Abstract
Objectives: The purpose of this study was to assess the impact of the injectable opioid drug shortage on analgesia and sedation management in the medical intensive care unit (MICU). Methods: A single-center, retrospective cohort study was conducted of mechanically ventilated patients during the injectable opioid shortage. Outcomes were compared between a cohort of patients during the intravenous (IV) opioid shortage (01/01/18-03/31/18) and a control cohort (01/01/17-03/31/17). Total IV opioids and alternative sedative administration were assessed. Richmond Agitation Sedation Score (RASS) and Clinical Pain Observation Score (CPOT) assessments were also evaluated. The primary outcome was percentage of RASS within goal. Secondary outcomes included duration of mechanical ventilation, hospital/ICU length of stay, and mortality. Results: One hundred patients were included (50 patients per cohort). In the shortage cohort, 23.2% fewer IV opioids were used (40 501.8 vs 52 713.8 oral morphine equivalents [OME]). No statistical differences were found in percentage of patients within goal RASS between the shortage and control (median 63.7% vs 74.8%; P = .094) or CPOT (median 49.7% vs 47.7%; P = .575). More patients received enteral opioids and propofol on day 1 in the shortage cohort when compared to the control (22% vs 4%; P = .007 and 76% vs 56%; P = .035) but there were no differences in benzodiazepine, dexmedetomidine, or antipsychotic use. No differences in mechanical ventilation, hospital/ICU length of stay, or mortality were found. Conclusions: Use of less IV opioids during the injectable opioid shortage did not affect achievement of goal RASS and CPOT scores or increase prescribing of sedative medications such as benzodiazepines in the MICU.
Keywords: drug shortage, injectable, opioid, intensive care unit, sedation, pain
Background
Over the past decade, drug shortages have become common and wide spread within the healthcare community. Various causes such as natural disasters, lack of raw materials, manufacturing quality issues, or inability to meet capacity have ignited detrimental and long-standing disruptions in drug supply. 1 The injectable opioid shortage that reached a critical peak in late 2017 left many hospitals concerned about the ability to provide patients with appropriate care as they were forced to redefine criteria for administrations of injectable opioids and ration supplies. The American Society of Health-System Pharmacists (ASHP) acknowledged the challenge institutions faced due to the multitude of indications for intravenous (IV) opioids and that interchangeability is not an option in all cases. 1 Specifically, ASHP noted the potential for increased medication errors as a result of improper dosing conversion to alternatives. 1 The American Society of Anesthesiologists has participated regularly in summits and issued statements regarding drug shortages, and the American Association of Nurse Anesthetists also responded to the Food and Drug Administration’s (FDA) public concerns on the IV opioid shortage, stating that it profoundly impacted their ability to provide quality patient care. 2 In a 2010 national survey conducted by the Institute for Safe Medication Practices of 1800 health care providers, 64% of respondents believed drug shortages posed risks, in addition to reporting over 1000 adverse events and near-misses associated with drug shortages. 3 Many respondents also believe there is a risk for increased mortality when forced to use alternative and potentially less effective medications, especially in vulnerable populations such as oncology, critically ill, and pediatric patients. 3
These responses demonstrate the reliance of the healthcare community on IV opioids for the provision of patient care. This includes the intensive care unit (ICU) where IV opioids are a major component of analgesia and sedation regimens, especially for mechanically ventilated patients. The Society of Critical Care Medicine’s (SCCM) 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) clinical practice guidelines acknowledge opioids remain a mainstay for pain management in most ICU settings, although recommendations now include a focus on utilizing multimodal pain therapy due to safety concerns with IV opioids. 4 The opioid epidemic has also increased pressure on clinicians to utilize alternative agents and practice opioid stewardship. Additionally, the PADIS guidelines recommend maintaining light levels of sedation, such as targeting a goal Richmond Agitation Sedation Scale (RASS) of −1 to +1. 4
At The Ohio State University Wexner Medical Center (OSUWMC), the IV opioid shortage impacted prescribing practices significantly. Beginning in December 2017, pharmacists were instructed to review all IV opioids for appropriateness and transition patients to enteral therapy or alternative non-opioid agents as soon as possible. Use of IV opioids was allowed for acute sedation needs in ICU patients, but clinicians were encouraged to limit utilization to the shortest duration necessary or order one time doses for procedures or acute pain crises. Additionally, the OSUWMC Drug Shortage Committee closely monitored available supply and rotated the preferred IV opioid agent throughout the shortage. Restrictions within the electronic medical record were also implemented to guide prescribing based on availability of specific injectable opioids (fentanyl, hydromorphone, or morphine). These alerts remained through May 2018.
