Summary
Background
Little is known regarding associations of conventional and emerging diseases and their multimorbidity with brain volumes.
Methods
This cross-sectional study included 36,647 European ancestry individuals aged 44–81 years with brain magnetic resonance imaging data from UK Biobank. Brain volumes were measured between 02 May 2014 and 31 October 2019. General linear regression models were used to associate 57 individual major diseases with brain volumes. Latent class analysis was used to identify multimorbidity patterns. A multimorbidity score for brain volumes was computed based on the estimates for individual groups of diseases.
Findings
Out of 57 major diseases, 16 were associated with smaller volumes of total brain, 14 with smaller volumes of grey matter, and six with smaller hippocampus volumes, and four major diseases were associated with higher white matter hyperintensity (WMH) load after adjustment for all other diseases. The leading contributors to the variance of total brain volume were hypertension (R2=0·0229), dyslipidemia (0·0190), cataract (0·0176), coronary heart disease (0·0107), and diabetes (0·0077). We identified six major multimorbidity patterns and multimorbidity patterns of cardiometabolic disorders (CMD), and CMD-multiple disorders, and metabolic disorders were independently associated with smaller volumes of total brain (β (95% CI): -6·6 (-8·9, -4·3) ml, -7·3 (-10·4, -4·1) ml, and -10·4 (-13·5, -7·3) ml, respectively), grey matter (-7·1 (-8·5, -5·7) ml, -9·0 (-10·9, -7·1) ml, and -11·8 (-13·6, -9·9) ml, respectively), and higher WMH load (0·23 (0·19, 0·27), 0·25 (0·19, 0·30), and 0·33 (0·27, 0·39), respectively) after adjustment for geographic, socioeconomic, and lifestyle factors (all P-values<0·0001). The percentage of the variance of total brain volume explained by multimorbidity patterns, multimorbidity defined by the number of diseases, and multimorbidity score was 1·2%, 3·1%, and 7·2%, respectively. Associations between CMD-multiple disorders pattern, and metabolic disorders pattern and volumes of total brain, grey matter, and WMH were stronger in men than in women. Associations between multimorbidity and brain volumes were stronger in younger than in older individuals.
Interpretation
Besides conventional diseases, we found an association between numerous emerging diseases and smaller brain volumes. CMD-related multimorbidity patterns are associated with smaller brain volumes. Men or younger adults with multimorbidity are more in need of care for promoting brain health. These findings are from an association study and will need confirmation.
Funding
The Fundamental Research Funds of the State Key Laboratory of Ophthalmology, Project of Investigation on Health Status of Employees in Financial Industry in Guangzhou, China (Z012014075), Science and Technology Program of Guangzhou, China (202,002,020,049).
Keywords: Major diseases, Multimorbidity, Brain volume, Grey matter, Hippocampus, White matter hyperintensity, Moderation analysis
Abbreviations: AD, Alzheimer’s disease; APOE4, Apolipoprotein E ε4; BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; CKD, chronic kidney disease; CMD, cardiometabolic disorders; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FDR, false discovery rate; OLS, ordinary least squares; WMH, white matter hyperintensity
Research in context.
Evidence before this study
A literature search was conducted using PubMed on 22 October, 2021, using the search terms “multiple diseases”, “multiple conditions”, “multiple chronic diseases”, “multiple chronic conditions”, “individual diseases”, “individual conditions”, “individual chronic diseases”, “individual chronic conditions”, “major diseases”, “major conditions”, “major chronic diseases”, “major chronic conditions”, “multimorbidity”, “multi-morbidity”, “comorbidit”, or “co-morbidit*”, combined with “magnetic resonance imaging”, “MRI”, “grey matter”, “white matter”, or “hippocamp*" with no language restriction. Numerous studies with small sample sizes have investigated associations between well-known risk factors for dementia including traumatic brain injury, hearing impairment, obesity, hypertension, stroke, diabetes, and depression and brain volume. Data on associations between other major diseases and brain volumes are limited whilst it is unknown regarding associations between multimorbidity patterns and brain volumes.
Added value of this study
To our knowledge, this is the first study to examine associations of a wide range of major diseases and their major multimorbidity patterns with brain volumes. In addition to conventional conditions, we found atrial fibrillation, heart failure, peripheral vascular disease, alcohol problems, psychoactive substance abuse, schizophrenia, chronic obstructive pulmonary disease, inflammatory bowel disease, chronic kidney disease, breast cancer, rectal cancer, glaucoma, and cataract were independently associated with smaller volumes of total brain, grey matter, and hippocampus, and higher white matter hyperintensity load. For the first time, we identified multimorbidity patterns that were linked to brain volumes and created a multimorbidity score that was strongly predictive of brain volumes. In moderation analysis, associations between multimorbidity and brain volumes were stronger in men than in women, in younger than in older individuals, and in lowly educated than in highly educated individuals.
Implications of all the available evidence
We identified numerous emerging diseases (ie, those not previously associated with reductions in brain volume) in addition to conventional diseases that may help identify individuals at higher risk of reductions in brain volume. Cardiometabolic disorders related multimorbidity patterns were associated with a larger reduction in brain volumes. Our findings provide evidence for intervention priorities for the prevention and screening of reduction in brain volume in middle-aged and older adults.
Alt-text: Unlabelled box
Introduction
It is estimated that the number of people aged ≥65 years is 727 million in 2020 and this number is expected to increase to 1·5 billion by 2050.1 Ageing is the primary risk factor for neurodegenerative diseases,2 whilst non-pathological brain damage is the prelude to these neurodegenerative diseases. Volumes of total brain, grey matter, white matter hyperintensity (WMH), and hippocampus have been shown to play an important role in the development of dementia.3 Neurodegenerative diseases including dementia are frequently seen, and disease-free brains are rarely seen in the older population.2 Identifying modifiable determinants for brain damage will provide evidence for intervention priorities that may help to prevent dementia. This is important as brain atrophy may precede the onset of dementia by many years.2
Many well-known modifiable risk factors for dementia including obesity, hypertension, high cholesterol, diabetes, hearing loss, psychiatric disorders, and smoking have been linked to brain atrophy.4, 5, 6, 7, 8 However, these conventional factors appear to explain a small amount of variance (≤2%) in excess of covariates for brain atrophy.5,6 Therefore, it is imperative to identify other determinants for brain atrophy considering the rapid decline in brain volumes in older adults.9 Other emerging chronic diseases including musculoskeletal disorders, respiratory diseases, digestive disorders, and chronic kidney disease (CKD), which are shown to be risk factors of dementia, have been linked to brain atrophy in only a few studies.10, 11, 12 Meanwhile, many individuals have not only one but several conditions (multimorbidity) in the ageing population. Although multimorbidity has been linked to increased dementia risk,13,14 it is unknown whether multimorbidity and its major patterns confer to the risk of brain atrophy.
Identifying whether conventional and emerging diseases, that are known to be risk factors for dementia, are associated with brain volumes is important for the prevention of brain damage. Using the UK Biobank cohort, we aimed to investigate associations of a wide range of individual major diseases and their multimorbidity/multimorbidity patterns/multimorbidity score with brain volumes. Whether these associations were moderated by age, gender, or education was also tested.
Methods
Study population
UK Biobank is a community-based cohort that recruited more than 500,000 participants aged 38–73 years at baseline between 2006 and 2010.15 Design and population have been detailed elsewhere.15 Of approximately 9·2 million eligible invited people who were registered with the National Health Service, 502,505 individuals were measured at baseline. Data were collected at baseline (2006–2010), first repeat assessment visit (2012–2013), imaging visit (2014–2019), and first imaging repeat visit (2019+). Individuals with data on both brain magnetic resonance imaging (MRI) and major diseases were included in the analysis. Those with dementia, Parkinson's disease, multiple sclerosis, epilepsy, stroke, head or neurological injury, or trauma, or other chronic neurological problems (nervous system infection, brain abscess, encephalitis, demyelinating disease, cerebral aneurysm, cerebral palsy, brain haemorrhage, or brain cancer) were excluded from the analysis. Imaging visit data were used in the present cross-sectional analysis. The present study was reported following the STROBE guidelines.16
The UK Biobank Study's ethical approval has been granted by the National Information Governance Board for Health and Social Care and the NHS North-West Multicenter Research Ethics Committee. All participants provided informed consent through electronic signature at recruitment. The present study was conducted under application number 62,443 of the UK Biobank resource.
Brain magnetic resonance imaging
The main outcomes of the study included volumes of total brain, grey matter, white matter, WMH, and hippocampus. Brain MRI data were acquired on a standard Siemens Skyra 3T scanner with a standard 32-channel radio-frequency receiver head coil.17,18 The T1- and T2-weighted scans were analysed with the Functional MRI of the Brain Software Library. Global and regional brain imaging-derived phenotypes included total brain, grey matter, white matter, WMH, and hippocampus. Total brain volume was computed by adding the grey matter and white matter volumes (excludes cerebrospinal fluid). Total brain and regional brain volumes were normalized for head size based on the external surface of the skull,17,18 using the ratio-corrected method.17 Given the positively skewed distribution, WMH was log-transformed in the analysis. Given the small sample size of the first imaging repeat visit (2019+), MRI data measured between 2 May, 2014 and 31 October, 2019 (imaging visit) were used in the main analysis.
