Abstract
Objective: Providers often admit patients with active outpatient prescriptions for levothyroxine. During an inpatient admission, providers may instruct critically ill patients to take nothing by mouth, or nil per os (NPO). Thus, they may prescribe the intravenous (IV) formulation of levothyroxine during this period. However, levothyroxine possesses a prolonged half-life of up to 7 days; therefore, immediate transition to IV levothyroxine may not be clinically necessary in the acute NPO setting. Intravenous levothyroxine is significantly more expensive than equivalent oral doses and may prove to be a financial burden for an institution. By understanding the pharmacokinetic properties of levothyroxine, we implemented a cost-saving initiative involving a 5-day therapeutic hold of IV levothyroxine. Methods: This was a retrospective evaluation in 2 intensive care units (ICU): a 20-bed surgical/trauma ICU and an 18-bed mixed medical/surgical ICU. Patient data, utilization data, and documented pharmacist interventions were collected for 6 months prior to implementation of the 5-day IV levothyroxine therapeutic hold and for 6 months post-implementation. All patients prescribed IV levothyroxine during these timeframes were included. Results: During the 6-month pre-implementation phase, 674 doses (691 vials) of IV levothyroxine for 77 unique patients were dispensed from the 2 ICUs. During the 6-month post-implementation phase, 168 doses (188 vials) of IV levothyroxine were dispensed for 44 unique patients. Of the 44 patients (48 orders) who still received IV levothyroxine, 22.9% of orders were deemed clinically necessary by the pharmacist and were not recommended to be held under the protocol, 64.6% were due to the verifying pharmacist being unaware of the protocol, 8.3% of orders were due to protocol non-compliance, and 4.2% were verified after the 5-day hold was complete as the patient remained NPO. This pharmacy-led initiative resulted in a 75% decrease in usage post-implementation and an estimated annualized savings of $80,000. Conclusion: A pharmacy-led initiative comprised of a 5-day therapeutic hold of IV levothyroxine was feasible and led to a 75% reduction in usage and cost over a 6-month period in 2 ICU’s. Future steps include additional staff education for improved protocol adherence and expanding the protocol institution-wide for an even greater cost-savings potential.
Keywords: pharmacoeconomics, drug, medical use evaluation, cost effectiveness, financial management, formulary management, P & T, metabolic, endocrine
Introduction
Hypothyroidism is one of the most common hormone disorders in the world, affecting 1% to 2% of the population in iodine-sufficient countries and rising to 7% in individuals aged 85 to 89 years. 1 Whereas environmental iodine deficiency is the most common cause of hypothyroidism on a global scale, chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) is the most prevalent cause in the United States. 2 Autoimmune thyroid diseases (AITDs) occur pathologically as sensitized T lymphocytes infiltrate the thyroid serologically through the circulation of thyroid autoantibodies. 2 This autoimmunity to the thyroid gland due to an inherited defect in immune surveillance leads to either an altered regulation of immune responsiveness or an alteration of presenting antigen in thyroid. 3 A less common cause of hypothyroidism in iodine-sufficient countries is central hypothyroidism, occurring from the insufficient production of active thyroid stimulating hormone (TSH) due to causes such as tumors, inflammatory diseases, or hemorrhagic necrosis, among others. 2
The primary function of the thyroid is endogenous production of thyroxine (T4) and triiodothyronine (T3). 4 As the production of these endogenous hormones decreases in hypothyroidism, treatment with exogenous thyroid hormones becomes necessary to alleviate clinical symptoms. The most prevalent treatment for hypothyroidism is levothyroxine, or synthetic T4. 4 While patients were historically supplemented with synthetic T3 in addition to levothyroxine, treatment with levothyroxine monotherapy has been shown to be as effective as combination therapy due to the rapid conversion of T4 to T3 in vivo. 4
In an acute inpatient setting, providers often admit patients with active outpatient prescriptions for levothyroxine. During their inpatient admission, patients may be instructed to take nothing by mouth, or nil per os (NPO), due to their clinical condition or in preparation for a procedure. Thus, providers may prescribe the intravenous (IV) formulation of levothyroxine during the period of NPO. However, levothyroxine possesses a prolonged elimination half-life (t1/2) of 7.5 days in hypothyroid patients and 6.2 days in euthyroid patients; therefore, immediate transition to IV administration may not be clinically necessary in the acute NPO setting. 5 Because IV levothyroxine is approximately 64 times more expensive than the comparable dose of the oral tablet, opportunities to spare the use of the IV formulation offer significant savings opportunities to both hospitals and the patients they serve. 6 One prior evaluation estimated that 56% of IV levothyroxine was prescribed inappropriately for NPO status without any other compelling indication. 7 By understanding the pharmacokinetic properties of levothyroxine, a cost-savings and medication stewardship evaluation was conducted to determine the impact of a pharmacist-led 5-day therapeutic hold of IV levothyroxine at an academic medical center.
