Abstract
Objective: Evidence shows that patients with opioid use disorder (OUD) have an increased rate of discharge against medical advice (DAMA) as well as higher rates of hospital readmission. Therefore, the objective of this study was to determine if inpatient initiation of buprenorphine/naloxone in patients with OUD is associated with decreased rates of DAMA. Methods: This was a single center retrospective cohort study conducted at a level 1, academic medical center. The study included patients with OUD admitted to the Internal Medicine service from January through May of both 2018 and 2019 for an admitting diagnosis other than opioid withdrawal. The primary endpoint was rate of DAMA among OUD patients not initiated on opioid agonist therapy compared to those initiated on buprenorphine/naloxone. The secondary endpoint was the association between factors of the initiation process on rates of DAMA. Patients were excluded if they were discharged in less than 24 hours or received intermittent administration of buprenorphine/naloxone. Results: The rate of DAMA in OUD patients not initiated on buprenorphine/naloxone was 13.85% compared to 2.56% in those initiated on buprenorphine/naloxone (P = .048). Conclusion: In OUD patients initiated on buprenorphine/naloxone, the rate of DAMA was significantly lower than those who were not. This data supports the importance of optimizing the opportunity to initiate buprenorphine/naloxone in the acute care setting to minimize withdrawal symptoms therefore reducing the rate of DAMA. Ultimately increasing the ability to adequately treat the primary reason for admission and potentially decreasing readmission rates. Further studies are needed to evaluate this impact as this study is limited to a small sample size therefore lacking adequate power.
Keywords: opioid use disorder, initiation, inpatient, buprenorphine/naloxone
Introduction
The latest National Survey on Drug Use and Health released in September 2018 showed that 2.1 million people in the United States had an opioid use disorder in the year 2017. 1 Opioid use disorder (OUD) is defined in the Diagnostic and Statistical Manual of Mental Disorders, fifth Edition (DSM-5) as a problematic pattern of opioid use leading to clinically significant impairment or distress. 2 Studies have shown a dramatic increase in the rate of opioid-related emergency department visits often leading to inpatient stays.3,4 Although overdose is a common cause of mortality in these patients, it is important to realize this population has a high rate of morbidity due to chronic illness, as well as acute illnesses such as infection. 5 Increased exposure to the inpatient healthcare system serves as a possible opportunity for providers to initiate medication assisted treatment (MAT) for the short term goal of suppressing withdrawal symptoms and ultimate goal of decreasing opioid dependence.
Medication assisted treatment for OUD is a well-established first-line approach to treating opioid use disorder. Neither the Controlled Substances Act, as amended by the Drug Addiction Treatment Act (DATA 2000), nor Drug Enforcement Administration (DEA) regulations, Title 21 Code of Federal Regulations (CFR) 1306.07 impose any limitations on a physician or other authorized hospital staff to maintain or detoxify a person with buprenorphine as an incidental adjunct to medical or surgical conditions other than opioid dependency.
Approved medications for MAT include methadone, buprenorphine, and naltrexone. Often clinicians must consider the impact of patient-specific factors and treatment setting to determine the most appropriate choice of therapy. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor, exhibiting less respiratory depression than methadone. 6 When co-formulated with naloxone, a pure opioid antagonist, the risk of abuse potential and divergence is also decreased in comparison with methadone. In addition, buprenorphine products have fewer drug interactions, a more straight-forward dosing titration, and do not carry the risk of cardiac adverse effects compared to methadone. Naltrexone is an opioid-receptor antagonist available in injection formulation with no abuse or diversion potential. However, when buprenorphine was compared to naltrexone for maintenance treatment in a randomized controlled trial, buprenorphine resulted in a significantly greater time to first heroin use and retention in MAT compared to naltrexone. 7
A Cochrane review of buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence was conducted including 31 studies ranging from moderate to strong quality of evidence. The authors concluded that buprenorphine prescribed at fixed doses (above 7 mg per day) was not statistically different from methadone prescribed at fixed doses (40 mg or more per day) in retaining people in treatment or in suppression of illicit opioid use. However, the review of trials did find that buprenorphine dosed at 2 to 6 mg per day was less effective compared to methadone in retaining patients in treatment. 8 A randomized controlled trial studied buprenorphine/naloxone initiation during medical hospitalization and found a statistically significant increase in continuation of opioid agonist therapy during outpatient follow up as well as decreased illicit opioid use at 6 months. 9
In addition to the aforementioned possible benefits, the short-term goal of initiating buprenorphine/naloxone in an inpatient setting is to help alleviate withdrawal symptoms in order to facilitate treatment of their unrelated acute illness. Patients with substance abuse disorder have an increased rate of discharge against medical advice (DAMA) as well as a higher readmission rate. 10 The rate of DAMA in the general population is typically less than 4% however patients with substance use disorder was found to be over 20%. 11 Data to confirm that optimizing the opportunity to initiate buprenorphine/naloxone while inpatient would decrease rates of DAMA would have an impact on the ability to adequately treat the primary reason for admission. The objective of this investigation was to determine if the initiation of buprenorphine/naloxone in patients with OUD admitted to a major academic medical center is associated with decreased rates of DAMA.