Definition of diseases and multimorbidity
A major disease was defined if participants reported that they had ever been told by a doctor that they had the corresponding disease. A total of 57 major diseases such as cardiovascular disease (CVD), cancer, diabetes, psychiatric diseases, CKD were included in the analysis (Field code is listed in Table S1). Additional disease cases at baseline were defined using inpatient data where the disease was initially diagnosed before MRI assessment. Inpatient hospital data for the UK Biobank participants were available since 1997. Body mass index (BMI) was calculated based on measured weight and height, and obesity was defined as BMI≥30 kg/m2. International Classification of Diseases codes for the remaining 57 diseases are listed in Table S2. Multimorbidity was defined by having ≥2 of 19 groups of these 57 chronic diseases (Table S3). Obesity, hypertension, high cholesterol, diabetes, CVD, hearing loss, traumatic brain injury, and psychiatric disorders that are well-known risk factors of dementia are considered conventional risk factors for greater brain atrophy.4, 5, 6, 7 Those diseases including painful conditions, respiratory disorders, digestive disorders, CKD, cancers, and eye diseases that are linked to brain volumes in only a few studies are considered as emerging factors.9, 10, 11
Covariates
Demographics including age (years), gender (men, women), education (0–5 years, 6–12 years, ≥13 years), ethnicity (whites, non-whites), and annual household income (<18,000 £, 18,000–30,999 £, 31,000–51,999 £, 52,000–100,000 £, >100,000 £) were self-reported. Sleep duration was assessed with the survey item “About how many hours sleep do you get in every 24 h?” We divided sleep duration into three groups: <7, 7–9, and >9 h.19 A short form of the International Physical Activity Questionnaire was used to assess physical activity. A healthy diet score was calculated based on seven commonly eaten food groups following recommendations on dietary priorities for cardiometabolic health.20 A higher score represented a healthier diet. Those factors measured at the survey of MRI assessment were used in the analysis. Genotyping was conducted by Affymetrix using a bespoke BiLEVE Axiom array or the UK Biobank Axiom array. Apolipoprotein E ε4 (APOE4) + dominant model of ε3/ε4 and ε4/ε4 was used to define the presence of APOE4.
Statistical analysis
Data of characteristics were expressed percentages (95% confidence intervals [CIs]) and means (95% CIs) by multimorbidity.
Both linear regression and spine curve models were used to test the association between age and brain. Associations between individual diseases and volumes of total brain, grey matter, white matter, hippocampus, and WMH were tested using general linear regression models volumes (these brain volumes were analyzed as a single multivariate outcome). We tested three models: 1) unadjusted model; 2) Model 2 was adjusted for age, gender, APOE4, income, education, alcohol consumption, physical activity, sleep duration, smoking, and diet score; 3) Model 3 was adjusted for Model 2 plus all other diseases for each disease. Benjamin-Hochberg's procedure was used to control false discovery rate (FDR) at a 5% level for multiple comparisons.21 Associations between multimorbidity (defined by the number of diseases) and brain volumes were also tested.
Multimorbidity patterns were identified using latent class analysis based on 19 groups of diseases (recategorized based on 57 major diseases, Table S3). Consistent Akaike Information Criterion (CAIC) and adjusted Bayesian Information Criterion (ABIC) were used to determine the optimal number of latent classes.22 Lower ABIC and CAIC values indicated better fitting models. Participants were assigned to one class according to the highest computed probability of membership. Latent classes were labelled according to those chronic conditions whose prevalence in latent classes was higher than that in the whole cohort. General linear regression models were then used to test associations between multimorbidity patterns and brain volumes.
We computed a multimorbidity score for brain volumes based on the estimates for 19 individual groups of diseases (Table S4). The multimorbidity score was calculated using the formula:
refers to whether individuals had the i group of diseases or not (yes, no), whilst is the coefficient for brain volumes associated with the i group of diseases. This analysis was conducted for volumes of total brain, grey matter, and hippocampus, separately. The formula for WMH volume was:
A higher multimorbidity score represents unhealthier brain health. Multimorbidity score was also calculated by accounting for variability in the estimated β for brain volumes associated with 19 individual groups of diseases (the calculation method is detailed in the Supplemental materials). The association between multimorbidity score in quintiles and brain volumes was then tested. Given that log transformation of WMH may induce bias in the estimates of coefficients for WMH associated with 19 individual groups of diseases using linear regression model, ordinary least squares (OLS) regression model was also used to estimate those coefficients. Multimorbidity scores calculated based on coefficients estimated using both linear and OLS regression models were then associated with WMH.
The amount of variance of brain volume accounted for by individual diseases and multimorbidity was computed by a baseline model R2 in which brain volume was modelled with covariates only subtracting from the R2 of the full model. To facilitate the comparison of associations across individual diseases, standardized coefficients are reported in the supplemental materials. The effect sizes can be classified as small, medium, or large according to Cohen, when the standardized coefficients are 0·1, 0·3, or 0·5, respectively.23
The potential modification effects of age (younger individuals aged <65 years, older individuals aged ≥65 years), gender (men, women), APOE4 (yes, no), and education (0–5 years, 6–12 years, ≥13 years) on associations between multimorbidity and brain volumes were tested using general linear regression models.
In order to test the risk of bias due to missing values, we examined associations between individual major diseases and brain volumes among those with complete data (covariates). A large proportion of individuals without MRI data were not included in the analysis. Given the potential differences between individuals with and without MRI data, associations between individual diseases and brain volumes were tested using the inverse probability weighting method. Inverse probability weighting is a method that can be used to account for missing data when subjects with missing data could not be included in the main analysis.24 In our analysis, individuals with complete data were weighted by the inverse of their probability of being a complete case. A sensitivity analysis was also conducted to examine associations between individual diseases defined by inpatient data (cases diagnosed from 0 to 5 years before MRI assessment) and total brain volume.
Missing values were replaced with data collected between 2012 and 2013, or 2006–2010. After replacement, there were no missing values in alcohol consumption or BMI. The percentage of participants with missing values in physical activity, household income, education, APOE4, diet, sleep duration, and smoking was14·7%, 9·5%, 7·8%, 2·4%, 0·6%, 0·05%, and 0·01%, respectively. Multiple imputations for missing data were conducted, and all covariates were included in the imputation models to create 10 imputed datasets.
Data analyses were conducted using SAS 9·4 for Windows (SAS Institute Inc.) and all P values were two-sided with statistical significance set at <0·05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication.
Results
Population selection and characteristics
Of 502,505 participants with baseline data, those of other ethnic background rather than European ancestry (n = 30,380), with missing values on MRI assessments (n = 433,728), or with prevalent dementia (n = 8), Parkinson's disease (n = 66), multiple sclerosis (n = 132), epilepsy (n = 248), stroke (n = 613), head or neurological injury, or trauma (n = 604), or other chronic neurological problems (n = 79) were excluded from the analysis. A total of 36,647 adults (53·4% females) aged 44–81 years (mean ± standard deviation: 63·7 ± 7·5) were included in the final analysis. Individuals with more diseases were more likely to be older, male, lowly educated, and physically inactive, and to have lower household income and diet quality (Table 1).
Table 1.
Baseline characteristics of participants.