Despite national media attention covering drug shortages, there have been limited studies to date published describing the impact of injectable opioid drug shortages on patient outcomes. Mechanically ventilated ICU patients specifically may be highly vulnerable to the effects of opioid drug shortages due to frequent use of IV opioids for analgesia and sedation. A study by Hughes et al. 5 conducted in a pediatric ICU found an increase in benzodiazepine prescribing, but no significant difference in the rate of medication errors during a 2012 injectable fentanyl drug shortage. Meanwhile Klaus et al. 6 demonstrated that the choice of opioid may have impacted the length of mechanical ventilation, ICU length of stay, and level of sedation in a perioperative ICU during a 2016 shortage of remifentanil. Identifying the impact of forced changes in prescribing during the IV opioid shortage is crucial to guiding care in the face of future shortages and may aid clinicians engaging in opioid stewardship. Therefore, the objective of this study was to assess the impact of the IV opioid drug shortage on prescribing practices and clinical outcomes in the Medical Intensive Care Unit (MICU).
Methods
A single-center, retrospective cohort study was conducted to compare sedation and analgesia practices in the OSUWMC MICU in an IV opioid shortage cohort (January 1, 2018-March 31, 2018) to a control cohort a year prior to the shortage (January 1, 2017-March 31, 2017). Institutional protocols did not differ between the 2 cohorts. Mechanically ventilated patients 18 to 89 years old admitted to the MICU at OSUWMC were included. Patients were assessed from day 1 following intubation through day 7, ICU discharge, transition to comfort care, or death. Only the patient’s first admission to the MICU was included. Patients were excluded if they were mechanically ventilated for less than 24 hours, mechanically ventilated for 24 hours or more prior to admission, received continuous neuromuscular blockade in the 7 day post-intubation period, comatose (defined as RASS −4 to −5 throughout the 7 day post-intubation period), received methadone or buprenorphine prior to or during ICU admission, had a tracheostomy present on admission, were incarcerated, or pregnant. The study was approved by the Institutional Review Board at OSUWMC.
The primary outcome was percentage of RASS assessments within sedation goal range (−1 to +1) during the 7 day post-intubation period. The percentage of RASS within goal was determined by taking the number of RASS assessments with goal and divided by the total number of RASS assessments for each day. Secondary outcomes included: total cumulative dose of IV opioids in oral morphine equivalents (OME), percentage of Critical Care Pain Observation Tool (CPOT) assessments in goal range (0-2), percentage of positive Confusion Assessment Method (CAM)-ICU assessments, transfer out of the ICU with an IV or enteral opioid order, hospital discharge with a new opioid prescription per discharge instructions, ICU and hospital length of stay (LOS), duration of invasive mechanical ventilation (MV), and in-hospital mortality. Additional secondary outcomes included number of patients receiving analgesics and sedatives on hospital day 1, 3, 5, and 7 of the following medications: IV opioids, enteral opioids, transdermal opioid, benzodiazepines (midazolam, diazepam, or lorazepam), ketamine, propofol, dexmedetomidine, and antipsychotics (haloperidol, quetiapine, and risperidone). Total daily doses of IV opioids (OME) were collected for the entire study period.
Patients in both study cohorts were identified via an admissions report for the OSUWMC MICU, a 36-bed unit within a large tertiary-care academic medical center. During both study cohorts, an institutional protocol for analgesia and sedation management was utilized in the MICU. RASS, CPOT, and CAM-ICU assessments were completed by beside nurses, and daily sedation awakening trials and spontaneous breathing trials were completed in mechanically ventilated patients per protocol. All demographic and clinical data was collected via retrospective chart review from the electronic medical record. Demographics, pertinent past medical history including documented history of active drug abuse or chronic opioid use (greater than 30 OME per day), and disease severity scores were collected at baseline. Diagnosis of Acute Respiratory Distress Syndrome (ARDS) based on progress note documentation was also collected. Comorbidities were characterized using the Charlson Comorbidity Index and severity of illness was captured via the Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) score.7,8 Doses of opioid and sedative medications as well as RASS, CPOT, and CAM-ICU assessments were collected from hospital day 1 following intubation through hospital day 7, ICU discharge, transition to comfort care, or death. The end of mechanical ventilation was defined as 24 consecutive hours without reintubation.
