Abstract
The million-dollar question that has been the talk of the day is how effective the COVID 19 vaccines are against the Omicron variant. Still, there is no clear-cut answer to this question but several studies have concluded that this Variant of Concern (VOC) successfully weakens the neutralizing capability of the antibodies acquired from the COVID 19 vaccines and prior infections, which indicates that Omicron can easily bypass an individual’s humoral immune response. However, the most significant confusion revolves around cell-mediated immunity tackling the Omicron variant. This paper aims to provide a clear idea about the status of the body’s immune surveillance concerning the infection caused by the Omicron variant by producing the effectivity of the humoral and cell-mediated immunity in handling the same. This work also provides complete detail of the various characteristics of the Omicron variant and how it may be a blessing in disguise. The effectiveness of the current vaccines, the transmissibility rate of the variant compared to the other variants, and the importance of administering a booster dose to prevent the spread of this variant are also discussed. Finally, this work aims to bridge the gap between the past and the current status of the Omicron infection and sheds light on the hypothetical idea that herd immunity developed from the SARS-COV2 infection may help tackle other dangerous variants.
Keywords: Omicron, Delta, Immunity, Mutation, Booster, Variants
1. Introduction
The latest variant of SARS-CoV-2, called B.1.1.529 variant or Omicron, was first discovered in the specimens collected in Botswana, a country in South Africa, on November 11, 2021 (Prevention, 2021). The Omicron variant has been reported to exhibit more than 50 mutations compared to the original wild-type strain. It has been identified in several countries worldwide (Sun et al., 2021). Initial epidemiological studies have demonstrated that the cases are increasing in South Africa; most of the PCR tests failed to detect the S-gene target (Keeton et al., 2021). The daily case numbers in South Africa quickly shifted from 273 on November 16 to more than 1200 by November 25. By November 29, cases were reported in Italy, Netherlands, Germany, France, and Portugal (Torjesen, 2021). According to the data from South Africa, the spreading rate of Omicron is more significant than any of the previously reported covid variants suggesting the immune escape of the variant in both vaccinated and previously infected people (Dyer, 2021, Rodger, 2022).
Some of the mutations present in this variant are a bit concerning as the studies suggest that the reinfection rate of this variant is higher. The PCR test results check the S gene target failure or S gene drop out, confirming the infection. To better understand the Omicron variant, WHO has requested the countries to upsurge their surveillance and genome sequencing, submit the associated data to the GISAID database, and report the initial VOC infections through the IHR mechanism. Field investigations and experimental assessments are performed to understand the effects of the VOC, its severity, antibody neutralization and immune responses, and the possible measures that can be taken. Social measures recommended by WHO include proper sanitation and ventilation, using a properly fitting mask, social distancing, avoiding social gatherings, and receiving the vaccination (WHO. 23rd).
Ferré et al. observed high correlation with the live virus neutralization assay, pseudo neutralization or variant-specific ELISA assays and they concluded that this may be beneficial for monitoring the humoral response. These findings demonstrated that the nature of the humoral response can be determined using simpler or automated techniques than live virus neutralization. Indeed, these assays could be useful for monitoring immunocompromised patients (Ferré et al., 2022).
As per several studies, the upsurge of Omicron-related COVID infections still provides hope. Even if the cases are increasing, the hospitalization and severity in cases are more diminutive. The studies suggest that Omicron is a less concerning phase in the pandemic. The Omicron variant is hypothesized to help silence the pandemic as it helps build immunity. The numerous mutations and the immunity achieved by the population have finally resulted in a less severe form than the previous forms. During the fourth wave dominated by the Omicron variant in South Africa, less than 73% of people hospitalized were more likely to survive severe diseases than in the third wave, which was dominated by the Delta variant (Standard, 2022).
1.1. Sublineages of the Omicron variant
Researchers have confirmed the presence of three more sublineages of Omicron. The common lineage is BA.1/B.1.1.529.1, and the two other sublineages are referred to as BA.2/B.1.1.529.2 and BA.3/B.1.1.529.3. Complete sequencing can detect all these lineages. The lineage BA.2 has been called ‘Stealth Omicron’ because it significantly differs from the original or standard variety. It is reported not to demonstrate the S gene target failure (SGTF). As a result, the PCR tests cannot detect an Omicron, or Alpha, variant (Control, 2022).
2. Mutations in the Omicron variant
The Omicron variant is found to have at least 50 mutations, of which 30 mutations occur in the S gene and are identical to the delta and alpha variants, many of which are unique to the Omicron variant (Thakur and Kanta Ratho, 2021). A total of 37 mutations are present in the Omicron variant spike protein. Six are deletion mutations, 30 mutations are substitutional, and 1 is an insertional mutation (Venkatakrishnan et al., 2021). The S protein’s receptor-binding domain (RBD) directly binds to the human ACE2 receptors. Several amino acid changes are seen in the RBD of the Omicron variant (Quarleri et al., 2021). Position 214 of the EPE spike contains three amino acid insertions. The impact of Spike protein mutations on disease transmission, pathogenesis, and vaccine efficacy is being closely studied (Gong et al., 2021). Compared to the previous B.1, B.1.1, and Delta SARS-CoV-2 variants, the Omicron SARS-CoV-2 virus has several sequence modifications. The mutations in all four structural proteins were investigated by Syed et al., using the SARS-CoV-2 virus-like particles (SC2-VLPs). Their findings suggest that the S and N protein mutations in Omicron increased cell permeability and the capsid assembly by three times more than the Delta virus. Compared to B.1/Delta, Omicron has one mutation in the E protein, and in the M protein, it contains three mutations (Table 1 ) (Syed et al., 2021).
Table 1.
A comparative summary of different Covid-19 variants. This table represents a detailed comparative overview of the most studied variants of Covid-19, first detection, changes in amino acids including amino acid mutation in S protein, number of mutations, transmissibility, and GISAID clade.
| Covid-19 Variants | Alpha (α) (B.1.1.7) | Beta (β) (B.1.351) | Gamma (γ) (P.1) | Delta (δ) (B.1.617.2) | Lambda (λ) (C.37) | Mu (μ) (B.1.621) | Omicron (ο) (B.1.1.529) |
|---|---|---|---|---|---|---|---|
| GISAID clade | GRY | GH/501Y.V2 | GR/501Y.V3 | G/478 K.V1 | GR/452Q.V1 | GH | GRA |
| Next strain clade | 20I (V1) | 20H (V2) | 20 J (V3) | 21A, 21I, 21 J | 21G | 21H | 21 K, 21L, 21 M |
| Monitored Amino acid changes | +S:484 K +S:452R |
+S: L18F | +S:681H | +S:417 N +S:484 K |
– | – | +S: R346K |
| First detection | United Kingdom (UK) | South Africa (SA) | Brazil | India (IN) | Peru | Colombia | Several Countries |
| Initially recorded samples | Sept 2020 | May 2020 | Nov 2020 | Oct 2020 | Dec 2020 | Jan 2021 | Nov 2021 |
| Date of Designation | Dec 18, 2020 | Dec 18, 2020 | Jan 11, 2021 | May 11, 2021 | Jun 14, 2021 | Aug 13, 2021 | Nov 26, 2021 |
| No. of Mutations in S protein | 7 Mutations and 2 Deletions | 9 Mutations and 1 Deletions | 12 Mutations | 17 Mutations and Mahor mutations | 7 nonsynonymous and 1 Deletion Mutations | 9 Mutation | 30 Substitutional, 6 Deletion, and 1 Insertional Mutations |
| Amino acid mutation in S protein | N501Y, A570D, D614G, P681H, T716I, S982A, D1118H, H69-V70del, Y144del |
L18F, D80A, D215G, R246I, K417N, E484K, N501Y, D614G, A701V, LAL 242-244del | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, V1176F | E484Q, L452R, T19R, T95I, G142D, E156-, F157-, R158G, L452R and more | Δ247-253, G75V, T76I, L452Q, F490S, T859N, ORF1a gene (Δ3675-3677) | T95I, Y144S, Y145N, R346K, E484K, N501Y, D614G, P681H, and D950N | G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, K417N, N440K and more |
| Transmissibility compares to Wild type | High | High | High | High | – | – | High |
Researchers studied the RBD-hACE2 binding of the Omicron variant by evaluating its Free energy of perturbation (FEP) data. They have found that mutations altered the RBD-hACE2 interactions near the Q498R and Q493R substitutions, which were the most contributing substitutions. They have shown that in addition to N501Y, the Q498R, Y505H, and G496S mutations significantly increased binding to hACE2. They increased binding by 98, 14, and 13 folds, respectively, converting the S1-RBD to a picomolar binder. In contrast to mice, the Q493R/K mutations, when combined with K417N and T478K, significantly reduced S1 RBD binding by over 100 folds. The Omicron variant’s N440K, G446S, and T478K substitutions had lesser contributions (Fratev, 2021). G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, K417N, and N440K are the amino acid mutations present in the RBD of the new variant (Control, 2022, Vitiello et al., 2022). Higher amounts of hydrophobic amino acids like phenylalanine and leucine are present in the Omicron variant compared to the delta variant, both in its RBD and its entire spike protein. The more stable structure of the Omicron variant is supported by the fact that it has a more alpha-helix structure both in its RBD and whole spike protein compared to the delta variant (Kumar et al., 2021).
