Abstract
Objectives. The objectives of this study were (1) to assess the impact of the 2016 Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain (GPOCP) on tramadol and opioid prescription rates in patients with chronic kidney disease (CKD), (2) to identify if tramadol was being properly dosed based on kidney function, and (3) to identify the number of clinically relevant drug-drug interactions related to tramadol. Design. Retrospective cohort study. Setting and Participants. Patients with a diagnosis of CKD stage IV or V or end-stage renal disease (ESRD) with a hospital discharge were identified. Participants were distributed into a pre-GPOCP cohort (January to December 2015) and post-GPOCP cohort (January 2017 to May 31, 2018) based on their hospital discharge date. Participants were then further divided into three categories: those who were discharged with a new prescription for tramadol, those who were discharged with a prescription for another opioid product, or those who were discharged with no new opioid or tramadol prescription. Outcome Measures. The primary outcome was incidence of new outpatient tramadol and opioid hospital discharge prescriptions. The secondary outcomes were the number of correctly dosed tramadol discharge prescriptions based on kidney function and incidence of clinically significant drug-drug interactions with tramadol. Results. New tramadol and opioid prescription rates upon hospital discharge for CKD stage IV and V and ESRD patients decreased from 76 (2.5%) to 54 (1.1%) and from 145 (4.7%) to 119 (2.5%), respectively (P < .001). Among the patients discharged with a new tramadol prescription, 113 (86.9%) patients did not have any clinically significant drug-drug interactions, and 94 (72.3%) patients were dosed correctly based on kidney function. Conclusion. The incidence of new outpatient tramadol and opioid prescriptions at discharge was significantly lower after the CDC GPOCP publication than before the publication.
Keywords: adverse drug reactions, adverse drug reactions reporting/monitoring, analgesics, disease management, education, medicaiton safety, monitoring drug therapy
Key Points
Background
Analgesic control for patients with chronic kidney disease often requires the use of opioids.
Numerous global and national initiatives are being proposed and reinforced to reduce opioid prescribing due to the Opioid Epidemic.
Tramadol provides better analgesic control than acetaminophen or non-steroidal anti-inflammatory drugs, but careful monitoring of drug-drug interactions and kidney function is required to minimize adverse effects, especially in patients with chronic kidney disease.
Findings
Tramadol and opioid hospital discharge prescription rates decreased in response to the 2016 Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain.
There is an opportunity to optimize tramadol dosing for patients with chronic kidney disease at hospital discharge.
Background
Pain management for patients with chronic kidney disease (CKD) is multifaceted and involves both biologic and psychological factors. 1 The prevalence of chronic pain in patients with advanced CKD is greater than 60%-70%, with most patients reporting moderate to severe pain. Common causes of pain in patients with severe CKD are kidney-related bone disease, peripheral artery disease, diabetic neuropathy, osteoarthritis, osteoporosis, and dialysis procedures. 2 A study in 2003 showed that approximately 75% of these patients were ineffectively treated for pain; 32% were prescribed no analgesics, 29% were prescribed nonopioid analgesics, and 26% were prescribed weak opioids. 3 However, a 2017 retrospective cohort study assessing opioid prescription rates in patients with end-stage renal disease (ESRD) between 2006 and 2010 showed that greater than 60% of patients with ESRD had at least one opioid prescription filled annually, of which approximately 20% were for chronic pain. 4 This trend indicated that opioid prescriptions in the CKD patient population were increasing during this time.
