Abstract
Objective: To analyze all chronic obstructive pulmonary disease (COPD) drugs-related articles that were indexed in the Web of Science Core Collection (WOSCC) database until August 28, 2021 using bibliometric analysis, in order to provide a reliable reference for the treatment of COPD.
Methods: A comprehensive search was conducted to analyze all COPD drugs-related articles using WOSCC database from inception to August 28, 2021. Abstracts and potentially eligible articles, which were retrieved during literature search, were screened by two reviewers. Besides, the CiteSpace (5.8.R1) software was utilized to analyze the overall structure of the network, the network clusters, the links between clusters, the key nodes or pivot points, and the pathways.
Results: A total of 2552 COPD-drugs related articles were retrieved. From the perspective of categorization of published articles based on country, the United States is the country with the largest number of published articles and completed clinical trials, highlighting the important role of this country in the treatment of COPD. However, in terms of the proportion of ongoing clinical trials, China has the highest proportion, suggesting that China will play a more pivotal role in the medication of COPD in the future. From the perspective of cooperation among countries, the cooperation among European countries was closer than that among Asian countries. In the recent three decades, the top 20 institutions, with a particular concentration on the treatment of COPD, were from North America and Europe. The co-citation analysis showed that, among 2,552 articles, 53154 citations were recorded, and the co-citation network indicated that 24 clusters could be achieved.
Conclusion: The administration of bronchodilators and pulmonary drug delivery systems, as well as consideration of elderly COPD patients remained the hotspots, while triple therapy and comorbidity of COPD, as well as the prevention and treatment of elderly COPD patients had been frontiers in recent years.
Keywords: COPD, medications, bibliometric analysis, pulmonary drug delivery systems, elderly
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease, characterized by persistent airflow limitation that is mainly progressive and is associated with an enhanced inflammatory response in the airways and lung to noxious particles or gases (GOLD, 2021). The pathology of COPD encompasses a variety of structural alterations, involving airways, lung parenchyma, and pulmonary vasculature (Decramer et al., 2012). An epidemiological survey showed that the prevalence of COPD among Chinese adults and smokers who aged >40 and >60 years old was 13.7% and 40%, respectively (Wang et al., 2018). A survey performed in South Korea revealed that the prevalence of COPD among adult non-smokers who aged ≥40 years old was 6.67% (Kim et al., 2014). COPD has gradually become a global public health crisis (Patel et al., 2019). The World Health Organization (WHO) announced that the prevalence of COPD will continue to increase in the upcoming 40 years, exceeding 5.4 million COPD and other related diseases patients who will annually die by 2060 (Chronic Obstructive Pulmonary Disease Group of Chinese, 2021). COPD generates substantial costs for the health system, mainly related to moderate to severe stages and the exacerbations and complications entailed, (Gutiérrez Villegas et al., 2021) and the biggest driver of these healthcare costs is hospitalization (Khakban et al., 2017). Thus, how to prevent or delay the progression of COPD and reduce the frequency of acute exacerbations not only improves patients’ quality of life and reduces mortality, but also saves medical costs. At present, traditional Chinese medicine, such as Tai Chi and Qigong, may be significant for the treatment of COPD patients in terms of enhancing lung function, relieving dyspnea, and improving patients’ quality of life (Ding et al., 2014). However, the most important treatment for chronic obstructive pulmonary disease is still to give patients the correct drug treatment through various channels. The current treatment of COPD is mainly based on different combinations of bronchodilators and inhaled corticosteroids, and the amount of drug have increased during the exacerbation of COPD. However, the therapeutic effect is sometimes not ideal, and the long-term use of some drugs will produce side effects with different manifestations. In recent years, with the gradual deepening of the etiology, pathogenesis and clinical research of COPD, the development of new drugs for the treatment of COPD has become possible. Bibliometrics is a statistical method which could quantitative analysis the research papers concerned about one special topic via mathematical ways (Chen et al., 2014). It could also access the quality of the studies, analysis the key areas of researches and predict the direction of future studies (Yu et al., 2020).
In this study, we employed bibliometric analysis to analyze all COPD drugs-related articles that were indexed in the Web of Science Core Collection (WOSCC) database until August 28, 2021, in order to provide a reliable reference for the treatment of COPD.
2 Methods
2.1 Search Strategy
A comprehensive search was conducted to analyze all COPD drugs-related articles using WOSCC database from inception to August 28, 2021. Abstracts and potentially eligible articles, which were retrieved during literature search, were screened by two reviewers. Any discrepancies between reviewers in the study selection were resolved via consultation with a third reviewer. Those articles that entitled the terms “COPD” or “chronic obstructive pulmonary disease” or “chronic obstructive pulmonary diseases” were included in our search. The search indices included Science Citation Index Expanded (SCI-Expanded), the Conference Proceedings Citation Index–Science (CPCI-S), and Current Chemical Reactions Expanded (CCR-Expanded). WOS Core Collection (formerly Institute for Scientific Information Web of Knowledge) is the most used and authoritative research literature search engine, providing comprehensive coverage of key research outputs from around the world. It is a multidisciplinary database with more than 100 subjects, including the major sciences, arts, humanities, and social sciences (e.g., political science, architecture, and philosophy) (Shao et al., 2021).
2.2 Data Extraction and Quality Assessment
Data extraction and quality evaluation were performed independently. After searching in the WOSCC database, the number of publications and the total and average citations for the authors and journals were recorded. For authors actively publishing on COPD-related drugs, the following indicators were measured: the h-index, which is the number of publications and the number of times the publication is cited; the R-index, which is the square-root of the total citation frequency in the h-core, defined by the h-index; the h(2)-index, or the number of publications h(2) that are cited at least [h(2)]2 times; and the i10-index, or number of publications cited at least 10 times. The higher the values of these bibliometric indicators, the greater the influence of the authors and their publications. Besides, the CiteSpace (Chen, 2006) (5.8.R1) software and VOSviewer (van Eck and Waltman, 2010) (1.6.17) was utilized to analyze the overall structure of the network, the network clusters, the links between clusters, the key nodes or pivot points, and the pathways. A node in the map represented the type of study being analyzed, and links between the nodes represented relationships or collaborations, co-occurrence, or co-citations. For literature analysis, the time slice was 1 year, and the correlation strength was cosine. The threshold for each time slice selected Top N = 50.
2.3 Number of Clinical Trials
Using the name of the country as the search term, the COPD clinical trials carried out in various countries since the establishment of the database were searched in the American clinical trials database (https://clinicaltrials.gov).
3 Results
3.1 General Data
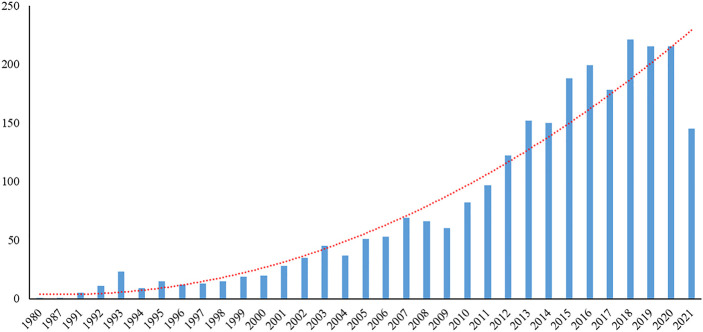
From 1980 to August 28, 2021, 2552 articles were published. From 1980 to 2004, the number of published articles was not noticeable, with an average of (18.06 ± 12.82) articles per year, and it rapidly increased in 2012. From 2012 to 2020, the average number of published articles was (182.22 ± 34.58), accounting for 66.76% of the total publications. The majority of articles were published in 2018 (n = 221) (Figure 1).
FIGURE 1.
The statistics of COPD drugs-related articles from 1980 to 2021.