RBD binding to ACE2 is strengthened by the compensating mutations in the binding site of ACE2. The apo form and RBD of the spike trimer of omicron variant are thermodynamically unstable and the open conformation is supported by the unusual RBD-RBD interaction inside the ACE2-spike complex which strengthens the ACE2′s binding to the spike trimer. The neutralizing activity against omicron is maintained by JMB2002 which is a broad-spectrum therapeutic antibody and has completed the clinical trail phase 1. The ACE2 binding is inhibited by this antibody by binding to the RBD (Yin et al., 2022).
The variants BA.4 and BA.5 were considered as variants of omicron lineage by UK Health Security Agency (UKHSA). The first case of BA.4 variant was reported from South Africa within GISAID in January 2022. BA.4 and BA.5 contain the same mutations; however, other features are also seen in BA.5 variant. 41 genome cases of BA.4 and 27 sequences of BA.5 were found in most parts of South Africa from February 2022 (WION Web Team, 2022). Both BA.4 and BA.5 share the same amino acid mutations called F486V which is located on the spike protein of the virus near the place where the protein binds to the cell’s ACE receptor to start an infection. The previous antibodies present in the body gained by infection to COVID-19 will hang on to this spot and will neutralise the infection. The immune escape of these two variants is studied by immunologist by exposing the variants to the blood taken from people with the history of SARS-COV-2 infection and vaccinated individuals (Maxmen, 2022). XE variant is a recombinant of BA.1 and BA.2 subvariants of omicron and has the ability to lead to community transmission. As on April 5 1,125 cases of XE has been reported in England. The mutations which are not seen in the sequences of BA.1 and BA.2 are also present in this variant. XD and XF recombinants of delta variant and BA.1 subvariant of omicron were also previously been identified by U.K(Ho, 2022). WHO considers XE variant to be ten times more infectious than BA.2 variant (Goshwam, 2022). The first case of XE was detected on January 19 in U.K and apart from U.K other countries like India and Japan have also reported XE cases. According to researchers’ vaccines especially after a booster shot gives protection against severe diseases from this variant (Ho, 2022).
3. The pathophysiology of the Omicron variant
The status of whether Omicron variants result in severe diseases is unclear. The symptoms of Omicron variants are similar to the previous variants (Jr., 2021, Sheikh et al., 2021). Due to many changes in the SARS-CoV-2 receptor binding domain, the Omicron variant display a greater affinity for human ACE2 than the Delta version, showing a more excellent capability for transmission (Kumar et al., 2021). More evidence from EU/EEA countries is required to evaluate the magnitude of the effect fully and whether it holds across all population groups to account for international differences in vaccination coverage and patient populations currently hospitalized. As of now, there is no concrete proof of any change in the impact on casualties. Whether the vaccines and treatment and the drugs available are effective against Omicron, whether it spreads faster or causes more severe diseases and mortality, should be immediately addressed (Petersen et al., 2022). Antibodies developed against previous forms of SARS-CoV-2 are less effective in variants like Omicron and Delta due to alterations. Moreover, when most of the world obtains resistance to the virus via infection, vaccination, or both, the factors driving this ‘antigenic change’ are expected to get stronger (Fig. 1 ) (Callaway, 2021).
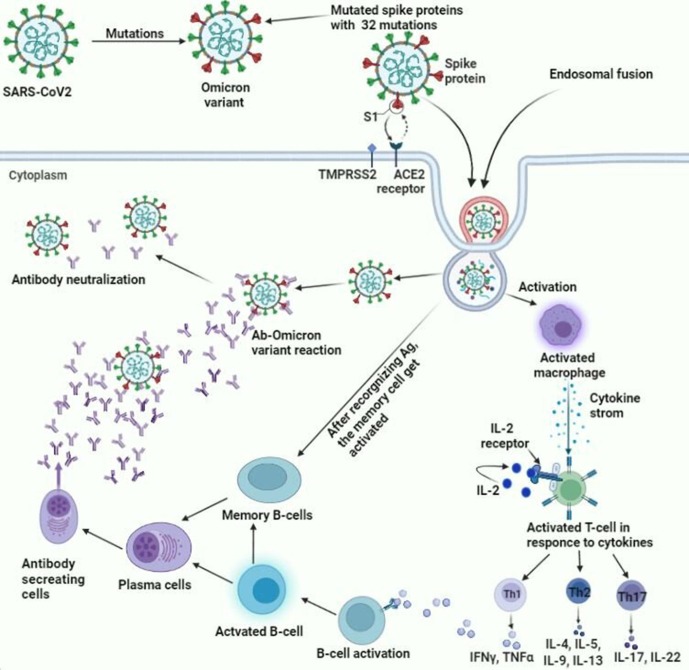
Fig. 1.
This figure demonstrates the overall pathophysiology and the immune responses associated with the Omicron variant. It shows the mechanism adopted by the Omicron variant to enter the body binding to the ACE 2 receptor and undergoing endosomal fusion to enter the cells. Upon entering the cells, it leads to several mechanistic pathways leading to the trigger of humoral and cell-mediated immunity, which coordinate to tackle the virus.
3.1. Threat to genomic surveillance
Genomic surveillance is based on mutations resulting from changes when the genetic material inside the genome replicates during reproduction. The rate of mutations in different species will be different and can be tracked. So, it would be easier to study how it is changing and evolving (Vancollie, (2021).) The genome sequencing of the positive samples is done in most countries as a part of their genomic surveillance to estimate the variants in the cases of super spread or breakthrough of the infection. Many countries use global genomic databases like GISAID to store the details of SARS-CoV-2 variants; more than 5.5 million samples have been submitted so far by different countries (Mohanty, 2021). While the Omicron variant appears to have increased the transmissibility of the Covid-19 infection, it is still unclear whether it will trigger a massive disease or evade vaccine-provided immunity (Service, 2021b, Service, 2021a). As most countries have relaxed their limitations on viral propagation, the chances for SARS-CoV-2 to make substantial evolutionary leaps also increase (Callaway, 2021). The modifications of the spike gene prevent the detection of Omicron in the test; hence positive results are only given by two of the genes in the samples carrying the variant (Callaway and Ledford, 2021).
3.2. Immune evasion and the mechanism of action
Omicron spreads faster as it can escape the first line of defense provided by the vaccines. The antibodies prevent infection by binding to the viral surface. However, the Omicron cannot cause severe conditions in vaccinated individuals because of the second line of defense provided by the vaccines, where the T and B cells work once the infection starts. Over time, or due to the spike protein mutations, the power of antibodies to prevent upper respiratory tract infections may diminish. However, the second line of defense provided by the vaccines does its job of fighting against the Omicron (Emily Head, 2021). You may even remain asymptomatic depending on how fast your T cells and B cells work (Brown, 2022, M, 2021). Omicron has numerous new mutations in the RBD of spike that vividly enhance the binding affinity in the RBDhACE2 complex while also mutating and causing rapid spread in humans all over the world (Dudas et al., 2021). It may have given the ancient SARS-CoV-2 and prior VOCs a distinct advantage in lung cells and primary human airway epithelial cells by enabling spike activation by the plasma membrane protease TMPRSS2, allowing for rapid cell surface fusion (Jackson et al., 2022).
Kumar S et al. studied the Delta and Omicron for the early prediction (at the molecular level) of a newly emerging viral variant of concern utilizing computational techniques (quick and cost-effective approach). Saxena SK et al. remark that dynamic transmission of Omicron appears to be greater than the earlier SARS-CoV-2 subtypes. The furin cleavage point is close to His655Tyr (speed up spike cleavage). The mutant His655Tyr residue could aid in the transmission and contribute to monoclonal antibody treatment resistance. Asn501Tyr increases the binding of ACE2 receptors (which may improve transmission). The proximity of Asn679Lys to the furin cleavage site (polybasic character of residue may aid spike cleavage) permits transmission (Saxena et al., 2021, Hoffman and Margolis, 2020, Peacock et al., 2021, Saxena et al., 2021). Kumar S et al. discovered that the Omicron variant had an enhanced affinity for human angiotensin-converting enzyme 2 than the Delta variant, indicating a higher expectation for transmission and significant impact on pathogenesis compared to the Delta variant. The mutational sites (Q493R, N501Y, S371L, S373P, S375F, Q498R, and T478K) contribute considerably too high binding affinity with the hACE2 receptor.
Compared to the Delta version, the whole spike protein and RBD of Omicron contain many hydrophobic amino acids such as LEU (leucine) and PHE (phenylalanine) (Kumar et al., 2021). Willett et al. found that the variant’s biological behavior has changed significantly; it now enters cells without the help of the host TMPRSS2 receptor protein, preferentially via the endosomal fusion pathway. To reduce syncytial development in infected cells, Omicron changes the mechanism of fusion activation to the host cell. It lowers the chances of recognition through cell-to-cell communication (Willett et al., 2022). These additional traits can significantly alter cellular tropism and disease etiology. Viruses persist with lower lethality and a high spread rate, posing a significant risk to persons with co-morbidities (two or more conditions), the elderly, and notably those immunosuppressed. Furthermore, research shows that SARS-CoV-2 has significant antigenic and functional changes. Furthermore, disease prediction is feasible due to fundamental shifts in transmission (Willett et al., 2022). When a highly transmissible variation is observed, it causes morphological modifications (e.g., Omicron). Vaccines mediate innate immune responses and virus evasion of immune and immunological responses. Omicron infections in young people are 40–70 % less severe than Delta infections (Saxena et al., 2021).
