Abstract
This article describes data related to the research paper entitled “ROMP of norbornene and oxanorbornene derivatives with pendant fluorophore carbazole and coumarin groups” [1]. Six novel norbornene and oxanorbornene dicarboximides derivatives functionalized with carbazole or coumarin moieties, are synthesized and investigated in the preparation of fluorescent polymers by Ring-Opening Metathesis Polymerization (ROMP). Herein, we report on the characterization of all these compounds by 1D and 2D Nuclear Magnetic Resonance (NMR), UV-Visible and fluorescence spectroscopy. The characterization data include information obtained from 1H, 13C, Homonuclear Correlation Spectroscopy (1H–1H COSY) and Heteronuclear Single Quantum Coherence (1H–13C HSQC). The absorbence and fluorescence spectra for all these compounds are given. This work provides useful characterization data for the design of new norbornene and oxanorbornene-based monomers with fluorescent carbazole and coumarin groups, which can be employed for the synthesis of functional materials via ROMP.
Keywords: NMR spectroscopy, Carbazole and coumarin groups, UV-Vis, Fluorescence, ROMP
Specifications Table
| Subject | Chemistry |
| Specific subject area | Organic and polymer chemistry, spectroscopic characterization |
| Type of data | Table Figure |
| How the data were acquired | NMR spectra were recorded on an NMR Bruker ASCEND 600 spectrometer or 400 MHz AVANCE spectrometer. Processing with TopSpin 4.1.0 software. UV–Vis measurements were performed by a Varian Cary 50 spectrophotometer and fluorescent measurements by a Varian Cary Eclipse spectrophotometer. Precision cell made of quartz SUPRASIL was used to house the samples. All figures of UV–Vis and emission spectra were generated using Origin 7.0 software. |
| Data format | Raw and analyzed |
| Description of data collection | NMR of all the compounds were measured in CDCl3. Chemical shifts were shown as δ-values and expressed in ppm with reference to tetramethylsilane (TMS) as an internal chemical shift reference. absorbence and fluorescence spectra were measured in tetrahydrofuran inhibitor-free, HPLC grade, ≥99.9% (UV-absorbence ≤0.02 at 300 nm) for solutions of carbazole compounds (1 × 10−4 M), and in Chloroform HPLC grade, ≥99.9% (UV-absorbence ≤0.01 at 400 nm, 0.01 at 290 nm, 0.01 at 270 nm) for solutions of coumarin compounds (1 × 10−6 M). |
| Data source location | Dipartimento di Chimica e Biologia “Adolfo Zambelli”, Università di Salerno, Via Giovanni Paolo II 132, I-84,084 Fisciano, Salerno, Italy. Latitude: 40° 46′ 9.59″ N Longitude: 14° 47′ 15.59″ E 40.769330256 14.787663516. Istituto di Scienze e Tecnologie Chimiche “G. Natta” (SCITEC), Consiglio Nazionale delle Ricerche, Via A. Corti, 12, 20,133 Milano, Italy Latitude: 45° 48′ 05.70″ N Longitude: 9° 23′ 18.20″ E 45.48065 9.23166 |
| Data accessibility | Repository name: Mendeley Data Data identification number (DOI): 10.17632/h3khzrtcg7.1 Direct URL to data: https://data.mendeley.com/datasets/h3khzrtcg7/1 |
| Related research article | R. Troiano, M. Carratù, S. Pragliola, A. C. Boccia, Fabia Grisi, ROMP of norbornene and oxanorbornene derivatives with pendant fluorophore carbazole and coumarin groups, Eur. Polym. J. 167 (2022) 111,065. 10.1016/j.eurpolymj.2022.111065 |
Value of the Data
-
•
The following data serve as characterization of six norbornene and oxanorbornene dicarboximide derivatives, presenting carbazole and coumarin as fluorophore pendant groups, that have great potential in materials sciences.
-
•
The comparison with various other ROMP monomers presenting similar fluorophore groups can help in understanding the role of the structure of the polymerizable unit in influencing fluorescence properties.
-
•
The data can be useful to researchers interested in the design of fluorescent norbornene and oxanorbornene-based monomers for the synthesis via ROMP of functional materials.
-
•
The data can be used for further modification of the structure norbornene and oxanorbornene monomers towards improved photoluminescence properties.