| Number of diseases |
P-value* | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n = 5388) | 1 (n = 8492) | 2 (n = 7808) | 3 (n = 6110) | 4 (n = 4013) | 5 (n = 2330) | ≥6 (n = 2506) | ||
| Age (years)† | 60·7 (60·5–60·9) | 62·2 (62·1–62·4) | 63·5 (63·3–63·7) | 64·7 (64·5–64·8) | 65·6 (65·4–65·8) | 66·4 (66·1–66·6) | 67·4 (67·1–67·7) | <0·0001 |
| Gender | <0·0001 | |||||||
| Women | 59·2 (57·9–60·5) | 55·9 (54·9–57·0) | 53·7 (52·6–54·8) | 51·6 (50·4–52·9) | 50·3 (48·7–51·8) | 47·4 (45·4–49·5) | 46·6 (44·6–48·5) | |
| Men | 40·8 (39·5–42·1) | 44·1 (43·0–45·1) | 46·3 (45·2–47·4) | 48·4 (47·1–49·6) | 49·7 (48·2–51·3) | 52·6 (50·6–54·6) | 53·4 (51·5–55·4) | |
| APOE4‡ | 0·31 | |||||||
| No | 73·7 (72·5–74·9) | 74·5 (73·6–75·4) | 74·4 (73·4–75·3) | 73·9 (72·8–75·0) | 75·4 (74·1–76·7) | 74·9 (73·1–76·7) | 74·5 (72·8–76·3) | |
| Yes | 24·0 (22·9–25·2) | 23·1 (22·2–24·0) | 23·3 (22·4–24·3) | 23·7 (22·6–24·8) | 22·0 (20·7–23·3) | 23·1 (21·3–24·8) | 22·6 (21·0–24·2) | |
| Missing | 2·3 (1·9–2·7) | 2·4 (2·1–2·7) | 2·3 (2·0–2·7) | 2·4 (2·0–2·8) | 2·6 (2·1–3·1) | 2·1 (1·5–2·6) | 2·9 (2·2–3·5) | |
| Education | <0·0001 | |||||||
| 0–5 years | 3·2 (2·7–3·6) | 4·7 (4·2–5·1) | 5·9 (5·4–6·4) | 6·6 (6·0–7·3) | 8·3 (7·5–9·2) | 9·0 (7·9–10·2) | 12·4 (11·1–13·7) | |
| 6–12 years | 43·1 (41·8–44·4) | 45·4 (44·3–46·4) | 46·1 (45·0–47·2) | 48·6 (47·4–49·9) | 47·4 (45·9–48·9) | 49·0 (47·0–51·0) | 50·8 (48·8–52·7) | |
| ≥13 years | 52·4 (51·1–53·8) | 48·7 (47·6–49·8) | 46·4 (45·3–47·6) | 43·1 (41·9–44·4) | 42·3 (40·8–43·9) | 40·0 (38·1–42·0) | 34·6 (32·8–36·5) | |
| Missing | 1·3 (1·0–1·6) | 1·3 (1·0–1·5) | 1·6 (1·3–1·9) | 1·6 (1·3–2·0) | 1·9 (1·5–2·4) | 1·9 (1·4–2·5) | 2·2 (1·7–2·8) | |
| Household income (pounds) | <0·0001 | |||||||
| <18,000 | 7·2 (6·5–7·9) | 8·1 (7·5–8·7) | 10·0 (9·3–10·6) | 10·9 (10·1–11·7) | 11·9 (10·9–12·9) | 14·2 (12·8–15·6) | 18·9 (17·3–20·4) | |
| 18,000–30,999 | 15·4 (14·4–16·3) | 17·4 (16·6–18·2) | 19·7 (18·8–20·6) | 20·9 (19·9–21·9) | 22·5 (21·2–23·8) | 24·8 (23·0–26·5) | 25·1 (23·4–26·8) | |
| 31,000–51,999 | 27·2 (26·0–28·4) | 28·1 (27·2–29·1) | 27·7 (26·7–28·7) | 27·9 (26·8–29·0) | 27·4 (26·1–28·8) | 26·5 (24·7–28·3) | 25·7 (24·0–27·5) | |
| 52,000–100,000 | 31·9 (30·7–33·2) | 28·9 (27·9–29·8) | 26·3 (25·4–27·3) | 24·7 (23·6–25·8) | 22·7 (21·4–24·0) | 19·8 (18·2–21·4) | 16·2 (14·8–17·7) | |
| >100,000 | 10·1 (9·3–10·9) | 8·1 (7·5–8·7) | 6·8 (6·2–7·4) | 5·8 (5·3–6·4) | 5·6 (4·9–6·3) | 4·2 (3·4–5·0) | 3·4 (2·7–4·1) | |
| Unknown | 1·3 (1·0–1·6) | 1·9 (1·6–2·2) | 1·9 (1·6–2·2) | 1·9 (1·5–2·2) | 2·0 (1·6–2·4) | 1·9 (1·3–2·4) | 2·2 (1·6–2·8) | |
| Not answered | 6·9 (6·2–7·6) | 7·6 (7·0–8·2) | 7·6 (7·0–8·2) | 8·0 (7·3–8·7) | 7·8 (7·0–8·7) | 8·7 (7·5–9·8) | 8·5 (7·4–9·6) | |
| Diet score†,§ | 4·27 (4·24–4·31) | 4·23 (4·2–4·26) | 4·16 (4·13–4·19) | 4·15 (4·11–4·18) | 4·07 (4·02–4·11) | 4·04 (3·99–4·09) | 3·95 (3·9–4) | <0·0001 |
| Physical activity (MET-minutes/week)† | 2551 (2492–2610) | 2552 (2503–2600) | 2478 (2430–2526) | 2448 (2392–2504) | 2389 (2322–2457) | 2419 (2329–2510) | 2447 (2354–2540) | <0·0001 |
| Smoking | <0·0001 | |||||||
| Never | 68·6 (67·3–69·8) | 66·1 (65·1–67·1) | 63·5 (62·4–64·5) | 61·2 (60·0–62·4) | 58·8 (57·2–60·3) | 54·5 (52·5–56·5) | 51·0 (49·0–52·9) | |
| Former | 28·1 (26·9–29·3) | 30·4 (29·5–31·4) | 33·3 (32·2–34·3) | 35·4 (34·2–36·6) | 37·7 (36·2–39·2) | 41·6 (39·6–43·6) | 45·8 (43·8–47·7) | |
| Current | 3·3 (2·8–3·8) | 3·5 (3·1–3·9) | 3·3 (2·9–3·7) | 3·4 (2·9–3·8) | 3·6 (3·0–4·2) | 3·9 (3·1–4·7) | 3·3 (2·6–4·0) | |
| Sleep duration (hours/day) | <0·0001 | |||||||
| <7 | 21·1 (20·0–22·1) | 22·0 (21·1–22·8) | 24·0 (23·0–24·9) | 25·0 (23·9–26·1) | 25·6 (24·3–27·0) | 26·1 (24·4–27·9) | 28·0 (26·3–29·8) | |
| 7–9 | 78·5 (77·4–79·6) | 77·4 (76·5–78·3) | 75·0 (74·1–76·0) | 73·7 (72·6–74·8) | 72·6 (71·2–74·0) | 71·9 (70·0–73·7) | 68·7 (66·9–70·5) | |
| >9 | 0·5 (0·3–0·7) | 0·7 (0·5–0·8) | 1·0 (0·8–1·2) | 1·3 (1·0–1·6) | 1·8 (1·4–2·2) | 2·0 (1·5–2·6) | 3·3 (2·6–4·0) | |
| Alcohol consumption | <0·0001 | |||||||
| Never | 2·2 (1·8–2·6) | 2·5 (2·2–2·9) | 2·6 (2·3–3·0) | 2·9 (2·5–3·3) | 3·4 (2·9–4·0) | 2·9 (2·2–3·6) | 4·3 (3·5–5·1) | |
| Previous | 2·1 (1·7–2·4) | 2·3 (1·9–2·6) | 2·9 (2·6–3·3) | 3·4 (3·0–3·9) | 4·4 (3·8–5·0) | 3·9 (3·1–4·7) | 5·6 (4·7–6·5) | |
| Current | 95·8 (95·2–96·3) | 95·2 (94·8–95·7) | 94·5 (94·0–95·0) | 93·7 (93·1–94·3) | 92·2 (91·4–93·0) | 93·2 (92·2–94·2) | 90·1 (88·9–91·3) | |
APOE4, apolipoprotein E ε4; MET, Metabolic equivalent of task.
ANOVA for continuous variables and Chi-square test for categorical variables were used to analyze the difference across the number of diseases.
Data are means (95% confidence intervals). Others are percentages (95% confidence intervals).
APOE4+ dominant model of ε3/ε4 and ε4/ε4 was used to define the presence of APOE4.
Diet score was computed based on seven commonly eaten food groups following recommendations on dietary priorities for cardiometabolic health with a higher score representing healthier diet quality.
Age and brain volumes
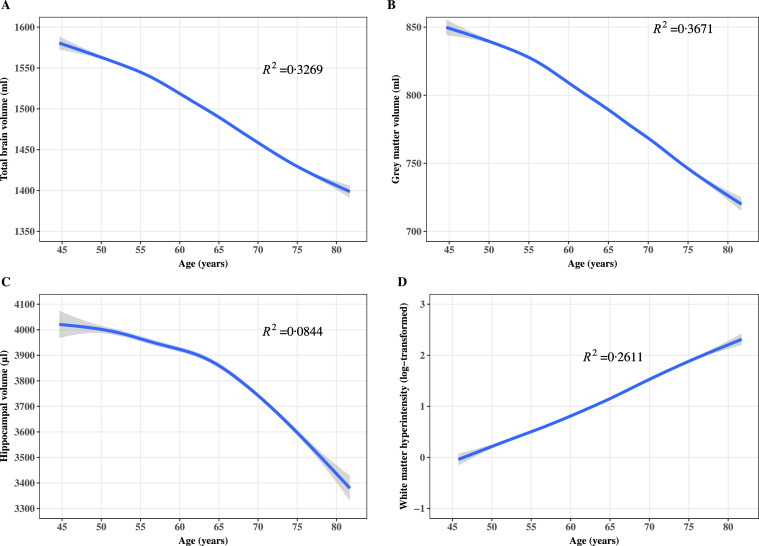
As shown in Figure 1, volumes of total brain (R2=0·3269), grey matter (R2=0·3671), and hippocampus (R2=0·0844) decreased with ageing. There was a sharper decrease in total brain and grey matter volumes after the age of 55 years and a sharper decrease in the hippocampal volume after the age of 65 years. A linear relationship between WMH and age was observed (R2=0·2611).