3.2 Categorization of Published Articles Based on Country, Region, and Institution
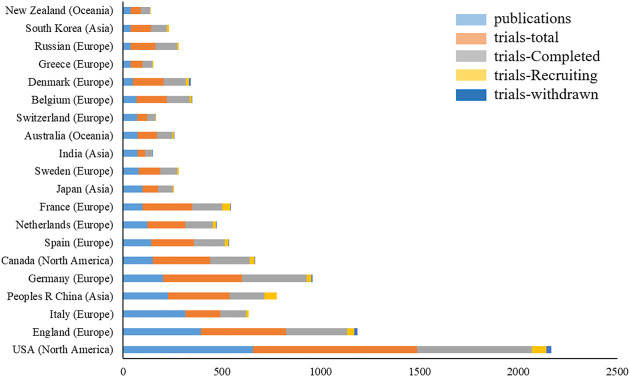
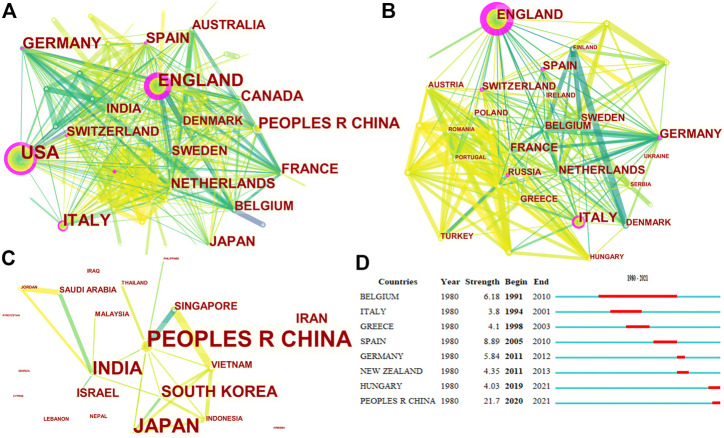
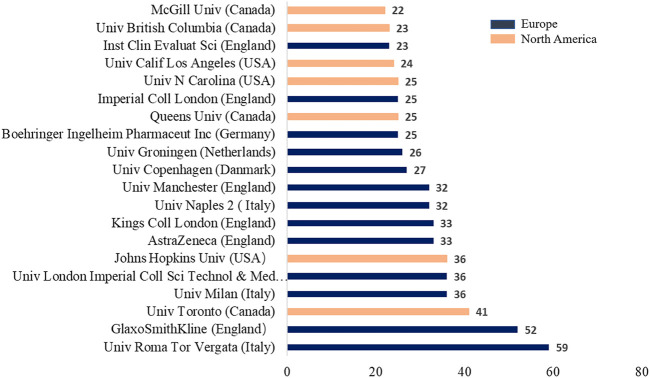
Authors were from 83 countries. Among the top 20 countries, including 12 from Europe, 4 from Asia, 2 from North America, and 2 from Oceania. In terms of proportion of each continent, 53.99%, 24.05%, and 15.83% of COPD drugs-related studies were published in Europe, North America, and Asia, respectively (Figure 2). The United States (n = 657, centrality = 0.27) and the UK (centrality = 0.41, n = 396) accounted for the highest number of articles published. The greatest number of completed clinical trials was recorded in the USA (n = 582), followed by Germany (n = 327), and the UK (n = 308). China (20.13%) has the highest proportion of ongoing clinical trials, followed by France (16.27%), and Greece (13.56%). Compared with Asian countries, a closer cooperation was found among European countries (Figure 3). Among the top 20 research institutions, 13 were located in Europe and 7 in North America. The majority of published articles were from Tor Vergata University of Rome (Italy). Of the top 20 pharmacological companies, GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim had the highest rates of contribution in the research projects (Figure 4). In terms of the annual number of articles published, among the top 6 countries, the USA ranked the first, and China was the country with a steady increase in the number of published articles (Figures 5, 6). A sudden increase in publications was seen from Univ Roma Tor Vergate, Univ Toronto, Kings Coll London, Johns Hopkins Univ, Univ Manchester, Univ Groningen between 2015 and 2021, Imperial Coll London, Karolinska Inst, Univ Tennessee between 2016 and 2021, and Astrazeneca, Univ Campania Luigi Vanvitelli, German Ctr Lung Res DZL between 2020 and 2021 (Table.1).
FIGURE 2.
Top 20 countries that published COPD drugs-related studies and the relevant clinical trials from 1980 to 2021.
FIGURE 3.
Cooperation among countries that published COPD drugs-related studies from 1980 to 2021. Note: (A): countries around the world; (B): European countries; (C): Asian countries; (D): top 8 countries with the strongest citation bursts.
FIGURE 4.
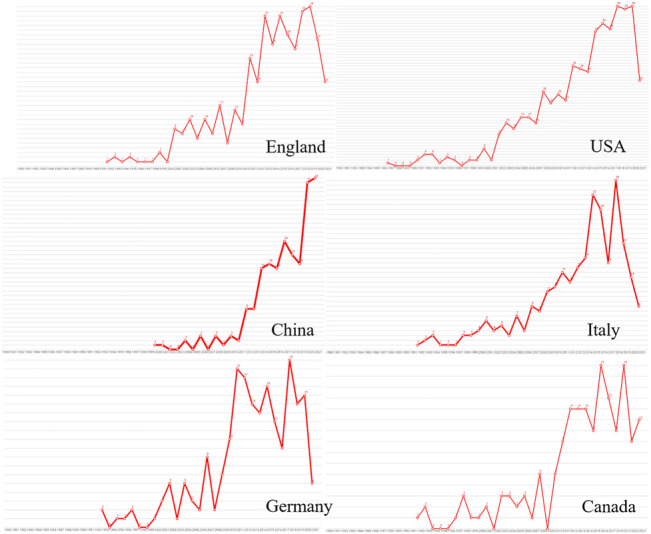
Annual number of COPD drugs-related studies in each country from 1980 to 2021.
FIGURE 5.
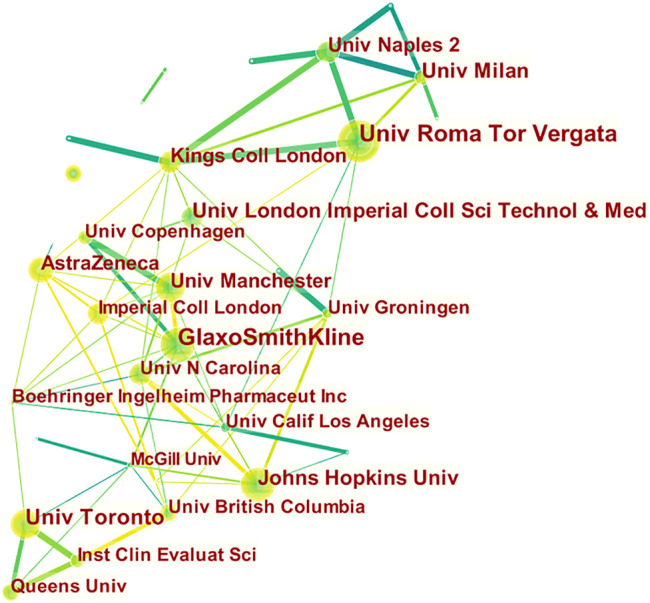
Cooperation among institutions that published COPD drugs-related studies from 1980 to 2021.
FIGURE 6.
Top 20 institutions that cooperated in publishing COPD drugs-related studies from 1980 to 2021.
TABLE 1.
