statistical analyses were performed using STATA version 15.1 (StataCorp LLC, Texas, USA).
3. Results
With similar target coverage, all IMPT plan techniques resulted in a significantly reduced dose to the OARs compared with IMXT. When the dose-volume parameters were compared between the IMPT plans, the IMPT-B plan achieved the lowest lung dose (MLD = 7.1 Gy(RBE)) and the IMPT-C plan resulted in the lowest heart dose (MHD = 12.8 Gy(RBE)), liver dose (mean liver dose = 3.9 Gy(RBE)), and spinal cord dose (D1cm3 = 36.8 Gy(RBE)). In eight patients with distal thoracic esophageal tumors, the left kidney dose was significantly lower in the IMPT plans (mean kidney dose = 8.6 Gy in IMXT vs. 3.1–3.3 Gy(RBE) in IMPT), whereas the right kidney dose was very low in all plans because the distal location and gastrohepatic lymph node deviated to the left side of the abdomen. The dose-volume parameters in the IMXT and three IMPT plans are presented in Table 1.
Table 1.
Dose-volume parameter comparison between the IMXT and IMPT plans.
| IMXT | IMPT-A | IMPT-B | IMPT-C | ||
|---|---|---|---|---|---|
| CTV-LR | D100%, Gy(RBE) | 54.0 | 54.9 | 53.9 | 54.4 |
| CTV-HR | D100%, Gy(RBE) | 62.5 | 63.4 | 63.2 | 63.3 |
| PTV-LR | D95%, Gy(RBE) | 54.4 | 55.2 | 54.4 | 54.9 |
| PTV-HR | D95%, Gy(RBE) | 62.1 | 63.6* | 63.6* | 63.5* |
| Lung | Mean, Gy(RBE) | 15.0 | 9.4* | 7.1* | 8.2* |
| V5 (%) | 69.0 | 39.2* | 21.7* | 29.0* | |
| V10 (%) | 50.6 | 29.5* | 18.3* | 23.2* | |
| V15 (%) | 37.5 | 22.7* | 16.0* | 18.9* | |
| V20 (%) | 27.5 | 17.4* | 14.1* | 16.2* | |
| V30 (%) | 14.6 | 10.3* | 10.6* | 10.8* | |
| V40 (%) | 7.8 | 6.3* | 7.6 | 7.3 | |
| Heart | Mean, Gy(RBE) | 27.1 | 13.2* | 14.5* | 12.8* |
| V10 (%) | 71.5 | 35.5* | 38.6* | 27.9* | |
| V20 (%) | 60.8 | 22.8* | 26.2* | 20.4* | |
| V30 (%) | 44.1 | 16.4* | 18.7* | 15.7* | |
| V40 (%) | 27.9 | 12.5* | 13.9* | 12.1* | |
| V50 (%) | 16.4 | 9.3* | 10.1* | 9.1* | |
| Liver | Mean, Gy(RBE) | 10.4 | 5.4* | 4.6* | 3.9* |
| V5 (%) | 42.9 | 19.3* | 14.4* | 12.4* | |
| V10 (%) | 29.9 | 15.8* | 12.7* | 10.2* | |
| V20 (%) | 17.4 | 9.6* | 10.0* | 7.7* | |
| V30 (%) | 11.4 | 6.3* | 7.3* | 5.9* | |
| Spinal cord | D1cm3, Gy(RBE) | 42.0 | 39.7 | 38.0* | 36.8* |
| Right kidney (N = 8) | Mean, Gy(RBE) | 2.0 | 0.9* | 0.7 | 0.1* |
| V10 (%) | 3.3 | 2.7 | 7.4 | 10.9 | |
| Left kidney (N = 8) | Mean, Gy(RBE) | 8.6 | 3.3* | 3.1* | 3.3* |
| V10 (%) | 30.7 | 10.4* | 10.9* | 10.7* | |
| V20 (%) | 11.4 | 3.3* | 4.9 | 4.8 | |
| V30 (%) | 7.7 | 1.9 | 2.4 | 2.4 |
*significance level, p-value < 0.05 (compared with IMXT).
Dose-volume parameters are presented as mean value and the paired t-test was used to compare the IMXT and IMPT results.