1. Data Description
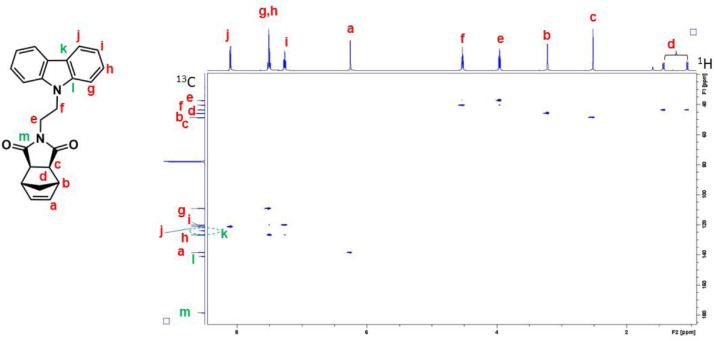
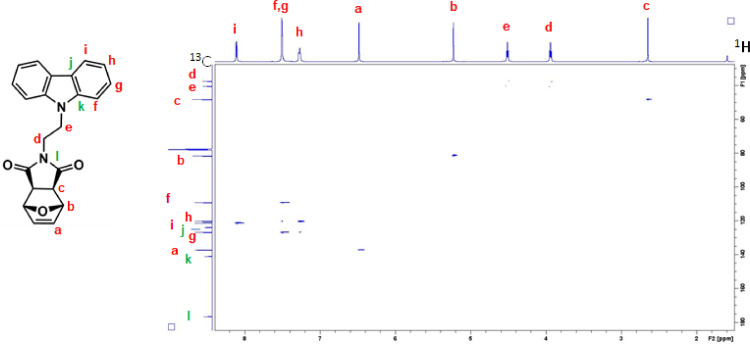
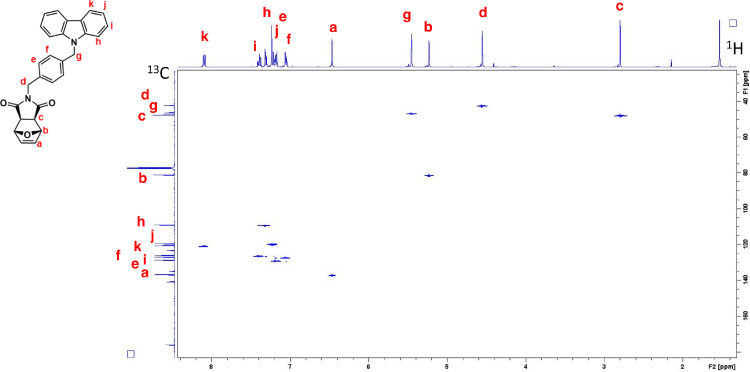
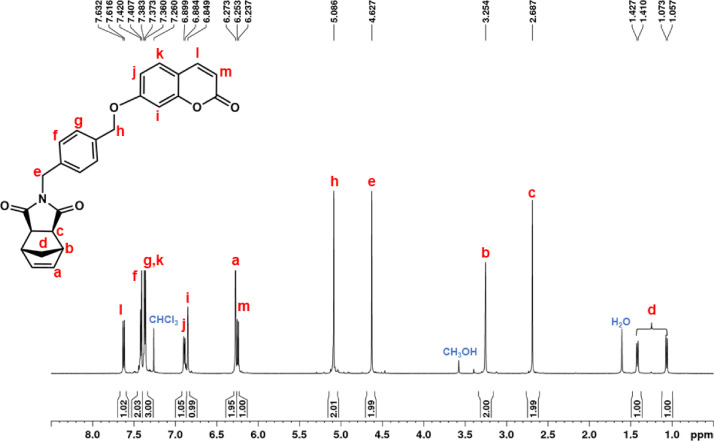
Six norbornene and oxanorbornene dicarboximide monomers containing carbazole and coumarin group were synthesized [1]. These compounds were characterized by using 1D and 2D solution NMR spectroscopy, UV-Visible absorption and photoluminescence spectroscopy, mass spectrometry and DSC. For each monomer, complete assignments of resonances in the 1H and 13C NMR spectra were obtained by means of homonuclear Correlation Spectroscopy (1H–1H COSY) and Heteronuclear single quantum coherence spectroscopy (1H–13C HSQC) experiments. 1H–1H COSY spectrum is an experiment of homonuclear correlation amongst protons that are coupled to each other and allows to define a spin system. Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons. With this experiment is possible establish who is near to whom. 1H–13C HSQC spectrum is an experiment of heteronuclear correlation amongst protons and carbons through single bond correlation. It is a very informative experiment as, the presence of a cross peak in the spectrum indicates which proton is directly bonded to which carbon.The 1H, 13C, 1H–1H COSY and 1H–13C HSQC spectra of all the monomers are presented, along with their structure showing the alphabetical labels