Top 27 institutions with the strongest citation bursts that published COPD drugs-related studies from 1980 to 2021.
| Begin | End | Strength | Year | Entity |
|---|---|---|---|---|
| 1998 | 2006 | 5.3941 | 1980 | A Cardarelli Hosp |
| 2005 | 2010 | 4.1669 | 1980 | Univ Calif Los Angeles |
| 2011 | 2013 | 4.1035 | 1980 | Univ Auckland |
| 2013 | 2015 | 5.8454 | 1980 | Keio Univ |
| 2015 | 2021 | 9.4482 | 1980 | Univ Roma Tor Vergata |
| 2015 | 2019 | 9.4252 | 1980 | GlaxoSmithKline |
| 2015 | 2021 | 9.1131 | 1980 | Univ Toronto |
| 2015 | 2018 | 7.3131 | 1980 | Inst Clin Evaluat Sci |
| 2015 | 2021 | 6.2404 | 1980 | Kings Coll London |
| 2015 | 2021 | 6.1765 | 1980 | Johns Hopkins Univ |
| 2015 | 2018 | 5.882 | 1980 | St Michaels Hosp |
| 2015 | 2021 | 5.7948 | 1980 | Univ Manchester |
| 2015 | 2016 | 5.6786 | 1980 | Univ Naples 2 |
| 2015 | 2019 | 5.5957 | 1980 | Univ N Carolina |
| 2015 | 2021 | 5.2239 | 1980 | Univ Groningen |
| 2015 | 2016 | 4.0515 | 1980 | Univ Ferrara |
| 2015 | 2019 | 4.0359 | 1980 | Univ Southern Denmark |
| 2016 | 2021 | 9.4024 | 1980 | Imperial Coll London |
| 2016 | 2019 | 6.5962 | 1980 | Queens Univ |
| 2016 | 2017 | 5.3541 | 1980 | GSK |
| 2016 | 2019 | 4.4053 | 1980 | Univ Alabama Birmingham |
| 2016 | 2021 | 4.0044 | 1980 | Karolinska Inst |
| 2016 | 2021 | 3.8956 | 1980 | Univ Tennessee |
| 2017 | 2021 | 10.4528 | 1980 | AstraZeneca |
| 2017 | 2021 | 9.1418 | 1980 | Univ Campania Luigi Vanvitelli |
| 2017 | 2019 | 7.5462 | 1980 | Harvard Med Sch |
| 2017 | 2021 | 3.8611 | 1980 | German Ctr Lung Res DZL |
3.3 Distribution of Fields of Study
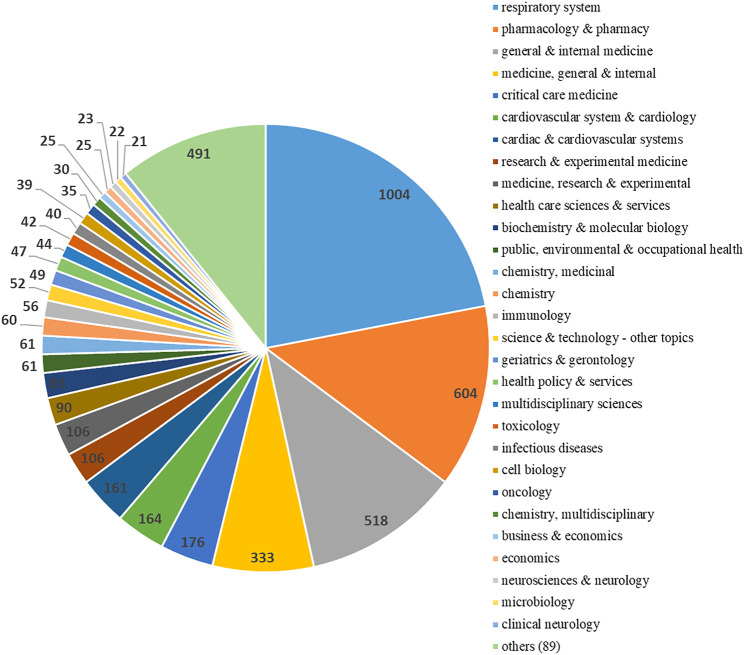
The respiratory system (n = 1,004), Pharmacology and pharmacy (n = 604), and general medicine (n = 518) totally accounted for 46.54% of the fields of study. Critical care medicine, Cardiovascular system and cardiology, Biochemistry and molecular biology, Public, environmental and occupational health, Chemistry, medicinal, Immunology, Geriatrics and gerontology, Health policy and services and Toxicology were also included (Figure 7).
FIGURE 7.
Research areas of COPD drugs-related studies that were published from 1980 to 2021.
3.4 Authors’ Collaborations
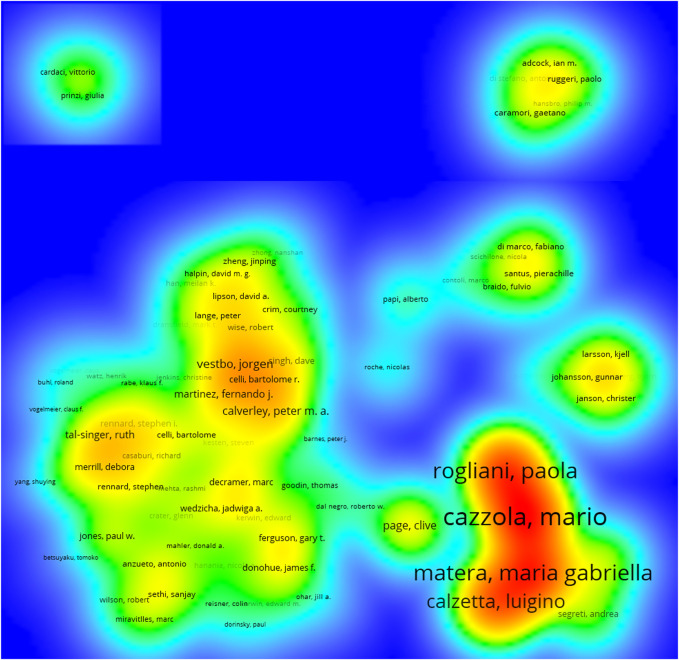
As displayed in Figure 8, the size of each circle indicates the number of articles produced by the author. The distance between any two circles demonstrates the relatedness of their co-authorship link, and the thickness of the connecting line indicates the strength of the link. We found that authors who published a large number of papers generally had fixed partners, and they accordingly created their own research team.
FIGURE 8.
Cooperation among authors that published COPD drugs-related studies from 1980 to 2021.
3.5 Citations
3.5.1 Author Co-Citation Analysis
Among the top 10 cited authors (Table 2), Barnes PJ and Celli BR had the highest participation in finalizing global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017–2019 Report (GOLD).
TABLE 2.
The top 10 authors with the highest citations of COPD drugs-related studies from 1980 to 2021.
| No | Cited author | Frequency | Author | N |
|---|---|---|---|---|
| 1 | Barnes PJ | 423 | Cazzola M | 53 |
| 2 | Calverley PMA | 392 | Matera MG | 38 |
| 3 | Celli BR | 372 | Rogliani P | 33 |
| 4 | Tashkn DP | 351 | Calzetta L | 28 |
| 5 | Cazzola M | 349 | Cazzola M | 20 |
| 6 | Vestbo J | 342 | Vestbo J | 16 |
| 7 | Jones PW | 269 | Singh D | 16 |
| 8 | Rabe KF | 258 | Vozoris NT | 15 |
| 9 | Mahler DA | 251 | Miravitlles M | 14 |
| 10 | Wedzicha JA | 244 | Donohue JF | 14 |
3.5.2 Journal Co-Citation Analysis
The top 10 cited journals and article published journals are listed in Table 3. The top 3 cited journals were European Respiratory Journal, American Journal of Respiratory and Critical Care Medicine, and Chest. The top 3 journals were International Journal Of Chronic Obstructive Pulmonary Disease, Respiratory Medicine and European Respiratory Journal. The top 10 and 3 cited journals accounted for 42.07% and 17.72% of the total number of COPD-related journals, The top 10 and 3 journals accounted for 25.45% and 12.45% of the total number of COPD-related journals, respectively, indicating that the top journals in the field of respiratory diseases were further concentrated on COPD-related medication.
TABLE 3.
