Abstract
Objective
To estimate the effect of extracorporeal membrane oxygenation (ECMO) compared with conventional mechanical ventilation on outcomes of patients with covid-19 associated respiratory failure.
Design
Observational study.
Setting
30 countries across five continents, 3 January 2020 to 29 August 2021.
Participants
7345 adults admitted to the intensive care unit with clinically suspected or laboratory confirmed SARS-CoV-2 infection.
Interventions
ECMO in patients with a partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio <80 mm Hg compared with conventional mechanical ventilation without ECMO.
Main outcome measure
The primary outcome was hospital mortality within 60 days of admission to the intensive care unit. Adherence adjusted estimates were calculated using marginal structural models with inverse probability weighting, accounting for competing events and for baseline and time varying confounding.
Results
844 of 7345 eligible patients (11.5%) received ECMO at any time point during follow-up. Adherence adjusted mortality was 26.0% (95% confidence interval 24.5% to 27.5%) for a treatment strategy that included ECMO if the PaO2/FiO2 ratio decreased <80 mm Hg compared with 33.2% (31.8% to 34.6%) had patients received conventional treatment without ECMO (risk difference –7.1%, 95% confidence interval –8.2% to –6.1%; risk ratio 0.78, 95% confidence interval 0.75 to 0.82). In secondary analyses, ECMO was most effective in patients aged <65 years and with a PaO2/FiO2 <80 mm Hg or with driving pressures >15 cmH2O during the first 10 days of mechanical ventilation.
Conclusions
ECMO was associated with a reduction in mortality in selected adults with covid-19 associated respiratory failure. Age, severity of hypoxaemia, and duration and intensity of mechanical ventilation were found to be modifiers of treatment effectiveness and should be considered when deciding to initiate ECMO in patients with covid-19.
Introduction
About 40% of patients with covid-19 admitted to the intensive care unit develop severe acute respiratory failure.1 2 In patients without covid-19 who develop progressive acute respiratory failure despite optimal support with conventional mechanical ventilation, extracorporeal membrane oxygenation (ECMO) reduces mortality by maintaining gas exchange and mitigating ventilator induced lung injury while the lungs recover.2 3 4 5 6 Reports of poor survival rates in case series of patients with covid-19 associated acute respiratory failure treated with ECMO, however, discouraged clinicians from using ECMO early in the pandemic and even led some to call for a moratorium on its use in patients with covid-19, especially given limited resources during a pandemic.7 However, an early report from the Extracorporeal Life Support Organization showed that the mortality of patients receiving ECMO for covid-19 associated acute respiratory failure might be closer to 40%.8 Moreover, subsequent observational studies reported outcomes with ECMO in covid-19 associated acute respiratory failure that were similar to previous observations on the effect of ECMO in patients with acute respiratory failure from other causes, although mortality rates have varied over time and across jurisdictions during the pandemic.9 10 11 12 13 14 Nevertheless, data to guide clinical decisions about patient selection for ECMO are lacking, and established protocols are largely based on the results from a recent randomised controlled trial in patients with acute respiratory failure without covid-19, which suggested a benefit of ECMO in patients with severe hypoxaemic acute respiratory failure, characterised by a partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio <80 mm Hg.3 4
Using observational data to emulate a target trial represents an established statistical approach to estimate treatment effectiveness across populations in an uncontrolled setting.15 16 This analysis approach provides an attractive complement to randomised controlled trials17 and can yield important and more generalisable information, especially when the conduct of a randomised trial is challenging (eg, slow enrolment rates, crossovers, restrictive inclusion criteria, and lack of equipoise), particularly when investigating a complex and resource intensive intervention such as ECMO during a global pandemic.3 18 19
In this registry based cohort study of adults with covid-19 related acute respiratory failure, we compared hospital mortality and the probability of being discharged alive between ECMO treatment in patients with a PaO2/FiO2 ratio <80 mm Hg and a treatment strategy where all patients received conventional mechanical ventilation without ECMO. In additional analyses, we investigated if age, pre-existing comorbidities, or the duration of mechanical ventilation preceding ECMO use was associated with modified treatment effectiveness. Finally, we estimated the effectiveness of ECMO when initiated on the basis of various ranges for markers accounting for the severity of acute respiratory failure or intensity of mechanical ventilation that change during a patient’s hospital admission.
Methods
We used data from the international, multicentre COVID-19 Critical Care Consortium registry.20 Study methods and design of the registry are published elsewhere.21 Briefly, adults with clinically suspected (determined by attending doctor) or laboratory confirmed SARS-CoV-2 infection (reverse transcriptase polymerase chain reaction or next generation sequencing, or both) were eligible for analysis if they were admitted to an intensive care unit between 3 January 2020 and 29 August 2021. We excluded patients from countries that did not provide ECMO during the observation period and patients who were missing all baseline and longitudinal measurements, as counterfactual outcomes could not be reliably determined.
Participating hospitals obtained approval from their local institutional review board. Because the study was observational, with only deidentified data recorded in a central repository, waivers of informed consent were granted for all patients.21
Primary analysis
We compared hospital mortality and the probability of being discharged alive between a treatment strategy where ECMO was initiated if the PaO2/FiO2 ratio dropped below 80 mm Hg and a treatment strategy where all patients had received conventional mechanical ventilation without ECMO. As we wanted to measure the causal effect of ECMO on outcomes (as in a per protocol analysis of a randomised controlled trial), adherence adjusted estimates were calculated for the primary analysis. We also conducted an “as treated” analysis and compared outcomes between treatment as received (which could have included ECMO) and treatment with conventional mechanical ventilation without ECMO. The primary outcome was hospital mortality, and patients were followed from admission to the intensive care unit (time point zero for the analysis) until either death, hospital discharge alive, or 60 days, whichever occurred first. Discharge alive was considered a competing event for hospital mortality because patients who were discharged could not then die in hospital.
Secondary analyses
In secondary analyses, we investigated if age, pre-existing comorbidities (diabetes, obesity, and arterial hypertension), and duration of mechanical ventilation were potential effect modifiers. Also, we estimated the effectiveness of ECMO when initiated based on different time varying markers of disease severity. The PaO2/FiO2 ratio was used as marker of the severity of acute respiratory failure. As a marker for the intensity of mechanical ventilation, we used static driving pressure, calculated as plateau airway pressure minus positive end expiratory pressure.22 In addition to the primary analysis, we investigated two additional ranges for the PaO2/FiO2 ratio as a basis to initiate ECMO: ≥80 and <120 mm Hg, ≥120 and <150 mm Hg. Four thresholds were used for static driving pressure: >12 cmH2O, >15 cmH2O, >17 cmH2O, and >20 cmH2O. The supplementary appendix provides further details.