3.3. Infectivity and mortality risk
Scientists show that the current treatment options against SARS-COV-2 like remdesivir, molnupiravir, and the antiviral drugs developed by Pfizer were also effective against the Omicron variant. Since the first reported case in November 2021, Omicron cases have increased worldwide, dominating the Delta variant. However, there is no increase in the hospitalization and mortality rates. It is hypothesized that Omicron is less efficient in counteracting the host cell interferon response (Willett et al., 2022). In that case, it may account for the milder severity of the disease. The loss of efficiency in suppressing interferon responses means that the innate immune system will recognize and fight the new variant more effectively. The vaccine efficiency in immunocompromised patients against the SARS-CoV-2 is less. Severe complications in high-risk groups can be prevented by using antiviral therapies. Moderate to less severe cases do not require hospitalization (Peacock et al., 2021, Saxena et al., 2021).
Scientists exposed the Calu-3 and Caco-2 cultures of cells to Omicron or Delta variant isolates. The infection rate of Omicron was less compared to the Delta variant, but the infection patterns were similar. The less number of infected Vero cells showed that in comparison to the Delta variant, the Omicron variant is not effective in blocking the signals of cellular interferon. Omicron was successful in stimulating the promoter of the interferon response factor in the A549 cells in culture. The Omicron variant has almost double the number of mutations as the Delta variant. The mutations in the virus’s spike protein help it escape from the immune response and increase its potential to cause infection. The membrane protein, NSP3, NSP6, NSP14, S protein, and nucleocapsid protein mutations are seen in the Omicron variant. They are found to inhibit the interferon response of the host cell (Solis-MoreiraJan, 2022).
Garrett et al., described the asymptomatic carriage among South African health care workers in a substudy of the Sisonke vaccination trial, which found 2.6 percent asymptomatic carriage during the Beta and Delta outbreaks, rising to 16 percent during the Omicron phase. These conclusions sturdily recommend that Omicron has a much higher rate of asymptomatic carriage than other VOC and that this high prevalence of asymptomatic infection is likely a major factor in the variant's widespread, rapid spread around the world, even among populations with high rates of SARS-COV-2 infection previously (Garret et al., 2021).
4. Immune responses concerning the Omicron variant
4.1. B-cell mediated response and the Omicron variant
Studies by Perugino et al. show that in earlier unvaccinated and uninfected people, non-Receptor Binding Domain (non-RBD) SARS-CoV-2 spike-specific B cells were evident in two different, intense, inactive, cross-reactive, “pre-existing” switched memory B cell compartments. They found that the pre-existing RBD-specific B cells were scarce in the uninfected and unvaccinated individuals who were later molded by vaccination and infection. It led to vaccine boosted RBD-specific B cells. Additionally, they found no change in the incidence of wild-type RBD-binding memory B cells that cross-react with the Omicron variant RBD whereas, after a boost, B cells recognize the full-length Omicron variant spike protein expanded, with pre-existing resting memory B cells differentiating into effector B cell populations. B-cells obtained from early or priorly existing memory cells that identify full-length wild-type spikes with enhanced affinity following boosting are the same cells that positively interact with the spike protein of the Omicron variant (Fig. 1) (Perugino et al., 2022).
4.2. T-cell Mediated response and the Omicron variant
4.2.1. Immunosequencing and epitope mapping
Cell-mediated immunity of T- cell-mediated responses plays a massive role in instigating humoral immunity and plays the chief role in fighting against viruses. So a relation has been established between the T- cell and the reason behind less hospitalization of the re-infected patients despite the failure of the humoral responses. So if this T-cell response is activated, it is hypothesized to help tackle the Omicron variant infections that can easily bypass the neutralizing antibodies. The greatest fear is that if the several mutations in Omicron result in immune evasion ultimately (evading T-cells also), it will completely limit or restrict the cell-mediated immunity, leading to an enhanced number of infections and severity rate of the disease (May et al., 2021).
May et al. demonstrated the effect of the several Omicron mutations on vaccine-induced cell-mediated immunity, including CD4 + and CD8 + cells. They combined the principle of epitope mapping to SARS- CoV-2 vaccines with the T cell receptors. They concluded a loss of around 30% of the CD4 + T cell immunity compared to only 20% loss in CD8 + T cells. These reductions are much more as compared to the other variants. Thus, their studies reported an apparent reduction in the on-target Tcell memory, but a large part, i.e., over two-thirds of the cellular immune response, is hypothesized to remain intact. They presented that the individuals with prior infection from other variants are expected to reduce the spike-specific cellular immune memory. The substantial loss in the efficiency of the neutralizing antibodies obtained from the vaccine will dramatically reduce the effectiveness of the vaccines.
Nevertheless, since most of the mutations in the Omicron variant are in the spike proteins, they expect little or no abolition of the cell-mediated T-cell response outside the spike protein. Around 95% conservation is established between the number of mutations involving the T cell epitopes in several different genes of the virus compared to other variants. They have also clarified that the CD8 + T-cell response may show much promise against the Omicron variant (May et al., 2021).
4.3. Abs neutralization
Studies show that the human polyclonal antibodies target various neutralizing epitopes in and around the RBD. In natural SARS-CoV-2 communities, the diverse spike sequences coincide with the antibody targets. Researchers have developed a pseudotype spike protein polymutant that can resist the polyclonal antibody neutralization to the same extent as the SARS-VOC-2. Polymutant was created by combining the plasma selected spike substitutions. They showed that 20 mutations found naturally in the spike protein of SARS-COV-2 are adequate to achieve pseudotypes having almost the same resistance to polyclonal neutralizing antibodies generated by individuals who had received the mRNA vaccination. They also observed that this extremely resistant SARS-COV-2 spike polymutant was neutralized by the plasma of infected people and then vaccinated with the mRNA vaccine (Fig. 1) (Syed et al., 2021, Schmidt et al., 2021).
4.4. Cell-cell fusion studies of the Omicron and Delta variant
According to the latest (in vitro) studies with transfected cell lines, SARS-CoV-2 S protein (spike protein) expressed on the surface of the virus-donor cells, cleavage of the RBD and fusion domain of S1 and S2, respectively, generate and expose the fusion peptide directly involved in the fusion of the viral and host cell membrane expressing ACE2 and TMPRSS2. Infected cells can fuse with non-infected cells, resulting in giant syncytia, which are derived from the host cell–cell fusion of infected alveolar macrophages, and which undoubtedly play a crucial role in the systemic hyper-inflammation known as macrophage activation (cytokine storm) seen in patients with critical disease indications (Fig. 2)(Leroy et al., 2020).
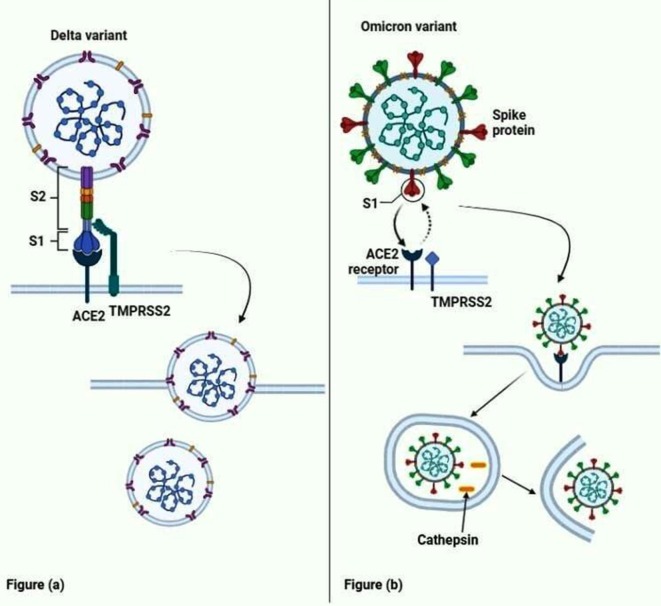
Fig. 2.
Comparison of the cell entry of the Omicron variant with the delta variant. Fig (a) shows the Delta variant, which requires a serine protease TMPRSS2 cleaves between the S1 and S2 part of the spike protein for cell entry. The S2 part of the spike protein then fuses with the cell membrane to release the viral mRNA into the cell. Fig (b) shows the entry of the Omicron variant by endocytosis. It requires only the ACE2 receptors, and the TMPRSS2 does not play a role in the entry of the Omicron variant. Once the virus undergoes endocytosis, the cathepsin present in the cell membrane liberates the viral mRNA from the phagosome.
According to Hanjun Zhao et al., the replication efficacy and cell–cell fusion activity of Omicron VOC and Delta VOC are substantially less dependent on TMPRSS2 and boosted in TMPRSS2/VeroE6 cells, respectively.TMPRSS 2 is an endothelial cell surface receptor protein that allows viruses to enter endothelial cells in the respiratory system. The viral genome (+ve sense ssRNA) can reach the cytoplasm by conformational changes in S protein, which is required to form the fusion pore, which mediates viral and cellular membrane fusion and allows the viral genome (+ve sense ssRNA). ACE2 (central receptor) and TMPRSS2 receptors must be expressed on nasal epithelial cells and alveolar type I and type II cells in the lung and other organ linings. These receptors prime the SARS-COV-2 virus’s S1 and S2 spike proteins. The endosomal cathepsin B/L, or TMPRSS2, cleaves the S2′ site and the S1/S2 junction by furin. The Omicron replication was unsuccessful in this study when using TMPRSS2 for viral replication. Previous animal research showed that TMPRSS2 enhanced viral replication of the Delta variant in alveolar pneumocytes. According to the findings, the Omicron has a lower replication efficacy in the lungs than the Delta variation of concern (Zhao et al., 2021).