Methods
This study was a retrospective, single-center, pre- and post-implementation evaluation of a 5-day therapeutic hold of IV levothyroxine which took place in 2 intensive care units (ICUs) at a large, academic medical center: a 20-bed surgical/trauma ICU and an 18-bed mixed medical/surgical ICU. Patient characteristics, utilization data, and documented pharmacist interventions were collected from the electronic medical record (EMR) for 6 months prior to implementation of the 5-day therapeutic hold (March to September 2018) and for 6 months post-implementation (December 2018-June 2019). A run-in period of 3 months was used prior to full implementation for adequate staff education and provider notification. The protocol was approved at an institutional multi-disciplinary ICU committee consisting of physicians, nurses, and pharmacists. Education was provided to attending physicians, resident physicians, fellows, nurse practitioners, and nursing staff in the study ICUs during in-service presentations, via e-mail communications, and during patient care discussions on daily interdisciplinary rounds. Prescribers were notified immediately at the time of prescribing by the verifying pharmacist to request the IV levothyroxine to be held during the post-implementation phase. Physicians had the option to override at any time if they felt that IV therapy was warranted based on the patient’s condition. Because this protocol was implemented as part of an initial pilot study at the institution, the expectation was that patients would only be evaluated for inclusion during shifts that the study pharmacists were present. For example, during the weekend or overnight shifts, pharmacists who were unaware of the pilot program would not be expected to evaluate patients for inclusion in the therapeutic hold protocol.
All patients who were prescribed IV levothyroxine were included. During the pre-implementation phase, there was no limit to the duration of therapy for patients who received IV levothyroxine. As the 5-day therapeutic hold was not yet established, there were no pharmacist interventions directly related to the protocol; however, pharmacists at the institution do intervene as needed to recommend IV to oral switches when appropriate. In the post-implementation phase, when a patient was NPO at the time of prescribing IV levothyroxine, the unit pharmacist would recommend a 5-day therapeutic hold to be placed on the order to the primary team. As soon as the patient was able to take oral medications or enteral access was established, oral levothyroxine would be restarted, regardless of whether the 5-day hold was completed. As a guideline-based standard, early enteral feeding is promoted for all patients at the institution as soon as clinically appropriate.8,9 In the event the patient still remained NPO after the 5-day therapeutic hold, the provider would initiate treatment with once daily administration of IV levothyroxine at 50% to 75% of the intended oral dose, and the patient would subsequently be routinely assessed for eligibility for an IV to oral switch of levothyroxine therapy. Patients were excluded from participation in the therapeutic hold if they were deemed to have a high acuity critical illness requiring IV therapy based on the provider’s discretion; the IV levothyroxine was recommended based on a formal consult from an endocrinologist; the patient was in a myxedema coma; the patient was receiving a continuous IV levothyroxine infusion for organ preservation; or the patient was exhibiting clinical hypothyroidism (TSH ≥ 10 mIU/ml or T4 < 4.5 µg/dL).