Patients were screened for study inclusion using a random number generator until 50 patients in each group were identified meeting criteria for study enrollment. Statistical analysis was completed comparing baseline characteristics and clinical outcomes between the IV opioid shortage cohort and the control cohort. Nominal data is presented as number (percentage) and was analyzed using the chi-squared or the Fisher’s exact test. Continuous parametric data is presented as a mean (±standard deviation) and continuous non-parametric data is presented as median [25%-75% interquartile range (IQR)] and was analyzed using the Student’s t-test or Mann–Whitney U test, respectively. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University.9,10 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. All data was analyzed using SPSS software for Windows, version 26 (Chicago, IL).
Results
A total of 433 patients were screened for study inclusion. Three hundred and thirty-three patients met exclusion criteria and the remaining 100 patients were included in the final analysis (50 patients in the shortage cohort and 50 patients in the control cohort). The most common reason for exclusion was mechanical ventilation less than 24 hours or never mechanically ventilated (n = 245). Other reasons for exclusion included: chronic tracheostomy (n = 23), mechanically ventilated for ≥24 hours prior to ICU admission (n = 21), coma defined as RASS −5 to −4 (n = 12), receiving continuous neuromuscular blockade (n = 12), incarceration (n = 7), receiving methadone/buprenorphine (n = 10), and pregnancy (n = 3).
The median age of patients in the overall cohort was 59 years [47-68]. For the overall cohort, the median APAHE II score and Charlson Comorbidity Index were 28 [24-32] and 5 [3-7], respectively. Baseline characteristics were mostly similar between groups (Table 1). The IV opioid shortage cohort did have a significantly higher number of patients with chronic opioid use, 9 (18%) versus 2 (4%), P = .025. Additionally, body mass index was significantly lower in the shortage cohort than the control (27.5 vs 33.1, P = .006). In the overall study cohort, the mean number of RASS assessments was 5.6 per patient per day, CPOT assessments was 1.7 per patient per day, and CAM-ICU assessments was 2.5 per patient per day.
Table 1.
Comparison of Baseline Characteristics between Injectable Opioid Shortage and Control Cohorts.
| Characteristic | Shortage (n = 50) | Control (n = 50) | P-value |
|---|---|---|---|
| Female | 23 (46.0) | 24 (48.0) | .841 |
| Age (years) | 54.0 ± 15.9 | 59.1 ± 15.6 | .106 |
| Height (cm) | 168.8 ± 11.6 | 170.7 ± 10.6 | .395 |
| Weight (kg) | 79.2 ± 22.4 | 97.1 ± 39.1 | .006 |
| BMI (kg/m2) | 27.5 ± 6.6 | 33.1 ± 12.1 | .006 |
| ARDS diagnosis | 1 (2.0) | 6 (12.0) | .112 † |
| Chronic opioid use >30 OME/day | 9 (18.0) | 2 (4.0) | .025 |
| Active drug abuse (heroin, cocaine, recreational opioids) | 4 (8.0) | 3 (6.0) | 1.000 † |
| APACHE II score | 28.0 [22.0-31.0] | 25.0 [23.0-27.0] | .220 |
| Charlson comorbidity index | 5.0 [4.5-6.0] | 5.5 [2.0-9.0] | .972 |
Note. Nominal data are presented as number (percentage). Continuous data are presented as mean ± standard deviation (SD) if parametric or median [interquartile range (IQR)] if non-parametric. BMI = body mass index; ARDS = acute respiratory distress syndrome, OME = oral morphine equivalents.
Fisher’s exact test.