Figure 1.
Age and volumes of total brain, grey matter, hippocampus, and white matter hyperintensity; Pannels A, B, C, and D show the results for total brain, grey matter, hippocampus, and white matter hyperintensity, respectively. The splines curve method was used to smooth the relationship between age and brain volumes. Blue lines refer to the fitted spline curves and grey ribbons refer to confidence intervals for the fitted lines.
Individual diseases and brain volumes
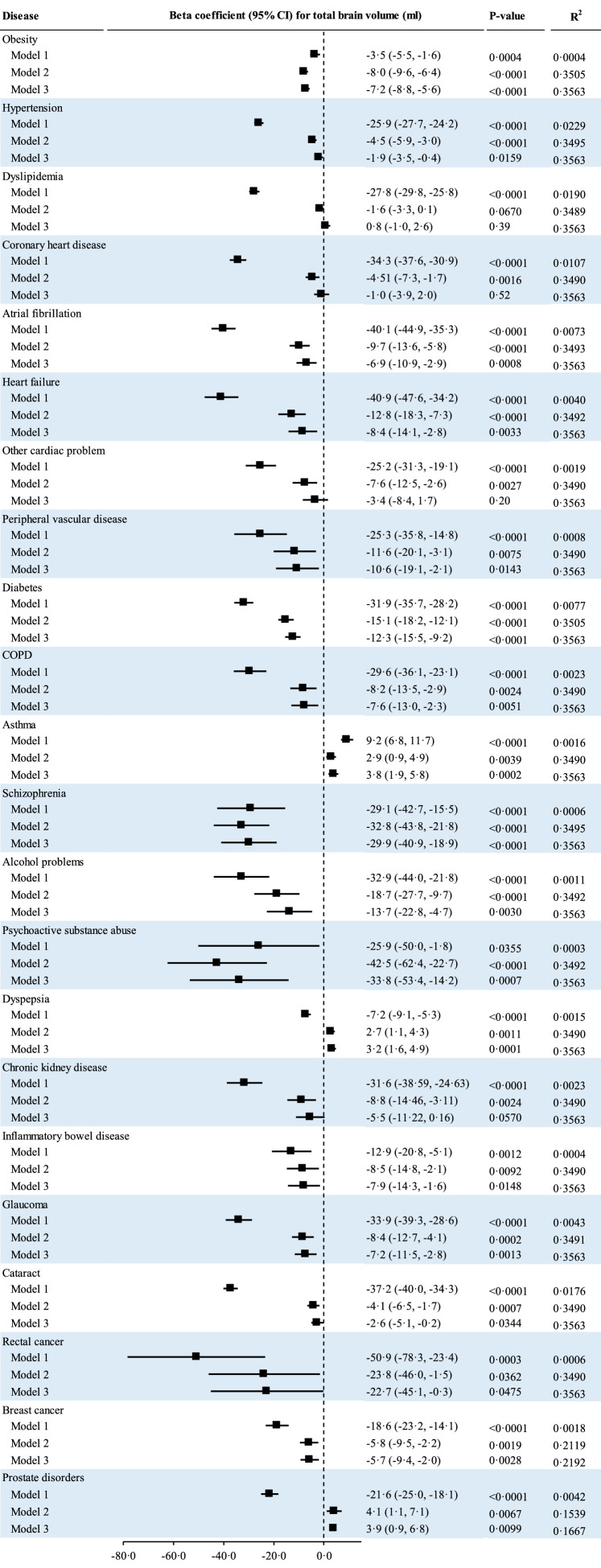
Of 57 observed diseases, 41 were significantly associated with total brain volume after controlling for FDR. Associations for 19 diseases were attenuated to be non-significant after adjustment for age and gender. Associations for 22 other diseases remained significant in the multivariable-adjusted analysis (Model 2). Both prostate disorder and dyspepsia were associated with smaller total brain volume in the unadjusted analysis but with larger total brain volume in the age- and gender-adjusted model. Asthma was associated with larger total brain volume in the unadjusted and multivariable-adjusted models. The remaining 19 diseases were individually associated with smaller total brain volume in unadjusted (Model 1) and multivariable-adjusted models (Model 2). The number of diseases, that were inversely associated with total brain volume, was reduced to 16 after additional adjustment for all other diseases (Model 3). The top five leading contributors to the variance of total brain volume were hypertension (R2=0·0229), dyslipidemia (0·0190), cataract (0·0176), coronary heart disease (CHD) (0·0107), and diabetes (0·0077). Coefficients (95% CIs) for total brain volume (ml) associated with hypertension, dyslipidemia, cataract, CHD, and diabetes were −25·9 (−27·7, −24·2), −27·8 (−29·8, −25·8), −37·2 (−40·0, −34·3), −34·3 (−37·6, −30·9), and −31·9 (−35·7, −28·2), respectively (Figure 2).
Figure 2.
Associations of individual major diseases with total brain volume; CI, confidence interval; COPD, chronic obstructive pulmonary disease; Coefficients for total brain volume associated with individual diseases were estimated using general linear regression models. The raw P-values and R2 for those diseases with significant associations with total brain volume are present in this figure. Model 1 was the unadjusted model; Model 2 was adjusted for age, gender, apolipoprotein E ε4, education, income, smoking, physical acidity, alcohol consumption, sleep duration, and diet; Model 3 was adjusted for Model 2 plus all other diseases for each disease. Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the mean of 0·0.
Potential effect sizes of psychiatric or mental disorders were larger compared with other diseases (Table S5).
Similarly, 18 diseases were individually associated with smaller grey matter volume in both unadjusted (Model 1) and multivariable-adjusted models (Model 2, Table S6). The top five leading contributors to the variance of grey matter volume were hypertension (R2=0·0415), dyslipidemia (0·0286), cataract (0·0188), CHD (0·0166), and diabetes (0·014). After adjustment for all other diseases and covariates, 14 diseases were significantly associated with smaller grey matter volume (Figure S1).
Eleven out of 57 observed diseases were associated with smaller hippocampal volume after adjustment for FDR. This number was reduced to six after the adjustment for all other diseases (Model 3, Table S7, Figure S2).
Eleven diseases were associated with higher WMH volume in both unadjusted (Model 1) and multivariable-adjusted models (Model 2, Table S8). Only four diseases were associated with higher WMH load independent of all other diseases (Model 3). Hypertension (R2=0·0299), and dyslipidemia (0·0125) were leading contributors to the total variance of WMH volume (Figure S3).
Multimorbidity and brain volumes
As shown in Table S9, the six-class model, which yielded the lowest CAIC (19,118·3), was used to identify multimorbidity patterns. Class one, characterized as a lower prevalence of all individual diseases, was labelled as “relatively healthy pattern”. Class two, characterized as a higher prevalence of hearing problems, digestive disorders, and cancer, was labelled as “cancer-degenerative disorders pattern”. Class three, characterized as a higher prevalence of obesity, hypertension, and diabetes, was labelled as “metabolic disorders pattern”. Class four, characterized as a higher prevalence of cardiometabolic disorders ([CMD]: CVD, hypertension, diabetes, high cholesterol, and obesity) and multiple other diseases, was labelled as “CMD-multiple disorders pattern”. Class five, characterized as a higher prevalence of mental disorders, digestive disorders, painful conditions, and multiple other diseases, was labelled as “multiple disorders pattern”. Class six with a higher prevalence of CVD, hypertension, dyslipidemia, and diabetes was labelled as “CMD pattern” (Table S10).
Compared with the relatively healthy multimorbidity pattern, all other patterns were associated with smaller volumes of total brain, grey matter, and hippocampus and higher WMH load. After adjustment for all covariates (Model 3), associations for patterns of CMD, CMD-multiple disorders, and metabolic disorders remained significant. Adjusted β (95% CI) for total brain volume associated with patterns of CMD, CMD plus multiple diseases, and metabolic disorders was −6·6 (−8·9, −4·3) ml, −7·3 (−10·4, −4·1) ml, and −10·4 (−13·5, −7·3) ml, respectively. The corresponding number for grey matter volume was −7·1 (−8·5, −5·7) ml, −9·0 (−10·9, −7·1) ml, and −11·8 (−13·6, −9·9) ml, respectively. Patterns of CMD, CMD-multiple disorders, and metabolic disorders were also associated with higher WMH load (0·23 (0·19, 0·27), 0·25 (0·19, 0·30), and 0·33 (0·27, 0·39), respectively) after adjustment for geographic socioeconomic, and lifestyle factors, and all other diseases (Model 3, all P-values<0·0001). The variance of volumes of total brain, grey matter, hippocampus, and WMH explained by multimorbidity patterns was 0·0120, 0·0186, 0·0012, and 0·0103, respectively (Table 2).
Table 2.