Methods
This was a single center retrospective observational cohort study conducted at University of Louisville Hospital (ULH), a 404-bed academic teaching hospital and the area’s only level 1 trauma center. The study included patients with an International Classification of Disease (ICD)-10 code for OUD who were admitted to one of the four Internal Medicine teaching teams for an admitting diagnosis other than opioid withdrawal. Patients were excluded if they were under 18 years of age, pregnant, discharged in less than 24 hours, or had documented hypersensitivity to buprenorphine, naloxone or any component of the formulation. Patients were also excluded if they received intermittent administration of buprenorphine/naloxone; instances such as being initiated on buprenorphine/naloxone and then therapy being temporary discontinued due to intubation or acute surgical management requiring intravenous opioids. Timeframe for inclusion was January through May of 2018, during which the use of buprenorphine products were restricted to continuation of home medication at ULH, and January through May of 2019, after an Internal Medicine initiative to initiate opioid agonist therapy with buprenorphine/naloxone in appropriate patients, regardless of use prior to hospitalization, was enacted. This included a grant through Kentucky Opioid Response Effort (KORE) that provided social workers to specifically address substance use, assess for MAT interest and connect with outpatient support.
The primary endpoint was rate of DAMA among OUD patients not initiated on opioid agonist therapy compared to those initiated on buprenorphine/naloxone. The secondary endpoint was the association between factors of the initiation process such as COWS score at time of buprenorphine/naloxone initiation, social work consult, titration of dose on day 1, stable dose in milligrams at discharge, and rates of DAMA.
Patient baseline characteristics at time of admission such as age, sex, race, and admitting diagnosis were collected. The following data was collected in regard to the buprenorphine/naloxone initiation process in addition to buprenorphine/naloxone dose administered and symptoms assessments: day buprenorphine/naloxone was initiated, if social work was consulted prior to initiation, daily dose in milligrams at discharge, reason for discharge (routine, left against medical advice, or expired), if routine discharge, was patient given prescription at discharge, and was outpatient opioid treatment program follow-up organized and included in the discharge summary.
The primary endpoint is a binomial response and was analyzed using a simple Chi-square test. Assuming a DAMA rate of 20% in OUD patients not initiated on buprenorphine/naloxone vs 5% in OUD patients initiated on buprenorphine/naloxone with a one-sided alpha of 0.05 to achieve 80% power, 75 patients were required in both groups. The secondary endpoints were analyzed using a Chi-square test for independence.
Results
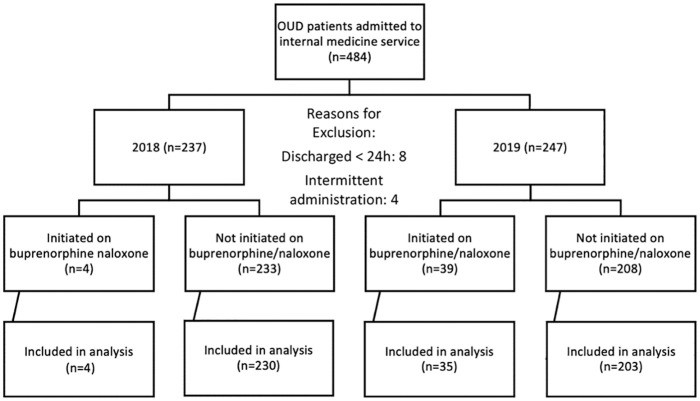
As seen in Figure 1, 237 patients with ICD-10 codes for OUD were admitted to an Internal Medicine service in 2018 and screened for inclusion. Of these patients, 4 patients initiated on buprenorphine/naloxone and 230 of the 233 not initiated on buprenorphine/naloxone met inclusion criteria for further analysis. In 2019, 247 patients with ICD-10 codes for OUD were admitted to an Internal Medicine service and screened for inclusion. Of these patients, 35 of the 39 patients initiated on buprenorphine/naloxone and 203 of the 208 not initiated on buprenorphine/naloxone met inclusion criteria for further analysis (Figure 1). Reasons for exclusion included 8 patients who were discharged within 24 hours and 4 patients who received intermittent administration of buprenorphine/naloxone secondary to being transferred to the intensive care unit and receiving intravenous opioids.
Figure 1.
Patient screening summary.
Baseline demographics are summarized in Table 1. Baseline demographics were similar between the 2 groups with a median age of 37 to 39 years old, balanced numbers of male and female, and majority Caucasian.
Table 1.