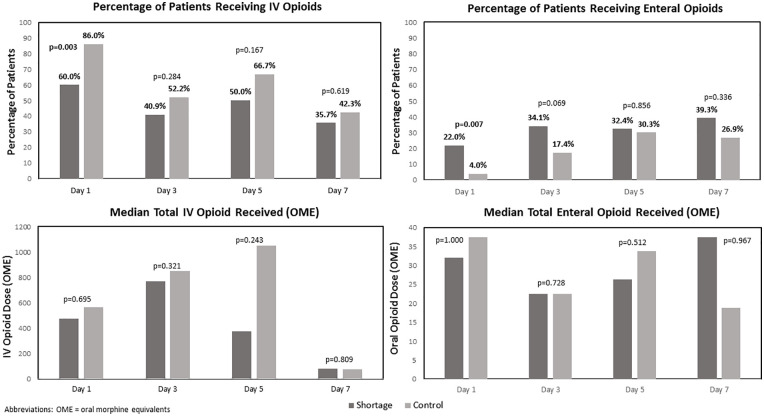
Overall, there was a 23.2% decrease in the total cumulative IV opioid dose administered from days 1 to 7 in the shortage cohort compared to the control cohort (40 501.8 OME vs 52 713.8 OME). Additionally, fewer patients received IV opioids in the shortage cohort than the control cohort (74% vs 94%; P = .006). Figure 1 demonstrates comparisons of IV and enteral opioid use on days 1, 3, 5, and 7 between cohorts. The shortage cohort had a lower percentage of patients receiving IV opioids on day 1 and a higher percentage of patients who received enteral opioids on day 1 compared to the control cohort, which were each statistically significant. Both the percentage of patients receiving IV opioids and median IV opioid dose was lower on all study days in the shortage cohort, although not statistically significant. Overall, the percentage of patients receiving enteral opioids and median enteral opioid dose was similar between the shortage cohort and control cohort during the study period. No patients received transdermal fentanyl in either cohort.
Figure 1.
Comparison of intravenous and enteral opioid utilization and dose between shortage cohort and control cohort.
There was not a statistically significant difference between the shortage and control cohort for the primary outcome of percentage of RASS within goal range of −1 to +1 from days 1 to 7 (median 63.7% vs 74.8%; P = .094). The shortage cohort trended toward having a higher percentage of patients below RASS goal (−2 to −5) from days 1 to 7 (median 25.9% vs 18.0%; P = .064). The percentage of patients above RASS goal (+2 to +4) from days 1 to 7 was similar between cohorts (median 4.0% vs 3.9%; P = .597). Additionally, there were no statistically significant differences in the median percentage of patients within goal RASS (−1 to +1) on study days 1, 3, 5, and 7 between cohorts (Figure 2). In both cohorts, there was an increase in percentage of patients within goal RASS over time. The percentage of CPOT scores within goal range 0 to 2 from days 1 to 7 (median 49.7% vs 47.7%; P = .575) and percentage of CAM-ICU positive from days 1 to 7 (median 80.2% vs 92.3%; P = .505) were both similar between the IV opioid shortage and control cohorts.
Figure 2.
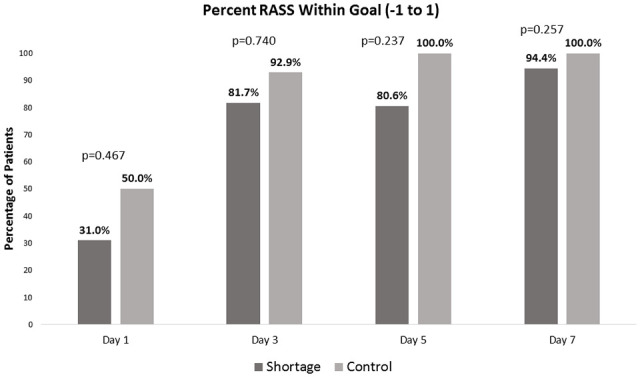
Comparison of median percentage of patients within goal RASS (−1 to +1) on days 1, 3, 5, and 7 between shortage cohort and control cohort.
Comparisons of use of other sedatives and antipsychotics between study cohorts can be seen in Table 2. Only one patient in the control cohort received ketamine during the study period. A statistically significant increase was found in the number of patients receiving propofol on day 1 of the study period in the shortage cohort. No differences were found in the selection of any of the other agents across days of the study period.
Table 2.
Comparison of Alternative Agent Use and Secondary Outcomes between Injectable Opioid Shortage and Control Cohorts.