used to assign the NMR data summarized in Table 1. Therefore, Figs. 1–4 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC NMR spectra of 2-(2-(9H-carbazol-9-yl)ethyl)−3a,4,7,7a-tetrahydro-1H-4,7 methanoisoindole-1,3(2H)-dione (NDI-EtCAR); Figs. 5–8 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC spectra of 2-(2-(9H-carbazol-9-yl)ethyl)−3a,4,7,7a-tetrahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione (ONDI-EtCAR); Figs. 9–12 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC spectra of 2-(4-((9H-carbazole-9-yl)methyl)benzyl)−3a,4,7,7a-tetrahydro-1H-4,7 methanoisoindole-1,3(2H)-dione (NDI-XyCAR); Figs. 13–16 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC spectra of 2-(4-((9H-carbazol-9-yl)methyl)benzyl)−3a,4,7,7a-tetrahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione (ONDI-XyCAR); Figs. 17–20 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC spectra of 3-methylene-2-(2-((2-oxo-2H-chromen-7-yl)oxy)ethyl)−2,3,3a,4,7,7a hexahydro-1H-4,7-methanoisoindol-1-one (NDI-EtCOU); Figs. 21–24 show 1H, 13C, 1H–1H COSY, and 1H–13C HSQC spectra of 2-(4-(((2-oxo-2H-chromen-7-yl)oxy) methyl)benzyl)−3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione (NDI-XyCOU).
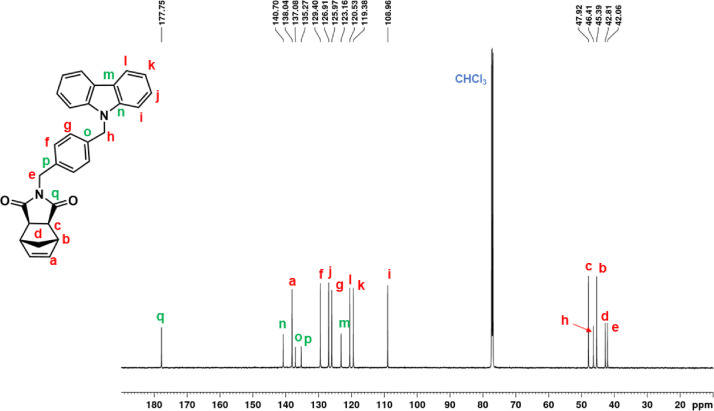
Fig. 2.
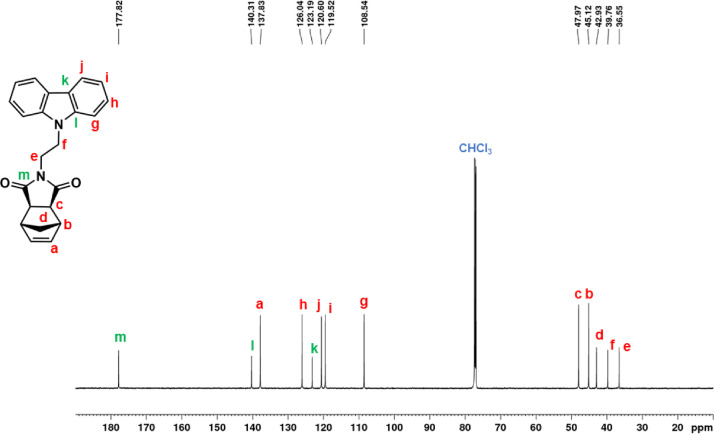
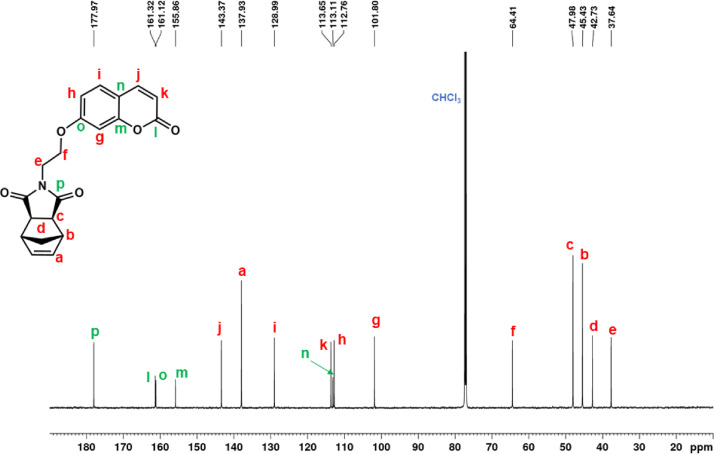
13C NMR spectrum of NDI-EtCAR (CDCl3, 150 MHz).