Analgesic control for patients with CKD can be problematic because they are more sensitive to medication changes, have altered pharmacokinetics, and are at an increased risk for adverse effects. 2 In addition, minimal guidance is available regarding the selection of an appropriate analgesic agent for patients with CKD, which could be another key reason why a majority of these patients are ineffectively treated for pain. Proper pharmacologic nociceptive analgesic management for CKD patients involves the World Health Organization (WHO) three-step analgesic ladder. This strategy starts with nonopioid treatment measures, such as acetaminophen, and then escalates to step two, which involves weak-opioid options, such as tramadol and low-dose oxycodone. High-dose opioids are used as last-line agents in step three. 2
In 2016, the Centers for Disease Control and Prevention (CDC) published the Guideline for Prescribing Opioids for Chronic Pain (GPOCP). 5 The rationale behind this document was to educate healthcare providers on a safe and effective approach to chronic pain management, which would ultimately lead to a reduction in the number of opioids prescribed. Since then, numerous initiatives and restrictions, both statewide and nationally, have been enforced to ensure safe opioid prescribing. 6 While beneficial in preventing adverse effects related to opioids, the GPOCP and other restrictions have made it more challenging to prescribe opioids for patients requiring them.
Prescribers, as a response to the GPOCP, may have started favoring tramadol for pain management, as it provides a better analgesic effect than acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) and is not associated with as much negative stigma as other pure opioid products, although this drug is also an opioid. 2 Tramadol is often viewed as a “safer” alternative to other low-dose opioid options, although no direct, comparative evidence exists confirming that tramadol produces less adverse effects than other opioid products in patients with CKD. A recent study demonstrated that tramadol had similar risks for prolonged opioid use compared to other short-acting opioids in postoperative patients, which puts into question the true safety profile of tramadol. 7 In addition, tramadol requires renal dose adjustments and is associated with significant drug-drug interactions due to its unique mechanism of action that inhibits the reuptake of serotonin and norepinephrine as well as the μ-opiate receptor blockade in the central nervous system (CNS). 8 Both of these caveats are concerning for patients with CKD since they are often on numerous medications and have impaired drug clearance of renally eliminated medications.
Objectives
The objectives of this study were (1) to assess the impact of the 2016 CDC GPOCP on tramadol and opioid prescription rates in patients with CKD, (2) to identify if tramadol was being properly dosed based on kidney function, and (3) to identify the number of clinically-relevant drug-drug interactions related to tramadol.
Methods
This study was approved by the Saint Joseph Mercy Hospital Institutional Review Board and was a retrospective cohort study. Patients 18 years of age or older who were discharged with a diagnosis of CKD stage IV or V or ESRD (glomerular filtration rate 0-29 mL/minutes/1.73 m2) were identified. Included participants had a discharge from 1 of the 4 included hospitals between January 2015 and May 2018 (Table 1). The study population was then divided into 2 cohorts: those patients discharged prior to (January to December 2015) and after (January 2017 to May 2018) the release of the GPOCP. No data from 2016 were included because it represented a ramp-up year in which our prescribers adopted the GPOCP. There was an email that was sent to the hospitalists that summarized the CDC recommendations in the Spring of 2016. The primary end point of this study was to compare the incidence rates of new outpatient opioid and tramadol prescriptions upon hospital discharge between the pre-GPOCP and post-GPOCP cohorts in patients with CKD stage IV and V and ESRD. There were no institutional or statewide restrictions on opioid prescribing during this study period.
Table 1.
Studied Hospitals.
| Hospital name | Location | Number of licensed beds |
|---|---|---|
| Saint Joseph Mercy | Ann Arbor, MI, USA | 537 |
| Saint Joseph Mercy | Chelsea, MI, USA | 133 |
| Saint Joseph Mercy | Howell, MI, USA | 136 |
| Saint Mary Mercy | Livonia, MI, USA | 304 |
Sample demographics were summarized for each cohort using means and standard deviations for interval-level variables and frequencies and percentages for categorical variables. Independent samples t-tests and chi-square tests were performed to determine if there were significant differences in the demographic characteristics between the 2 cohorts. The rates of tramadol and opioid prescriptions in the pre-GPOCP cohort were assessed to verify that there were no downward or upward trends in prescription practices prior to the GPOCP that would confound the comparison.