Statistical analyses
To answer the research questions, we emulated a pragmatic randomised controlled trial using observational data and calculated adherence adjusted estimates using a three step analytical procedure (see supplementary figure S1), similar to a per protocol analysis of randomised controlled trial. This robust analysis approach, which eliminates immortal time bias in the estimation of absolute and relative risks, has been well described.16 23 24
Firstly, we created clones of each patient and assigned these clones to the different treatment strategies: ECMO, defined as ECMO being initiated if the PaO2/FiO2 ratio was <80 mm Hg, or conventional mechanical ventilation (without ECMO). Secondly, we censored clones that were non-adherent to their assigned treatment strategy during follow-up (eg, initiation of ECMO in the group treated with conventional mechanical ventilation). To mitigate the risk of violating the positivity assumption (patients rarely receive a cannula if they have already undergone a prolonged course of mechanical ventilation), clones were eligible for censoring during the first 21 days in the intensive care unit. Thirdly, we used inverse probability weighting to deal with selection bias introduced by censoring non-adherent clones. For each day we calculated the probability of not being censored, based on factors that might have been considered by the treatment team to decide whether ECMO should be initiated or not, such as physiological characteristics, disease severity, ventilation variables, specific treatments, and time (see supplementary appendix for additional details). Weighted marginal structural models were used to calculate absolute risks, differences in absolute risks, and risk ratios. The results were reported for the primary outcome (direct effect on hospital mortality) and for the competing event (indirect effect mediated by hospital discharge on mortality), thereby giving a better understanding of the total effect of ECMO on outcomes.25 Percentile based 95% confidence intervals were obtained using non-parametric bootstrapping with 500 resamples.
We conducted sensitivity analyses to detect residual confounding, control for country specific heterogeneity in treatment, and measure the influence of missing data and multiple imputation. Supplementary table S1 provides details on covariates included in the models, calculations of the inverse probability weights, and sensitivity analyses, as well as information on missing data and how such data were handled. The article is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.26 Analyses were performed in R version 4.0.4.
Patient and public involvement
No patient representatives or members of the public were involved in the design, conduct, or reporting plans of this research project. We aim to involve patients and members of the public in the development of an appropriate method of dissemination.
Results
Data on 7847 patients were recorded in the registry. We excluded 502 (6.4%) patients—150 were from countries that did not enrol patients requiring ECMO during the observation period and 352 had no baseline or longitudinal measurements. A total of 7345 (93.6%) patients were included in the analysis (see supplementary figure S2). The median age was 59 (interquartile range 49-68) years, and 2265 (30.8%) patients were women. The median PaO2/FiO2 ratio at baseline was 130 (interquartile range 88-193) mm Hg. The median length of hospital stay was 16 (interquartile range 6-30) days. The cumulative probability of death at 60 days was 35.3% (95% confidence interval 33.9% to 36.7%). A total of 844 patients (11.5%) received ECMO at any time point during follow-up. The median duration of ECMO was 13 (interquartile range 5-26) days. For patients who received ECMO during follow-up, median length of hospital stay was 29 (interquartile range 15-49) days, the cumulative probability of death at 60 days was 50.0% (95% confidence interval 46.2% to 54.1%), and the cumulative probability of remaining in hospital at 60 days was 16.0% (95% confidence interval 13.0% to 19.7%). Table 1 and supplementary tables S2 to S8 provide details on patient characteristics and ventilation variables at baseline.
Table 1.
Patient characteristics at baseline, treatments, and outcomes. Values are median (interquartile range) unless stated otherwise
| Characteristics | Estimate (n=7345) |
|---|---|
| No (%) women | 2265 (30.8) |
| Age (years) | 59 (49-68) |
| SOFA score | 4 (3-6) |
| APACHE II score | 14 (10-18) |
| Ventilation variables at baseline: | |
| Tidal volume (mL/kg predicted bodyweight) | 6.4 (5.8-7.1) |
| FiO2 (%) | 60 (49-76) |
| Positive end expiratory pressure (cmH2O) | 10 (8-12) |
| Airway plateau pressure (cmH2O) | 22 (20-25) |
| Respiratory rate per minute | 24 (20-28) |
| Gas exchange at baseline: | |
| PaO2/FiO2 ratio (mm Hg) | 130 (88-193) |
| PaO2 (mm Hg) | 79 (64-100) |
| SaO2 (%) | 94 (91-96) |
| Arterial pH | 7.41 (7.35-7.45) |
| PaCO2 (mm Hg) | 39 (34-47) |
| Serum bicarbonate (mmol/L) | 24 (22-27) |
| Lactate (mmol/L) | 1.4 (1.1-1.9) |
| No (%) of specific treatments recorded for at least one day during follow-up: | |
| Prone position | 1117 (15.2) |
| Neuromuscular blockade agent | 1707 (23.2) |
| Inhaled nitric oxide | 72 (1.0) |
| Vasoactive drugs | 1590 (21.6) |
| Renal replacement therapy | 738 (10.0) |
| Corticosteroids | 2961 (40.3) |
SOFA=sequential organ failure assessment; APACHE=acute physiology and chronic health evaluation; FiO2=fraction of inspiratory oxygen; PaO2=arterial partial pressure of oxygen; SaO2=arterial oxygen saturation; PaCO2=arterial partial pressure of carbon dioxide.
Primary analysis
Adherence adjusted hospital mortality was 26.0% (95% confidence interval 24.5% to 27.5%) for a treatment strategy that included ECMO if the PaO2/FiO2 ratio dropped below 80 mm Hg compared with 33.2% (31.8% to 34.6%) had patients received conventional treatment without ECMO (risk difference –7.1%, 95% confidence interval –8.2% to –6.1%; risk ratio 0.78, 95% confidence interval 0.75 to 0.82) (fig 1). The probability of being discharged alive was 67.5% (95% confidence interval 65.7% to 69.3%) for patients in the treatment strategy with ECMO compared with 60.6% (59.0% to 62.2%) had patients received conventional treatment without ECMO (risk difference 6.9%, 95% confidence interval 5.9% to 8.0%; risk ratio 1.11, 95% confidence interval 1.10 to 1.13). Estimated hospital mortality at 60 days under treatment as received was 34.8% (95% confidence interval 33.4% to 36.1%), and the estimated probability of being discharged alive was 58.3% (95% confidence interval 56.8% to 59.7%), similar to treatment with conventional mechanical ventilation without ECMO (fig 2 and supplementary table S9).
Fig 1.