The list of top 10 cited journals that published COPD drugs-related studies from 1980 to 2021.
| Cited-journal | N | Journal | N |
|---|---|---|---|
| Eur Respir J | 1,619 | Int J Chronic Obstr | 111 |
| Am J Resp Crit Care | 1,582 | Resp Med | 108 |
| CHEST | 1,562 | Eur Respir J | 99 |
| Thorax | 1,322 | CHEST | 83 |
| New Engl J Med | 1,193 | COPD | 56 |
| Resp Med | 1,103 | Pulm Pharmacol Ther | 56 |
| Lancet | 1,076 | Am J Resp Crit Care | 38 |
| Resp Res | 673 | Resp Res | 36 |
| Int J Chronic Obstr | 621 | Cochrane Db Syst Rev | 32 |
| COPD | 559 | Thorax | 31 |
3.5.3 Co-Citation Analysis
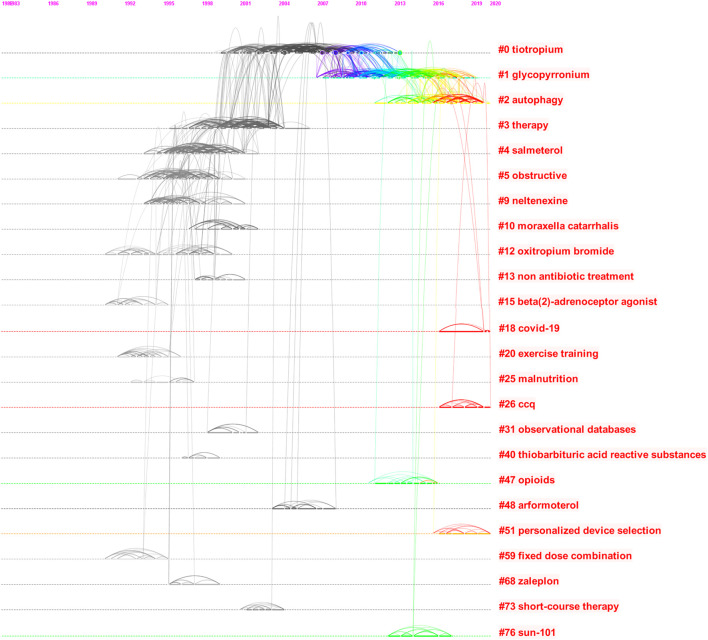
The co-citation analysis showed that, among 2552 articles, 53154 citations were recorded, and the co-citation network is shown in Figure 9. It can be seen that 24 clusters could be achieved, with a Q-value and a silhouette value of 0.902 and 0.954, respectively. The size of the circle represents the size of the surge index. The clusters in the co-citation network are presented in Table 4, including tiotropium, glycopyrronium, salmeterol, neltenexine, and other drugs. The five most frequently cited articles and the most frequently cited researches published in 2021 are listed in Table 5. Research involves Tiotropium, QVA149 [indacaterol/glycopyrronium (Matera et al., 2015)], single-inhaler combination of an extra fine formulation of beclometasone dipropionate, formoterol fumarate, and glycopyrronium bromide and triple therapy (corticosteroid, fluticasone furoate, and vilanterol). The most frequently cited article in 2021 is a real-world clinical evidence.
FIGURE 9.
Co-citation analysis of COPD drugs-related studies that were published from 1980 to 2021.
TABLE 4.
Co-citation clustering of COPD drugs-related studies that were published from 1980 to 2021.
| Cluster | Size | Silhouette | Mean (Year) | Label (LLR) |
|---|---|---|---|---|
| 1 | 278 | 0.947 | 2005 | Tiotropium |
| 2 | 252 | 0.934 | 2013 | Glycopyrronium |
| 3 | 173 | 0.915 | 2016 | Autophagy |
| 4 | 165 | 0.945 | 2000 | Therapy |
| 5 | 138 | 0.967 | 1997 | Salmeterol |
| 6 | 126 | 0.931 | 1996 | Obstructive |
| 7 | 65 | 0.988 | 1996 | Neltenexine |
| 8 | 45 | 0.995 | 2000 | Moraxella catarrhalis |
| 9 | 40 | 0.985 | 1995 | Oxitropium bromide |
| 10 | 34 | 0.997 | 1998 | Non antibiotic treatment |
| 11 | 30 | 1 | 1991 | Beta (2)-adrenoceptor agonist |
| 12 | 28 | 1 | 2019 | COVID-19 |
| 13 | 27 | 0.999 | 1992 | Exercise training |
| 14 | 22 | 1 | 1994 | Malnutrition |
| 15 | 21 | 1 | 2018 | CCQ |
| 16 | 20 | 0.996 | 2000 | Observational databases |
| 17 | 15 | 1 | 1997 | Thiobarbituric acid reactive substances |
| 18 | 14 | 0.998 | 2014 | Opioids |
| 19 | 13 | 0.997 | 2005 | Arformoterol |
| 20 | 11 | 1 | 2017 | Personalized device selection |
| 21 | 10 | 0.998 | 1996 | Fixed dose combination |
| 22 | 8 | 1 | 1996 | Zaleplon |
| 23 | 7 | 1 | 2002 | Short-course therapy |
| 24 | 1 | 1 | 2014 | Sun-101 |
TABLE 5.
The top 5 cited COPD drugs-related studies that were published from 1980 to 2021 and the most cited papers in 2021.
| No | Author | Cited frequency | Drug | Condition or disease | Conclusion |
|---|---|---|---|---|---|
| 1 | (Vogelmeier et al., 2011) | 455 | Tiotropium | Moderate-to-very-severe COPD | In patients with moderate-to-very-severe COPD, tiotropium is more effective than salmeterol in preventing exacerbations |
| 2 | (Wedzicha et al., 2013) | 364 | QVA149 | COPD stages III-IV, and one or more moderate COPD exacerbation in the past year | The dual bronchodilator QVA149 was superior in preventing moderate to severe COPD exacerbations compared with glycopyrronium, with concomitant improvements in lung function and health status |
| 3 | (Vogelmeier et al., 2013) | 238 | QVA149 | COPD stages II-III, without exacerbations in the previous year | Once-daily QVA149 provides significant, sustained, and clinically meaningful improvements in lung function versus twice-daily salmeterol-fluticasone, with significant symptomatic benefit |
| 4 | (Vestbo et al., 2016) | 231 | Corticosteroid, fluticasone furoate, and vilanterol | Moderate COPD and heightened cardiovascular risk | In patients with moderate COPD and heightened cardiovascular risk, treatment with fluticasone furoate and vilanterol did not affect mortality or cardiovascular outcomes, reduced exacerbations, and was well tolerated |
| 5 | (Singh et al., 2016) | 216 | Single-inhaler combination of an extra fine formulation of beclometasone dipropionate, formoterol fumarate, and glycopyrronium bromide (BDP/FF/GB) | COPD had post-bronchodilator FEV1 of lower than 50%, one or more moderate-to-severe COPD exacerbation in the previous 12 months, CAT ≥10, and a Baseline Dyspnea Index focal score of 10 or less | This paper provide evidence for the clinical benefits of stepping up patients with COPD from an inhaled corticosteroid/long-acting β2-agonist combination treatment to triple therapy using a single inhaler |
| 6 | (Izquierdo et al., 2021) | 13 | Clinical Management of COPD in a Real-World Setting | COPD | This study identifies the main features of an unselected COPD population and the major errors made in the management of the disease |
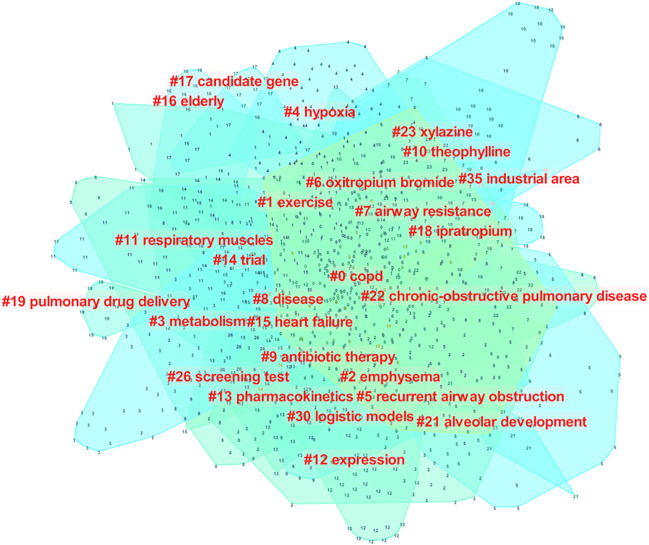
3.6 Analysis of Keywords
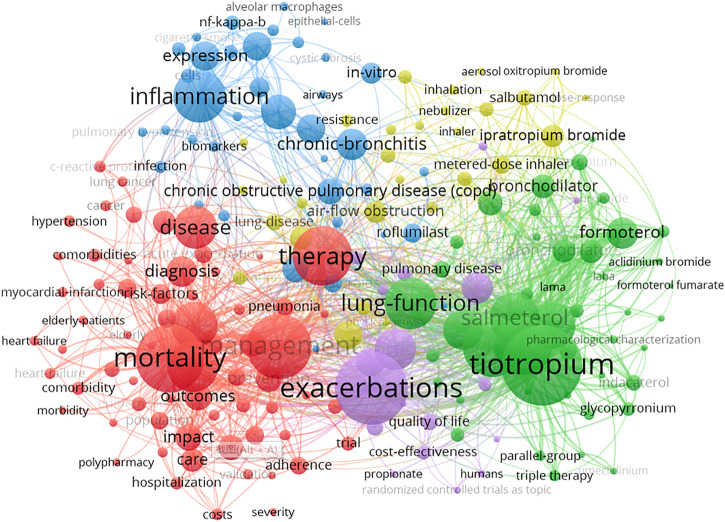
When the keywords with the same meaning were combined, the frequency of the published keywords was statistically analyzed, and the top 20 keywords are summarized in Table 6. A total of 26 items could be achieved by cluster analysis (Table 7), including interventions, pathogenesis of the disease, complications, etc. Interventions for COPD included exercise, Oxitropium bromide, antibiotic therapy, theophylline, ipratropium. The pathogenesis of the disease included metabolic pathways, hypoxia, recurrent airway obstruction, airway resistance, respiratory muscles, alveolar development and other factors. Heart failure was the major complication. Susceptible groups of people (e.g., elderly) and different risk factors (e.g., living in industrial areas) were recorded as well. In addition to the disease name, the most frequently cited keywords were related to exacerbation, tiotropium, mortality, management, therapy, double blind and efficacy, reflecting the importance of medications in the management of COPD, prevention of acute attacks, and reduction of mortality (Figures 10, 11).