Although both VeroE6/TMPRSS2 and Calu3 cells exhibit high quantities of TMRPSS2, there are variances in Omicron variant RNA replication in these two cell lines. The viral load in VeroE6/TMPRSS2 cell lines is identical when the Omicron and Delta variants are compared. The Omicron form had a substantially lower Calu-3 cell than the Delta variant (Zhao et al., 2021). According to Meng B et al., examining single-cell RNA-sequence data (nasal cells), Lung cells have larger ACE2 and TMPRSS2 mRNA transcripts than upper airway cell types. The findings of Meng B et al. from ex-vivo tissue showed reduced Omicron spike cleavage, and Omicron spike poorly utilizes TMPRSS2 surface protein-dependent cell membrane fusion. A limited frequency of viral incursions into target lower airway artificial organ cells or Calu-3 lung cells expressing TMPRSS2 serine protease and ACE2 was also linked to the Omicron variant spike (Meng et al., 2021). Meng B et al. used molecular dynamic modeling to find mutations in many SARS-CoV-2 lineages, particularly the B.1.1. The fusogenic potential of Alpha and Delta was much more significant than that of a D614G Wuhan-1 altered spike. The presence of three mutations in Omicron at P681H, 679, and 655 should favor spike S1&S2 cleavage; the observed cleavage efficiency is comparable to wild-type Wuhan-1 D614G mutation but lower than delta. According to the researchers, the delta had a higher fusion potency than the D614G Wuhan-1 spike. The low fusogenicity of the Omicron spike was detected, as expected from subpar cleavage and cell–cell fusing (alveolar or lung cell) required TMPRSS2. A low cell–cell fusion process could facilitate infectious cell–cell spread, resulting in small plaque sizes. Omicron compromised cell entry in lung cells and the syncytial formation ability. At the same time, it gained access to immune evasion, with possible implications for pathogenicity that may cause milder disease, as shown in preliminary epidemiological studies (Meng et al., 2021).
4.5. The status of monoclonal antibody treatment against Omicron
Laboratory tests indicate that most of the monoclonal antibodies used to avoid acute diseases are ineffective against the Omicron variant. The antibody therapy manufacturing companies admit that their products would not be adequate for the Omicron variant compared to the other variants. According to the preprints, only two antibodies display solid evidence of retaining some potential to prevent the variant; these include sotrovimaband DXP-604, which is presently facing clinical trials in China. The prevalence of Omicron, the number of cases, and hospitalizations will be the deciding factors for distributing sotrovimab across the states in the United States. Many countries fail to meet the demands for sotrovimab, which again stresses the health care system.
Either a single antibody or a combination of antibodies is present in the monoclonal antibody treatment. These monoclonal antibodies prevent viral infection by attaching to the spike protein of the virus. It is found to reduce the severity of disease by 85%. To study efficacy, artificial pseudoviruses or live SARS-CoV-2 were mixed with varied antibody treatment dosages. The antibody virus mixture was used to test their effectiveness against the ACE2 expressing cells which act as the receptor for the entrance of the virus into the cells. The researchers then measured the concentration at which the viral replication was reduced to half. Regeneron Pharmaceuticals in Tarrytown, New York, declared that their antibodies had reduced efficiency against Omicron. Of all the antibody treatments available, sotrovimab is considered effective against Omicron compared to the other antibodies even though it shows decreased potency compared to action against other variants because it specifically targets the portion of spike protein which usually remain unchanged across the other variants. The clinical neutralization data of the AstraZeneca-developed antibodies should be completed to confirm their neutralizing power. Scientists are working hard to understand the severity of diseases possessed by the Omicron variant. The mechanism of action of oral antiviral drugs such as molnupiravir and paxlovid are found to be effective against the Omicron variant. These oral drugs are cheaper than the other antibody treatments available. To determine the effectiveness of a treatment, it is necessary to identify the variant that had caused the disease, which is a bit challenging in the present situation (Kozlov, 2021).
4.6. The efficacy of antiviral drugs against Omicron
The sensitivity of the variants to antiviral drugs like favipiravir, ribavirin, camostat, PF-07321332, remdesivir, and aprotinin, which had distinct action mechanisms, were studied by researchers. The sensitivity shown by both variants was similar. The RNA-dependent RNA polymerase Nsp12 mutations found on the other variant of concerns like Alpha, Beta, and Gamma had no effect on the antiviral medications, particularly those focusing on the RNA-dependent RNA polymerase and viral genome replication. Another variant-defining mutation on the exonuclease is the nsp14 substitution, which results in an I42V alteration near the interface region with nsp10. The researchers discovered that one of the mutation’s two short hydrophobic side chains directly contacts V40 and N41, contacting nsp10 (Solis-MoreiraJan, 2022).
5. The efficiency of vaccines against the Omicron variant
5.1. Studies on Johnson and Johnson vaccine (Ad26.CoV2.S), Pfizer–BioNTech COVID-19 vaccine (BNT162b2), and unvaccinated patients
Keeton et al. directed a study to assess the capability of the T- cells to interact with the Omicron variant. For this study, they considered Ad26.CoV2.S or BNT162b2 vaccinated candidates and even in many unvaccinated convalescent COVID-19 patients. Their study demonstrates constant maintenance of around 70–80% of the CD4 and CD8 T cell response to S protein. Despite Omicron possessing more mutations than the other variants, the extent of Omicron cross-reactive T cells was similar to other variants. Keeton et al. also concluded that they noted a comparable T-cell action to the traditional spike, nucleocapsid, and membrane proteins in patients infected with the Omicron variant similar to that observed in the previous other variants. It indicates that despite the numerous mutations in Omicron, and decreased response to neutralizing antibodies, a significant portion of the T-cell response acquired from vaccination or typical infection still cross-identifies the variant. This study paves the way even to strengthen the concept that T-cell immunity may be the reason behind a fewer number of hospitalization and deaths (Keeton et al., 2021). In another similar kind of study by Liu et al. on Ad26.COV2.S or BNT162b2 vaccinated individuals displayed sturdy CD8 + and CD4 + T cell responses, indicating a substantial cross-reactivity against Omicron and the various other variants despite a notable reduction in the Omicron specific neutralizing antibodies. They have studied the consistency of 82–84% cross-reactivity of CD8 + T cell responses to Omicron. This study adds that vaccines may aid in reducing the severity of the disease to a great extent by activating cellular immunity (Liu et al., 2022).
Another study by Cele et al. describes that boosting and a combination of infection and vaccination elicit an enhanced neutralization capacity and show good effectiveness against Omicron. A decreasing neutralization response will diminish the effectiveness of the vaccines. However, since severe disease mainly involves T-cell immunity, the protection may be maintained against the Omicron variant despite reducing or lowering neutralization levels (Cele et al., 2021).
5.2. The status of the fully vaccinated individuals against Omicron
The majority of vaccinations target the virus’s spike protein region. It is that portion of the coronavirus that helps it to penetrate a human cell. Vaccines function by conditioning the human immune system to recognize and target the SARS-CoV-2 spike protein when the virus attempts to enter the body (Renu et al., 2020a, Renu et al., 2020b). The spike protein attaches to a human cell through its RBD portion. When carried by the mutated RBD, the Omicron variant may remain unrecognized by the body’s immune system. The antibodies and T cells generated in the body after infection or vaccination memorize the pathogen and effectively against the new SARs-CoV-2 variants (Desk, 2021).
The two vaccine shots provided 33 % protection from infection and 70 % from hospitalization. The delta variant was 80 % and 90 %, respectively (Sakay, 2022). The Pfizer vaccine’s two doses still prove effective in preventing the severity of the disease because most of the epitopes in the spike protein identified by the CD8 + T cells remain unchanged in the Omicron variant (Pfizer, 2021). As the current scenario concerns, being fully immunized meant receiving one vaccine shot from Johnson & Johnson’s or two shots of vaccine either from Moderna Inc. or Pfizer Inc. and partner BioNTech SE. After six months of the second dose, the people who received Pfizer-BioNTech and Moderna shots are eligible for the third or booster dose. Those who received J&J can go for their booster dose two months after their first dose (Schwartz, 2021). According to reports from South Africa, vaccinated individuals who got infected with Omicron did not have any complications, and about 90% of the people admitted to hospitals from the disease were unvaccinated (Crist, 2021).