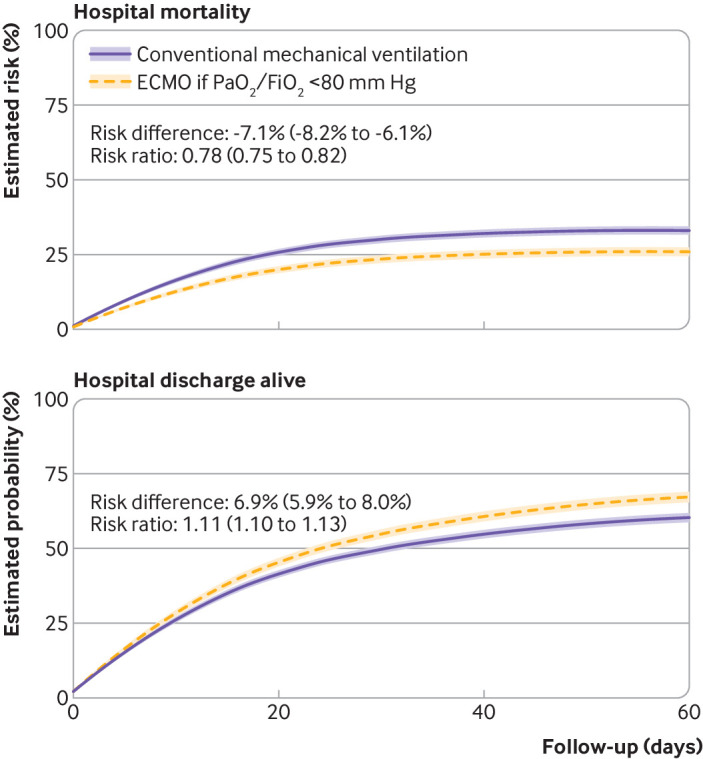
Treatment with extracorporeal membrane oxygenation (ECMO) if ratio of partial pressure of arterial oxygen to fraction of inspiratory oxygen (PaO2/FiO2) was <80 mm Hg, was compared with treatment with conventional mechanical ventilation without ECMO. Adherence adjusted estimates are reported for differences in hospital mortality and probability of hospital discharge alive in 7345 patients with covid-19 associated acute respiratory failure. Shaded areas represent 95% confidence intervals
Fig 2.
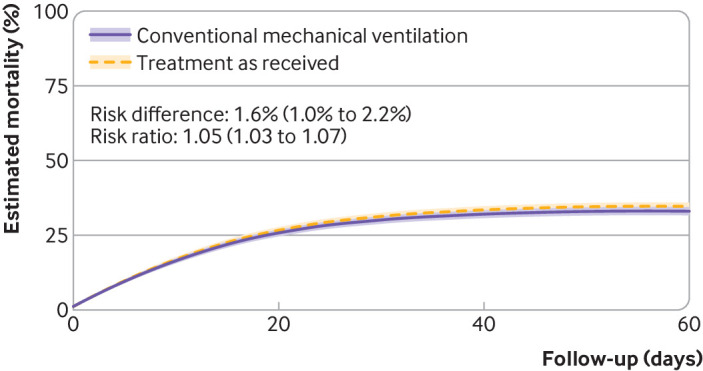
As treated analysis in 7345 patients, with hospital mortality compared between treatment as received, which could have included treatment with extracorporeal membrane oxygenation (ECMO), and treatment with conventional mechanical ventilation without ECMO. Shaded areas represent 95% confidence intervals
Secondary analyses
Treatment effectiveness of ECMO was shown to decrease with increasing patient age. In patients aged <50 years, the risk ratio for mortality was 0.71 (95% confidence interval 0.62 to 0.81) when ECMO was compared with conventional mechanical ventilation. In contrast, the risk ratio for mortality in patients aged ≥65 years was 0.84 (0.81 to 0.88) (fig 3 and supplementary table S10).
Fig 3.
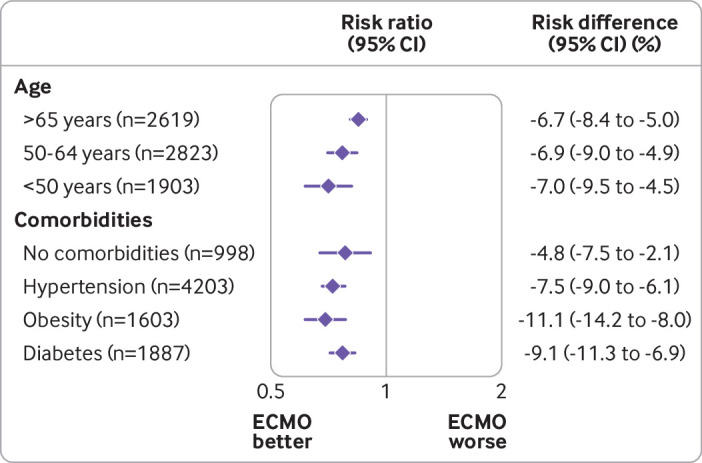
Treatment with extracorporeal membrane oxygenation (ECMO) if ratio of partial pressure of arterial oxygen to fraction of inspiratory oxygen (PaO2/FiO2) was <80 mm Hg compared with conventional mechanical ventilation without ECMO. Adherence adjusted effects (95% confidence intervals) on hospital mortality within 60 days reported by age groups and baseline comorbidities
For patients with pre-existing comorbidities, we estimated the following risk ratios when mortality was compared between ECMO and conventional mechanical ventilation: for arterial hypertension 0.75 (95% confidence interval 0.71 to 0.80), for diabetes 0.79 (0.74 to 0.84), and for obesity 0.72 (0.65 to 0.80) (fig 3 and supplementary tables S11 and S12). The risk ratio for mortality of patients without any comorbidities was 0.80 (95% confidence interval 0.70 to 0.91).
Treatment effectiveness was seen to decrease with increasing duration of mechanical ventilation before cannulation (fig 4). In patients who were mechanically ventilated for one day or less the risk ratio for mortality was 0.91 (0.88 to 0.94) when ECMO was compared with conventional mechanical ventilation. In patients who were mechanically ventilated for seven days, effectiveness of ECMO was observed to decrease, and the risk ratio for mortality was 0.97 (0.95 to 0.98) (supplementary table S13).
Fig 4.