Within the two cohorts, the samples were further divided into 3 treatment groups: those prescribed tramadol with or without another opioid prescription at discharge; those prescribed an opioid without a new prescription for tramadol; and those with no new opioid or tramadol prescription. The primary endpoint was assessed using a chi-square test of independence. Counts of patients in the 3 treatment groups were conducted for the pre- and post-GPOCP timeframes, and the chi-square test was performed to determine if the distribution of patients in the 3 treatment groups was significantly different between the 2 cohorts.
Secondary end points of the study were the number of clinically significant drug-drug interactions involving tramadol in CKD stage IV and V and ESRD patients and the percentage of new tramadol prescriptions at discharge that were dosed correctly based on kidney function for only those patients whose creatinine clearance (CrCL) was less than 30 mL/minutes. Clinically significant drug-drug interactions were defined as interactions that warranted increased monitoring of the patient for adverse effects or altered efficacy, as determined by our disease state experts, drug databases, and package inserts. Specific medications that were screened for included serotonergic agents, monoamine oxidase inhibitors, CNS depressants, and carbamazepine. The CrCL was calculated based on the ideal body weight (IBW), however, actual body weight (ABW) was used if the patient’s actual weight was less than IBW or adjusted body weight (AdjBW) was used when the body mass index (BMI) was greater than 30 kg/m2. CrCL (mL/minutes) = [(140 − Age) × IBW or ABW or AdjBW]/(Scr × 72), multiplied by 0.85 if female. Patients who had a diagnosis of ESRD or who were receiving hemodialysis or peritoneal dialysis had a CrCL of 10 mL/minutes assigned to them, allowing for inclusion in the secondary end point analysis. A tramadol dose was classified as correct if the maximum daily dose was less than or equal to 200 mg/day, regardless of the dosing frequency or timing of administration for patients on dialysis. 8 All secondary outcomes were summarized descriptively using means and standard deviations for interval-level variables and frequencies and percentages for categorical variables.
Results
A total of 7867 patients were enrolled in our study, 3079 participants were included in the pre-GPOCP cohort, and 4788 patients were included in the post-GPOCP cohort. Our data indicated that the baseline demographics and health histories between the 2 cohorts were significantly different (Table 2). The distribution of race was significantly different (P = .004), and the percentage of cases with diabetes mellitus (60% pre-GPOCP vs. 62% post-GPOCP| P = .023), peripheral vascular disease (17% vs 8% | P < .001), hemodialysis (42% vs 49% | P < .001), cancer (1% vs 4% | P < .001), liver disease (4% vs 6% | P < .001), chronic obstructive pulmonary disease (14% vs 26% | P < .001), and substance use disorders (12% vs. 4% | P < .001) varied substantially between cohorts. These differences were thought to be partially attributed to the coding switch between the International Classifications of Diseases Ninth Revision (ICD-9) and ICD Tenth Revision (ICD-10) that occurred during this study period.
Table 2.
Baseline Characteristics.