5.3. The efficacy and need for booster doses
The current laboratory studies done by Pfizer and BioNTech suggest that after the third booster dose of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2), the serum antibodies induced by the vaccines neutralize the SARS-CoV-2 Omicron variant. A third booster dose of BNT162b2 increases the neutralizing antibody levels by 25 times more effective for the Omicron variant than the two doses; this is similar to the immunity provided by the two doses of the original SARs-CoV2 variant (Pfizer, 2021). Similarly, the latest study conducted by the University of Oxford shows that the third dose of the AstraZeneca vaccine will also provide some protection against Omicron as it could increase the titer value of the neutralizing antibodies. It was similar to the protection offered by the two-dose vaccination against the delta variant (Service, 2021). Some researchers believe that there is no necessity to take a booster dose as the two doses of vaccine will also effectively manage the virus as it causes only mild diseases; however, booster doses should be administered in immunosuppressed individuals to get sufficient immunity. The immunity provided by two vaccination doses or previous infection diminishes after 4–9 months; hence getting a booster dose would help (Dey, 2021). Moderna also claimed that their authorized booster doses neutralizing antibody titers were 37 times greater than the pre-booster levels. The approved dosage of the booster is 50 µg. However, the company claims that a 100-microgram shot would increase the neutralizing antibody levels by 83 times. The company’s vaccine named mRNA-1273 was less effective against the Omicron variants without a booster dose (Turak, 2021). However, the third booster dose will help increase the quantity and quality of the immunity possessed by the individual from vaccination or infection and reduce the chances of hospitalization (Schwartz, 2021).
The findings indicate that Omicron has the potential to affect vaccine efficacy significantly. Sigal’s team discovered that higher levels of neutralizing antibodies against Omicron were seen in people infected with the virus before vaccination than in people with no infection record. Neutralizing antibody titers were seen in individuals who received the third booster shot compared to the other SARS-CoV-2 variants induced by the two doses of the vaccine, according to the Study by Pfizer-BioNTech (Callaway, 2021). The initial findings suggest that compared to the Delta variant, vaccine effectiveness against the symptoms of Omicron infection is considerably lower. According to the data available, the protection against viral infection and hospitalization is achieved by the mRNA vaccines like Pfizer-BioNTech or Moderna. The Pfizer booster provides 70–75 % age protection in the beginning. After two weeks of a booster dose, the protection offered by the AstraZeneca vaccine was 71 %. In people who received Pfizer-BioNTech vaccine initially was 76 %. Initial studies show that the vaccine effectivity against Omicron after six months of the second dose dropped to 52 %. It can be brought up to 88 % using the booster dose. The Moderna vaccine also provides similar protection. Two doses of the Moderna vaccine give about 72 % protection, increasing to 88 % by a booster dose. A single dose of the J&J vaccine does not prove to be effective in the antibodies produced against Omicron, and enough data is not available to evaluate its effectiveness (Sakay, 2022). No peer-reviewed research is available suggesting the vaccine efficacy, and also only limited data is available to date (Organisation., December 23, 2021).
5.4. Engineering to develop an updated vaccine
The live Omicron variant causes human lung cell infection through ACE2 receptors, which the laboratory studies on this variant proved. It shows that the existing vaccines can still effectively tackle this variant (Collins, 2021). Omicron shows sensitivity to antiviral drugs such as EIDD-1931, camostat, aprotinin, PF-07321332, remdesivir, favipravir, nafamostat and ribavirin (Solis-MoreiraJan, 2022). Pfizer Inc. combined two antiviral pills, one which blocks the enzyme used by coronavirus to replicate itself with another antiviral pill, an HIV medication ritonavir, which aids in slowing the breakdown of the other medicine to create an oral treatment called Paxlovid. Molnupiravir, developed by Merck & Co. and Ridgeback Biotherapeutics LP, is the other treatment initially developed for the treatment of influenza. The lethal mutagenesis mechanism of the pill prevents viral replication (Lauerman, 2021). The current vaccines available are based on viral vectors and mRNA. Research in developing new vaccines based on the old protein-based technology targets the SARS-COV-2. These vaccines are considered safe and effective and have low side effects. The virtual proteins are used instead of the tiny viral components or RNA pieces. If the virus does not have considerable genetic changes, the vaccines which attack the entire protein will offer protection. These vaccines were also made in the last two years and are entering clinical studies (Dhawan, 2021).
6. Omicron, Delmicron, and Florona
The Omicron variant has about 26–32 spike protein mutations, increasing its transmissibility and potential to escape humoral immunity. Omicron variant has been reported across 110 countries by December 22, 2021. According to the available data, Omicron has a significant growth benefit over Delta (Organisation., December 23, 2021). The data available suggest that in the EU/EEA countries, community transmission has taken place and will contribute to an increased number of Omicron cases within a couple of months. Different countries implemented travel restrictions to tackle the SARS-COV-2 pandemic. All the countries have encouraged their citizens to get vaccinated to fight against the virus. The progress in vaccination continues to stay uneven across nations. Still, less than 50% of the total population is vaccinated in four EU/EEA countries (European Centre for Disease Prevention and Control, 2021).
Delmicron was named, combining the names of Delta and Omicron variants which are seen worldwide (Staff, December 23, 2021). However, Delmicron, on the other hand, is not a new variant; it is a condition in which both the Omicron and Delta variants work together to increase the COVID cases. Reports suggest that individuals with a weakened immune system like older people and others with chronic conditions are more likely to get infected with both variants at the same time. Experts have a different opinions on whether this combination would result in a super strain (Ray, December 24, 2021). Delmicron rarely occurs in the form of reinfection in people who are careless with their safety precautions. Delmicron-specific symptoms are not yet reported so far. The symptoms are the same as SARS-COV-2, including fever, cough, loss of taste and smell, and fatigue. Scratchy throat, mild fever, extreme body ache, and sneezing were also reported in Omicron patients (India, 2021, India, 2022).
Florona is a condition in which the patient suffers from both Influenza and Covid-19 and is not considered a covid variant. The first Florona case was reported in Israel in a hospitalized pregnant woman who was not vaccinated. Florona arises when a person gets infected with both the flu virus and the SARS-CoV-2. High temperature, persistent chest pain or constriction, difficulty breathing, and decreased appetite are all symptoms of Florona. It might also cause feelings of confusion and anxiousness. Inflammation in heart muscles, pneumonia, and myocarditis, are the symptoms that appear in extreme cases of Florona (Desk, 2022). Florona is spread from an infected individual to a healthy individual by inhaling this contaminated air or touching a surface contaminated with the virus. Once Florona enters the body of a healthy individual, it shows symptoms within 2-10 days, and the virus spreading rate will also be high during the first few days. Even during the pandemic last year, there were concerns about double infection. However, due to the restrictions in many countries, no cases were reported. Now, as the restrictions imposed are lifted, and due to the decreased temperature, the concerns about the spread of Florona have also increased. The treatment of Florona can be delayed because of the common symptoms shown by the two viruses (India, 2022). Florona does not have any treatment as of now. The flu virus symptoms are only treated, and Pfizer antiviral drugs are used to treat corona. To prevent complications, the high-risk groups are preferred to get both the flu and COVID-19 vaccination (Mulko, 2022).
7. Can Omicron be a blessing in disguise?
According to several studies, serum after two vaccine doses exhibits minor Omicron neutralizing effects than other Beta and Delta peptides (VOC). The neutralizing effects of Wu-Hu-1 and Omicron are increased when vaccination booster doses are increased, especially in recipients of an adenoviral vector vaccine, such as ChAdOx1. The influence of diminished neutralizing responses in vaccine recipients is evaluated in this study to determine the rate of vaccine potency. According to the neutralization results after two doses in recipients, the likelihood of infection with Omicron vs. the Delta version was much higher (Willett et al., 2022).
Willett et al. claim that Omicron can evade the immunological response by obtaining access as an endosomal fusion of the host cell membrane, avoiding immune cell detection. There is no need for neutralizing antibodies because a complete escape is possible. It boosts transmission and may cause tissue tropism to shift (Willett et al., 2022). More research is needed to infer that the Omicron can operate as a natural vaccine against COVID 19 mutations. Omicron invaders cannot defy the intelligence of our immune cells that have well adapted for survival. The higher transmission of RNA viruses is highly vulnerable to “antigenic drift” due to their absence of an in-built proof-reading mechanism during genome replication.
It evades the host’s immune system and susceptibility to the vaccine, leading to rapidly ongoing mutations in the life cycle of a virus and the COVID-19 virus. However, such types of viral mutations majorly produce weaker variants and lose the ability of transmissibility. These weaker strains cause less symptomatic infections that contribute to herd immunity, somewhat like vaccination. This attenuated form may lead to herd immunity, which can be achieved much faster if the mutated strains have a higher transmission rate but a low severity rate. Hence this will be beneficial for the newly raised mutants of COVID-19 because of the herd immunity in previous exposure, therefore decline in the fatality rate of the population. Parameters to check the severity of viral infections include the case fatality rate (CFR) and Infection fatality rate (IFR). The number of COVID deaths is divided by the number of people carrying SARS-CoV-2 antibodies by comparing the infectivity and mortality in the first and second waves (Ozer, 2022, Firoz and Talwar, 2022, Venugopal et al., 2020, Mahalaxmi et al., 2021, Subramaniam et al., 2020). There are only two ways to measure antibodies, first in SARS-CoV-2 infected people and second in vaccinated people; these suggest that antibodies load in sera is due to the viral infection or vaccination with viral infection or only vaccination (Chaturvedi and Badwe, 2021).