Baseline Demographics.
| 2018 (n = 234) | 2019 (n = 238) | |
|---|---|---|
| Age—years, mean (SD) | 37.4 (12.5) | 39.89 (12.9) |
| Gender, n (%) | ||
| >Male | 125 (53.4) | 132 (55.4) |
| >Female | 109 (46.6) | 106 (44.6) |
| Race, n (%) | ||
| >Caucasian | 198 (84.6) | 209 (87.8) |
| >African American | 36 (15.4) | 27 (11.3) |
| >Other | 0 (0) | 2 (0.9) |
Of the total 472 included patients, 433 were not initiated on buprenorphine/naloxone. Of these, 60 patients were discharged against medical advice corresponding to a rate of 13.85%. The overall rate of DAMA was similar between the 2 years, in 2018 it was 14.1% (33/234) compared to 11.76% (28/238) in 2019 (P = .506). Thirty-nine of the included patients were initiated on buprenorphine/naloxone. Of those patients, only 1 patient was discharged against medical advice corresponding to a rate of 2.56%. The difference in rate of DAMA among those not initiated on buprenorphine/naloxone of 13.85% compared to those initiated on buprenorphine/naloxone of 2.56% was found to be statistically significant with a P value of .048 corresponding to a number needed to treat (NNT) of 9 (Table 2).
Table 2.
Primary Outcome Data.
| Routine discharge | DAMA | Rate of DAMA | P value | NNT | |
|---|---|---|---|---|---|
| Initiated on buprenorphine/naloxone (n = 39) | 38 | 1 | 2.56% | .048 | 9 |
| Not initiated on buprenorphine/naloxone (n = 433) | 373 | 60 | 13.85% |
Table 3 includes the admitting diagnosis along with factors of the initiation process of the 25 patients initiated on buprenorphine/naloxone in 2019. Of patients with OUD admitted to an Internal Medicine service in 2019, 81.8% were due to an infectious cause. The average length of stay in patients initiated on buprenorphine/naloxone that were not DAMA was 9 days. Social work was consulted in 90.9% of the patients that were initiated on buprenorphine/naloxone. The mean COWS score at time of buprenorphine/naloxone initiation was 10, and a mean dose of 4 mg was initially administered. Repeat doses were administered as needed for continuing withdrawal for a mean total day 1 dose of 6.46 mg (±2.68). The mean dose at discharge was 9.18 mg (±4.11) for patients that did not leave DAMA. Patients were discharged with a 72-hour prescription for buprenorphine/naloxone with an outpatient follow-up appointment or sent directly to inpatient rehabilitation facility in 91% of patients. Two patients refused inpatient rehabilitation and did not have health insurance to cover the prescription, and 1 patient was transferred to another facility for valve replacement surgery due to endocarditis. The single patient that was DAMA received 1 dose of buprenorphine/naloxone immediately prior to leaving for acute withdrawal and uncontrolled back pain.
Table 3.
Secondary Outcome Data in Patients Initiated on Buprenorphine/Naloxone.
| Endpoint | DAMA (n = 1) | Not DAMA (n = 34) |
|---|---|---|
| Admitting diagnosis | ||
| ● Infectious, n (%) | 1 (100) | 28 (82.4) |
| ● Other, n (%) | 0 (0) | 6 (17.6) |
| Length of stay—days, mean (SD) | 5 | 9.06 (8.92) |
| Social work consulted, n (%) | 1 (100) | 31 (91) |
| Clinical opioid withdrawal scale score at time of first dose, mean (SD) | 10 | 10.4 (3.88) |
| Initial dose received—mg, mean (SD) | 4 | 4.14 (1.19) |
| Total dose received on day 1 of initiation—mg, mean (SD) | 4 | 6.46 (2.68) |
| Dose at discharge—mg, mean (SD) | N/A | 9.18 (4.11) |
Discussion
Failure to treat opioid withdrawal symptoms can exacerbate underlying medical problems and can result in patients leaving against medical advice without receiving adequate care. Inadequately treated comorbidities are associated with a threefold increase in one-year mortality. 12 Patients that have not completed treatment prior to discharge are often readmitted, usually with a more serious form of illness requiring a lengthier stay, which places a strain on the healthcare system. 12 Inpatient settings provide a unique opportunity to initiate opioid agonist therapy to manage acute withdrawal symptoms and engage those with opioid use disorder in treatment.
At University of Louisville Hospital, the use of buprenorphine containing products were restricted to continuation of a patient’s home regimen or initiation by psychiatry team. In June of 2018, after an Internal Medicine Faculty Grand Rounds on this topic, these faculty requested buprenorphine/naloxone be added to formulary without restriction. In October of 2018, Internal Medicine hospitalist physicians made initiation of buprenorphine/naloxone in patients with OUD a strategic planning aim and created guidelines as well as education for resident physicians and nursing staff. The physician teams have built close relationships with the KORE-grant funded substance use disorder social workers and case managers that assisted in in transitions of care to outpatient rehabilitation or medication assisted treatment programs.