Abbreviations: IMXT = intensity-modulated radiation therapy; IMPT = intensity modulated proton therapy; CTV = clinical target volume; PTV = planning target volume; LR = low-risk; HR = high-risk; RBE = relative biological effectiveness; Dx = dose that × volume received; Vx = volume receiving × Gy(RBE).
For the superior tumor location (above the contoured heart), the IMPT-B plan had better lung-sparing (MLD = 6.0 Gy(RBE)) compared with the other plans (IMXT = 12.5 Gy(RBE), IMPT-A = 7.2 Gy(RBE), and IMPT-C = 6.5 Gy(RBE)). For the inferior location, the IMPT-C plan generated the lowest heart dose parameters (MHD = 13.6 Gy(RBE) vs. IMXT = 32.9 Gy(RBE), IMPT-A = 16.0 Gy(RBE), and IMPT-B = 17.5 Gy(RBE)) with a slightly higher lung dose than the IMPT-B plan (MLD = 8.7 Gy(RBE) in IMPT-C vs. 7.4 Gy(RBE) in IMPT-B). The dose-volume data between each treatment plan on the lung and heart dose are shown in Table 2.
Table 2.
Comparison of the lung and heart doses between the IMXT and IMPT plans according to tumor location.
|
Superior location (N = 5) |
Inferior location (N = 20) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IMXT | IMPT-A | IMPT-B | IMPT-C | IMXT | IMPT-A | IMPT-B | IMPT-C | ||
| Heart | Mean, Gy(RBE) | 3.9 | 2.3* | 2.4* | 9.7 | 32.9 | 16.0* | 17.5* | 13.6* |
| V10 (%) | 10.8 | 7.5 | 8.0 | 7.3 | 86.6 | 42.4* | 46.2* | 33.1* | |
| V20 (%) | 5.1 | 4.3 | 4.7 | 4.1 | 74.8 | 27.4* | 31.5* | 24.5* | |
| V30 (%) | 2.1 | 2.3 | 2.6 | 2.3 | 54.6 | 20.0* | 22.7* | 19.1* | |
| V40 (%) | 1.0 | 1.1 | 1.2 | 1.1 | 34.7 | 15.3* | 17.1* | 14.8* | |
| V50 (%) | 0.3 | 0.4 | 0.4 | 0.4 | 20.4 | 11.6* | 12.5* | 11.3* | |
| Lung | Mean, Gy(RBE) | 12.5 | 7.2* | 6.0* | 6.5* | 15.6 | 10.0* | 7.4* | 8.7* |
| V5 (%) | 52.2 | 27.3* | 16.9 * | 23.1* | 73.3 | 42.2* | 22.9* | 30.4* | |
| V10 (%) | 41.2 | 20.6* | 14.8* | 16.4* | 53.0 | 31.7* | 19.1* | 24.8* | |
| V15 (%) | 32.6 | 16.1* | 13.1* | 13.4* | 38.7* | 24.4* | 16.7* | 20.3* | |
| V20 (%) | 24.3 | 13.1* | 11.6* | 11.6* | 28.3* | 18.5* | 14.8* | 17.3* | |
| V30 (%) | 12.7 | 8.7 | 8.9* | 8.7* | 15.1 | 10.7* | 11.1* | 11.3* | |
| V40 (%) | 6.9 | 5.8 | 6.5 | 6.2 | 8.0 | 6.5* | 7.9 | 7.6 | |
*significance level, p-value < 0.05 (as compared with IMXT).
Dose-volume parameters are presented as mean value and the paired t-test was used to compare the IMXT and IMPT results.
Abbreviations: IMXT = intensity-modulated radiation therapy; IMPT = intensity modulated proton therapy.