The findings in a recently published Danish study explain why Omicron is spreading more rapidly. It shows that the Omicron variant is finding a way around vaccinating peoples’ immunity than the Delta variant. Out of 100%, Danes fully vaccinated are 78%, and 48% of those received a third “booster” shot of Pfizer-BioNTech’s vaccine. Danish data indicates that out of 93 people admitted to the hospital, less than five received intensive care due to COVID-19 from Omicron in late December (Reuters, 2022). The study also found that the virus is less likely to transmit in booster-vaccinated people, regardless of the variant, than in the unvaccinated. Researchers conducted the study at the University of Copenhagen, Statistics Denmark, and Statens Serum Institute (SSI), suggesting the virus is spreading more speedily because it is better at circumventing immunity received from vaccines (Reuters, 2022).
This study answers some of the questions that have been raised in the pandemic era. The early period of viral infection following administration of a booster with vaccine shows moderate-to-high effectiveness, about 70–75% against symptomatic COVID-19 disease (Bradu et al., 2022, Andrews et al., 2021). These consequences strongly support the complete introductory vaccination course followed by administering a booster dose to confer protection against the Omicron Variant of concern causing symptomatic disease.
The Population seroprevalence gave evidence of increasing population seropositivity. The study conducted in Finland, among the older age group who were the first to be vaccinated, reported the highest seropositivity results, estimates exceeding 90% (Oliveira et al., 2020). Various serological assays used in the studies conducted on June 1 and July 7, 2021, in the European region (Geneva), reported total seropositivity of 66.1% and seroprevalence of 29.9% in infection-induced people (Charlotte Bermingham, 2021). More recent data in the UK from October 4 to November 28, 2021, reported seropositivity estimates of 22.7% induced antibodies for infection and 97.8% for antibodies acquired from the vaccine or natural infection immunity (Agency, 2021). However, it is ascertained and challenging without specific studies, estimated reports based on infection frequency to understand the contribution of naturally-acquired immunity from previous exposure in the total population (Agency, 2021, Nicolay et al., 2021, Meslé et al., 2021, Tenforde et al., 2021, Haas et al., 2021, Mattiuzzi et al., 2021, Sacco et al., 2021, Control, E.C.f.D.P.a. December 7, 2021).
The Israel health officials claim that the country will achieve herd immunity against covid as the Omicron infections increase. However, the Israeli physician Nachman Ash said they should attain herd immunity through vaccination and not wait for cases to increase. Herd immunity is used to describe a community immune to a disease because either sufficient people have been vaccinated or already infected people have acquired antibodies from the disease. Salman Zarka, head of the Coronavirus task force, also said that herd immunity could not be guaranteed as recovered individuals were also getting re-infected. Towards the end of January, almost four million people are estimated to get infected, according to data analysts. As per reports, the medical personnel and senior citizens will be administered a fourth dose of the COVID-19 vaccine, as Prime Minister Naftali Bennett stated. He also did not eliminate the possibility of another shutdown as the number of daily cases has increased to over 3500; however, the number of deaths remained the same. Eran Segal from the Weizmann Institute predicted that the cases would increase beyond the country’s testing capacity; however, the hospitalization would be lower than in the previous wave. Over nine million of Israel’s population have taken either two doses or three doses of the vaccine. However, the vaccination rate among Palestinians is just over a quarter. The country also approved a fourth dose of vaccine to be administered to immunecompromised individuals (News, 2022).
8. Gaps and probable solutions
Omicron has at least 50 mutations (Thakur and Kanta Ratho, 2021); mutations in S and N increase the cell permeability and the capsid assembly three times more than the Delta virus. Initially, when a PCR test is made with the S target gene, it leads to S gene target failure; this feature is used to differentiate it from the other variant (WHO. 23rd). The membrane protein, NSP3, NSP6, NSP14, S, and nucleocapsid protein mutations are seen in the Omicron variant. They are found to inhibit the interferon response of the host cell. A VOC like Omicron exhibits traits linked to one or more substantial changes in global public health.
According to Willet et al., there is no need for neutralizing antibodies because a complete escape is possible by avoiding recognition by an immune cell. Hence, complete escape can be achieved, which increases the transmission and may change its tissue tropism (Willett et al., 2022). Cleavage of S1 and S2 generates and exposes the fusion peptide, infusing the viral and host cell membrane to express ACE2 and TMPRSS2 surface receptor proteins. Omicron compromised cell entry in lung cells and the syncytial formation ability (Meng et al., 2021). Fusion of Infected cells with non-infected cells (host cell–cell fusion of infected alveolar macrophages), resulting in giant syncytia. Hypersensitive macrophages lead to (systemic hyper-inflammation) macrophage activation syndrome that produces an enormous cytokine called cytokine storm is seen in patients with critical cases. Researchers conducted the study at the University of Copenhagen, Denmark, and Statens Serum Institute (SSI), suggesting the virus is spreading more speedily because it is better at circumventing immunity received from vaccines. Some researchers believe that booster doses should be administered in immunosuppressed patients to get sufficient immunity, which is unnecessary in vaccinated individuals. It has been found that this variant’s mortality rate is less than the other variants (Dyer, 2021). The third booster dose is required to get immunity in vaccinated individuals to reduce the risk of hospitalization (Schwartz, 2021). However, the third dose of mRNA vaccine is needed to significantly lower the risk of infection, but it does not restore complete immunity. The third booster dose should be of mRNA vaccines like Pfizer-BioNTech or Moderna. Omicron and Delta variants work together to increase the COVID cases, and the combination would result in a super strain (Ray, 2021). Reports suggest that individuals with a weakened immune system like older people and others with chronic conditions are more likely to get infected with both variants at the same time. Florona is a condition in which the patient suffers from both Influenza and Covid-19 can also work in combination to give severe disease threats (Ray, 2021).
Some antiviral drugs like favipiravir, ribavirin, camostat, PF-07321332, remdesivir, and aprotinin, which had distinct action mechanisms, are effective against the VOC. The sensitivity shown by both variants was similar. Some other oral antiviral drugs such as sotrovimab, molnupiravir, and paxlovid are effective against the Omicron variant. Regeneron Pharmaceuticals in Tarrytown, New York, declared that their antibodies had reduced efficiency against Omicron. Among all the antibody treatments available, sotrovimab is considered effective against Omicron. The mechanism of action of oral antiviral drugs such as molnupiravir and paxlovid are found to be effective against the Omicron variant. These oral drugs are cheaper than the other antibody treatments available. Pfizer claims that the Paxloid drug developed by them would reduce the hospitalized cases by 89% and reduce the chances of death if administered soon after the symptoms appear (Torjesen, 2021, Ray, 2021, Tenforde et al., 2021).
9. Conclusion
The Omicron variant has now been reported in many countries and was declared by WHO as a variant of concern. However, the disease severity is less compared to the other variants. The cell permeability and the capsid assembly were increased three times in the Omicron variant compared to the Delta variant due to the S and N protein mutations. The more stable structure of the Omicron variant is supported by the fact that it has a more alpha-helix structure both in its RBD and whole spike protein compared to the delta variant (Kumar et al., 2021, Jr., 2021). While the Omicron variant appears to have increased the transmissibility of the Covid-19 infection, it is still unclear whether it will trigger a massive disease or evade vaccine-provided immunity (Desk, 2021, Sakay, 2022). The third vaccine, or a booster dose, is now being administered to the people, increasing neutralizing antibody levels. As the restrictions imposed during the pandemic are also being relaxed, the spread of infection has also increased. The inherent intelligence of our immune cells shows an effect against Omicron invaders. This attenuated form is hypothesized to lead to herd immunity because of its high transmissibility and lower mortality rate. Therefore, it is quite right to say that Omicron may be a blessing in disguise as Omicron VOC is now gaining much attention in current conditions. However, it also affects the vaccinated individual, making it more doubtful. In our actual knowledge of the Omicron variant, these assumptions implicit a wide range of uncertainty. At the same time, there is considerable uncertainty about several characteristics of Omicron. The urgency to administer booster doses has gained much attention due to the swift upsurge in the egression of the Omicron variant worldwide. There has been much confusion about the beneficiary and utility of vaccines against the Omicron variant. Although several studies indicate that the effectiveness of the COVID 19 vaccines is low concerning the Omicron variant, people need to get vaccinated as vaccinations help reduce the severity of the disease to an enormous extent. The Omicron variant is still a very new concept, and hence much research is required to understand it clearly. Therefore, administering a booster COVID-19 vaccine dose can be adapted to vaccination strategies. Focusing on hurrying up the vaccine booster programs and re-introducing stricter and preventive measures that lessen the spread and transmissibility of the virus should be carried out effectively.
The biggest question is whether the next variant will reverse this trend and cause more severe sickness, or will it be the introduction of SARS-CoV-2 variants with lower pathogenicity, Omicron level or better? While we have completely failed to predict the course of the pandemic thus far, perhaps it is time to reconsider how new variants evolve, keeping in mind that various variants may evolve in different ways, so that we can anticipate what comes next (Sigal, 2022).
Funding
This work was supported by the “VIT SEED GRANT” and ICMR-National Task Force Project [F.No.5/7/482/2010-RBMH&CH].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the VIT, Vellore, Tamilnadu, India, for supporting this work.
References
- Agency, U.H.S.a. 2021. COVID-19 vaccine surveillance report – week 49.