The initiation of opioid agonist therapy for patients with opioid use disorder admitted to the hospital for another diagnosis is a relatively new practice. Liebschutz and colleagues studied the effects of administering buprenorphine during hospitalization and linking patients at discharge to outpatient clinics on engagement in outpatient opioid agonist therapy and decreased illicit opioid use. This study is the first, to our knowledge, to address the inpatient initiation of buprenorphine/naloxone and its impact on rates of DAMA. 8
The reduction in the primary outcome, rate of DAMA, in patients with opioid use disorder initiated on buprenorphine/naloxone compared to those that were not initiated was statistically significant. This result supports the benefit of initiating opioid agonist therapy in patients with OUD that are hospitalized for a diagnosis other than OUD. The secondary outcome of this trial, factors of the initiation process, that were to be tested for association on rates of DAMA could not be statistically analyzed as only 1 patient initiated on buprenorphine/naloxone was DAMA. However, this data was evaluated as an institution to analyze how our initiation process aligns with guideline recommendations and will inform future quality improvement projects.
Limitations of this study include its single-center, retrospective design with a small sample size that did not meet power. Secondly, there are many potential confounders as to why an individual would leave against medical advice other than to alleviate active withdrawal. However, the rate of DAMA in patients with OUD not initiated on buprenorphine/naloxone were similar between 2018 and 2019, 14.1% versus 13.85% respectively. This is consistent with previous trials that found the rate of DAMA in OUD patients to be 14%. 13 Lastly, this trial did not address whether patients not initiated on buprenorphine/naloxone were receiving opioid analgesia. However, if patients with OUD where receiving opioid analgesia it is possible that this would decrease withdrawal symptoms, subsequently reducing the probability of DAMA, which would ultimately decrease the difference in primary outcome.
As this study only focused on patients admitted to the internal medicine service, future studies are needed to evaluate the initiation of buprenorphine/naloxone in other populations such as in critical care areas. Another future area of exploration is the emerging interest in utilizing a different dosing scheme to optimize acute pain management in this population.
Conclusion
The rate of DAMA in patients with OUD decreased when initiated on buprenorphine/naloxone which was found to be statistically significant. This association supports the importance of optimizing the opportunity to initiate buprenorphine/naloxone in the acute care setting to minimize withdrawal symptoms, therefore reducing the rate of DAMA and ultimately increasing the ability to adequately provide optimal treatment for these patients. As this study is limited to a small sample size, therefore lacking adequate power, further studies are needed to evaluate the impact of buprenorphine/naloxone initiation on rates of DAMA in patients with OUD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lindsey B. Kays  https://orcid.org/0000-0001-9984-1616
https://orcid.org/0000-0001-9984-1616
References
- 1. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53); 2018. [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5.™ 5th ed. American Psychiatric Publishing, Inc. [Google Scholar]
- 3. Weiss AJ, Bailey MK, O’Malley L. Patient characteristics of opioid-related inpatient stays and emergency department visits nationally and by state, 2014. Healthcare Cost Utiliz Proj. Brief #224. 2017:2-17. [Google Scholar]
- 4. Brown AM, DeFrances C, Crane E, Cai R, Naeger S. Identification of substance-involved emergency department visits using data from the national hospital care survey. Natl Health Stat Rep. 2018;114:1-15. [PubMed] [Google Scholar]
- 5. Hser Y, Mooney LJ, Saxon AJ, et al. High mortality among patients with opioid use disorder in a large healthcare system. J Addic Med. 2017;11(4):315-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kampman K, Jarvis M. American society of addiction medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addic Med. 2015;9(5):358-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;371(9631):2192-2200. doi: 10.1016/s0140-6736(08)60954-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattick R, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2): CD002207. doi: 10.1002/14651858.cd002207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liebschutz JM, Crooks D, Herman D, et al. “Buprenorphine treatment for hospitalized, opioid-dependent patients.” JAMA Intern Med. 2014;174(8):1369. doi: 10.1001/jamainternmed.2014.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraut A, Fransoo R, Olafson K, Ramsey CD, Yogendran M, Garland A. A population-based analysis of leaving the hospital against medical advice: incidence and associated variables. BMC Health Serv Res. 2013;13: 415. doi: 10.1186/1472-6963-13-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franks P, Meldrum S, Fiscella K. Discharges against medical advice: are race/ethnicity predictors? J Gen Intern Med. 2006;21:955-960. doi: 10.1111/j.1525-1497.2006.00505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PloS One. 2011;6:e24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu H, Wu L. National trends and characteristics of inpatient detoxification for drug use disorders in the United States. BMC Pub Health. 2018;18:1073. doi: 10.1186/s12889-018-5982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]