Fig. 3.
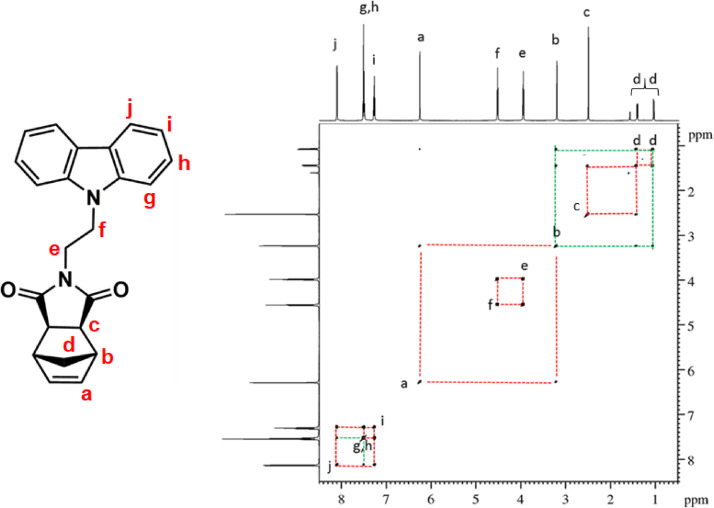
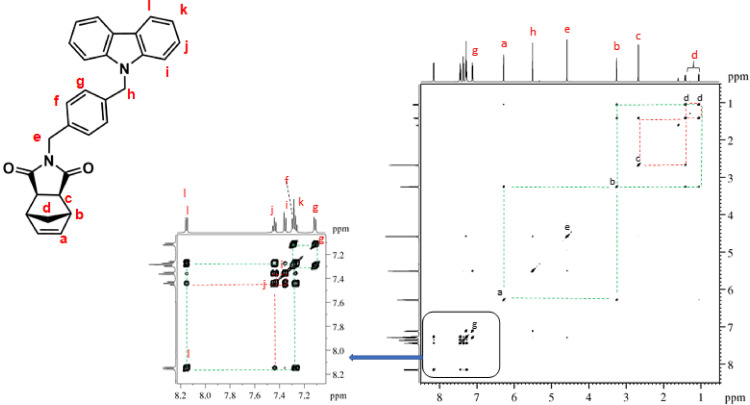
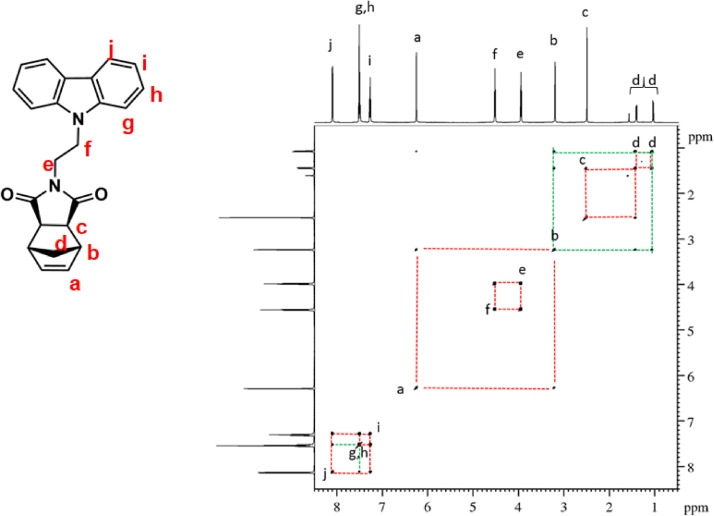
1H–1H COSY NMR spectrum of NDI-EtCAR (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Fig. 6.