- Andrews, N., Stowe, J., Kirsebom, F., Toffa, S., Rickeard, T., Gallagher, E., Gower, C., Kall, M., Groves, N., O'Connell, A.-M. 2021. Effectiveness of COVID-19 vaccines against the Omicron (B. 1.1. 529) variant of concern. MedRxiv. [DOI] [PMC free article] [PubMed]
- Bradu P., Biswas A., Nair C., Sreevalsakumar S., Patil M., Kannampuzha S., Mukherjee A.G., Wanjari U.R., Renu K., Vellingiri B. Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ. Sci. Pollut. Research. 2022:1–32. doi: 10.1007/s11356-022-20024-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- European Centre for Disease Prevention and Control. Assessment of the further emergence and potential impact of the SARS-CoV-2 Omicron variant of concern in the context of ongoing transmission of the Delta variant of concern in the EU/EEA, 18th update - 15 December 2021. ECDC: Stockholm; 2021.
- Brown, K.V. January 2, 2022 Can Omicron Escape Vaccines and Boosters? Bloomberg.
- Callaway E. Beyond Omicron: what’s next for COVID’s viral evolution. Nature. 2021;600:204. doi: 10.1038/d41586-021-03619-8. [DOI] [PubMed] [Google Scholar]
- Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600:367. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- Callaway E., Ledford H. How bad is Omicron? What scientists know so far. Nature. 2021;600:197. doi: 10.1038/d41586-021-03614-z. [DOI] [PubMed] [Google Scholar]
- Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. MedRxiv. 2021 [Google Scholar]
- Charlotte Bermingham, J.M., Vahé Nafilyan. 2021. Deaths involving COVID-19 by vaccination status, England: deaths occurring between January 2 and July 2 2021.
- Chaturvedi P., Badwe R.A. With the emergence of Omicron, SARS-CoV-2 is getting weaker and human species is getting stronger due to our inherent cellular intelligence. Cancer Res. Stat. Treatment. 2021;4:608. [Google Scholar]
- D.F. Collins Latest on Omicron Variant and COVID-19 Vaccine Protection 2021.
- Control, E.C.f.D.P.a. 2022. SARS-CoV-2 variants of concern as of January 5 2022.
- Control, E.C.f.D.P.a. December 7 2021. EMA and ECDC recommendations on heterologous vaccination courses against COVID-19.
- Crist, C. 2021. Vaccinated Patients with Omicron Variant Don’t Have Complications: Doctors.
- Desk, I.T.W. 2021. Double-vaccinated? Are you safe from Omicron variant? . India Today.
- Desk, O.W. January 3 2022. Is ‘Florona’ Another Covid-19 Variant? What We Know About The New Disease From Israel.
- Dey S. The Times of India; 2021. Expert groups considering scientific evidence on justification for covid vaccine booster dose. [Google Scholar]
- Dhawan, B. December 31, 2021. Two years of coronavirus: COVID-19 vaccines, Omicron, treatment and everything we know. Financial Express.
- Dudas G., Hong S.L., Potter B.I., Calvignac-Spencer S., Niatou-Singa F.S., Tombolomako T.B., Fuh-Neba T., Vickos U., Ulrich M., Leendertz F.H. Emergence and spread of SARS-CoV-2 lineage B. 1.620 with variant of concern-like mutations and deletions. Nat. Commun. 2021;12:1. doi: 10.1038/s41467-021-26055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer O. British Medical Journal Publishing Group; South African data show: 2021. Covid-19: Omicron is causing more infections but fewer hospital admissions than delta. [DOI] [PubMed] [Google Scholar]
- Emily Head, D.S.L.v.E.x., 2021. Omicron largely evades immunity from past infection or two vaccine doses. Imperial College London news.
- Ferré V.M., Lebourgeois S., Chenane H.R., Menidjel R., Masson C., Collin G., Visseaux B., Descamps D., Fidouh N., Charpentier C. Vaccine Ab neutralization against Omicron and SARS-CoV-2 variants using neutralization and specific ELISA assays. J. Infect. 2022:1–4. doi: 10.1016/j.jinf.2022.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoz Arman, Talwar Priti. COVID-19 and Retinal Degenerative Diseases: Promising link. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratev, F. 2021. The High Transmission of SARS-CoV-2 Omicron (B. 1.1. 529) Variant is Not Only Due to Its hACE2 binding: A Free Energy of Perturbation Study. bioRxiv.
- Garret N., Tapley A., Andriesen J., Seocharan I., Fisher L.H., Bunts L., Espy N., Wallis C., Randhawa A.K., Goga A. High rate of asymptomatic carriage associated with variant strain Omicron. MedRxiv. 2021:1–6. [Google Scholar]
- Gong S.Y., Chatterjee D., Richard J., Prévost J., Tauzin A., Gasser R., Bo Y., Vézina D., Goyette G., Gendron-Lepage G. Contribution of single mutations to selected SARS-CoV-2 emerging variants spike antigenicity. Virology. 2021;563:134. doi: 10.1016/j.virol.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshwam, S. (17 April 2022). Omicron XE variant symptoms, severity, treatment. cases so far. Retrieved from https://dmerharyana.org/omicron-xe-variant/.
- Haas E.J., McLaughlin J.M., Khan F., Angulo F.J., Anis E., Lipsitch M., Singer S.R., Mircus G., Brooks N., Smaja M. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet. Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S. (14 April 2022). Omicron XE: What we know about the new subvariant. CTV News.
- Hoffman J.M., Margolis K.G. Building community in the gut: a role for mucosal serotonin. Nat. Rev. Gastroenterol. Hepatol. 2020;17:6. doi: 10.1038/s41575-019-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- India, T.o. December 31, Delmicron versus Omicron: What should you be more scared of? 2021 Signs to watch out for Times of India.
- India, T.o. January 3, The First Case OfFlorona (Flu + Coronavirus) Detected In Israel: Here’s All You Need To Know About Symptoms And Severity Times of India. 2022.
- Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jr., E.E.a.B.L. Omicron symptoms: What we know about illness caused by the new variant NCBNEWS. 2021.
- Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., Khan K., Cele S., Bernstein M., Karim F. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. MedRxiv. 2021 [Google Scholar]
- Kozlov M. Omicron overpowers key COVID antibody treatments in early tests. Nature (Lond.) 2021 doi: 10.1038/d41586-021-03829-0. [DOI] [PubMed] [Google Scholar]
- Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J. Med. Virol. 2021 doi: 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- Lauerman, J.G.a.J. 2021. How Does Omicron Challenge the Treatments for Covid? . How Does Omicron Challenge the Treatments for Covid? .
- Leroy H., Han M., Woottum M., Bracq L., Bouchet J., Xie M., Benichou S. Virus-mediated cell-cell fusion. Int. J. Mol. Sci. 2020;21:9644. doi: 10.3390/ijms21249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chandrashekar A., Sellers D., Barrett J., Lifton M., McMahan K., Sciacca M., VanWyk H., Wu C., Yu J. Vaccines Elicit Highly Cross-Reactive Cellular Immunity to the SARS-CoV-2 Omicron Variant. MedRxiv. 2022 doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M, k.s. 50% of Omicron cases were double jabbed, masks and surveillance must for breaking transmission chain The Indian Express. 2021.
- Mahalaxmi I., Jayaramayya K., Venkatesan D., Subramaniam M.D., Renu K., Vijayakumar P., Narayanasamy A., Gopalakrishnan A.V., Kumar N.S., Sivaprakash P., Sambasiva Rao K.R.S., Vellingiri B. Mucormycosis: an opportunistic pathogen during COVID-19. Environ. Res. 2021;201:111643. doi: 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzi, C., Henry, B.M., Lippi, G. 2021. COVID-19 vaccination uptake strongly predicts averted deaths of older people across Europe. [DOI] [PMC free article] [PubMed]
- Maxmen, A. (15 April 2022). Are new Omicron subvariants a threat? Here's how scientists are keeping watch. Nature news. Retrieved from https://www.nature.com/articles/d41586-022-01069-4. [DOI] [PubMed]
- May D.H., Rubin B.E., Dalai S.C., Patel K., Shafiani S., Elyanow R., Noakes M.T., Snyder T.M., Robins H.S. Immunosequencing and epitope mapping reveal substantial preservation of the T cell immune response to Omicron generated by SARS-CoV-2 vaccines. medRxiv. 2021:1. [Google Scholar]
- Meng, B., Ferreira, I., Abdullahi, A., Kemp, S.A., Goonawardane, N., Papa, G., Fatihi, S., Charles, O., Collier, D., Choi, J. 2021. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. bioRxiv.
- Meslé M.MI., Brown J., Mook P., Hagan J., Pastore R., Bundle N., Spiteri G., Ravasi G., Nicolay N., Andrews N., Dykhanovska T., Mossong J., Sadkowska-Todys M., Nikiforova R., Riccardo F., Meijerink H., Mazagatos C., Kyncl J., McMenamin J., Melillo T., Kaoustou S., Lévy-Bruhl D., Haarhuis F., Rich R., Kall M., Nitzan D., Smallwood C., Pebody R.G. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Eurosurveillance. 2021;26(47) doi: 10.2807/1560-7917.ES.2021.26.47.2101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, K. 2021. EXPLAINED: As Omicron Surfaces In India, How Genomic Surveillance Can Help Us Stay A Step Ahead Of Variants.
- Mulko, M. January 7, 2022. What We Know About Florona: the New COVID-19 and Influenza Compound Infection. . Interesting Engineering.