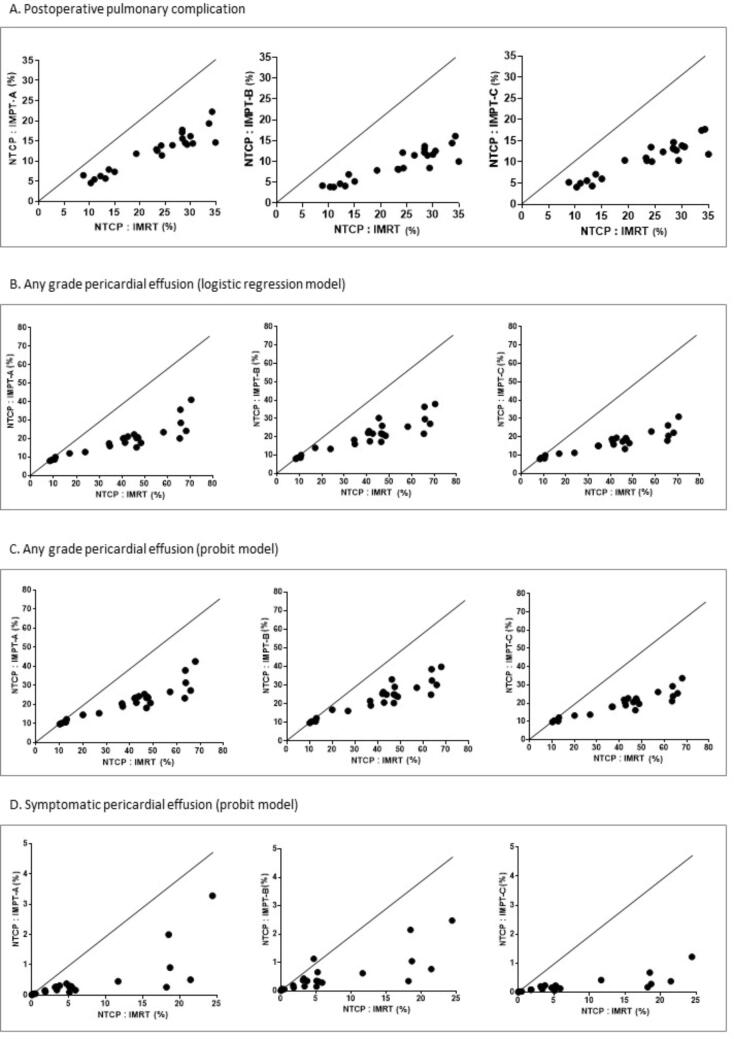
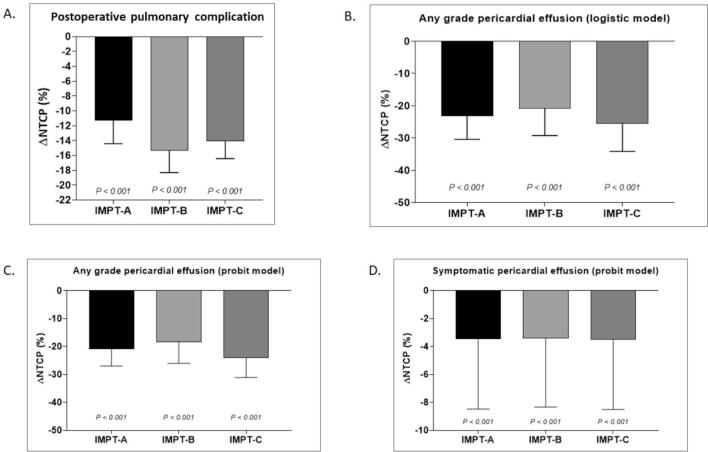
The IMPT plans resulted in a significantly lower risk of lung and heart toxicity compared with IMXT treatment based on the NTCP model. The risk for postoperative pulmonary complication and any grade pericardial effusion was reduced by more than 10% whereas the risk for symptomatic (grade ≥ 3) pericardial effusion was slightly reduced. The NTCP values and NTCP differences (ΔNTCP) for the IMXT and three IMPT plans for heart and lung are as summarized in Table 3 and illustrated in Supplement Figs. 2S and 3S.
Table 3.