TABLE 6.
The top 20 keywords of COPD drugs-related studies that were published from 1980 to 2021.
| Rank | Frequency | Centrality | Key words |
|---|---|---|---|
| 1 | 1,312 | 0.04 | COPD |
| 2 | 717 | 0.03 | Obstructive pulmonary disease |
| 3 | 286 | 0.02 | Exacerbation |
| 4 | 244 | 0.03 | Tiotropium |
| 5 | 206 | 0.06 | Mortality |
| 6 | 192 | 0.05 | Management |
| 7 | 176 | 0.03 | Therapy |
| 8 | 173 | 0.05 | Double blind |
| 9 | 170 | 0.12 | Efficacy |
| 10 | 169 | 0.01 | Lung function |
| 11 | 155 | 0.02 | Inflammation |
| 12 | 147 | 0.02 | Risk |
| 13 | 143 | 0.01 | Salmeterol |
| 14 | 136 | 0.01 | Safety |
| 15 | 131 | 0.11 | Disease |
| 16 | 131 | 0.05 | Prevalence |
| 17 | 115 | 0.03 | Bronchodilator |
| 18 | 105 | 0.03 | Quality of life |
| 19 | 102 | 0.04 | Chronic bronchiti |
| 20 | 101 | 0.03 | Acute exacerbation |
TABLE 7.
Clustering of keywords in COPD drugs-related studies that were published from 1980 to 2021.
| Cluster | Size | Silhouette | Mean (Year) | Label (LLR) |
|---|---|---|---|---|
| 0 | 203 | 0.564 | 2002 | COPD |
| 1 | 94 | 0.824 | 1997 | Exercise |
| 2 | 85 | 0.902 | 2000 | Emphysema |
| 3 | 76 | 0.901 | 1998 | Metabolism |
| 4 | 71 | 0.921 | 1996 | Hypoxia |
| 5 | 62 | 0.939 | 1998 | Recurrent airway obstruction |
| 6 | 58 | 0.861 | 1996 | Oxitropium bromide |
| 7 | 58 | 0.879 | 2000 | Airway resistance |
| 8 | 58 | 0.938 | 1997 | Disease |
| 9 | 56 | 0.896 | 2000 | Antibiotic therapy |
| 10 | 55 | 0.866 | 2000 | Theophylline |
| 11 | 54 | 0.937 | 1998 | Respiratory muscles |
| 12 | 46 | 0.939 | 1999 | Expression |
| 13 | 43 | 0.934 | 1996 | Pharmacokinetics |
| 14 | 33 | 0.907 | 2000 | Trial |
| 15 | 31 | 0.962 | 2000 | Heart failure |
| 16 | 20 | 0.958 | 1997 | Elderly |
| 17 | 16 | 0.999 | 1999 | Candidate gene |
| 18 | 14 | 0.986 | 2000 | Ipratropium |
| 19 | 10 | 0.999 | 2002 | Pulmonary drug delivery |
| 21 | 10 | 0.992 | 2001 | Alveolar development |
| 22 | 9 | 0.998 | 2003 | COPD |
| 23 | 8 | 0.996 | 1998 | Xylazine |
| 26 | 7 | 1 | 1991 | Screening test |
| 30 | 4 | 0.995 | 2004 | Logistic models |
| 35 | 3 | 1 | 2000 | Industrial area |
FIGURE 10.
Keywords analysis of COPD drugs-related studies that were published from 1980 to 2021.
FIGURE 11.
Keywords co-occurrence analysis of COPD drugs-related studies that were published from 1980 to 2021.
So-called “burst words” represent words that are cited frequently over a period of time, (Liang et al., 2017) Burst keywords show the frontier topics and key areas of research. This article focuses on the keywords with the strongest citation bursts that continue to 2021, keywords suddenly increased: “Safety” suddenly increase between 2014 and 2021. “risk,” “oxidative stress,” “risk factor” suddenly increase between 2015 and 2021. “Drug delivery” suddenly increase between 2016 and 2021. “impact,” “device” suddenly increase between 2017 and 2021. “Prevalence,” “triple therapy,” “prevention,” “parallel group,” “health,” “comorbidity” suddenly increase between 2018 and 2021. “adherence,” “in vitro,” “association,” “resistance,” “nf kappa b,” “COPD exacerbation,” “drug,” “inhaler,” “tuberculosis,” “metered dose inhaler” suddenly increase between 2019 and 2021. “COPD,” “depression,” “burden,” “COVID-19,” “intervention,” “diagnosis,” “older adult” suddenly increase between 2020 and 2021 (Table 8).
TABLE 8.
Keywords with the strongest citation bursts of COPD drugs-related studies from 1980 to 2021.
| Begin | End | Strength | Year | Entity |
|---|---|---|---|---|
| 2014 | 2021 | 8.30 | 1980 | Safety |
| 2015 | 2021 | 12.16 | 1980 | Risk |
| 2015 | 2021 | 7.98 | 1980 | Oxidative stress |
| 2015 | 2021 | 5.36 | 1980 | Risk factor |
| 2016 | 2021 | 10.72 | 1980 | Drug delivery |
| 2017 | 2021 | 10.65 | 1980 | Impact |
| 2017 | 2021 | 5.31 | 1980 | Device |
| 2018 | 2021 | 14.09 | 1980 | Prevalence |
| 2018 | 2021 | 10.64 | 1980 | Triple therapy |
| 2018 | 2021 | 9.60 | 1980 | Prevention |
| 2018 | 2021 | 8.40 | 1980 | parallel group |
| 2018 | 2021 | 8.08 | 1980 | Health |
| 2018 | 2021 | 6.50 | 1980 | Comorbidity |
| 2019 | 2021 | 8.98 | 1980 | Adherence |
| 2019 | 2021 | 8.58 | 1980 | In vitro |
| 2019 | 2021 | 6.96 | 1980 | Association |
| 2019 | 2021 | 6.55 | 1980 | Resistance |
| 2019 | 2021 | 5.92 | 1980 | nf kappa b |
| 2019 | 2021 | 5.12 | 1980 | Copd exacerbation |
| 2019 | 2021 | 4.92 | 1980 | Drug |
| 2019 | 2021 | 4.82 | 1980 | Inhaler |
| 2019 | 2021 | 4.18 | 1980 | Tuberculosis |
| 2019 | 2021 | 4.01 | 1980 | Metered dose inhaler |
| 2020 | 2021 | 7.93 | 1980 | COPD |
| 2020 | 2021 | 6.18 | 1980 | Depression |
| 2020 | 2021 | 5.67 | 1980 | Burden |
| 2020 | 2021 | 5.38 | 1980 | COVID-19 |
| 2020 | 2021 | 5.28 | 1980 | Intervention |
| 2020 | 2021 | 5.12 | 1980 | Diagnosis |
| 2020 | 2021 | 3.77 | 1980 | Older adult |
4 Discussion
In recent years, significant progress has been made in the development of new pharmacological and surgical tools to treat COPD, while the rates of prevalence and mortality owing to COPD are still noticeable. Through the visual bibliometric analysis by the CiteSpace software, we could understand the progression of research on COPD drugs, enabling us to more accurately predict the study direction in the future. The number of publications can reflect the overall development trend. The number of COPD drugs-related articles has been elevated from 1980 to 2021, especially an explosive growth was recorded in 2012, indicating that the treatment of COPD has markedly attracted clinicians’ attention in the recent decades.