No sample size calculations were performed. The analysis population included all patients who were prescribed IV levothyroxine in the 2 ICUs during the study period from March 2018 until June 2019. Fisher’s exact test was used to compare the proportion of patients prescribed IV levothyroxine during pre- and post-implementation of IV levothyroxine therapeutic hold protocol and an unpaired Student’s t-test was used to compare total IV levothyroxine doses dispensed during pre- and post-implementation of IV levothyroxine therapeutic hold. All analyses were performed using Microsoft Excel® and IBM SPSS® Statistics Version 26.
Levothyroxine doses were prepared using a 100 microgram (µg) reconstituted vial. Wholesale acquisition cost (WAC) was used as the reference for medication costs of the IV levothyroxine 100-µg reconstituted vial and the levothyroxine oral tablets. The WAC price of levothyroxine sodium as of June 2019 was $78.59 for the 100-µg vial and $1.22 for the 125-µg tablet, representing a cost difference of over 640% (McKesson Corporation, 2019). Cost savings for the study were calculated based on the difference in total utilization of IV levothyroxine in the 2 study ICUs during the pre- and post-implementation periods. The difference in the number of IV levothyroxine vials utilized during the 6 month pre-implementation phase compared to the 6 month post-implementation phase represented the change in utilization. The total doses prevented from being dispensed was calculated by counting the number of days after the intervention until the patient was prescribed levothyroxine again (either IV or an oral tablet). Results for categorical variables are summarized with frequencies and percentages.
Results
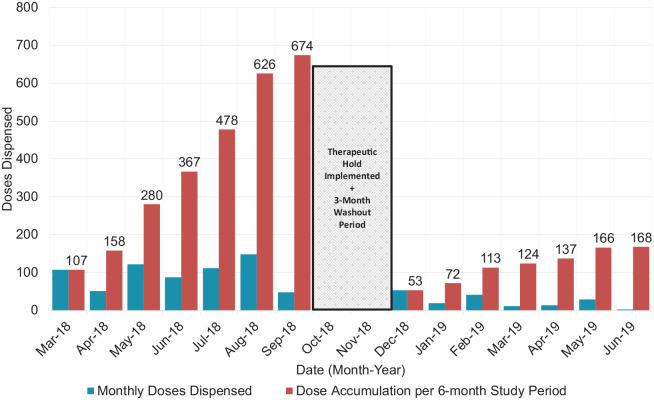
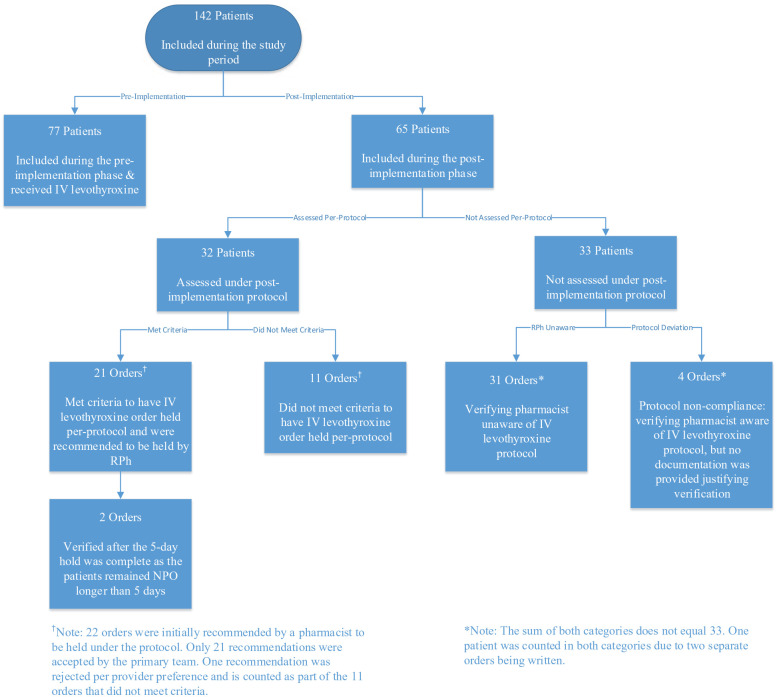
During the 6-month pre-implementation phase, 77 patients were prescribed IV levothyroxine, compared to 65 patients during the post-implementation phase. Of these 65 patients, 21 patients had their IV levothyroxine orders held as part of the pharmacist-led 5-day therapeutic hold protocol (P < .001). Thus, the intervention was associated with a statistically significant decrease in the number of patients who received IV levothyroxine pre- and post-implementation (Table 1). Similarly, 674 doses (691 vials) of IV levothyroxine were dispensed from the 2 ICUs pre-implementation (mean, 96.3 vials per month; standard deviation, 36.8; range, 48-148 vials per month). During the 6-month post-implementation phase, 168 doses (188 vials) of IV levothyroxine were dispensed for 44 unique patients (mean, 24 vials per month; standard deviation, 18; range, 2-53 vials per month). There was a significant decrease in the number of doses dispensed post-implementation of the pharmacist-led 5-day therapeutic hold (P = .001). See Figure 1 for monthly dispensation data. Of the documented pharmacist interventions recommending