| Outcome | Shortage | Control | P-value |
|---|---|---|---|
| Antipsychotics | |||
| Day 1 | 8/50 (16.0) | 4/50 (8.0) | .218 |
| Day 3 | 7/43 (16.3) | 13/46 (28.3) | .173 |
| Day 5 | 11/33 (33.3) | 14/32 (43.8) | .388 |
| Day 7 | 12/27 (44.4) | 13/26 (50.0) | .685 |
| Benzodiazepines | |||
| Day 1 | 14/50 (28.0) | 21/50 (42.0) | .142 |
| Day 3 | 8/44 (18.2) | 14/46 (30.4) | .176 |
| Day 5 | 3/34 (8.8) | 6/33 (18.2) | .305* |
| Day 7 | 4/28 (14.3) | 4/26 (15.4) | 1.000* |
| Dexmedetomidine | |||
| Day 1 | 6/50 (12.0) | 6/50 (12.0) | 1.000 |
| Day 3 | 6/44 (13.6) | 7/46 (15.2) | .831 |
| Day 5 | 7/34 (20.6) | 3/33 (9.1) | .305* |
| Day 7 | 7/28 (25.0) | 2/26 (7.7) | .144* |
| Propofol | |||
| Day 1 | 38/50 (76.0) | 28/50 (56.0) | .035 |
| Day 3 | 16/44 (36.4) | 10/46 (21.7) | .126 |
| Day 5 | 7/34 (20.6) | 6/33 (18.2) | .803 |
| Day 7 | 5/28 (17.9) | 4/26 (15.4) | 1.000* |
| Active IV opioid order at time of transfer from ICU to floor (n = 50) | 3/37 (8.1) | 1/34 (2.9) | .515 |
| Active enteral opioid order at time of transfer from ICU to floor (n = 50) | 20/37 (54.1) | 15/35 (42.8) | .577 |
| Discharge from hospital with new opioid prescription (n = 50) | 10 (20.0) | 10 (20.0) | 1.000 |
| Hospital LOS (days) (n = 50) | 16.0 [10.0-29.5] | 20.5 [13.0-33.3] | .258 |
| ICU LOS (days) (n = 50) | 8.0 [4.0-12.25] | 8.0 [4.0-12.0] | .785 |
| Duration of MV (hours) (n = 50) | 114.9 [47.4-198.5] | 73.1 [47.1-131.1] | .236 |
| In-hospital mortality (n = 50) | 10 (20.0) | 9 (18.0) | .799 |
Note. Nominal data are presented as number (percentage). Continuous data are presented as mean ± standard deviation (SD) if parametric or median [interquartile range (IQR)] if non-parametric. LOS = length of stay; MV = mechanical ventilation; IV = intravenous.
Fisher’s exact test.
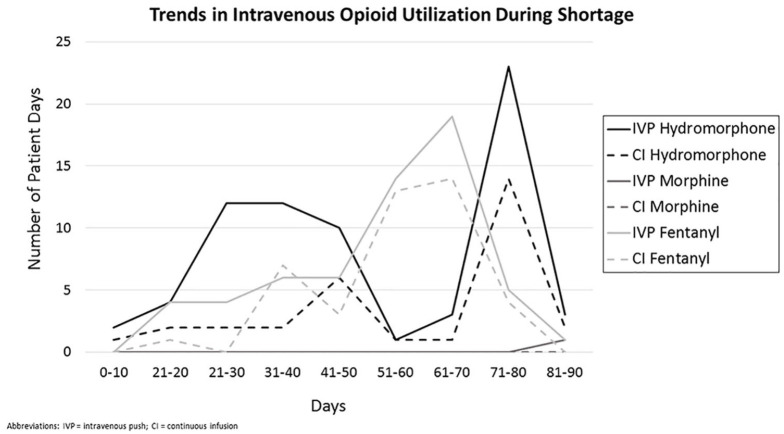
Figure 3 describes the trends in use of specific IV opioids throughout the 90-day shortage period based on supply and purchasing availability. Up to day 21, there was no preferred agent. From day 21 through day 50, IV hydromorphone was the preferred agent. Day 50 through day 60, there was a spike in fentanyl use and decrease in hydromorphone use that occurred concurrent with changes in institutional availability of hydromorphone. On day 78, fentanyl supply became critically low and a restriction was implemented, at which time usage in the MICU dropped drastically. Few patients received IV morphine throughout the shortage period. Overall, IV push opioid administration use was higher than continuous infusion opioid administration.
Figure 3.
Trends in intravenous opioid utilization during shortage period.
There were no significant differences found between the 2 cohorts for the other secondary outcomes (Table 2). The median duration of mechanical ventilation was 114.9 hours in the shortage cohort compared to 73.1 hours in the control cohort (P = .236). No difference in mortality was observed (20% vs 18%; P = .799). Overall, 20% of patients were discharged from the hospital with a new opioid prescription and this was similar between cohorts.