NTCP and NTCP difference between the IMXT and IMPT plans.
| Median of NTCP (IQR) (%) | Median of ΔNTCP (IQR) (%) | p-value | |
|---|---|---|---|
| NTCP postoperative pulmonary complication | |||
| IMXT | 26.5 (15–30) | reference | |
| IMPT-A | 13.8 (7.9–15.5) | −11.3 (−14.4 to −7.5) | <0.001 |
| IMPT-B | 9.9 (6.9–12.1) | −15.4 (−18.2 to −9.8) | <0.001 |
| IMPT-C | 11.7 (7.1–13.5) | −14.1 (−16.2 to −8.9) | <0.001 |
| NTCP any grade pericardial effusion (logistic model) | |||
| IMXT | 42.6 (23.8–48.4) | reference | |
| IMPT-A | 20.0 (12.7–21.0) | –23.2 (−30.1 to −11.2) | <0.001 |
| IMPT-B | 21.4 (14.0–25.6) | −20.8 (−29.1 to −10.4) | <0.001 |
| IMPT-C | 17.4 (11.3–19.4) | −25.5 (–33.0 to −12.6) | <0.001 |
| NTCP any grade pericardial effusion (MHD-based model) | |||
| IMXT | 43.9 (27–48.8) | reference | |
| IMPT-A | 23.2 (15.3–24.2) | −20.9 (−25.9 to −11.6) | <0.001 |
| IMPT-B | 24.6 (16.8–28.6) | −18.5 (−25.2 to −10.8) | <0.001 |
| IMPT-C | 20.5 (13.7–22.5) | −24.0 (−31.0 to −13.3) | <0.001 |
| NTCP symptomatic pericardial effusion (MHD-based model) | |||
| IMXT | 3.8 (0.5–5.9) | reference | |
| IMPT-A | 0.3 (0–0.3) | −3.4 (−5.7 to −0.4) | <0.001 |
| IMPT-B | 0.3 (0.1–0.6) | −3.4 (−5.6 to −0.4) | <0.001 |
| IMPT-C | 0.2 (0–0.2) | −3.5 (−5.8 to −0.5) | <0.001 |
Abbreviations: NTCP = normal tissue complication probability; ΔNTCP = NTCP difference; IMXT = intensity-modulated radiation therapy; IMPT = intensity modulated proton therapy; IQR = interquatile range.
When the patients were stratified based on tumor location, the IMPT plans reduced the risk of postoperative pulmonary complication by half in both subgroups. Although the IMPT-C plan slightly reduced the cardiac risk for superior tumors, it demonstrated remarkable improvement for inferior tumors. The ≥10% risk reduction of any grade pericardial effusion was seen in 100% of the patients in the inferior tumor location group (Supplement Table 2S and 3S).
4. Discussion
The results of the present study revealed the dosimetric and potential clinical benefit of IMPT for treating thoracic esophageal cancer using high-dose (≥60 Gy) radiation compared with IMXT. Among the three different beam configurations, the IMPT-B plan generated the lowest lung dose and the IMPT-C plan resulted in the optimal OARs sparing with the lowest heart dose.
The proton therapy beam configurations recommended by several studies included anteroposterior opposing beams, anterior or posterior with posterior oblique beams, or posterior with left lateral beams. However, most studies evaluated patients with cancer of the distal esophagus or EGJ and prescribed a standard radiation dose (50.4 Gy or less) [13], [17], [24], [25]. Compared with Western countries, in the Asian population many esophageal cancer tumors are not considered resectable and receive a high OARs dose due to the relatively larger tumor size, upper-mid thoracic location, and supraclavicular region irradiation. A meta-analysis found that a radiation dose ≥60 Gy improved treatment outcomes in esophageal carcinoma patients [35]. Therefore, dose escalation using advanced radiation techniques to improve tumor control and spare OARs is very challenging in Asian patients. The IMPT-C plan was designed for tumors that contained PTVs below the level of the heart. The PTVs were divided into superior and inferior portions using the top of the heart contour and were treated anteriorly with anterior oblique beams and posteriorly with posterior oblique beams. The superior anterior with anterior oblique beams treated the supraclavicular region without a radiation dose passing through the heart. We did not use a posterior beam for the supraclavicular region because of the heterogeneity along the beam path in the upper lung region and potential set up errors.