Associations between multimorbidity pattern and brain volumes.
| Relatively healthy pattern* | Cancer-degenerative disorders | Metabolic disorders | CMD-multiple diseases | Multiple disorders | CMD | P-value | R2 | |
|---|---|---|---|---|---|---|---|---|
| Total brain | ||||||||
| Participants | 22036 | 2475 | 1502 | 1482 | 6169 | 2983 | ||
| Volume (ml) | 1504 ± 72 | 1467 ± 70 | 1486 ± 70 | 1463 ± 69 | 1500 ± 72 | 1466 ± 69 | <0·0001 | |
| β (95% CI), Model 1† | Reference | -36·3 (-39·2, -33·3) | -17·3 (-21·0, -13·6) | -40·4 (-44·2, -36·7) | -3·3 (-5·4, -1·3) | -37·6 (-40·3, -34·9) | <0·0001 | 0·0120 |
| β (95% CI), Model 2 | Reference | 1·3 (-1·3, 3·8) | -10·0 (-13·0, -6·9) | -7·0 (-10·2, -3·8) | 1·5 (-0·2, 3·2) | -6·6 (-8·9, -4·2) | <0·0001 | 0·3403 |
| β (95% CI), Model 3 | Reference | 1·5 (-1·0, 4·1) | -10·4 (-13·5, -7·3) | -7·3 (-10·4, -4·1) | 1·4 (-0·3, 3·0) | -6·6 (-8·9, -4·3) | <0·0001 | 0·3459 |
| Grey matter | ||||||||
| Participants | 22036 | 2475 | 1502 | 1482 | 6169 | 2983 | ||
| Volume (ml) | 800 ± 46 | 770 ± 45 | 783 ± 47 | 766 ± 46 | 798 ± 47 | 768 ± 46 | <0·0001 | |
| β (95% CI), Model 1 | Reference | -29·5 (-31·4, -27·6) | -17·3 (-19·7, -14·9) | -34·1 (-36·5, -31·7) | -1·7 (-3·0, -0·4) | -31·8 (-33·5, -30·0) | <0·0001 | 0·0186 |
| β (95% CI), Model 2 | Reference | -0·3 (-1·8, 1·2) | -11·6 (-13·5, -9·8) | -9·2 (-11·1, -7·3) | 0·2 (-0·8, 1·2) | -7·2 (-8·6, -5·8) | <0·0001 | 0·4438 |
| β (95% CI), Model 3 | Reference | -0·1 (-1·6, 1·4) | -11·8 (-13·6, -9·9) | -9·0 (-10·9, -7·1) | 0·2 (-0·8, 1·2) | -7·1 (-8·5, -5·7) | <0·0001 | 0·4503 |
| White matter | ||||||||
| Participants | 22036 | 2475 | 1502 | 1482 | 6169 | 2983 | ||
| Volume (ml) | 704 ± 41 | 697 ± 42 | 704 ± 39 | 697 ± 41 | 702 ± 40 | 698 ± 42 | <0·0001 | |
| β (95% CI), Model 1 | Reference | -6·8 (-8·5, -5·1) | 0·01 (-2·1, 2·1) | -6·3 (-8·5, -4·2) | -1·6 (-2·8, -0·5) | -5·8 (-7·4, -4·3) | <0·0001 | 0·0012 |
| β (95% CI), Model 2 | Reference | 1·6 (-0·1, 3·2) | 1·7 (-0·3, 3·7) | 2·2 (0·2, 4·3) | 1·3 (0·2, 2·4) | 0·7 (-0·9, 2·2) | 0·0333 | 0·1088 |
| β (95% CI), Model 3 | Reference | 1·6 (-0·04, 3·3) | 1·4 (-0·7, 3·4) | 1·7 (-0·3, 3·8) | 1·1 (0·1, 2·2) | 0·5 (-1·0, 2·0) | 0·10 | 0·1114 |
| Hippocampus | ||||||||
| Participants | 22036 | 2475 | 1502 | 1482 | 6169 | 2983 | ||
| Volume (µl) | 3865 ± 436 | 3805 ± 453 | 3835 ± 452 | 3729 ± 440 | 3823 ± 438 | 3797 ± 466 | <0·0001 | |
| β (95% CI), Model 1 | Reference | -60·1 (-78·4, -41·8) | -29·8 (-52·8, -6·8) | -136·2 (-159·4, -113·0) | -42·3 (-54·7, -29·8) | -68·3 (-85·2, -51·5) | <0·0001 | 0·0034 |
| β (95% CI), Model 2 | Reference | -14·7 (-32·3, 2·8) | -20·4 (-41·8, 1·0) | -73·7 (-95·5, -51·9) | -1·5 (-13·1, 10·1) | -38·6 (-54·6, -22·5) | <0·0001 | 0·1412 |
| β (95% CI), Model 3 | Reference | -10·6 (-28·1, 6·8) | -7·3 (-28·7, 14·1) | -52·8 (-74·7, -30·9) | 6·6 (-5·0, 18·2) | -28·4 (-44·5, -12·4) | <0·0001 | 0·1527 |
| WMH‡ | ||||||||
| Participants | 13907 | 1568 | 922 | 938 | 3719 | 1800 | ||
| Volume (ml) | 0·97 ± 0·98 | 1·44 ± 0·96 | 1·38 ± 0·98 | 1·61 ± 0·96 | 1·03 ± 0·99 | 1·55 ± 0·96 | <0·0001 | |
| β (95% CI), Model 1 | Reference | 0·47 (0·42, 0·52) | 0·41 (0·35, 0·48) | 0·65 (0·58, 0·71) | 0·06 (0·02, 0·10) | 0·58 (0·53, 0·63) | <0·0001 | 0·0103 |
| β (95% CI), Model 2 | Reference | 0·04 (-0·00, 0·09) | 0·34 (0·29, 0·40) | 0·27 (0·21, 0·33) | -0·01 (-0·04, 0·03) | 0·24 (0·20, 0·29) | <0·0001 | 0·1736 |
| β (95% CI), Model 3 | Reference | 0·04 (-0·01, 0·08) | 0·33 (0·27, 0·39) | 0·25 (0·19, 0·30) | -0·01 (-0·05, 0·02) | 0·23 (0·19, 0·27) | <0·0001 | 0·1729 |
CI, confidence interval; CMD, cardiometabolic disorders; WMH, white matter hyperintensity.
Multimorbidity patterns were identified using latent class analysis. The six-class model, which yielded the lowest value of adjusted Consistent Akaike Information Criterion, were used to derive multimorbidity patterns in the analysis.
Coefficients for brain volumes associated with multimorbidity patterns were estimated using general linear regression models. Model 1 was the unadjusted model; Model 2 was adjusted for age and gender; Model 3 was adjusted for Model 2 plus APOE4, education, income, smoking, physical acidity, alcohol consumption, sleep duration, and diet quality. Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the mean of 0·0.
WMH was log-transformed in the analysis given its positively skewed distribution.
Multimorbidity defined by the number of major diseases was associated with smaller volumes of total brain, grey matter, and hippocampus and larger WMH volume. Adjusted β (95% CI) for volumes of total brain, grey matter, hippocampus, and WMH associated with multimorbidity (≥ 6 diseases) was −6·5 (−9·3, −3·6) ml, −9·4 (−11·1, −7·6) ml, −31·5 (−51·4, −11·6) µl, and 0·25 (0·19, 0·30) ml, respectively (Model 3). The R2 for total brain, grey matter, hippocampus, and WMH volumes were 0·0305, 0·0474, 0·0073, and 0·0244, respectively (Table 3).
Table 3.
Associations of multimorbidity defined by the number of diseases with brain volumes.