From the perspective of categorization of published articles based on country, the USA is the country with the largest number of published articles and completed clinical trials, highlighting the important role of this country in the treatment of COPD. However, in terms of the proportion of ongoing clinical trials, China has the highest proportion, suggesting that China will play a more pivotal role in the medication of COPD in the future. A sudden increase in publications was seen from Hungary between 2019 and 2021, and China between 2020 and 2021 also proved this. From the perspective of cooperation among countries, the cooperation among European countries was closer than that among Asian countries. In the recent three decades, the top 20 institutions, with a particular concentration on the treatment of COPD, were from North America (n = 7) and Europe (n = 13). The identification of core journals with high publication and co-citation counts provide important information for authors to select high-quality journals (Wang et al., 2020). The present study revealed that a variety of journals have published COPD-drugs related articles. High cited journals were relatively concentrated, and the top 10 journals accounted for 42.07% of the total number of journals related to the medication of COPD, suggesting that the importance of COPD was acknowledged by these journals.
The co-citation analysis showed that, among 2552 articles, 53154 citations were recorded, and the co-citation network indicated that 24 clusters could be achieved, with a Q-value and a silhouette value of 0.902 and 0.954, respectively. Bronchodilators play a pivotal role in the treatment of symptomatic patients with COPD. Inhaled short-acting bronchodilators are currently recommended for relieving symptoms of patients with mild COPD, whereas inhaled long-acting bronchodilators are recommended as first-line agents for maintenance therapy of patients with moderate and severe COPD (Steiropoulos et al., 2012). Tiotropium resulted in a higher FEV1 than placebo at 24 months and ameliorated the annual decline in the FEV1 after bronchodilator use in patients with COPD of GOLD stage 1 or 2 (Zhou et al., 2017). This also reveals the significant role of drug delivery systems, for example, SUN-101 is a combination of glycopyrrolate delivered through an innovative, electronic nebulizer (Kerwin et al., 2017). Meanwhile, fixed-dose combination and personalized medicine are also important topics. One study (Hu et al., 2020) showed that acute exacerbation and hospitalization of COPD patients were infrequent during the coronavirus disease 2019 (COVID-19) pandemic. However, COVID-19 patients with pre-existing COPD had a higher risk of all-cause mortality.
Based on the keyword analysis of COPD-drugs related articles, we summarize the following four keywords: 1) Management: the target of COPD management is to improve a patient’s functional status and quality of life by preserving optimal lung function, improving symptoms, and preventing the recurrence of exacerbations. 2) Anticholinergics, such as ipratropium bromide and tiotropium bromide, are the most effective group of bronchodilators in the treatment of COPD. Tiotropium bromide was the first long-acting muscarinic antagonist (LAMA) available for COPD in clinical practice. There are two pulmonary drug delivery modes: delivery of inhalation powder via a dry powder inhaler (DPI) and drug delivery via a soft mist inhaler (SMI). Tiotropium was found comparable to inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) in improving lung function and reducing exacerbations and had a greater influence on exacerbation rates than LABAs. Hence, fixed-dose LAMA/LABA combinations have also been developed. Studies showed that tiotropium and olodaterol dual bronchodilator therapy may improve lung function and quality of life and reduce exacerbations in patients with COPD in early stages (Criner and Duffy, 2021). The co-formulation of indacaterol and glycopyrronium can be usefully utilized to optimize and maximize bronchodilation in many COPD patients, who do not experience an adequate airflow increase by using a single bronchodilator (Pelaia et al., 2014). Oxitropium bromide can improve the exercise capacity of patients with stable COPD (Ikeda et al., 1994). Our results showed that, tiotropium has not only attracted clinicians’ attention in the treatment of COPD previously, but also it plays a significant role in the development of further effective therapies for COPD. 3) Pulmonary drug delivery is a compelling noninvasive technique to deliver systemic drugs into circulation. Nowadays, most inhaled drugs are delivered by pressurized metered dose inhaler, (Newman and Dhand, 2015) dry powder inhaler (Hickey and Dhand, 2015) or nebulizer (Fink et al., 2015). The advantage of COPD pulmonary drug delivery is to use a relatively low dose, a low incidence of systemic side effects and a rapid onset of action. And pulmonary drug delivery is a very complicated process. First, the respiratory tract has evolved defense mechanisms that are intended to keep inhaled materials out of the lungs, as well as removing or inactivating them once they have been deposited (Labiris and Dolovich, 2003). Second, it is necessary for a patient to use an inhaler device, and to use it correctly (Newman, 2014). Handling errors of inhaler devices are underestimated in real life and are associated with an increased rate of severe COPD exacerbation (Molimard et al., 2017). Meeting the challenge of delivering drugs to the lungs requires selection of an appropriate inhaler and formulation (Newman, 2017). A review article on tiotropium/olodaterol in the treatment of COPD showed that once-daily delivery of fixed-dose combinations of tiotropium and olodaterol via a very efficient and simple-to-use inhaler device such as Respimat significantly contributes to enhance the therapeutic efficacy of dual bronchodilation, as well as to increase patient adherence to inhaled treatment (Pelaia et al., 2015). Therefore, it is highly essential to further concentrate on the development of COPD drugs in terms of components of drug, drug delivery systems, as well as training patients to take medications safely. 4) COPD in elderly patients: Khakban A predict, the total number of patients diagnosed with COPD will increase by 155%, and COPD-related hospitalization will increase by 210% in 2010–2030. By 2030, 55% of the patients with COPD will be 75 years and older. (Khakban et al., 2017) Frontier studies on the treatment of COPD should particularly involve elderly populations. COPD is a common disease among elderly patients, in which treatment of elderly patients with COPD is highly challenging, and randomized controlled trials may underestimate the risk of adverse effects of interventions. Although age is an important factor in the incidence of COPD, there is a lack of age-based research on COPD drugs. This may be one of the next efforts in COPD drug research.
Diagnosis and treatment of COPD are complicated because COPD may manifest as multiple phenotypes. Research showed that the phenotypical approach is crucial in the management of COPD as it allows to individualize the therapeutic strategy and to obtain more effective clinical outcomes (Dal Negro et al., 2021). According to the analysis of keywords, we summarize five research directions in the following. 1) Identification of the risk factors of COPD: exposure to cigarette smoke worsens lung edema and inflammation, (Hou et al., 2019) and smoking is the main risk factor for COPD (Mannino and Buist, 2007). However, there are still numerous unknown risk factors for COPD, which should be explored through epidemiological assessment. 2) Triple therapy: research showed that addition of fluticasone-salmeterol to tiotropium therapy did not statistically influence rates of COPD exacerbation but did improve lung function, quality of life, and hospitalization rates in patients with moderate to severe COPD (Aaron et al., 2007). Subsequent studies of this kind have gradually become the hotspot and frontier of COPD drug research. The route of administration should be mainly pulmonary inhalation, and attention should be paid to the development and utilization of drug delivery devices. Study showed that, once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) was non-inferior to twice-daily budesonide/formoterol via metered-dose inhaler plus once-daily tiotropium via HandiHaler (BUD/FOR + TIO) for weighted mean change from baseline in 0–24 h FEV1 at Week 12 in patients with COPD. Greater improvements in trough and serial FEV1 measurements at Week 12 with FF/UMEC/VI versus BUD/FOR + TIO, together with similar health status improvements and safety outcomes including the incidence of pneumonia (Ferguson et al., 2020). And for patients with frequent and/or severe acute exacerbations in the past, although these patients have received triple, LABA/ICS, single bronchodilator or double bronchodilator, they used closed triple inhalation therapy compared with fixed-dose double bronchodilator therapy, can still benefit from mortality (GOLD, 2021). The main administration route should be pulmonary inhalation, and further attention should be paid to the research and development of drug delivery systems. 3) COPD and comorbidity: the Global Initiative for GOLD guidelines (2011) has recommended that, in general, the presence of co-morbidities should not alter COPD treatment, and comorbidities should be treated urgently, and a new version has emphasized the role of acute exacerbation and complications of COPD in the disease assessment. The most common comorbidities are ischaemic heart disease, diabetes, skeletal muscle wasting, cachexia, osteoporosis, depression, and lung cancer (Corlateanu et al., 2016). It was found that, the relationship between complications of COPD and treatment is still in infancy, and depression and COVID-19 are worthy of consideration to better explore the mentioned relationship. 4) Elderly patients with COPD: further research should be conducted to improve medications more effectively for elderly patients with COPD. 5) Pharmacoeconomics analysis: pharmacoeconomics analysis of COPD better clarifies the economic benefits of the proposed medications. Revealing the appropriate initial and maintenance drug regimens for patients with different phenotypes or subtypes of COPD, which is expected to control medical costs. Study showed that promptly initiating triple therapy after two moderate or one severe exacerbation is associated with decreased morbidity and economic burden in COPD, (Tkacz et al., 2022) for example.