Discussion
Healthcare systems continue to face frequent and sustained drug shortages, which can present many challenges. The IV opioid drug shortage that took place in late 2017 and persisted through May 2018 drastically decreased the national supply of these medications forcing institutions to ration supplies and utilize alternatives when feasible. Results of this study show that prescribing practices in a MICU setting during the IV opioid shortage were impacted compared to a historical control, without negatively affecting patient outcomes. This study revealed that during the IV opioid shortage, 23.2% fewer IV opioids were used total compared to the control cohort, indicating that clinicians did alter their practice in response to the shortage. However, when assessing the percentage of mechanically ventilated patients within goal RASS and CPOT, there was no significant difference found between the 2 cohorts. The decrease in use of IV opioids did not significantly affect the duration of mechanical ventilation, ICU or hospital length of stay.
Even though there was less cumulative IV opioid use during the 7-day study period, the most profound differences in prescribing practices were seen on day 1 following intubation. Day 1 was the only individual study day with a statistically significant lower number of patients receiving IV opioids in the shortage cohort than the control cohort (60% vs 85%, P = .003). This same study day, there was a statistically significant higher number of patients who received propofol (76% vs 56%, P = .035) and enteral opioids in the shortage cohort (22% vs 4%, P = .007). It is not surprising that more differences were seen on day 1 since the analgesic and sedation needs may be more challenging in the immediate post-intubation period. This is evident by the trend in the increasing percentage of patients within goal RASS as patients progressed from study day 1 to study 7 as seen in Figure 2. No differences were found for overall prescribing of other sedative or adjunctive medications including benzodiazepines, dexmedetomidine, or antipsychotics. It is important to note that the prescribing of benzodiazepines did not increase to compensate for decrease availability of IV opioids, since they are known to be harmful with known impact on delirium, duration of mechanical ventilation, and ICU length of stay. 4
There are only a few published studies to date evaluating outcomes during IV opioid drug shortages, none of which were conducted in the MICU population. Hughes et al. were the first to investigate the impact of an opioid drug shortage on patient outcomes in a pediatric ICU in 2012. 3 Similar to our study, Hughes et al. observed a decrease in prescribing of the shortage medications by 10%. 5 They also found a significant decrease in number of days on ventilator per patient and reduction of ICU length of stay in the shortage group. Additionally, Klaus et al. analyzed the effect of a remifentanil shortage on a perioperative population. 6 The study identified the shortage group’s duration of mechanical ventilation was about 12 hours longer. After a regression analysis, adjusting for confounders, this was found to be statistically significant (RR 2.19; 95% CI [2.14–2.24]; P < .001). This suggests the remifentanil shortage may have had a negative impact on patient outcomes. These findings may be attributed to the longer duration of action of the alternative agents, preventing immediate post-operative extubations in this patient population. More recently, Bouwma et al. 11 demonstrated comparable results to ours in a surgical intensive care unit during the 2017 injectable opioid shortage. They reported adequate pain management with less overall use of IV opioids without an increase in adverse outcomes. 11 Evaluating the available literature as a whole, it is evident that drug shortages influence ICU patients across various patient populations; however, their impact on patient outcomes is less clear.
With drug shortages becoming increasingly prevalent, institutions must be prepared to quickly and effectively respond to maintain supply for patients in whom use is essential. ASHP and the FDA routinely track and report these shortages. They recommend optimizing quantities in automated dispensing cabinets based on location usage, restricting use for specific populations or indications, and switching to enteral therapies often. 1 The study institution employed multiple approaches during the IV opioid shortage. These were implemented by the institutional Drug Shortage Committee which is comprised of specialty practice pharmacists, pharmacy purchasing, drug information pharmacists, and informatics representatives to ensure IV opioids were available for patient populations the committee felt was most vulnerable. The electronic health record was immediately programmed to alert clinicians with a notification of the shortage and recommend alternative agents, such as enteral opioids. Pharmacists were actively involved in reducing the use of IV opioids and encouraging the use of smaller doses, shorter durations, IV pushes, or enteral agents. The Drug Shortage Committee rotated preferred IV opioids based upon purchasing and supply availability. Our results revealed that these drug shortage management strategies were effective as IV opioids were still available and used in 76% of mechanically ventilated MICU patients during the shortage period. This availability appears to have been augmented through IV opioid rotation, as evidenced by fluctuations in usage of hydromorphone and fentanyl throughout the 90-day shortage period.