| Number of diseases* |
P-value | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |||
| Total brain | |||||||||
| Participants | 5388 | 8492 | 7808 | 6110 | 4013 | 2330 | 2506 | ||
| Volume (ml) | 1513 ± 71 | 1505 ± 71 | 1497 ± 72 | 1489 ± 72 | 1483 ± 72 | 1476 ± 72 | 1469 ± 71 | ||
| β (95% CI), Model 1† | Reference | −8·3 (−10·8, −5·9) | −16·1 (−18·6, −13·6) | −23·9 (−26·5, −21·3) | −29·9 (−32·8, −27·0) | −37·4 (−40·9, −33·9) | −44·2 (−47·6, −40·8) | <0·0001 | 0·0305 |
| β (95% CI), Model 2 | Reference | 0·3 (−1·7, 2·3) | −0·2 (−2·3, 1·8) | −1·4 (−3·6, 0·8) | −2·0 (−4·5, 0·4) | −5·0 (−7·9, −2·1) | −6·3 (−9·1, −3·4) | <0·0001 | 0·3407 |
| β (95% CI), Model 3 | Reference | 0·2 (−1·9, 2·2) | −0·4 (−2·5, 1·6) | −1·6 (−3·8, 0·6) | −2·2 (−4·6, 0·3) | −5·1 (−8·0, −2·2) | −6·5 (−9·3, −3·6) | <0·0001 | 0·3463 |
| Grey matter | |||||||||
| Participants | 5388 | 8492 | 7808 | 6110 | 4013 | 2330 | 2506 | ||
| Volume (ml) | 807 ± 46 | 801 ± 45 | 794 ± 47 | 788 ± 47 | 783 ± 48 | 778 ± 48 | 770 ± 49 | ||
| β (95% CI), Model 1 | Reference | −6·4 (−8·0, −4·8) | −12·9 (−14·5, −11·3) | −19·0 (−20·8, −17·3) | −23·9 (−25·8, −21·9) | −29·6 (−31·9, −27·4) | −37·1 (−39·3, −34·9) | <0·0001 | 0·0474 |
| β (95% CI), Model 2 | Reference | −0·1 (−1·4, 1·1) | −1·4 (−2·6, −0·1) | −2·8 (−4·1, −1·5) | −3·7 (−5·2, −2·3) | −6·1 (−7·9, −4·3) | −9·6 (−11·3, −7·9) | <0·0001 | 0·4453 |
| β (95% CI), Model 3 | Reference | −0·2 (−1·4, 1·0) | −1·4 (−2·6, −0·1) | −2·8 (−4·1, −1·5) | −3·7 (−5·1, −2·2) | −5·8 (−7·6, −4·1) | −9·4 (−11·1, −7·6) | <0·0001 | 0·4517 |
| White matter | |||||||||
| Participants | 5388 | 8492 | 7808 | 6110 | 4013 | 2330 | 2506 | ||
| Volume (ml) | 706 ± 40 | 704 ± 40 | 703 ± 41 | 701 ± 41 | 700 ± 41 | 698 ± 41 | 699 ± 41 | ||
| β (95% CI), Model 1 | Reference | −1·9 (−3·3, −0·5) | −3·3 (−4·7, −1·8) | −4·9 (−6·4, −3·4) | −6·1 (−7·7, −4·4) | −7·7 (−9·7, −5·8) | −7·2 (−9·1, −5·3) | <0·0001 | 0·0031 |
| β (95% CI), Model 2 | Reference | 0·5 (−0·9, 1·8) | 1·1 (−0·2, 2·5) | 1·4 (−0·1, 2·8) | 1·7 (0·1, 3·3) | 1·1 (−0·8, 3·0) | 3·4 (1·5, 5·2) | 0·0149 | 0·109 |
| β (95% CI), Model 3 | Reference | 0·3 (−1·0, 1·7) | 1·0 (−0·4, 2·3) | 1·2 (−0·3, 2·6) | 1·5 (−0·1, 3·1) | 0·8 (−1·1, 2·7) | 2·9 (1·0, 4·8) | 0·0642 | 0·1115 |
| Hippocampus | |||||||||
| Participants | 5388 | 8492 | 7808 | 6110 | 4013 | 2330 | 2506 | ||
| Volume (µl) | 3888 ± 428 | 3875 ± 434 | 3847 ± 439 | 3820 ± 448 | 3811 ± 452 | 3802 ± 445 | 3746 ± 448 | ||
| β (95% CI), Model 1 | Reference | −12·8 (−27·9, 2·2) | −40·4 (−55·7, −25·1) | −67·5 (−83·7, −51·4) | −76·6 (−94·6, −58·6) | −85·7 (−107·1, −64·3) | −141·2 (−162·1, −120·3) | <0·0001 | 0·0073 |
| β (95% CI), Model 2 | Reference | 5·7 (−8·4, 19·7) | −4·9 (−19·2, 9·4) | −16·8 (−32·0, −1·7) | −12·4 (−29·3, 4·6) | −15·0 (−35·2, 5·2) | −55·0 (−74·8, −35·3) | <0·0001 | 0·1416 |
| β (95% CI), Model 3 | Reference | 9·8 (−4·2, 23·7) | 3·0 (−11·2, 17·3) | −6·5 (−21·7, 8·7) | 0·6 (−16·4, 17·5) | 1·8 (−18·4, 22·0) | −31·5 (−51·4, −11·6) | 0·0019 | 0·1528 |
| WMH‡ | |||||||||
| Participants | 5388 | 8492 | 7808 | 6110 | 4013 | 2330 | 2506 | ||
| Volume (ml) | 0·81 ± 0·96 | 0·98 ± 0·99 | 1·06 ± 0·98 | 1·18 ± 0·98 | 1·28 ± 1·00 | 1·41 ± 0·98 | 1·52 ± 0·98 | ||
| β (95% CI), Model 1 | Reference | 0·17 (0·13, 0·21) | 0·26 (0·21, 0·30) | 0·37 (0·33, 0·42) | 0·47 (0·42, 0·53) | 0·61 (0·54, 0·67) | 0·71 (0·66, 0·77) | <0·0001 | 0·0244 |
| β (95% CI), Model 2 | Reference | 0·06 (0·02, 0·09) | 0·07 (0·03, 0·11) | 0·11 (0·07, 0·15) | 0·15 (0·10, 0·19) | 0·24 (0·18, 0·29) | 0·27 (0·22, 0·33) | <0·0001 | 0·1754 |
| β (95% CI), Model 3 | Reference | 0·05 (0·02, 0·09) | 0·06 (0·02, 0·10) | 0·10 (0·06, 0·14) | 0·14 (0·09, 0·18) | 0·22 (0·17, 0·27) | 0·25 (0·19, 0·30) | <0·0001 | 0·1744 |
CI, confidence interval; WMH, white matter hyperintensity.
Number of diseases was calculated based on the 19 groups of major diseases in Table S3.
Coefficients for brain volumes associated with multimorbidity were estimated using general linear regression models. Model 1 was the unadjusted model; Model 2 was adjusted for age and gender; Model 3 was adjusted for Model 2 plus APOE4, education, income, smoking, physical acidity, alcohol consumption, sleep duration, and diet. Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the mean of 0·0.
WMH was log-transformed in the analysis given its positively skewed distribution.
A higher multimorbidity score was associated with smaller volumes of total brain, grey matter, and hippocampus and higher WMH load. The variance of total brain volume explained by multimorbidity score was 0·0537 (Table 4). In the multivariable-adjusted analysis (Model 3), the coefficient for volumes of total brain, grey matter, hippocampus, and WMH associated with each standard deviation increment of multimorbidity score was −2·6 (−3·3, −2·0) ml, −3·2 (−3·6, −2·8) ml, −12·6 (−17·1, −8·1) µl, and 0·10 (0·09, 0·11) ml respectively. Percentage of variance of volumes of total brain, grey matter, hippocampus, and WMH explained by multimorbidity score was 7·2%, 11·0%, 1·6%, and 7·4%, respectively (Figure S4). There was minimal difference between the results with (Table S11) and without (Table 4) accounting for the variation in beta coefficients for brain volumes associated with 19 individual groups of diseases.
Table 4.
Associations between multimorbidity score and brain volumes.
| Multimorbidity score* |
P-value | R2 | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | for trend | ||
| Total brain | |||||||
| Participants | 8295 | 6332 | 7205 | 7475 | 7340 | ||
| Volume (ml) | 1516 ± 71 | 1508 ± 71 | 1498 ± 78 | 1486 ± 70 | 1468 ± 70 | ||
| β (95% CI), Model 1† | Reference | −8·1 (−10·5, −5·8) | −17·3 (−19·5, −15·1) | −30·0 (−32·3, −27·8) | −47·9 (−50·1, −45·7) | <0·0001 | 0·0537 |
| β (95% CI), Model 2 | Reference | 0·7 (−1·3, 2·6) | −0·8 (−2·6, 1·1) | −2·3 (−4·2, −0·4) | −6·1 (−8·0, −4·1) | <0·0001 | 0·3408 |
| β (95% CI), Model 3 | Reference | 0·5 (−1·5, 2·4) | −0·8 (−2·6, 1·1) | −2·4 (−4·3, −0·4) | −6·2 (−8·2, −4·2) | <0·0001 | 0·3464 |
| Grey matter | |||||||
| Participants | 8295 | 6332 | 7205 | 7475 | 7340 | ||
| Volume (ml) | 809 ± 46 | 800 ± 46 | 796 ± 47 | 786 ± 46 | 773 ± 47 | ||
| β (95% CI), Model 1 | Reference | −8·5 (−10·0, −7·0) | −12·8 (−14·2, −11·3) | −22·4 (−23·8, −20·9) | −35·5 (−37·0, −34·0) | <0·0001 | 0·0652 |
| β (95% CI), Model 2 | Reference | −0·5 (−1·7, 0·7) | −1·3 (−2·5, −0·2) | −3·4 (−4·5, −2·2) | −7·7 (−8·9, −6·5) | <0·0001 | 0·4453 |
| β (95% CI), Model 3 | Reference | −0·6 (−1·8, 0·5) | −1·2 (−2·4, −0·1) | −3·4 (−4·5, −2·2) | −7·6 (−8·8, −6·4) | <0·0001 | 0·4518 |
| Hippocampus | |||||||
| Participants | 7617 | 6222 | 7864 | 7567 | 7364 | ||
| Volume (µl) | 3898 ± 432 | 3887 ± 436 | 3859 ± 442 | 3820 ± 438 | 3749 ± 444 | ||
| β (95% CI), Model 1 | Reference | −10·9 (−25·6, 3·8) | −39·2 (−53·0, −25·3) | −78·10–92·1, −64·2) | −149·0 (−163·0, −134·9) | <0·0001 | 0·0136 |
| β (95% CI), Model 2 | Reference | 5·8 (−7·9, 19·6) | −0·3 (−13·3, 12·67) | −5·4 (−18·7, 7·9) | −32·8 (−46·6, −19·1) | <0·0001 | 0·1414 |
| β (95% CI), Model 3 | Reference | 7·8 (−5·9, 21·4) | 3·7 (−9·2, 16·7) | 0·7 (−12·6, 14·0) | −20·7 (−34·4, −6·9) | 0·0006 | 0·1528 |
| WMH‡ | |||||||
| Participants | 5470 | 3578 | 4734 | 4568 | 4504 | ||
| Volume (ml) | 0·83 ± 0·97 | 0·89 ± 0·95 | 1·02 ± 0·98 | 1·24 ± 0·98 | 1·53 ± 0·96 | ||
| β (95% CI), Model 1 | Reference | 0·06 (0·02, 0·10) | 0·18 (0·15, 0·22) | 0·41 (0·37, 0·45) | 0·69 (0·65, 0·73) | <0·0001 | 0·0600 |
| β (95% CI), Model 2 | Reference | 0·04 (0·01, 0·08) | 0·06 (0·03, 0·09) | 0·14 (0·10, 0·17) | 0·29 (0·26, 0·33) | <0·0001 | 0·2694 |
| β (95% CI), Model 3 | Reference | 0·04 (−0·00, 0·07) | 0·05 (0·02, 0·09) | 0·13 (0·09, 0·16) | 0·28 (0·24, 0·31) | <0·0001 | 0·2718 |
CI, confidence interval; WMH, white matter hyperintensity.