Our results demonstrated that the IMPT-B plan generated the lowest lung dose regardless of tumor location, which was consistent with other studies [17], [24]. The IMPT-C plan resulted in the best cardiac sparing, particularly when the PTVs partially covered the posterior part of the heart or inferior tumor location. The specific advantages of each IMPT plan indicate that selecting the optimal plan should be individualized based on the anatomy of the tumor and the patient’s condition. For patients with lung disease, the anteroposterior opposing beam configuration (IMPT-B) might be most appropriate to minimize lung toxicity. For inferior tumors or patients with underlying cardiac disease, we recommend the IMPT-C plan for a lower heart dose.
The anteroposterior opposing beam arrangement was used in several studies because this arrangement generated the least lung dose; however, these beams resulted in an increased heart dose compared with posterior beam angles [12], [13], [17], [30]. The single posterior beam and posterior with lateral or oblique beam usually resulted in the optimal lung and heart dose [13], [16], [17], [30], [31]. Wang et al. stratified their patients based on tumor location and found that PSPT lowered the mean heart dose in mid and distal esophageal lesions from 24.5 Gy and 19.7 Gy using the IMXT plan to 5.1 GyRBE and 12.8 GyRBE using the PSPT plan [18]. Compared with similar tumor locations in our study, the mean heart dose in the IMPT-C plan was 13.6 GyRBE, which was slightly higher than in Wang et al., despite the PTV dose escalation in our study (60–64 Gy).
For more modern PBT techniques, an early IMPT study performed in 10 patients at the MD Anderson Cancer Center escalated the radiation dose to the tumor (50.4 GyRBE to the PTV and simultaneously integrated the GTV boost to 65.8 GyRBE) [17]. Three beam configurations were used. The anteroposterior opposing beams resulted in the lowest lung dose, the posterior oblique beams had the best heart sparing, and the anterior with posterior oblique beams were optimal for sparing the heart and lung. The authors concluded that selecting a unique IMPT plan for each patient should be based on the patient’s anatomy and preexisting pulmonary or cardiac disease. However, the patients in their study had EGJ tumors. In contrast, our patients had tumors in the upper and mid thoracic location that had a risk for increased mean heart dose, compared with EGJ tumors [12]. Another high-dose PBT study reported a lower lung dose, but a higher heart dose, compared with our study [10]. However, because the data of the PBT technique or beam arrangement and the use of an in-house treatment planning system were not available, it was difficult to directly compare their results with ours. Several dosimetric studies of proton treatment for esophageal carcinoma are presented in Supplement Table 4S.
The NTCP model is a valuable tool to translate the dosimetric advantage of IMPT into a clinical benefit. The NTCP model-based approach has been used as a strategy to select patients who will potentially benefit from PBT [32], [33], [34]. Our results confirmed the clinical benefit of IMPT because there was a significant reduction in NTCP for the heart and lungs, which was consistent with previous studies [11], [25]. Unlike other prior studies, the strength of this study is the use of NTCP models that were derived from esophageal cancer patients [28], [29]. Applying a common threshold of ΔNTCP > 10 percentage points to select PBT [32], [35], at least two-thirds of the patients would have benefited from IMPT based on NTCP reduction of the lung or heart toxicity. The risk of pericardial effusion was reduced for all patients, and especially for the inferior tumor location, regardless of IMPT beam configuration (Supplement Table 3S).
The limitations of this study were the small number of patients and the clinical outcome of the proposed IMPT beam configurations could not be determined. Furthermore, the effects of the proton therapy uncertainties, including range and setup uncertainty in a highly heterogeneous area of the chest were not evaluated. However, accurate radiation doses were safely delivered to the patients with robust optimization and proper motion management. The potential benefits of high-dose IMPT for esophageal cancer will be investigated in a prospective randomized trial at our institution (Thai Clinical Trials Registry: TCTR 20200310006).
In conclusion, IMPT provided good target coverage, better surrounding organ sparing, and less NTCP compared with IMXT when high-dose radiation was given. Selecting anteroposterior opposing (IMPT-B) or superior anterior with anterior oblique beams and inferior posterior with posterior oblique beams (IMPT-C) to treat thoracic esophageal cancer depends on tumor location.