| Variable | Subcategory | Pre-GPOCP (N = 3079) | Post-GPOCP (N = 4788) | P-value |
|---|---|---|---|---|
| Categorical | N (%) | N (%) | ||
| Gender | Female | 1591 (51.67%) | 2444 (51.04%) | .602 |
| Male | 1488 (48.33%) | 2344 (48.96%) | ||
| Race | Asian | 33 (1.07%) | 52 (1.09%) | .004 |
| Black | 750 (24.36%) | 1134 (23.68%) | ||
| Hawaiian/Pacific Islander | 0 (0%) | 13 (0.27%) | ||
| Multiracial | 22 (0.71%) | 14 (0.29%) | ||
| Native American/Alaskan | 2 (0.06%) | 5 (0.1%) | ||
| Unknown | 28 (0.91%) | 35 (0.73%) | ||
| White | 2244 (72.88%) | 3535 (73.83%) | ||
| Cancer | 26 (0.84%) | 201 (4.2%) | <.001 | |
| Chronic obstructive pulmonary disease | 438 (14.23%) | 1269 (26.5%) | <.001 | |
| Dementia | 370 (12.02%) | 574 (11.99%) | .998 | |
| Diabetes mellitus | 1846 (59.95%) | 2994 (62.53%) | .023 | |
| Heart failure | 1687 (54.79%) | 2511 (52.44%) | .044 | |
| Hemodialysis | 1292 (41.96%) | 2354 (49.16%) | <.001 | |
| Hypertension | 2891 (93.89%) | 4541 (94.84%) | .081 | |
| Liver disease | 114 (3.7%) | 306 (6.39%) | <.001 | |
| Peripheral vascular disease | 530 (17.21%) | 374 (7.81%) | <.001 | |
| Peritoneal dialysis | 40 (1.3%) | 18 (0.38%) | <.001 | |
| Substance use disorder | 352 (11.5%) | 174 (3.6%) | <.001 | |
| Surgical admission | 107 (3.48%) | 201 (4.2%) | .12 | |
| Continuous | Mean (SD) | Mean (SD) | ||
| Age (y) | 71.658 (13.921) | 71.161 (14.97) | .133 | |
| Body mass index (kg/m2) | 31.443 (59.27) | 38.423 (318.272) | .141 | |
| Creatinine clearance (mL/min) | 18.297 (12.439) | 17.972 (12.723) | .264 | |
| Serum creatinine (mg/dL) | 4.067 (2.581) | 4.231 (2.822) | .008 | |
| Height (cm) | 167.704 (13.342) | 167.903 (16.152) | .555 | |
| Weight (kg) | 84.54 (25.117) | 85.758 (30.512) | .054 |
Note. GPOCP = guideline for prescribing opioids for chronic pain; SD = standard deviation.
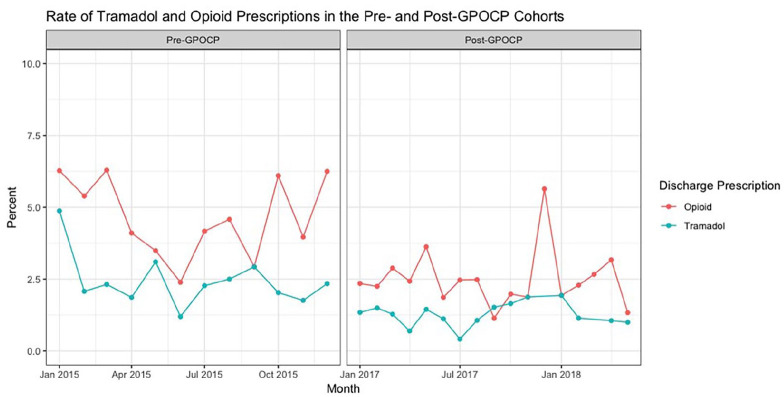
With respect to the primary endpoint (Figure 1 and Table 3), new opioid prescription rates upon hospital discharge for patients with CKD stage IV and V and ESRD decreased from 145 (4.7%) in the pre-GPOCP cohort to 119 (2.5%) in the post-GPOCP cohort (P < .001). A similar reduction in regular-release tramadol prescription rates was also observed between the pre-GPOCP and post-GPOCP cohorts, showing a decrease from 76 (2.5%) to 54 (1.1%) tramadol prescriptions, respectively (P < .001). No discharge prescriptions for extended-release tramadol were noted. A spike in opioid prescriptions at discharge was noted in December of 2015 and 2017. In 2015, opioid prescription rates increased from 9 (4%) in November to 16 (6%) in December. In 2017, they increased from 5 (2%) in November to 15 (6%) in December. These increases were attributed to the large number of elective surgeries performed at the end of the year.
Figure 1.