The two important limitations of the current study should be pointed out. Firstly, we only analyzed studies indexed in the WOSCC database. Secondly, the Matthew effect, which might influence the results of bibliometric analysis, was not considered (Jiang et al., 2021).
In summary, the administration of bronchodilators and pulmonary drug delivery systems, as well as consideration of elderly COPD patients require further attention in frontier studies on medications for COPD, and exact mechanisms underlying the pathogenesis of COPD should be explored as well.
Author Contributions
GZ designed the study, DJC directed the design of this study. GZ and LYY collected and verified the data. GZ performed software analysis. GZ and LYY drafted the first vision. GZ, LYY, and DJC revised and approved the final version of the manuscript.
Funding
This study is supported by the Shanghai 3 year Action Plan for further speeding up the development of Traditional Chinese Medicine (2018–2020) [No.ZY (2018–2020)-CCCX-4002], Shanghai Science and Technology Commission scientific research project (No.19401931400), the Chinese Medicine Innovation Project of the Shanghai Health Committee (No.ZYKC201601023) and National Natural Science Foundation of China (no. 81760901).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aaron S. D., Vandemheen K. L., Fergusson D., Maltais F., Bourbeau J., Goldstein R., et al. (2007). Tiotropium in Combination with Placebo, Salmeterol, or Fluticasone-Salmeterol for Treatment of Chronic Obstructive Pulmonary Disease: a Randomized Trial. Ann. Intern. Med. 146 (8), 545–555. Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. 10.7326/0003-4819-146-8-200704170-00152 [DOI] [PubMed] [Google Scholar]
- Chen C., Dubin R., Kim M. C. (2014). Emerging Trends and New Developments in Re-generative Medicine: A Scientometric Update (2000–2014). Expert Opin. Biol. Ther. 14, 1295–1317. 10.1517/14712598.2014.920813 [DOI] [PubMed] [Google Scholar]
- Chen C. M. (2006). Citespace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 57, 359–377. 10.1002/asi.20317 [DOI] [Google Scholar]
- Chronic Obstructive Pulmonary Disease Group of Chinese (2021). Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic Society; Chronic Obstructive Pulmonary Disease Committee of Chinese Association of Chest Physician. [Guidelines for the Diagnosis and Management of Chronic Obstructive Pulmonary Disease (Revised Version 2021)]. Zhonghua Jie He He Hu Xi Za Zhi 44 (3), 170–205. Chinese. [DOI] [PubMed] [Google Scholar]
- Corlateanu A., Covantev S., Mathioudakis A. G., Botnaru V., Siafakas N. (2016). Prevalence and burden of Comorbidities in Chronic Obstructive Pulmonary Disease. Respir. Investig. 54 (6), 387–396. 10.1016/j.resinv.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Criner G., Duffy S. (2021). Reducing and Managing Chronic Obstructive Pulmonary Disease Exacerbations with Tiotropium + Olodaterol. Curr. Med. Res. Opin. 37 (2), 275–284. 10.1080/03007995.2020.1841615 [DOI] [PubMed] [Google Scholar]
- Dal Negro R. W., Carone M., Cuttitta G., Gallelli L., Pistolesi M., Privitera S., et al. (2021). Prevalence and Clinical Features of Most Frequent Phenotypes in the Italian COPD Population: the CLIMA Study. Multidiscip Respir. Med. 16 (1), 790. 10.4081/mrm.2021.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decramer M., Janssens W., Miravitlles M. (2012). Chronic Obstructive Pulmonary Disease. Lancet 379 (9823), 1341–1351. 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Zhang W., Li K., Chen X. (2014). Effectiveness of T'ai Chi and Qigong on Chronic Obstructive Pulmonary Disease: a Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 20 (2), 79–86. 10.1089/acm.2013.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G. T., Brown N., Compton C., Corbridge T. C., Dorais K., Fogarty C., et al. (2020). Once-daily Single-Inhaler versus Twice-Daily Multiple-Inhaler Triple Therapy in Patients with COPD: Lung Function and Health Status Results from Two Replicate Randomized Controlled Trials. Respir. Res. 21 (1), 131. 10.1186/s12931-020-01360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. B., Stapleton K. W. (2015). “Nebulizers,” in ISAM Textbook of Aerosol Medicine. International Society for Aerosols in Medicine. Editor Dhand R. (New Rochelle, NY: online publication; ), 617–655. [Google Scholar]
- GOLD (2021). Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals. Available at: www.goldcopd.org .
- Gutiérrez Villegas C., Paz-Zulueta M., Herrero-Montes M., Parás-Bravo P., Madrazo Pérez M. (2021). Cost Analysis of Chronic Obstructive Pulmonary Disease (COPD): a Systematic Review. Health Econ. Rev. 11 (1), 31. 10.1186/s13561-021-00329-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey A. J. (2015). “Dry Powder Inhalers: an Overview,” in ISAM Textbook of Aerosol Medicine. International Society for Aerosols in Medicine, Online Publication. Editor Dhand R. (WerneGermany: North Rhine-Westphalia; ), 469–489. [Google Scholar]
- Hou W., Hu S., Li C., Ma H., Wang Q., Meng G., et al. (2019). Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. Biomed. Res. Int. 2019, 2025636. 10.1155/2019/2025636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Dong M., Xiong M., Zhao D., Zhao Y., Wang M., et al. (2020). Clinical Courses and Outcomes of Patients with Chronic Obstructive Pulmonary Disease during the COVID-19 Epidemic in Hubei, China. Int. J. Chron. Obstruct Pulmon Dis. 15, 2237–2248. 10.2147/COPD.S265004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A., Nishimura K., Koyama H., Sugiura N., Izumi T. (1994). Oxitropium Bromide Improves Exercise Performance in Patients with COPD. Chest 106 (6), 1740–1745. 10.1378/chest.106.6.1740 [DOI] [PubMed] [Google Scholar]
- Izquierdo J. L., Morena D., González Y., Paredero J. M., Pérez B., Graziani D., et al. (2021). Clinical Management of COPD in a Real-World Setting. A Big Data Analysis. Arch. Bronconeumol (Engl Ed. 57 (2), 94–100. 10.1016/j.arbres.2019.12.025 [DOI] [PubMed] [Google Scholar]
- Jiang S. Y., Huang X. Q., Chen S. Y. (2021). Hotspots and Frontiers of Cirrhosis with portal Vein Thrombosis: a Visual analysis[[J]. Chin. J. Evid Based. Med. 21 (3), 298–302. Chinese. 10.7507/1672-2531.202008137 [DOI] [Google Scholar]
- Kerwin E., Donohue J. F., Goodin T., Tosiello R., Wheeler A., Ferguson G. T. (2017). Efficacy and Safety of glycopyrrolate/eFlow® CS (Nebulized Glycopyrrolate) in Moderate-To-Very-Severe COPD: Results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 3 and 4 Randomized Controlled Trials. Respir. Med. 132, 238–250. 10.1016/j.rmed.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Khakban A., Sin D. D., FitzGerald J. M., McManus B. M., Ng R., Hollander Z., et al. (2017). The Projected Epidemic of Chronic Obstructive Pulmonary Disease Hospitalizations over the Next 15 years. A Population-Based Perspective. Am. J. Respir. Crit. Care Med. 195 (3), 287–291. 10.1164/rccm.201606-1162PP [DOI] [PubMed] [Google Scholar]