Funding
This Research was funded by the Thailand Science research and Innovation Fund Chulalongkorn University CU_FRB65_hea (30)_037_30_18.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
English editing service, Research Affairs, Faculty of Medicine, Chulalongkorn University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2022.04.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49. doi: 10.3322/caac.21660. [DOI] [PubMed]
- 2.Shapiro J., van Lanschot J.J.B., Hulshof M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Eyck B.M., van Lanschot J.J.B., Hulshof M., van der Wilk B.J., Shapiro J., van Hagen P., et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. doi: 10.1200/JCO.20.03614. [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Budach W. Definitive chemoradiotherapy. J Thorac Dis 2017;9:S792-s8. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed]
- 5.Welsh J., Settle S.H., Amini A., Xiao L., Suzuki A., Hayashi Y., et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–2640. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CY, Li CC, Chien CR. Does higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? a population based propensity-score matched analysis. Radiother Oncol 2016;120:136-9. doi:10.1016/j.radonc.2016.04.042. [DOI] [PubMed]
- 7.Hulshof MCCM, Geijsen D, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, et al. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J Clin Oncol 2021;39:2816-24.doi:10.1200/JCO.20.03697. [DOI] [PubMed]
- 8.Lertbutsayanukul C., Tharavej C., Klaikeaw N., Prayongrat A., Lowanitchai C., Sriuranpong V. High dose radiation with chemotherapy followed by salvage esophagectomy among patients with locally advanced esophageal squamous cell carcinoma. Thorac Cancer. 2017;8:219–228. doi: 10.1111/1759-7714.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Wang L, Wang Y, Kang J, Jiang W, Men Y, et al. High vs. low radiation dose of concurrent chemoradiotherapy for esophageal carcinoma with modern radiotherapy techniques: a meta-analysis. Front Oncol 2020;10:1222. doi: 10.3389/fonc.2020.01222. [DOI] [PMC free article] [PubMed]
- 10.Hirano Y, Onozawa M, Hojo H, Motegi A, Zenda S, Hotta K, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol 2018;13:23. doi: 10.1186/s13014-018-0966-5. [DOI] [PMC free article] [PubMed]
- 11.Makishima H., Ishikawa H., Terunuma T., Hashimoto T., Yamanashi K., Sekiguchi T., et al. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res. 2015;56:568–576. doi: 10.1093/jrr/rrv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol 2017;125:48-54. doi: 10.1016/j.radonc.2017.07.034. [DOI] [PubMed]
- 13.Wang J., Palmer M., Bilton S., Vu K., Greer S., Frame R., et al. Comparing proton beam to intensity modulated radiation therapy planning in esophageal cancer. Int J Part Ther. 2015;1:866–877. doi: 10.14338/IJPT-14-00018.1. [DOI] [Google Scholar]
- 14.Bhangoo RS, DeWees TA, Yu NY, Ding JX, Liu C, Golafshar MA, et al. Acute toxicities and short-term patient outcomes after intensity-modulated proton beam radiation therapy or intensity-modulated photon radiation therapy for esophageal carcinoma: a Mayo Clinic experience. Adv Radiat Oncol 2020;5:871-9. doi: 10.1016/j.adro.2020.04.026. [DOI] [PMC free article] [PubMed]
- 15.Lin SH, Merrell KW, Shen J, Verma V, Correa AM, Wang L, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol 2017;123:376-81. doi: 10.1016/j.radonc.2017.04.013. [DOI] [PubMed]
- 16.Prayongrat A, Xu C, Li H, Lin SH. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: a single institutional experience. Adv Radiat Oncol 2017;2:301-7. doi: 10.1016/j.adro.2017.06.002. [DOI] [PMC free article] [PubMed]
- 17.Welsh J., Gomez D., Palmer M.B., Riley B.A., Mayankkumar A.V., Komaki R., et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81:1336–1342. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xi M., Xu C., Liao Z., Chang J.Y., Gomez D.R., Jeter M., et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: a retrospective, single-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99:667–676. doi: 10.1016/j.ijrobp.2017.06.2450. [DOI] [PubMed] [Google Scholar]