Multimorbidity score for brain volumes was calculated based on the estimates for 19 individual groups of diseases.
General linear regression models were used to examine associations between multimorbidity score and brain volumes. Model 1 was the unadjusted model; Model 2 was adjusted for age and gender; Model 3 was adjusted for Model 2 plus APOE4, education, income, smoking, physical acidity, alcohol consumption, sleep duration, and diet.
WMH was log-transformed in the analysis given its skewed distribution.
Moderation analysis
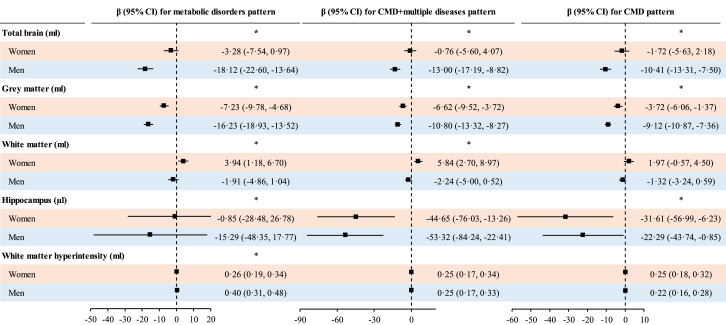
Associations between CMD-multiple disorders, metabolic disorders multimorbidity patterns and volumes of total brain, and grey matter were stronger in men than in women (Figure 3). The association between CMD-multiple disorders pattern and total brain volume was stronger in younger than in older individuals. Similar results were observed for metabolic disorders pattern and WMH load (Figure S5).
Figure 3.
Interaction between multimorbidity patterns and gender for brain volume; CI, confidence interval; CMD, cardiometabolic disorder.; General linear regression models were used to examine associations between multimorbidity patterns and brain volumes stratified by gender. Analysis was adjusted for age, apolipoprotein E ε4, education, income, diet, smoking, physical acidity, alcohol consumption, and sleep duration. Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the mean of 0·0. *Indicates significant interaction.
Associations between multimorbidity and total brain and WMH volumes were stronger in younger than in older individuals. Associations between multimorbidity and volumes of total brain and grey matter were stronger in men than in women. A stronger association between multimorbidity and total brain volume was observed among those with lower education (Table S12). No significant interaction between multimorbidity and APOE4 was found (not shown).
Sensitivity analysis
As shown in Table S13, there was minimal difference in the coefficients for WMH associated with multimorbidity score between the two calculation methods (linear and OLS regression models). The results among individuals with complete data (Table S14) and for individual diseases diagnosed from zero to five years before MRI assessment using inpatient data only (Table S15) were similar to the main findings. Similar results for analysis using the inverse probability weighting method were also observed (Table S16).
Discussion
We found obesity, diabetes, hypertension, high cholesterol, and depression were associated with smaller brain volumes. In addition to these conventional diseases, CVD (heart failure, atrial fibrillation, other cardiac diseases, and peripheral vascular disease), mental/psychiatric disorders (alcohol problems, psychoactive substance abuse, and schizophrenia), chronic obstructive pulmonary disease (COPD), CKD, breast cancer, rectal cancer, and some ocular diseases were associated with smaller brain volumes independent of all other diseases. CMD-related multimorbidity patterns may confer risk to reductions in brain volume. Associations between multimorbidity and brain volumes were stronger in men than in women, in younger than in older individuals, and in lowly educated than in highly educated individuals.
Total and regional brain volumes have been shown to be reliable predictors of Alzheimer's disease (AD).3 Hippocampal volume loss is associated with accelerated memory decline and shows higher diagnostic accuracy for AD than other regional brain volumes do.3 Recent research has demonstrated that grey matter measures may be used to track disease progression in AD.25 Whilst higher WMH load is associated with an increased risk of cognitive impairment and dementia.26 Therefore, identifying important determinants for these brain volumes may help prevent and screen dementia/AD.
As brain volumes are highly related to age,2 age-related chronic diseases may be associated with brain MRI markers. A cross-analysis has demonstrated that a greater number of vascular risk factors was associated with greater brain atrophy, and poorer white matter health,5 however, a small proportion of the variance (≤1·8%) can be accounted for by these vascular risk factors. Our findings agree with this study showing that CMDs including obesity, dyslipidemia, hypertension, and diabetes are associated with smaller total brain, grey matter, and hippocampal volumes, and higher WMH load. In addition, other CVDs such as atrial fibrillation, heart failure, other cardiac diseases, and peripheral vascular disease are associated with smaller brain volumes. The potentially harmful effect of CMD on brain damage may be partly explained by the fact that the reduction of blood flow and increase of cerebrovascular reactivity in participants with CMD27 may result in cerebral vasculature and brain damage. Multiple psychiatric disorders were linked to smaller volumes of total brain, grey matter, or hippocampus in our study. Likely, previous evidence has shown that high alcohol consumption,28 psychoactive substance abuse,29 and schizophrenia30 were risk factors for brain damage. It has been inconsistent regarding associations between depression, anxiety, and brain volumes in previous studies,31,32 which may be attributed to the age difference between individuals with depression and controls. In our analysis matched by age and gender, depression was associated with smaller volumes of total brain and grey matter and higher WMH load (not shown). Our findings underline the importance of CMD and psychiatric disorders in brain health.
Data on associations between other chronic diseases and brain structure are limited. A cross-sectional analysis of 30 patients with COPD and 30 controls demonstrated that COPD was associated with smaller hippocampal volumes but not significantly associated with total brain, or grey matter volume.10 We found COPD was associated with smaller volumes of grey matter and hippocampus and higher WMH load. Our analysis is also consistent with a previous study demonstrating that CKD was associated with greater brain atrophy.11 Although hearing impairment was among the leading risk factors for dementia,33 the association between hearing impairment and brain volumes was attenuated to be non-significant after adjustment for age. In contrast, we found cataract and glaucoma were associated with smaller brain volumes independent of covariates (including all other diseases). These ocular diseases have been linked to dementia risk in a growing number of studies.34 Our study confirmed potential emerging risk factors for reductions in brain volume with inconsistent findings in previous studies. We found asthma, and prostate disorders (not cancer) were also associated with larger white matter volume but not grey matter volume. Whilst white matter was not a reliable indicator of neurological disorders,3 which suggests that asthma and prostate disorders might not be beneficial for brain and cognitive health. Previous studies reported a weak inverse association between cancer and AD,35 which might be attributed to shared inverse etiological mechanisms. However, our study is consistent with several studies of small sample sizes demonstrating that breast cancer was associated with smaller brain volumes.36,37 The inverse association between rectal cancer and brain volumes observed in our study needs to be confirmed by longitudinal studies.