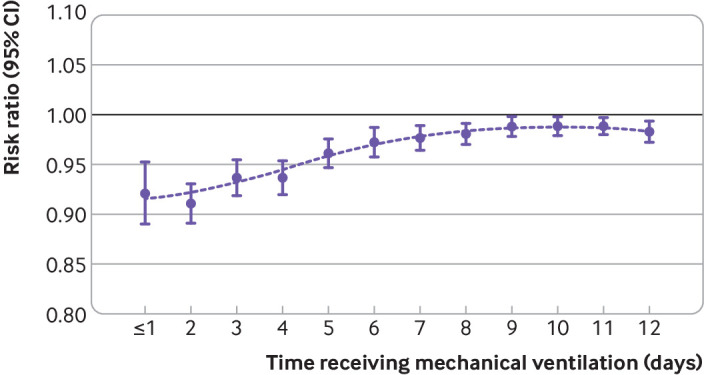
Treatment with extracorporeal membrane oxygenation (ECMO) if ratio of partial pressure of arterial oxygen to fraction of inspiratory oxygen (PaO2/FiO2) was <80 mm Hg compared with conventional mechanical ventilation without ECMO. The influence of duration of mechanical ventilation preceding ECMO is illustrated using risk ratios with 95% confidence intervals. Dots represent a loess curve
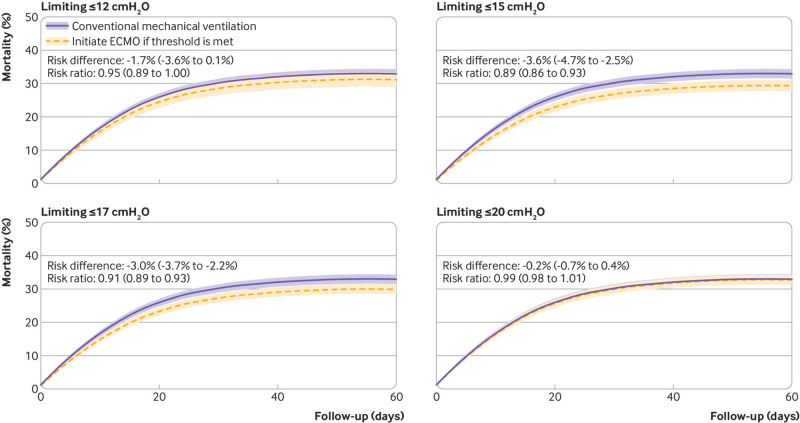
We compared different treatment strategies, when ECMO had been initiated according to either time dependent PaO2/FiO2 ratio or driving pressure levels, with conventional mechanical ventilation without ECMO. The risk ratio for mortality was 0.87 (0.84 to 0.91) for ECMO initiated in patients with a PaO2/FiO2 ratio of 80-119 mm Hg (fig 5). In contrast, the risk ratio for mortality was 1.05 (1.02 to 1.08) if ECMO had been initiated in patients with a PaO2/FiO2 ratio of 120-149 mm Hg, suggesting reduced effectiveness of ECMO in patients with less severe hypoxaemic respiratory failure (see supplementary table S14). We also compared ECMO treatment strategies initiated in patients with higher time dependent driving pressure levels with conventional mechanical ventilation without ECMO. The following risk ratios for mortality were measured if ECMO had been initiated according to the four prespecified thresholds for driving pressure: >12 cmH2O, 0.95 (0.89 to 1.00); >15 cmH2O, 0.89 (0.86 to 0.93); >17 cmH2O, 0.91 (0.89 to 0.93), and >20 cmH2O, 0.99 (0.98 to 1.01) (fig 6 and supplementary table S15).
Fig 5.
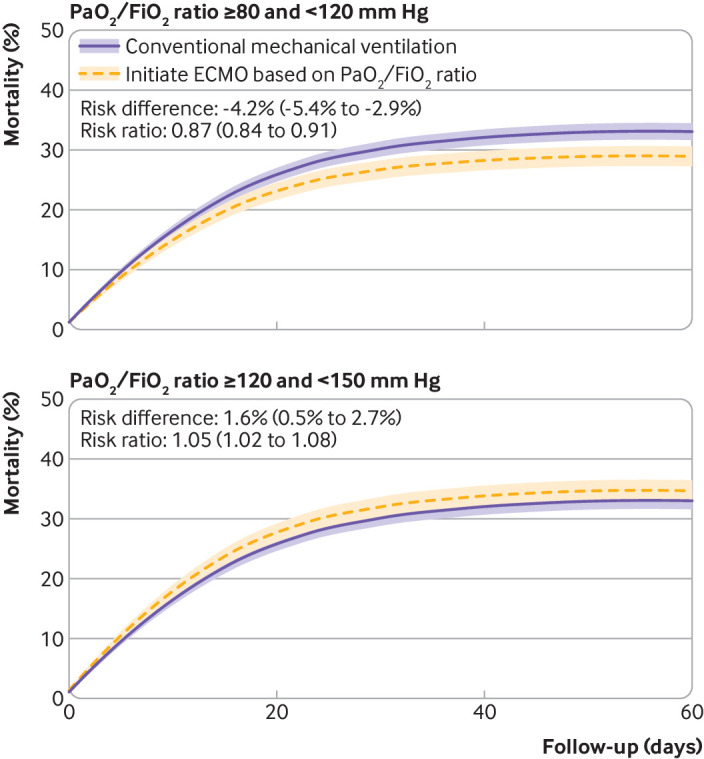
Treatment with extracorporeal membrane oxygenation (ECMO) if ratio of partial pressure of arterial oxygen to fraction of inspiratory oxygen (PaO2/FiO2) was <80 mm Hg compared with conventional mechanical ventilation without ECMO. The primary analysis was repeated with different PaO2/FiO2 ratio thresholds for initiation of ECMO. Shaded areas represent 95% confidence intervals
Fig 6.
Treatment with extracorporeal membrane oxygenation (ECMO) if ratio of partial pressure of arterial oxygen to fraction of inspiratory oxygen (PaO2/FiO2) was <80 mm Hg compared with conventional mechanical ventilation without ECMO. Treatment strategies, when ECMO was initiated based on various thresholds for driving pressure, were compared with conventional mechanical ventilation. Shaded areas represent 95% confidence intervals
Sensitivity analyses
Our findings were robust in several sensitivity analyses. Firstly, no effect was observed in an analysis using a random outcome variable with a 50:50 probability for which by design no causal relationship with ECMO existed (see supplementary figure S3). Detection of a treatment effect in such an analysis would have suggested important uncontrolled confounding. Secondly, similar results to the primary analysis were found in an analysis using an alternative set of covariates for the estimation of the inverse probability weights (see supplementary figure S4). Thirdly, estimates consistent with the primary analyses were measured in sensitivity analyses to detect the potential influence of country specific heterogeneity (see supplementary figure S5). Finally, supplementary figure S6 and table S16 describe the missing data patterns. Similar results to the primary analyses were measured in a complete case analysis without multiple imputation (see supplementary figure S7).