- 19.Lin S.H., Hobbs B.P., Verma V., Tidwell R.S., Smith G.L., Lei X., et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bert C., Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol. 2011;56:R113–R144. doi: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- 21.Thomas M., Defraene G., Levis M., Sterpin E., Lambrecht M., Ricardi U., et al. A study to investigate the influence of cardiac motion on the robustness of pencil beam scanning proton plans in oesophageal cancer. Phys Imaging Radiat Oncol. 2020;16:50–53. doi: 10.1016/j.phro.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Møller DS, Alber M, Nordsmark M, Nyeng TB, Lutz CM, Hoffmann L. Validation of a robust strategy for proton spot scanning for oesophageal cancer in the presence of anatomical changes. Radiother Oncol 2019;131:174-8. doi: 10.1016/j.radonc.2018.09.018. [DOI] [PubMed]
- 23.Celik E, Baus W, Baues C, Schröder W, Clivio A, Fogliata A, et al. Volumetric modulated arc therapy versus intensity-modulated proton therapy in neoadjuvant irradiation of locally advanced oesophageal cancer. Radiat Oncol 2020;15:120. doi: 10.1186/s13014-020-01570-y. [DOI] [PMC free article] [PubMed]
- 24.Zhang X., Zhao K.L., Guerrero T.M., McGuire S.E., Yaremko B., Komaki R., et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng H., Sio T.T., Rule W.G., Bhangoo R.S., Lara P., Patrick C.L., et al. Beam angle comparison for distal esophageal carcinoma patients treated with intensity-modulated proton therapy. J Appl Clin Med Phys. 2020;21:141–152. doi: 10.1002/acm2.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Liao Z., Jin J., Ajani J., Chang J.Y., Jeter M., et al. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:656–664. doi: 10.1016/j.ijrobp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Li H, Li Y, Li Y, Chang Y, Li Q, et al. Statistical evaluation of worst-case robust optimization intensity-modulated proton therapy plans using an exhaustive sampling approach. Radiat Oncol 2019;14:129. doi: 10.1186/s13014-019-1335-8. [DOI] [PMC free article] [PubMed]
- 28.Thomas M, Defraene G, Lambrecht M, Deng W, Moons J, Nafteux P, et al. NTCP model for postoperative complications and one-year mortality after trimodality treatment in oesophageal cancer. Radiother Oncol 2019;141:33-40. doi: 10.1016/j.radonc.2019.09.015. [DOI] [PubMed]
- 29.Fukada J, Fukata K, Koike N, Kota R, Shigematsu N. Mean heart dose-based normal tissue complication probability model for pericardial effusion: a study in oesophageal cancer patients. Sci Rep 2021;11:18166. doi: 10.1038/s41598-021-97605-9. [DOI] [PMC free article] [PubMed]
- 30.Zeng YC, Vyas S, Dang Q, Schultz L, Bowen SR, Shankaran V, et al. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: clinical outcome, dosimetry, and feasibility. Strahlenther Onkol 2016;192:913-21. doi: 10.1007/s00066-016-1034-4. [DOI] [PubMed]
- 31.Ling T.C., Slater J.M., Nookala P., Mifflin R., Grove R., Ly A.M., et al. Analysis of intensity-modulated radiation yherapy (IMRT), proton and 3D conformal radiotherapy (3D-CRT) for reducing perioperative cardiopulmonary complications in esophageal cancer patients. Cancer. 2014;6:2356–2368. doi: 10.3390/cancers6042356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267–73. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed]
- 33.Prayongrat A., Umegaki K., van der Schaaf A., Koong A.C., Lin S.H., Whitaker T., et al. Present developments in reaching an international consensus for a model-based approach to particle beam therapy. J Radiat Res. 2018;59:i72–i76. doi: 10.1093/jrr/rry008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirato H., Le Q.T., Kobashi K., Prayongrat A., Takao S., Shimizu S., et al. Selection of external beam radiotherapy approaches for precise and accurate cancer treatment. J Radiat Res. 2018;59:i2–i10. doi: 10.1093/jrr/rrx092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobi A., Bandurska-Luque A., Stutzer K., Haase R., Lock S., Wack L.J., et al. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup normal tissue complication probability analysis. Int J Radiat Oncol Biol Phys. 2015;92:1165–1174. doi: 10.1016/j.ijrobp.2015.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.