This graph illustrates the opioid (red) and tramadol (blue) prescription rates in the pre-GPOCP and post-GPOCP cohorts. No data was abstracted from 2016 to allow time for guideline adoption at our institutions. A decline in both opioid and tramadol prescriptions at hospital discharge can be seen from the pre-GPOCP cohort to the post-GPOCP cohort.
Table 3.
Incidence of Opioid and Tramadol Prescriptions.
| Cohort | Pre-GPOCP (n [%]) | Post-GPOCP (n [%]) | P-value |
|---|---|---|---|
| Control (no tramadol or opioid prescription at discharge) | 2858 (92.8%) | 4615 (96.4%) | <.001 |
| New opioid prescription (without a new tramadol prescription) | 145 (4.7%) | 119 (2.5%) | <.001 |
| New tramadol prescription | 76 (2.5%) | 54 (1.1%) | <.001 |
Note. GPOCP = guideline for prescribing opioids for chronic pain.
Among the patients discharged with a new tramadol prescription, 113 (87%) did not have any clinically significant drug-drug interactions (Table 4). Most of the drug-drug interactions identified in the other 17 (13%) patients were related to serotonergic modifiers, such as anti-depressants, or CNS depressive agents, such as benzodiazepines and other analgesic medications. There was no concomitant use of carbamazepine, which is not recommended with tramadol use. 8 For patients with a CrCL less than 30 mL/minutes, 94 (72.3%) patients had their tramadol dosed correctly based on kidney function (Table 4). For patients who were prescribed greater than 200 mg/day of tramadol, the total daily tramadol dose ranged between 300 and 800 mg/day.
Table 4.
Incidence of Drug-Drug Interactions and Correctly Dosed Tramadol Discharge Prescriptions.
| Studied outcome | Subcategory | N (%) |
|---|---|---|
| Number of clinically significant drug-drug interactions involving tramadol | 0 Interactions | 113 (87%) |
| 1 Interaction | 15 (11.5%) | |
| 2 Interactions | 2 (1.5%) | |
| Number of new tramadol discharge prescriptions dosed based on kidney function | Correctly | 94 (72%) |
| Incorrectly | 36 (28%) |
Discussion
A decline in opioid and tramadol prescription rates at discharge was seen in patients with CKD stage IV and V and ESRD after prescriber awareness of the GPOCP within our health system in 2016. Prescription rates fell by 56% for tramadol and by 47% for all other opioids. In addition, these results demonstrated that providers did not shift their prescribing habits towards more tramadol prescriptions in lieu of other opioid products for analgesic control. This goes against our initial supposition that tramadol prescription rates would increase to prevent the prescribing of other opioid agonists in response to the GPOCP.
For patients who were discharged with tramadol, providers were able to successfully prevent drug-drug interactions in the majority of patients (87%); most of the interactions were with other CNS depressants and serotonergic agents. However, a significant number of patients (28%) were prescribed more than the recommended maximum daily dose of tramadol when their CrCL was less than 30 mL/minutes, with the highest amount prescribed being 800 mg/day. This puts the patient at an increased risk for adverse effects, including altered mental status, seizures, respiratory depression, and falls.8,9 Further education and a pharmacist review at discharge could be beneficial to ensure that optimal renal dose adjustments are being made, especially in a population with an increased risk for adverse events.
Our study indicates that, in general, opioid and tramadol prescription rates are declining in the CKD patient population. This could be beneficial considering the large number of patients with CKD who were prescribed opioids between 2006 and 2010, putting them at an increased risk for adverse effects. 4 A recent study assessed the risks associated with opioids in ESRD patients. 9 In the study, they compared no opioid use to low-dose (eg, tramadol and less than 60 mg oral morphine equivalence (OME)) and high-dose opioid (eg, >60 mg OME) use to see which cohort had a higher risk for altered mental status, falls, and fractures. In their study, they showed positive statistical evidence that using opioids put hemodialysis patients at an increased risk for all 3 outcomes. 9 This reinforces the idea that opioid use should be decreased in CKD patients, and other nonopioid treatment modalities should be further explored to mitigate the amount of opioids required to provide adequate pain control.