- Kim W. J., Song J. S., Park D. W., Kwak H. J., Moon J. Y., Kim S. H., et al. (2014). The Effects of Secondhand Smoke on Chronic Obstructive Pulmonary Disease in Nonsmoking Korean Adults. Korean J. Intern. Med. 29 (5), 613–619. 10.3904/kjim.2014.29.5.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiris N. R., Dolovich M. B. (2003). Pulmonary Drug Delivery. Part I: Physiological Factors Affecting Therapeutic Effectiveness of Aerosolized Medications. Br. J. Clin. Pharmacol. 56 (6), 588–599. 10.1046/j.1365-2125.2003.01892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. D., Li Y., Zhao J., Wang X. Y., Zhu H. Z., Chen X. H. (2017). Study of Acupuncture for Low Back Pain in Recent 20 years: a Bibliometric Analysis via CiteSpace. J. Pain Res. 10, 951–964. 10.2147/JPR.S132808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino D. M., Buist A. S. (2007). Global burden of COPD: Risk Factors, Prevalence, and Future Trends. Lancet 370 (9589), 765–773. 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- Matera M. G., Rogliani P., Cazzola M. (2015). QVA149 (Indacaterol/glycopyrronium) for the Treatment of Chronic Obstructive Pulmonary Disease. Expert Opin. Pharmacother. 16 (7), 1079–1090. 10.1517/14656566.2015.1032247 [DOI] [PubMed] [Google Scholar]
- Molimard M., Raherison C., Lignot S., Balestra A., Lamarque S., Chartier A., et al. (2017). Chronic Obstructive Pulmonary Disease Exacerbation and Inhaler Device Handling: Real-Life Assessment of 2935 Patients. Eur. Respir. J. 49 (2), 1601794. 10.1183/13993003.01794-2016 [DOI] [PubMed] [Google Scholar]
- Newman S. (2014). Improving Inhaler Technique, Adherence to Therapy and the Precision of Dosing: Major Challenges for Pulmonary Drug Delivery. Expert Opin. Drug Deliv. 11 (3), 365–378. 10.1517/17425247.2014.873402 [DOI] [PubMed] [Google Scholar]
- Newman S. P. (2017). Drug Delivery to the Lungs: Challenges and Opportunities. Ther. Deliv. 8 (8), 647–661. 10.4155/tde-2017-0037 [DOI] [PubMed] [Google Scholar]
- Newman S. P. (2015). “Pressurized Metered Dose Inhalers,” in ISAM Textbook of Aerosol Medicine. International Society for Aerosols in Medicine, Online Publication. Editor Dhand R. (WerneGermany: North Rhine-Westphalia; ), 445–468. [Google Scholar]
- Patel A. R., Patel A. R., Singh S., Singh S., Khawaja I. (2019). Global Initiative for Chronic Obstructive Lung Disease: The Changes Made. Cureus 11 (6), e4985. 10.7759/cureus.4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaia G., Maselli R., Gallelli L. (2014). Pharmacologic Rationale, Efficacy and Safety of the Fixed-Dose Co-formulation of Indacaterol and Glycopyrronium. Multidiscip Respir. Med. 9 (1), 64. 10.1186/2049-6958-9-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaia G., Vatrella A., Busceti M. T., Gallelli L., Calabrese C., Terracciano R., et al. (2015). Pharmacologic Rationale Underlying the Therapeutic Effects of Tiotropium/olodaterol in COPD. Ther. Clin. Risk Manag. 11, 1563–1572. 10.2147/TCRM.S84151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Kim G., Li Q., Newman G. (2021). Web of Science-Based Green Infrastructure: A Bibliometric Analysis in CiteSpace. Land (Basel) 10 (7), 711. 10.3390/land10070711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Papi A., Corradi M., Pavlišová I., Montagna I., Francisco C., et al. (2016). Single Inhaler Triple Therapy versus Inhaled Corticosteroid Plus Long-Acting β2-agonist Therapy for Chronic Obstructive Pulmonary Disease (TRILOGY): a Double-Blind, Parallel Group, Randomised Controlled Trial. Lancet 388 (10048), 963–973. 10.1016/S0140-6736(16)31354-X [DOI] [PubMed] [Google Scholar]
- Steiropoulos P., Papanas N., Nena E., Bouros D. (2012). Indacaterol: a New Long-Acting β2-agonist in the Management of Chronic Obstructive Pulmonary Disease[J]. Expert Opin. Pharmacother. 13 (7), 1015–1029. 10.1517/14656566.2012.674513 [DOI] [PubMed] [Google Scholar]
- Tkacz J., Evans K. A., Touchette D. R., Portillo E., Strange C., Staresinic A., et al. (2022). PRIMUS - Prompt Initiation of Maintenance Therapy in the US: A Real-World Analysis of Clinical and Economic Outcomes Among Patients Initiating Triple Therapy Following a COPD Exacerbation. Int. J. Chron. Obstruct Pulmon Dis. 17, 329–342. 10.2147/COPD.S347735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck N. J., Waltman L. (2010). Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 84 (2), 523–538. 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J., Anderson J. A., Brook R. D., Calverley P. M. A., Bartolome R. C., Courtney C., et al. (2016). SUMMIT Investigators. Fluticasone Furoate and Vilanterol and Survival in Chronic Obstructive Pulmonary Disease with Heightened Cardiovascular Risk (SUMMIT): a Double-Blind Randomised Controlled Trial. Lancet 387 (10030), 1817–1826. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Hederer B., Glaab T., Beeh K. M., Rabe K. F., Fabbri L. M., et al. (2011). Tiotropium versus Salmeterol for the Prevention of Exacerbations of COPD. N. Engl. J. Med. 364 (12), 1093–1103. POET-COPD Investigators. 10.1056/NEJMoa1008378 [DOI] [PubMed] [Google Scholar]
- Vogelmeier C. F., Bateman E. D., Pallante J., Vijay K. T. A., D'Andrea P., Chen H., et al. (2013). Efficacy and Safety of Once-Daily QVA149 Compared with Twice-Daily Salmeterol-Fluticasone in Patients with Chronic Obstructive Pulmonary Disease (ILLUMINATE): a Randomised, Double-Blind, Parallel Group Study. Lancet Respir. Med. 1 (1), 51–60. 10.1016/S2213-2600(12)70052-8 [DOI] [PubMed] [Google Scholar]
- Wang C., Xu J., Yang L., Xu Y., Zhang X., Bai C., et al. (2018). Prevalence and Risk Factors of Chronic Obstructive Pulmonary Disease in China (The China Pulmonary Health [CPH] Study): a National Cross-Sectional Study. Lancet 391 (10131), 1706–1717. 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- Wang S. Q., Gao Y. Q., Zhang C., Xie Y-J., Wang J-X., Xu F-Y. (2020). A Bibliometric Analysis Using CiteSpace of Publications from 1999 to 2018 on Patient Rehabilitation after Total Knee Arthroplasty. Med. Sci. Monit. 26, e920795. 10.12659/MSM.920795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzicha J. A., Decramer M., Ficker J. H., Niewoehner D. E., Sandström T., Taylor A. F., et al. (2013). Analysis of Chronic Obstructive Pulmonary Disease Exacerbations with the Dual Bronchodilator QVA149 Compared with Glycopyrronium and Tiotropium (SPARK): a Randomised, Double-Blind, Parallel-Group Study. Lancet Respir. Med. 1 (3), 199–209. 10.1016/S2213-2600(13)70052-3 [DOI] [PubMed] [Google Scholar]
- Yu Y., Li Y., Zhang Z., Gu Z., Han Z, Zha Q., et al. (2020). A Bibliometric Analysis Using VOSviewer of Publications on COVID-19. Ann. Transl Med. 8 (13), 816. 10.21037/atm-20-4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhong N. S., Li X. (2017). Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 377 (10), 923–935. 10.1056/NEJMoa1700228 [DOI] [PubMed] [Google Scholar]