- News, B. 2022. Covid: Israel Omicron spike could bring herd immunity but with risks - health boss.
- Nicolay N., Innocenti F., Beauté J., Učakar V., Grgič Vitek M., Poukka E., Hannila-Handelberg T., Gauci C., Melillo T., Georgakopoulou T., Jarkovsky J., Slezak P., Delgado-Sanz C., Olmedo-Lucerón C., Suija H., Liausediene R., O’Lorcain P., Murphy N., Peralta-Santos A., Casaca P., Gregoriou I., Bundle N., Spiteri G., Ravasi G. Initial assessment of the COVID-19 vaccination’s impact on case numbers, hospitalisations and deaths in people aged 80 years and older, 15 EU/EEA countries, December 2020 to May 2021. Eurosurveillance. 2021;26(48) doi: 10.2807/1560-7917.ES.2021.26.48.2101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, L.M.d.S., Tiyo, B.T., Silva, L.T.d., Fonseca, L.A.M., Rocha, R.C., Santos, V.A.d., Ceneviva, C., Bedin, A.A., Almeida, A.d., Duarte, A.J.d.S. 2020. Prevalence of anti-SARS-CoV-2 antibodies in outpatients of a large public university hospital in Sao Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 62. [DOI] [PMC free article] [PubMed]
- Ozer E.A. Coronavirus blind spots in Nigeria. Nat. Commun. 2022;13 [Google Scholar]
- Peacock T.P., Goldhill D.H., Zhou J., Baillon L., Frise R., Swann O.C., Kugathasan R., Penn R., Brown J.C., Sanchez-David R.Y. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature. Microbiology. 2021;1 doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- Perugino, C.A., Liu, H., Feldman, J., Hauser, B.M., Jacob-Dolan, C., Nathan, A., Zhou, Z., Kaseke, C., Tano-Menka, R., Getz, M.A. 2022. Preferential expansion upon boosting of cross-reactive “pre-existing” switched memory B cells that recognize the SARS-CoV-2 Omicron variant Spike protein. medRxiv, 2021.12. 30.21268554.
- Petersen, E., Ntoumi, F., Hui, D.S., Abubakar, A., Kramer, L.D., Obiero, C., Tambyah, P.A., Blumberg, L., Yapi, R., Al-Abri, S. 2022. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B. 1.1. 529)-highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. International Journal of Infectious Diseases 114, 268. [DOI] [PMC free article] [PubMed]
- Pfizer. 2021. Pfizer and BioNTech Provide Update on Omicron Variant [Press release].
- Prevention, C.o.D.C.a. Dec.20,2021. Omicron Variant: What You Need to Know.
- Quarleri J., Galvan V., Delpino M.V. Springer; 2022. Omicron variant of the SARS-CoV-2: a quest to define the consequences of its high mutational load; pp. 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, M. December 24, 2021. ‘Delmicron’ could be driving Covid surge in US, Europe. Know more about it. . Hindustan Times.
- Renu K., Prasanna P.L., Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage–a review. Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu K., Subramaniam M.D., Chakraborty R., Myakala H., Iyer M., Bharathi G., Siva K., Vellingiri B., Valsala Gopalakrishnan A. The role of Interleukin-4 in COVID-19 associated male infertility–A hypothesis. J. Reprod. Immunol. 2020;142:103213. doi: 10.1016/j.jri.2020.103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUTERS. 2022. Omicron evades immunity better than Delta, Danish study finds.
- Rodger, D., Blackshaw, B.P. COVID-19 vaccination should not be mandatory for health and social care workers. The New Bioethics. [DOI] [PubMed]
- Sacco C., Mateo-Urdiales A., Petrone D., Spuri M., Fabiani M., Vescio M.F., Bressi M., Riccardo F., Del Manso M., Bella A., Pezzotti P. Estimating averted COVID-19 cases, hospitalisations, intensive care unit admissions and deaths by COVID-19 vaccination, Italy, January− September 2021. Eurosurveillance. 2021;26(47) doi: 10.2807/1560-7917.ES.2021.26.47.2101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakay Y.N. By the Numbers: COVID-19 Vaccines and Omicron. Healthline. 2022 [Google Scholar]
- Saxena S.K., Kumar S., Ansari S., Paweska J.T., Maurya V.K., Tripathi A.K., Abdel-Moneim A.S. Characterization of the novel SARS‐CoV‐2 Omicron (B. 1.1. 529) Variant of Concern and its global perspective. J. Med. Virol. 2021:1. doi: 10.1002/jmv.27524. [DOI] [PubMed] [Google Scholar]
- Schmidt F., Weisblum Y., Rutkowska M., Poston D., DaSilva J., Zhang F., Bednarski E., Cho A., Schaefer-Babajew D.J., Gaebler C. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature. 2021;600:512. doi: 10.1038/s41586-021-04005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz F. As Omicron Spreads, What It Means to Be Fully Vaccinated Is Changing. Wall Street J. 2021 [Google Scholar]
- Service, E.N. 2021. Booster dose with AstraZeneca vaccine found to work against Omicron. .
- Service., E.N. 2021. Omicron threat: 30 samples from Maharashtra sent for genomic sequencing so far. The Indian Express.
- Sheikh, A., Kerr, S., Woolhouse, M., McMenamin, J., Robertson, C. 2021. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. [DOI] [PMC free article] [PubMed]
- Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat. Rev. Immunol. 2022;22(2):69–71. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-MoreiraJan, J. 2022. Study identifies current mechanism of action behind reduced severity of Omicron variant COVID-19 infection.
- Staff, F. December 23, 2021. What is Delmicron? How is it different from Omicron? All your questions answered. Health.
- standard, B. 5th Jan 2022. Omicron: Data shows Covid-19 pandemic's end, US logs 1 million cases.
- Subramaniam M.D., Venkatesan D., Iyer M., Subbarayan S., Govindasami V., Roy A., Narayanasamy A., Kamalakannan S., Gopalakrishnan A.V., Thangarasu R., Kumar N.S., Vellingiri B. Biosurfactants and anti-inflammatory activity: A potential new approach towards COVID-19. Current Opin. Environ. Sci. Health. 2020;17:72–81. doi: 10.1016/j.coesh.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Lin W., Dong W., Xu J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J. Biosafety Biosecur. 2022;4(1):33–37. doi: 10.1016/j.jobb.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A.M., Ciling A., Khalid M.M., Sreekumar B., Kumar G.R., Silva I., Milbes B., Kojima N., Hess V., Shacreaw M. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. MedRxiv. 2021 doi: 10.1073/pnas.2200592119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W., Adams K., Olson S.M., Kobayashi M., Verani J.R., Patel M.M., Price A.M., Zambrano L.D., Campbell A.P., Kim S.S. Filtered By. MMWR Morb. Mortal. Wkly Rep. 2021;10:1483. [Google Scholar]
- Thakur V., Ratho R.K. OMICRON (B.1.1.529): A new SARS‐CoV‐2 variant of concern mounting worldwide fear. J. Med. Virol. 2022;94(5):1821–1824. doi: 10.1002/jmv.27541. [DOI] [PubMed] [Google Scholar]
- Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear, British Medical Journal Publishing Group. 2021 doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- Turak N. Moderna says Covid booster appears to protect against Omicron. CNBC. 2021 [Google Scholar]
- Vancollie, V. (2021). What is Genomic Surveillance?.
- Venkatakrishnan, A., Anand, P., Lenehan, P.J., Suratekar, R., Raghunathan, B., Niesen, M.J., Soundararajan, V. 2021. Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin.
- Venugopal A., Ganesan H., Sudalaimuthu Raja S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K.S.M., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr. Opin. Environ. Sci. Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A., Ferrara F., Auti A.M., Di Domenico M., Boccellino M. Advances in the Omicron variant development. J. Intern. Med. 2022:1–10. doi: 10.1111/joim.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett B.J., Grove J., MacLean O., Wilkie C., Logan N., De Lorenzo G., Furnon W., Scott S., Manali M., Szemiel A. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. MedRxiv. 2022 [Google Scholar]
- WHO. 23rd Dec 2021. Enhancing readiness for Omicron (B.1.1.529): Technical brief and priority actions for Member States.
- WION Web Team (Producer). (2022). WHO tracks two new Omicron subvariants: What are BA.4 and BA.5 strains? Retrieved from https://www.wionews.com/world/who-tracks-two-new-omicron-subvariants-what-are-ba4-and-ba5-strains-470395.
- Yin W., Xu Y., Xu P., Cao X., Wu C., Gu C., He X., Wang X., Huang S., Yuan Q., Wu K., Hu W., Huang Z., Liu J., Wang Z., Jia F., Xia K., Liu P., Wang X., Song B., Zheng J., Jiang H., Cheng X.i., Jiang Y.i., Deng S.-J., Xu H.E. Structures of the Omicron Spike trimer with ACE2 and an anti-Omicron antibody. Science. 2022;375(6584):1048–1053. doi: 10.1126/science.abn8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Lu L., Peng Z., Chen L.-L., Meng X., Zhang C., Ip J.D., Chan W.-M., Chu A.-W.-H., Chan K.-H. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with delta variant in TMPRSS2-expressed cells: Omicron variant replication kinetics. Emerg. Microb. Infect. 2021:1. doi: 10.1080/22221751.2021.2023329. [DOI] [PMC free article] [PubMed] [Google Scholar]