Further, it is pertinent to note our study demonstrated less IV opioid usage while still achieving goal RASS. The results from our study suggest implementation of opioid stewardship practices in mechanically ventilated MICU patients is feasible without compromising safety or efficacy of sedation regimens. In the era of the opioid crisis, it is imperative the healthcare community employ opioid stewardship practices. This is particularly important as regulatory bodies are reducing manufacturing of opioids. In 2018, the FDA proposed to reduce opioid manufacturing by 20%. 12 However, evaluation of opioid stewardship was not the focus of this study and further studies assessing opioid stewardship interventions with more robust analysis of their impact are still needed. A majority of patients did still receive IV opioids within 7 days following intubation despite the 23.2% less cumulative doses administered. Despite evidence of some opioid stewardship, our results also indicate that additional opportunities for improvement remain. Additionally, in both cohorts 20% of patients were discharged from the hospital with a new opioid prescription which is concerning.
There are several limitations to this study. First, these results should be interpreted with caution due to the retrospective study design and small sample size. Larger studies will be needed in the future to confirm our findings and identify any long-term impact of opioid shortages. Additionally, not all enteral opioids and alternative analgesics that may be included in multimodal pain regimens (ie, acetaminophen, gabapentin, ibuprofen, etc.) were collected due to limited use during the study period. This could potentially affect the interpretation of the RASS, CPOT, and CAM-ICU scores. Also, we did not include other self-reported pain assessment scores.
In conclusion, the opioid drug shortage did have a significant impact on prescribing practices. Extremely vulnerable populations, such as the critically ill, leave clinicians uneasy when IV opioids are no longer available which have historically been the mainstay of analgesic regimens. However, study findings support that institutions can effectively ration supplies during a shortage with a strategic drug shortage management process. IV opioids were still able to be utilized in MICU patients although opioid stewardship resulted in a 23.2% decrease in use during the shortage. Similar RASS and CPOT scores were maintained with use of less IV opioids without an increase in prescribing of harmful sedative medications such as benzodiazepines. Larger trials are needed to confirm our findings on the impact of drug shortages on patient outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-hpx-10.1177_0018578721999805 for Impact of the Injectable Opioid Drug Shortage on Analgesia and Sedation Management in the Medical Intensive Care Unit: A Retrospective Cohort Study by Kayla John, Kari Cape, Lauren Goodman and Jessica Elefritz in Hospital Pharmacy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Center for Advancing Translational Sciences, Grant UL1TR002733.
ORCID iDs: Kayla John  https://orcid.org/0000-0001-8701-8008
https://orcid.org/0000-0001-8701-8008
Lauren Goodman  https://orcid.org/0000-0001-5589-875X
https://orcid.org/0000-0001-5589-875X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Injectable Opioids Shortage FAQ. ASHP. Published 2018. Accessed August 19, 2019. https://www.ashp.org/Drug-Shortages/Shortage-Resources/Injectable-Opioid-Shortages-FAQ [Google Scholar]
- 2. Brydges G. RE: FDA–2018–N–3272, Identifying the root causes of drug shortages and finding enduring solutions; public hearing; request for comments. AANA. Letter. January 2019. [Google Scholar]
- 3. Ventola CL. The drug shortage crisis in the United States: causes, impact, and management strategies. Pharm Ther. 2011; 36(11):740-757. [PMC free article] [PubMed] [Google Scholar]
- 4. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873. [DOI] [PubMed] [Google Scholar]
- 5. Hughes KM, Goswami ES, Morris JL. Impact of a drug shortage on medication errors and clinical outcomes in the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2015;20(6):453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klaus DA, De bettignies AM, Seemann R, Krenn CG, Roth GA. Impact of a remifentanil supply shortage on mechanical ventilation in a tertiary care hospital: a retrospective comparison. Crit Care. 2018;22(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 8. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Conde, Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. Published online May 9, 2019. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouwma A, Mlynarek M, Peters M, Procopio V, Martz C. Surgical intensive care unit pain management in the era of intravenous opioid shortages. Crit Care Med. 2020;48(1):23. doi: 10.1097/01.ccm.0000618684.42663.b5 [DOI] [Google Scholar]
- 12. Hollingsworth H, Herndon C. The parenteral opioid shortage: causes and solutions. J Opioid Manag. 2018;14(2):81-82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-hpx-10.1177_0018578721999805 for Impact of the Injectable Opioid Drug Shortage on Analgesia and Sedation Management in the Medical Intensive Care Unit: A Retrospective Cohort Study by Kayla John, Kari Cape, Lauren Goodman and Jessica Elefritz in Hospital Pharmacy