Discussion
In this registry based cohort study of 7345 adults with covid-19 associated acute respiratory failure, treatment with ECMO in those with PaO2/FiO2 <80 mm Hg was associated with a 7.1% reduction in hospital mortality (95% confidence interval 6.1% to 8.1%) compared with conventional mechanical ventilation without ECMO (risk ratio 0.78, 95% confidence interval 0.75 to 0.82). In secondary analyses, we found that ECMO would have been most effective if consistently provided to well selected patients with more severe hypoxaemia (ie, PaO2/FiO2 <80 mm Hg) or with exposure to higher intensities of mechanical ventilation (ie, driving pressure levels >15 cmH2O).
Primary analysis
In the primary analysis, we reported adherence adjusted estimates as in a per protocol analysis of a randomised trial. Adherence adjusted mortality was lower than observed mortality under the natural course or mortality of patients treated with a strategy that did not include ECMO. This finding suggests that adherence to our treatment strategy (initiation of ECMO if the PaO2/FiO2 ratio was <80 mm Hg) was associated with a reduction in mortality compared with conventional mechanical ventilation without ECMO. Our analysis approach assigned clones for all observed patients to each of the two treatment strategies, censoring only when the assigned treatment was violated (assignment to the other treatment). In both treatment strategies, we considered patients fully adherent in the primary analysis if they maintained a PaO2/FiO2 ratio ≥80 mm Hg and were not treated with ECMO. Both treatment groups comprised patients whose PaO2/FiO2 ratio never dropped below 80 mm Hg or received ECMO during follow-up (ie, they only developed a mild to moderate but never a severe form of covid-19 acute respiratory distress syndrome), which explains the difference between adherence adjusted estimates for mortality reported in the target trial analysis and survival probabilities conditional on the receipt of ECMO at any time point during follow-up.
As treated analysis
We also conducted an as treated analysis comparing treatment as received (which could have included ECMO) with conventional mechanical ventilation that could have included ECMO. Interestingly, treatment under real world conditions resulted in similar outcomes compared with a treatment strategy in which none of the patients had received ECMO. This finding might be explained by factors related to patient selection for ECMO and by influences related to country specific and temporal heterogeneity in decision making and treatment capacity.27 28 For example, clinicians might have been hesitant to use ECMO early in the pandemic after reports of poor survival rates in the initial case series of patients with covid-19 associated acute respiratory failure. Moreover, many countries may have been overwhelmed by the sheer volume of critically ill patients requiring admission to an intensive care unit during the pandemic, resulting in resource intensive interventions such as ECMO being restricted to those patients who it was thought would benefit the most. This as treated analysis should be interpreted with caution, however, because one treatment strategy does not represent an active intervention but reflects the real world situation (ie, treatment as received). This analysis might be more susceptible to bias from uncontrolled confounding than the primary analysis where two active interventions were compared.16 For the inverse probability weighting, we modelled the decision making process for the initiation of ECMO (ie, the daily probability that a patient would receive a cannula for ECMO) rather than individual patient outcomes, considering the challenges to model the different course of the pandemic in different countries. Importantly, we excluded from the analysis those patients from countries that did not provide ECMO during the observation period. While baseline risk levels for the multiple countries and centres might vary, we report estimates for the effect of ECMO that are consistent with previous findings. The ECMO to Rescue Lung Injury in Severe ARDS trial reported a risk ratio of 0.76 (95% confidence interval 0.55 to 1.04) for patients with acute respiratory distress syndrome from causes other than covid-19,3 whereas another study reported an adjusted hazard ratio of 0.55 (95% confidence interval 0.41 to 0.74) for the same duration of follow-up in patients with covid-19 associated acute respiratory failure,9 suggesting that the identified range of values for the causal effect estimated in our analysis aligns well with evidence from previous studies. Also, the results of our primary analysis were confirmed in a sensitivity analysis, adjusted for country specific heterogeneity in treatment, as well as in an analysis that excluded patients from the United States, underlining the robustness of our findings and methodological approach.
Secondary analyses
In secondary analyses, we investigated if age, pre-existing comorbidities, and duration of mechanical ventilation preceding ECMO initiation were associated with modified treatment effectiveness. Furthermore, we estimated the effectiveness of ECMO when initiated based on markers accounting for the severity of acute respiratory failure or intensity of mechanical ventilation that change during a patient’s admission. The use of ECMO was highly effective in patients younger than 65 years, and to a lesser extent potentially also in older patients. Interestingly, although comorbidities such as obesity, diabetes, and arterial hypertension are associated with more severe covid-19,29 ECMO was also observed to be effective at reducing mortality in these patients. Although hypothesis generating, our analyses question whether ECMO should be strictly limited to patients with covid-19 who have a PaO2/FiO2 <80 mm Hg for at least six hours and only during the first seven days of mechanical ventilation, as recommended by the current guidelines from the Extracorporeal Life Support Organization, which are based on the results of the ECMO to Rescue Lung Injury in Severe ARDS trial.3 4 We found a potential beneficial effect of ECMO in patients with a PaO2/FiO2 ratio <120 mm Hg, and even if support was initiated up to 10 days after the start of mechanical ventilation. In addition, we found that ECMO was associated with improved survival in patients with potentially injurious intensities of mechanical ventilation (ie, a driving pressure >15 cmH2O), likely mitigating the risk of ventilator induced lung injury.22
Comparison with other studies
Our study agrees with and builds on findings from previous reports on the effectiveness of ECMO in patients with acute respiratory distress syndrome from causes other than covid-19,3 as well as the results of a previous single country cohort study from the US9 investigating the effectiveness of ECMO in patients with covid-19 and more severe acute respiratory failure. Moreover, our study expands the current knowledge on clinical criteria that might be considered before initiating ECMO and on factors influencing the effectiveness of such treatment in patients with covid-19. Importantly, our results should be confirmed in future randomised controlled trials and should be seen as complementary information recorded in an uncontrolled setting under real world conditions. However, the conduct of such randomised controlled trials might be challenging owing to constraints on time and resources, along with the changing epidemiology of the pandemic and potential transition to endemicity of covid-19 as effective vaccines become more widely available.30 31 Finally, our findings might also provide valuable insights for future study design, as well as the potential utility of ECMO in patients with acute respiratory failure of other causes, such viral pneumonia from seasonal influenza viruses or Middle East respiratory syndrome coronavirus.32
Limitations of this study
Our study has several important limitations. Firstly, residual confounding, limitations in modelling complex interventions such as ECMO, missing data, and imputation of missing values might have influenced the results of our analyses. Daily measurements were routinely collected during the pandemic and made available for our analysis. These measurements might not necessarily reflect the worst or best value of the day. Also, the measurements might or might not align with the time point of cannulation for ECMO. Similar to findings in patients with acute respiratory distress syndrome of causes other than covid-19, airway plateau pressures and driving pressures were often missing and might only have been measured in patients with more severe illness.33 Our secondary analyses on thresholds of driving pressures should therefore be interpreted with caution. Our results, however, were robust in sensitivity analyses with control outcomes, in an analysis with alternative sets of covariates to calculate the inverse probability weights, and in a complete case analysis.16 Secondly, some of the patients might have been transferred to long term acute care facilities after hospital discharge. The mortality of patients after transfer to long term acute care hospitals might be substantial.34 For this reason, we considered discharge alive to be a competing event for hospital mortality in our analysis and not treated as a censoring event. Also, a major portion of patients remained in hospital and at risk of experiencing the primary outcome at the end of follow-up. Finally, the estimated effects of ECMO in patients with prolonged preceding duration of mechanical ventilation or in elderly patients, should be interpreted with caution considering that only highly selected patients in our cohort had received ECMO under such circumstances. The results of these secondary analyses are therefore hypothesis generating and require further validation.