Although more vascular risk factors have been linked to greater brain atrophy,5 little is known regarding associations between multimorbidity and brain volumes. A recent study has shown that 1·8% of the variance of WMH was explained by vascular risk factors,5 whilst we found that a much larger proportion of variance in WMH (6·0%) was attributed to multimorbidity score. This suggests, in addition to CMD, psychiatric disorders, COPD, CKD, some cancers, and eye diseases also play an important role in brain health. It is not surprising that CMD-related multimorbidity patterns are significantly associated with smaller brain volumes in our analysis. Associations of multimorbidity patterns of CMD-multiple diseases, and metabolic disorders with brain volumes were stronger in men than in women. The smaller effect size of multimorbidity on brain atrophy in women may be due to the fact that women with multimorbidity are more likely to accommodate healthy lifestyle habits and to seek health care than men with multimorbidity. We also found a stronger association between multimorbidity defined by the number of major diseases and brain volume was observed in younger than in older adults. This is consistent with previous studies showing that metabolic disorders diagnosed at a younger age were associated with a larger excessive relative risk for dementia.5,38 We also found the association between multimorbidity and total brain volume was stronger among individuals with lower education. This is in line with previous studies showing that individuals with higher education are more likely to seek health care and less likely to lose brain volume with ageing.33,39
Although multimorbidity or its risk score provides a larger amount of variance in brain volumes compared with previous studies,5,6 the variance explained is still relatively small. Age alone plays a detrimental role in the variance of brain volume. We found associations between numerous diseases examined and brain volumes were attenuated to be non-significant after adjustment for age. Associations with other diseases remained significant, but effect sizes were substantially reduced. The effect size for multimorbidity was even reduced from large to small scale when age and gender were adjusted for. Further analysis showed the changes were largely attributed to age (not shown). This may be partly explained by the fact that both major diseases and brain volumes are highly related to age. Brain atrophy increases with age, whilst individual major diseases and multimorbidity may accelerate this rate. Age and major diseases together may be used to identify individuals at higher risk of reductions in brain volume.
To our knowledge, this study is the first to examine associations between a wide range of diseases and their multimorbidity patterns and brain volumes. This study has several potential limitations. Firstly, because of the cross-sectional design, causal relationships cannot be established based on our findings. Secondly, a relatively small proportion of variance in brain volumes was explained by multimorbidity in our study, therefore, other important determinants are needed to explore in future studies. Thirdly, our analysis was restricted to a sub-cohort of UK Biobank with MRI data. Individuals with MRI data were more likely to be younger, male, highly educated, and never smokers and more likely to have higher household income and diet quality compared to those without MRI data (Table S17). Thus, our findings may not be generalized to the whole UK population. Finally, older adults were less likely to be included in this analysis because of mortality, which might have biased the associations.
In conclusion, we found more than 20 well-known and emerging diseases that are associated with smaller brain volumes. CMD-related multimorbidity patterns are associated with a reduction in brain volumes. Both multimorbidity defined by the number of diseases and multimorbidity score are strongly predictive of brain volumes. Men or younger adults with multimorbidity are more in need of care for the prevention of reductions in brain volume.
Contributors
XS, and MH conceived and designed the study. XS conducted data analysis, performed data interpretation and drafted the initial manuscript. XS, XLZ, YH, ZZ, XYZ, JL, HY, WW, ZG, ST, XY, and MH made critical revisions of the manuscript for important intellectual content. All authors read the manuscript and approved the final draft. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. XS ZZ, and WW had accessed and verified the data.
Data sharing statement
Data are available in a public, open access repository (https://www.ukbiobank.ac.uk/).
Funding
The Fundamental Research Funds of the State Key Laboratory of Ophthalmology, Project of Investigation on Health Status of Employees in Financial Industry in Guangzhou, China (Z012014075), Science and Technology Program of Guangzhou, China (202,002,020,049).
Declaration of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Acknowledgments
This research was conducted using the UK Biobank resource. We thank the participants of the UK Biobank. HY receives support from the National Natural Science Foundation of China (81870663, 82171075), the Outstanding Young Talent Trainee Program of Guangdong Provincial People's Hospital (KJ012019087), Guangdong Provincial People's Hospital Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province (KJ012019457), Talent Introduction Fund of Guangdong Provincial People's Hospital (Y012018145). MH receives support from the University of Melbourne at Research Accelerator Program and the CERA Foundation. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101413.
Contributor Information
Xianwen Shang, Email: andy243@126.com.
Mingguang He, Email: mingguang_he@yahoo.com.
Appendix. Supplementary materials
References
- 1.United Nations Department of Economic and Social Affairs, Population Division . United Nations Department of Economic and Social Affairs; New York: United Nations: 2020. World Population Ageing 2020 Highlights: Living arrangements of Older Persons.https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd-2020_world_population_ageing_highlights.pdf Accessed 23 September 2020. [Google Scholar]
- 2.Hou Y., Dan X., Babbar M., et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi G., Crescioli G., Cavedo E., et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database Syst Rev. 2020;3(3) doi: 10.1002/14651858.CD009628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekkers I.A., Jansen P.R., Lamb H.J. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology. 2019;291(3):763–771. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 5.Cox S.R., Lyall D.M., Ritchie S.J., et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290–2300. doi: 10.1093/eurheartj/ehz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmaal L., Veltman D.J., van Erp T.G., et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2016;21(6):806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opel N., Goltermann J., Hermesdorf M., Berger K., Baune B.T., Dannlowski U. Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega- and meta-analytical findings from the ENIGMA consortium. Biol Psychiatry. 2020;88(9):678–686. doi: 10.1016/j.biopsych.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Shang X., Zhu Z., Zhang X., et al. Association of a wide range of chronic diseases and apolipoprotein E4 genotype with subsequent risk of dementia in community-dwelling adults: a retrospective cohort study. eClinicalMedicine. 2022;45 doi: 10.1016/j.eclinm.2022.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elobeid A., Libard S., Leino M., Popova S.N., Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol. 2016;75(4):316–325. doi: 10.1093/jnen/nlw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esser R.W., Stoeckel M.C., Kirsten A., et al. Structural brain changes in patients with COPD. Chest. 2016;149(2):426–434. doi: 10.1378/chest.15-0027. [DOI] [PubMed] [Google Scholar]
- 11.Tsuruya K., Yoshida H. Brain atrophy and cognitive impairment in chronic kidney disease. Contrib Nephrol. 2018;196:27–36. doi: 10.1159/000485694. [DOI] [PubMed] [Google Scholar]
- 12.Barroso J., Vigotsky A.D., Branco P., et al. Brain gray matter abnormalities in osteoarthritis pain: a cross-sectional evaluation. Pain. 2020;161(9):2167–2178. doi: 10.1097/j.pain.0000000000001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Hassen C., Fayosse A., Landré B., et al. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-068005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grande G., Marengoni A., Vetrano D.L., et al. Multimorbidity burden and dementia risk in older adults: the role of inflammation and genetics. Alzheimer's Dement J Alzheimer's Assoc. 2021;17(5):768–776. doi: 10.1002/alz.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Schulz K.F., Simera I., Altman D.G. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2) doi: 10.1371/journal.pmed.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S.M., Zhang Y., Jenkinson M., et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 18.Alfaro-Almagro F., Jenkinson M., Bangerter N.K., et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrie J.E., Shipley M.J., Cappuccio F.P., et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser. 1995;57:289–300. [Google Scholar]
- 22.Olaya B., Moneta M.V., Caballero F.F., et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC Geriatr. 2017;17(1):186. doi: 10.1186/s12877-017-0586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Hernán M.A., Robins J.M. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dicks E., Vermunt L., van der Flier W.M., Barkhof F., Scheltens P., Tijms B.M. Grey matter network trajectories across the Alzheimer's disease continuum and relation to cognition. Brain Commun. 2020;2(2):fcaa177. doi: 10.1093/braincomms/fcaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debette S., Schilling S., Duperron M.G., Larsson S.C., Markus H.S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76(1):81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferson A.L. Midlife consequences of cumulative blood pressure exposure: importance of a lifespan approach. Circulation. 2020;141(9):725–727. doi: 10.1161/CIRCULATIONAHA.120.044447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topiwala A., Allan C.L., Valkanova V., et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. doi: 10.1136/bmj.j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zehra A., Burns J., Liu C.K., et al. Cannabis addiction and the brain: a review. J Neuroimmune Pharmacol. 2018;13(4):438–452. doi: 10.1007/s11481-018-9782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Erp T.G., Hibar D.P., Rasmussen J.M., et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frodl T., Jäger M., Smajstrlova I., et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- 32.Irie F., Masaki K.H., Petrovitch H., et al. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia aging study. Arch Gen Psychiatry. 2008;65(8):906–912. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):P413–P446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang X., Zhu Z., Wang W., Ha J., He M. The association between vision impairment and incidence of dementia and cognitive impairment: a systematic review and meta-analysis. Ophthalmology. 2021;128(8):1135–1149. doi: 10.1016/j.ophtha.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Ospina-Romero M., Glymour M.M., Hayes-Larson E., et al. Association between alzheimer disease and cancer with evaluation of study biases: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppelmans V., de Ruiter M.B., van der Lijn F., et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 37.Chen B.T., Sethi S.K., Jin T., et al. Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res. 2018;20(1):38. doi: 10.1186/s13058-018-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang X., Hill E., Zhu Z., et al. The association of age at diagnosis of hypertension with brain structure and incident dementia in the UK Biobank. Hypertension. 2021;78(5):1463–1474. doi: 10.1161/HYPERTENSIONAHA.121.17608. [DOI] [PubMed] [Google Scholar]
- 39.Brayne C., Ince P.G., Keage H.A., et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.