the 5-day therapeutic hold (n = 22), 95.5% (n = 21) were accepted preventing 39 total doses from being dispensed, while 4.5% (n = 1) was rejected for clinical necessity due to low TSH and hemodynamic instability. Of the 44 patients who still received IV levothyroxine post-implementation (for 48 total orders), 22.9% (n = 11) of orders were deemed clinically necessary, 64.6% (n = 31) of orders were due to the verifying pharmacist being unaware of the therapeutic hold protocol, 8.3% (n = 4) of orders were verified due to protocol non-adherence by the study pharmacists, and 4.2% (n = 2) of orders were verified after the 5-day therapeutic hold was complete as the patient remained NPO (see Figure 2).
Table 1.
Patients Prescribed IV Levothyroxine Pre- and Post-Implementation.
| Pre-implementation | Post-implementation | Subtotal | |
|---|---|---|---|
| IV levothyroxine orders placed and dispensed | 77 | 44 | 121 |
| IV levothyroxine orders placed and held as part of therapeutic hold protocol | 0 | 21 | 21 |
| Subtotal | 77 | 65 |
Figure 1.
IV Levothyroxine doses dispensed pre- and post implementation of a pharmacy-led 5-day therapeutic hold.
Figure 2.
Pre- and post-implementation patient flow diagram.
The pharmacist-led 5-day therapeutic hold led to a cost savings of $39,767 (506 vials) during the 6-month post-implementation phase. The annualized savings across the 2 study ICUs is $79,534.
Discussion
This pilot study evaluated the effect of a pharmacist-led, 5-day therapeutic hold of IV levothyroxine at an academic medical center to determine the impact on usage of this high-cost medication. Due to the long pharmacokinetic half-life of levothyroxine of up to 7 days, 5 it may be clinically reasonable to delay the administration of this medication during the initial period that the patient is NPO. Pharmacists may lead the therapeutic hold initiative and secure agreement from the interdisciplinary team, as was done at our institution.
The recommendation to hold IV levothyroxine for up to 5 days or until the patient was no longer NPO was accepted 95.5% of the time, based on documented interventions, with 1 patient continuing to receive IV levothyroxine due to a concern for clinically low TSH levels and hemodynamic instability after discussions with the primary team. Two patients in the study resumed IV levothyroxine after a period of 5 days due to remaining NPO. All other patients were able to begin receiving oral or enteral medications within the 5-day period. This high rate of acceptance can be in part attributed to the education provided to primary teams prior to implementation of the therapeutic hold as to the clinical and pharmacokinetic rationale. It is likely that this acceptance rate would be consistent across all patient units within the institution, including general care areas where admitted patients may be of lower clinical acuity and duration of NPO may be shorter.