Conclusions
In this international, registry based cohort study of patients with covid-19 associated acute respiratory failure, ECMO was associated with a reduction in mortality by 7.1% (95% confidence interval 6.1% to 8.1%) compared with conventional mechanical ventilation without ECMO. Furthermore, ECMO would have improved outcomes if consistently provided to well selected patients with more severe hypoxaemia or with exposure to higher intensities of mechanical ventilation. Age, severity of hypoxaemia, intensity of mechanical ventilation, and duration of mechanical ventilation should be considered when deciding to initiate ECMO to mitigate the risk of ECMO associated harm and to maximise effectiveness of ECMO in patients with covid-19.
What is already known on this topic
The optimal indications for extracorporeal membrane oxygenation (ECMO) and modifiers of treatment effectiveness in patients with acute respiratory failure from covid-19 are currently unknown
Established protocols for the initiation of ECMO are largely based on a randomised controlled trial in patients without covid-19
What is this study adds
In this registry based cohort study of 7345 patients with covid-19 associated acute respiratory failure, ECMO in those with partial pressure of arterial oxygen to fraction of inspired oxygen ratio <80 mm Hg was associated with a reduction in hospital mortality by 7.1% compared with conventional mechanical ventilation without ECMO
Age and severity of hypoxaemia, as well as duration and intensity of mechanical ventilation were modifiers of treatment effectiveness and should be considered when deciding to initiate ECMO in patients with covid-19
Acknowledgments
This study is supported by the COVID-19 Critical Care Consortium. We thank the clinicians, patients, and families who contribute data to this study. We recognise the importance of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and Short Period Incidence Study of Severe Acute Respiratory Infection (SPRINT-SARI) networks for the development and expansion of the COVID-19 Critical Care Consortium. We thank the generous support we received from the Extracorporeal Life Support Organization and the International ECMO Network. Finally, we acknowledge all members of the COVID-19 Critical Care Consortium and various collaborators. The supplementary appendix includes a list of members and collaborators.
Web extra.
Extra material supplied by authors
Supplementary information: Additional information, tables, figures, and lists of collaborators and contributors
Contributors: MU, JFF, and EF designed and planned the study. MU wrote the analysis code. AGB ran the analysis. AGB and EF supervised the analysis. MU, AGB, NDF, and EF interpreted the data. MU and EF wrote the initial manuscript draft. All authors were involved in critical revision of the final manuscript. MU, EF, AGB, and JFF are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The COVID-19 Critical Care Consortium received funding from the University of Queensland, Wesley Medical Research, the Prince Charles Hospital Foundation, Queensland Department of Health, the Health Research Board of Ireland, Biomedicine international training research programme for excellent clinician-scientists, European Union’s research and innovation programme (Horizon 2020), the Extracorporeal Life Support Organization, the International ECMO Network, La Caixa Foundation, and Fisher and Paykel Healthcare. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/coi_dischlosure.pdf. MU is supported by a Vanier Canada graduate scholarship from the Canadian Institutes of Health Research. AGB is supported by a National Health and Medical Research Council fellowship. SH is supported by a PhD scholarship from the Prince Charles Hospital Foundation. EF reports personal fees from ALung Technologies, Aerogen, Baxter, Boehringer-Ingelheim, GE Healthcare, Inspira, and Vasomune outside the submitted work; he is the chair of the research committee, Extracorporeal Life Support Organization (ELSO) and chair of the data committee, International ECMO Network (ECMONet). DB receives research support from ALung Technologies. He reports personal fees from Abiomed, Medtronic, Inspira, Cellenkos, and Xenios; he is the president-elect of ELSO and chair of the executive committee of ECMONet. GLB is a recipient of the Biomedicine international training research programme for excellent clinician-scientists (BITRECS) fellowship; the BITRECS project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement No 754550) and from the La Caixa Foundation (ID 100010434), under agreement LCF/PR/GN18/50310006. JFF reports grant support from Fisher and Paykel Healthcare, Xenios, Getinge, Cynata, CSL Behring (Australia), and Queensland Health outside the submitted work. CLH is supported by a National Health and Medical Research Council investigator grant from Australia. KS acknowledges research support from Metro North Hospital and Health Service. JYS is supported by the Advance Queensland Fellowship from the Queensland government.