Although having fewer opioids prescribed may prevent adverse drug reactions in CKD patients, it can also negatively affect the level of pain control they achieve. New studies are warranted to assess the current status of analgesic control in patients with CKD, especially since our study showed such a drastic decrease in the number of opioids prescribed. These studies will be able to indicate whether restricting opioids to this degree was overall beneficial for patients with CKD in terms of balancing pain control with adverse effects. In addition, these studies could offer more information regarding which analgesic regimens provided optimal pain control with the least amount of consequences.
There were several limitations to our study. First, the retrospective nature of the study did not allow us to prove that the GPOCP was the direct cause of the decreased opioid and tramadol prescription rates in our studied population. This decrease is likely multifaceted. Second, our patient baseline demographics were different between the pre- and post-GPOCP cohorts. This could be due to differences in coding between ICD-9 and ICD-10, which occurred during the study’s timeframe. In addition, some of these discrepancies may have also been attributed to inaccurate documentation in the electronic medical record, which is another confounder we were unable to completely account for. As a result, the differences between cohorts and the possibility of inaccurate documentation decreased the validity of our results. Last, although we adjusted for multiple confounders, numerous other confounders exist that could alter the primary endpoint. For example, while oncologic patients are at a much higher risk for pain than the general population, the severity of pain differs depending on the type of cancer; this was something that was unable to be accounted for in our study. 10 Moreover, while we were able to delineate between surgical admissions and nonsurgical admissions, the type of surgery was unable to be identified. The type of surgery can significantly affect the amount of postoperative pain a patient experiences. In addition, surgical pain is classified as acute pain, which is not addressed by the GPOCP.
Conclusion
Our study showed that adoption of the 2016 CDC GPOCP in our health system was associated with a decrease in opioid and tramadol prescriptions in CKD stage IV and V and ESRD patients at hospital discharge. For a patient population already at an increased risk for adverse effects, especially from opioids, this could prove to be a safe trend. However, additional studies are needed to ensure adequate pain control is still being achieved in this patient population. With respect to tramadol, drug-drug interactions were minimal, but a significant number of patients were not properly dosed based on their kidney function. This reinforces the importance of having greater oversight at the time of discharge to ensure proper renal dose adjustments, especially for high-risk medications.
Acknowledgments
We would like to acknowledge Jeremy Albright and Caleb Scheidel for their assistance with the statistical analysis and Amy Burghardt for her assistance in meeting local standards for conducting research.
Footnotes
Authors’ Note: This project was presented as a poster at the Midyear Clinical Meeting 2018—ASHP.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Noah Leja  https://orcid.org/0000-0003-2988-333X
https://orcid.org/0000-0003-2988-333X
References
- 1. Patel SS. Treating pain to improve quality of life in end-stage renal disease. Semin Dial. 2013;26(3):268-273. [DOI] [PubMed] [Google Scholar]
- 2. Pham PC, Khaing K, Sievers TM, et al. 2017 Update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017;10(5):688-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42(6):1239-1247. [DOI] [PubMed] [Google Scholar]
- 4. Kimmel PL, Fwu CW, Abbott KC, et al. Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol. 2017;28(12):3658-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. [DOI] [PubMed] [Google Scholar]
- 6. Blackman K, Smith E. Prescribing policies: states confront opioid overdose epidemic; 2018. Washington, DC: National Conference of State Legislatures. [Google Scholar]
- 7. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ultram (tramadol) package insert. Janssen Pharmaceuticals; 2020. [Google Scholar]
- 9. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol. 2018;13(5):746-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Cancer Society. Facts about cancer pain. January 2019. Accessed May 29, 2019. www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/pain/facts-about-cancer-pain.html#written_by