Total usage and cost across the 2 ICUs participating in the study decreased by 75% after implementation of the therapeutic hold. With an estimated annualized cost savings of $80,000 across the 2 study ICUs, the pilot study yielded a positive financial impact. When this data is extrapolated to the entire institution, the potential financial implications are significant. In a time when many health-systems are facing increased financial pressure to control rising medication expenditures, this pharmacist-led initiative shows a promising opportunity to steward high-cost drug usage with the principles of pharmacokinetics.
This study has several limitations. Sixty-three percent of IV levothyroxine doses that were administered in the 6-month post-implementation period were verified and dispensed during off-hours by pharmacists who were unaware of the 5-day therapeutic hold protocol. This limitation is difficult to control, as this was designed as a pilot study intended to show initial cost-savings associated with the protocol prior to institution-wide implementation. Pharmacists covering the 2 ICUs on weekend or overnight shifts were not expected to assess patients or intervene as part of this pilot protocol. Second, due to the nature of this quality improvement study, clinical patient data such as severity scores and prevalence of outpatient levothyroxine therapy were not collected for the overall inpatient population during the pre- and post-implementation phases. Although it is likely that patient populations were similar throughout both phases, potential differences in clinical characteristics could account for variations in usage of IV levothyroxine during the pre- and post-implementation phases. Third, due to the retrospective nature of this evaluation, the study is at risk for selection bias. Lastly, the lack of a formalized protocol required the provider to either accept or deny the pharmacist’s therapeutic hold recommendation, rather than having an automatic 5-day hold be placed upon pharmacist verification. Providers who were unfamiliar with the clinical and pharmacokinetic rationale of the therapeutic hold may be more likely to reject the recommendation.
Overall, this study showed that a 5-day therapeutic hold of IV levothyroxine was an effective method for reducing usage by 75% at our institution over a 6-month period, while taking advantage of the drug’s favorable pharmacokinetic properties. Hospitals and health-systems may consider incorporating this cost-saving initiative into their clinical practices, given our institution’s high acceptance rate and the ease of implementation. Future steps to consider for an even greater cost-savings potential include formalizing a pharmacist-initiated protocol, expanding it across all units within the institution, and additional staff education to improve adherence. Although this study was not designed to detect clinical differences between patient populations, future studies should consider correlating the reduction in IV levothyroxine utilization with clinical severity scores and patient demographics.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Blake T. Barlow  https://orcid.org/0000-0001-5692-1253
https://orcid.org/0000-0001-5692-1253
References
- 1. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316. doi: 10.1038/nrendo.2018.18 [DOI] [PubMed] [Google Scholar]
- 2. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235. doi: 10.1089/thy.2012.0205 [DOI] [PubMed] [Google Scholar]
- 3. Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E, Tomer Y. Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93(9):3589-3596. doi: 10.1210/jc.2008-0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hennessey JV. The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine. 2017;55(1):6-18. doi: 10.1007/s12020-016-1199-8 [DOI] [PubMed] [Google Scholar]
- 5. Colucci P, Yue CS, Ducharme M, Benvenga S. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur Endocrinol. 2013;9(1):40-47. doi: 10.17925/EE.2013.09.01.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway S, Brotman D, Pinto B, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. doi: 10.12788/jhm.2779 [DOI] [PubMed] [Google Scholar]
- 7. DeSalvo K, McDermott M, Barber G, Reynolds P. MON-004 The effect of a pharmacist led training intervention on reducing inappropriate use of intravenous levothyroxine in hospitalized patients. J Endocr Soc. 2019;4(Suppl_1):MON-004. doi:0.1210/js.2019-MON-004 [Google Scholar]
- 8. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi: 10.1016/j.clnu.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 9. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi: 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]