The lead authors (MU and EF) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participant and related patient and public communities: The result of this work will be disseminated among the members of the COVID-19 Critical Care Consortium, Extracorporeal Life Support Organization, and International ECMO Network, as well as through our Twitter and other social media accounts.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: COVID-19 Critical Care Consortium Investigators, Tala Al-Dabbous, Huda Alfoudri, Subbarao Elapavaluru, Ashley Berg, Christina Horn, Stephan Schroll, Jorge Velazco, Wanda Fikes, Ludmyla Ploskanych, Dan Meyer, Maysoon Shalabi-McGuire, Trent Witt, Ashley Ehlers, Lorenzo Grazioli, E Wilson Grandin, Jose Nunez, Tiago Reyes, Mark Joseph, Brook Mitchell, Martha Tenzer, Ryuzo Abe, Yosuke Hayashi, Hwa Jin Cho, In Seok Jeong, Nicolas Brozzi, Jaime Hernandez-Montfort, Omar Mehkri, Stuart Houltham, Jerónimo Graf, Rodrigo Perez, Roderigo Diaz, Camila Delgado, Joyce González, Maria Soledad Sanchez, Diego Fernando Bautista Rincón, Melissa Bustamante Duque, Angela Maria Marulanda Yanten, Desy Rusmawatiningtyas, Gabrielle Ragazzo, Azhari Taufik, Margaretha Gunawan, Vera Irawany, Muhammad Rayhan, Elizabeth Yasmin Wardoyo, Mauro Panigada, Silvia Coppola, Sebastiano Colombo, Giacomo Grasselli, Michela Leone, Alberto Zanella, Massimo Antonelli, Simone Carelli, Domenico Grieco, Motohiro Asaki, Kota Hoshino, Leonardo Salazar, Laura Duarte, Joseph McCaffrey, Allison Bone, David Thomson, Christel Arnold-Day, Jerome Cupido, Zainap Fanie, Malcom Miller, Lisa Seymore, Dawid van Straaten, Ibrahim Hassan, Ali Ait Hssain, Jeffrey Aliudin, Al-Reem Alqahtani, Khoulod Mohamed, Ahmed Mohamed, Darwin Tan, Joy Villanueva, Ahmed Zaqout, Ethan Kurtzman, Arben Ademi, Ana Dobrita, Khadija El Aoudi, Juliet Segura, Gezy Giwangkancana, Shinichiro Ohshimo, Koji Hoshino, Saito Hitoshi, Yuka Uchinami, Javier Osatnik, Anne Joosten, Antoni Torres, Ana Motos, Minlan Yang, Carlos Luna, Francisco Arancibia, Virginie Williams, Alexandre Noel, Nestor Luque, Trieu Huynh Trung, Sophie Yacoub, Marina Fantini, Ruth Noemi Jorge García, Enrique Chicote Alvarez, Anna Greti, Oscar Lomeli, Adrian Ceccato, Angel Sanchez, Ana Loza Vazquez, Ferran Roche-Campo, Divina Tuazon, Toni Duculan, Hiroaki Shimizu, Marcelo Amato, Luciana Cassimiro, Flavio Pola, Francis Ribeiro, Guilherme Fonseca, Mehul Desai, Erik Osborn, Hala Deeb, Antonio Arcadipane, Claudia Bianco, Raffaele Cuffaro, Gennaro Martucci, Giovanna Occhipinti, Matteo Rossetti, Chiara Vitiello, Sung-Min Cho, Kate Calligy, Glenn Whitman, Naoki Moriyama, Jae-Burm Kim, Nobuya Kitamura, Takashi Shimazui, Abdullah Al-Hudaib, Alyaa Elhazmi, Johannes Gebauer, Toshiki Yokoyama, Abdulrahman Al-Fares, Esam Alamad, Fatma Alawadhi, Kalthoum Alawadi, Sarah Buabbas, Hiro Tanaka, Satoru Hashimoto, Masaki Yamazaki, Tak-Hyuck Oh, Mark Epler, Cathleen Forney, Jared Feister, Katherine Grobengieser, Louise Kruse, Joelle Williamson, Eric Gnall, Mara Caroline, Sasha Golden, Colleen Karaj, Sherry McDermott, Lynn Sher, Timothy Shapiro, Lisa Thome, Mark Vanderland, Mary Welch, Luca Brazzi, Tawnya Ogston, Dave Nagpal, Karlee Fischer, Roberto Lorusso, Maria de Piero, Mariano Esperatti, Diarmuid O’Briain, Edmund G Carton, Ayan Sen, Amanda Palacios, Deborah Rainey, Cassandra Seefeldt, Lucia Durham, Octavio Falcucci, Amanda Emmrich, Jennifer Guy, Carling Johns, Emily Neumann, Nina Buchtele, Michael Schwameis, Stephanie-Susanne Stecher, Delila Singh, Michaela Barnikel, Lukas Arenz, Akram Zaaqoq, Lan Anh Galloway, Caitlin Merley, Marc Csete, Luisa Quesada, Isabela Saba, Daisuke Kasugai, Hiroaki Hiraiwa, Taku Tanaka, Eva Marwali, Yih-Sharng Chen, John Laffey, Marlice VanDyk, Sarah MacDonald, Ian Seppelt, Indrek Ratsep, Lauri Enneveer, Kristo Erikson, Getter Oigus, Andra-Maris Post, Piret Sillaots, Effe Mihelis, Mamoru Komats, S Veena Satyapriya, Amar Bhatt, Marco Echeverria, Juan Fiorda, Alicia Gonzalez, Nahush A Mokadam, Johnny McKeown, Joshua Pasek, Haixia Shi, Alberto Uribe, Rita Moreno, Bishoy Zakhary, Hannah Johnson, Nolan Pow, Marco Cavana, Alberto Cucino, Giuseppe Foti, Marco Giani, Vincenzo Russotto, Davide Chiumello, Valentina Castagna, Andrea Dell’Amore, Hoi-Ping Shum, Alain Vuysteke, Asad Usman, Andrew Acker, Blake Mergler, Nicolas Rizer, Federico Sertic, Benjamin Smood, Alexandra Sperry, Madhu Subramanian, Navy Lolong, Ernita Akmal, Erlina Burhan, Menaldi Rasmin, Bhat Naivedh, Peter Barrett, Julia Daugherty, David Dean, Antonio Loforte, Irfan Khan, Olivia DeSantis, Mohammed Abraar Quraishi, Gavin Salt, Dominic So, Darshana Kandamby, Jose M Mandei, Hans Natanael, Eka Yudha Lantang, Anastasia Lantang, Anna Jung, Terese Hammond, George Ng, Wing Yiu Ng, Pauline Yeung, Shingo Adachi, Pablo Blanco, Ana Prieto, Jesús Sánchez, Meghan Nicholson, Michael Farquharson, Warwick Butt, Alyssa Serratore, Carmel Delzoppo, Pierre Janin, Elizabeth Yarad, Richard Totaro, Jennifer Coles, Robert Balk, Samuel Fox, James Hays, Esha Kapania, Pavel Mishin, Andy Vissing, Garrett Yantosh, Saptadi Yuliarito, Kohar Hari Santoso, Susanthy Djajalaksana, Arie Zainul Fatoni, Masahiro Fukuda, Keibun Liu, Paolo Pelosi, Denise Battaglini, Juan Fernando Masa Jiménez, Sérgio Gaião, Roberto Roncon-Albuquerque, Jessica Buchner, Young-Jae Cho, Sang Min Lee, Su Hwan Lee, Tatsuya Kawasaki, Pranya Sakiyalak, Prompak Nitayavardhana, Tamara Seitz, Rakesh Arora, David Kent, Swapnil Parwar, Andrew Cheng, Jennene Miller, Daniel Marino, Jillian E Deacon, Shigeki Fujitani, Naoki Shimizu, Jai Madhok, Clark Owyang, Hergen Buscher, Claire Reynolds, Olavi Maasikas, Aleksandr Beljantsev, Vladislav Mihnovits, Takako Akimoto, Mariko Aizawa, Kanako Horibe, Ryota Onodera, Meredith Young, Timothy Smith, Cheryl Bartone, Timothy George, Kiran Shekar, Niki McGuinness, Lacey Irvine, Brigid Flynn, Abigail Houchin, Keiki Shimizu, Jun Hamaguchi, Leslie Lussier, Grace Kersker, John Adam Reich, Gösta Lotz, Maximilian Malfertheiner, Esther Dreier, Lars Maier, Neurinda Permata Kusumastuti, Colin McCloskey, Al-Awwab Dabaliz, Tarek B Elshazly, Josiah Smith, Konstanty S Szuldrzynski, Piotr Bielański, Yusuff Hakeem, Keith Wille, Rebecca Holt, Ken Kuljit S Parhar, Kirsten M Fiest, Cassidy Codan, Anmol Shahid, Mohamed Fayed, Timothy Evans, Rebekah Garcia, Ashley Gutierrez, Tae Song, Rebecca Rose, Suzanne Bennett, Denise Richardson, Dalia Lopez-Colon, Lovkesh Arora, Kristina Rappapport, Kristina Rudolph, Zita Sibenaller, Lori Stout, Alicia Walter, Daniel Herr, Nazli Vedadi, Lace Sindt, Cale Ewald, Julie Hoffman, Sean Rajnic, Shaun Thompson, Ryan Kennedy, Matthew Griffee, Anna Ciullo, Yuri Kida, Ricard Ferrer Roca, Cynthia Alegre, Sofia Contreras, Jordi Riera, Christy Kay, Irene Fischer, Elizabeth Renner, Hayato Taniguci, Gabriella Abbate, Halah Hassan, Varun A Karnik, Katrina Ki, Hollier F O'Neill, Nchafatso Obonyo, Leticia Pretti Pimenta, Janice D Reid, Kei Sato, Aapeli Vuorinen, Karin S Wildi, Emily S Wood, and Stephanie Yerkovich
Ethics statements
Ethical approval
Participating hospitals obtained approval from their local institutional review board and waivers of informed consent were granted for all patients, since the study was observational, with only deidentified data recorded in a central repository.
Data availability statement
An independent research team collects data of the international, multicentre COVID-19 Critical Care Consortium registry. Deidentified registry data may be obtained for research purposes on approval of a formal proposal. Derived data supporting the findings of this study may be available from the corresponding author upon request.
References
- 1. Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934-43. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Guo Z, Li B, et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J 2020;66:475-81. 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Combes A, Hajage D, Capellier G, et al. EOLIA Trial Group, REVA, and ECMONet . Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018;378:1965-75. 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 4. Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO Guidance Document: ECMO for COVID-19 Patients with Severe Cardiopulmonary Failure. ASAIO J 2020;66:472-4. 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peek GJ, Mugford M, Tiruvoipati R, et al. CESAR trial collaboration . Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 6. Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 2019;7:163-72. 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 7. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J Crit Care 2020;58:27-8. 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal Life Support Organization . Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020;396:1071-8. 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaefi S, Brenner SK, Gupta S, et al. STOP-COVID Investigators . Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med 2021;47:208-21. 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt M, Hajage D, Lebreton G, et al. Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université. Paris-Sorbonne ECMO-COVID investigators . Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med 2020;8:1121-31. 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mustafa AK, Alexander PJ, Joshi DJ, et al. Extracorporeal Membrane Oxygenation for Patients With COVID-19 in Severe Respiratory Failure. JAMA Surg 2020;155:990-2. 10.1001/jamasurg.2020.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care 2021;25:211. 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gannon WD, Stokes JW, Francois SA, et al. Association Between Availability of ECMO and Mortality in COVID-19 Patients Eligible for ECMO: A Natural Experiment. Am J Respir Crit Care Med 2022. 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karagiannidis C, Strassmann S, Merten M, et al. High In-Hospital Mortality Rate in Patients with COVID-19 Receiving Extracorporeal Membrane Oxygenation in Germany: A Critical Analysis. Am J Respir Crit Care Med 2021;204:991-4. 10.1164/rccm.202105-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med 2021;384:1412-23. 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016;183:758-64. 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gershon AS, Jafarzadeh SR, Wilson KC, Walkey AJ. Clinical Knowledge from Observational Studies. Everything You Wanted to Know but Were Afraid to Ask. Am J Respir Crit Care Med 2018;198:859-67. 10.1164/rccm.201801-0118PP. [DOI] [PubMed] [Google Scholar]
- 18. Gattinoni L, Vasques F, Quintel M. Use of ECMO in ARDS: does the EOLIA trial really help? Crit Care 2018;22:171. 10.1186/s13054-018-2098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janiaud P, Axfors C, Ioannidis JPA, Hemkens LG. Recruitment and Results Reporting of COVID-19 Randomized Clinical Trials Registered in the First 100 Days of the Pandemic. JAMA Netw Open 2021;4:e210330. 10.1001/jamanetworkopen.2021.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubin R. Global Effort to Collect Data on Ventilated Patients With COVID-19. JAMA 2020;323:2233-4. 10.1001/jama.2020.8341. [DOI] [PubMed] [Google Scholar]
- 21. Li Bassi G, Suen J, Barnett AG, et al. COVID-19 Critical Care Consortium Investigators . Design and rationale of the COVID-19 Critical Care Consortium international, multicentre, observational study. BMJ Open 2020;10:e041417. 10.1136/bmjopen-2020-041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med 2020;8:905-13. 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ 2018;360:k182. 10.1136/bmj.k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotton CA, Heagerty PJ. Evaluating epoetin dosing strategies using observational longitudinal data. Ann Appl Stat 2014;8:2356-77. 10.1214/14-AOAS774. [DOI] [Google Scholar]
- 25. Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, Hernán MA. A causal framework for classical statistical estimands in failure-time settings with competing events. Stat Med 2020;39:1199-236. 10.1002/sim.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27. MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA 2020;323:1245-6. 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 28. Nkhata SG, Ngoma TN, Chilenga PM. SARS-CoV 2 (Covid-19) Heterogeneous Mortality Rates across Countries May Be Partly Explained by Life Expectancy, Calorie Intake, and Prevalence of Diabetes. Hum Ecol Interdiscip J 2020;48:633-8. 10.1007/s10745-020-00191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson S, Hirsch JS, Narasimhan M, et al. the Northwell COVID-19 Research Consortium . Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021;397:875-7. 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021;371:741-5. 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med 2021;47:83-5. 10.1007/s00134-020-06320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellani G, Laffey JG, Pham T, et al. LUNG SAFE Investigators. ESICM Trials Group . Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 34. Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA 2010;303:2253-9. 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional information, tables, figures, and lists of collaborators and contributors
Data Availability Statement
An independent research team collects data of the international, multicentre COVID-19 Critical Care Consortium registry. Deidentified registry data may be obtained for research purposes on approval of a formal proposal. Derived data supporting the findings of this study may be available from the corresponding author upon request.