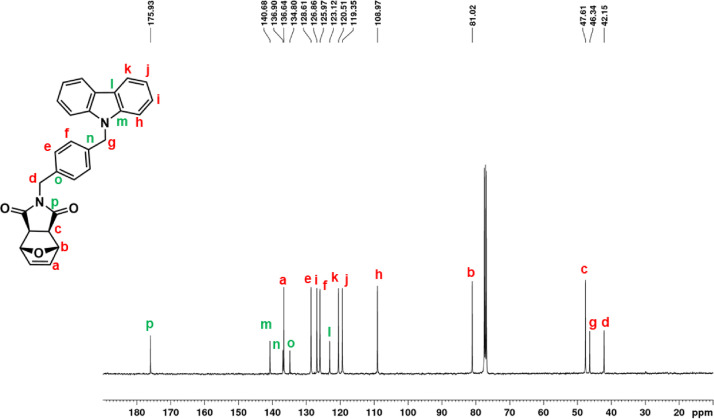
13C NMR spectrum of ONDI-EtCAR (CDCl3, 150 MHz).
Fig. 7.
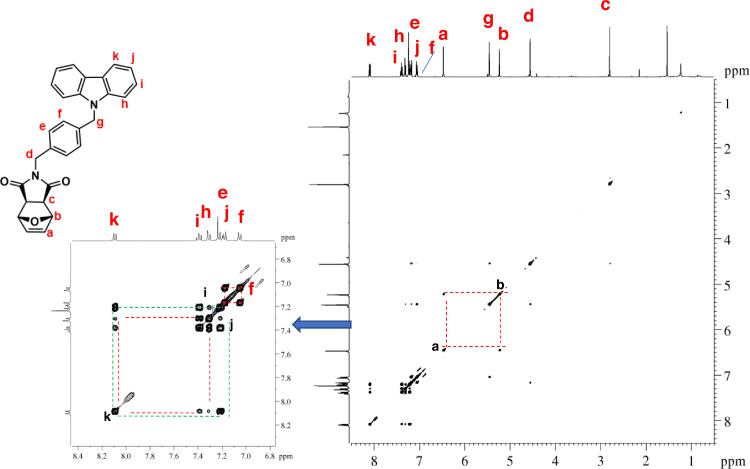
1H–1H COSY NMR spectrum of ONDI-EtCAR (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Fig. 10.
13C NMR spectrum of NDI-XyCAR (CDCl3, 150 MHz).
Fig. 11.
1H–1H COSY NMR spectrum of NDI-XyCAR (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Fig. 14.
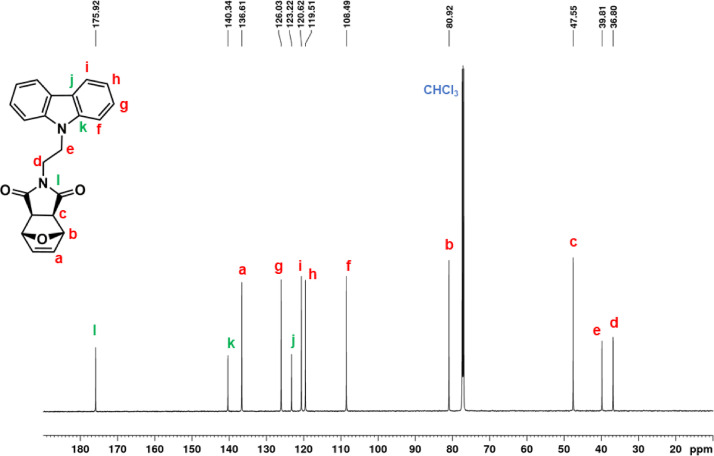
13C NMR spectrum of ONDI-XyCAR (CDCl3, 100 MHz).
Fig. 15.
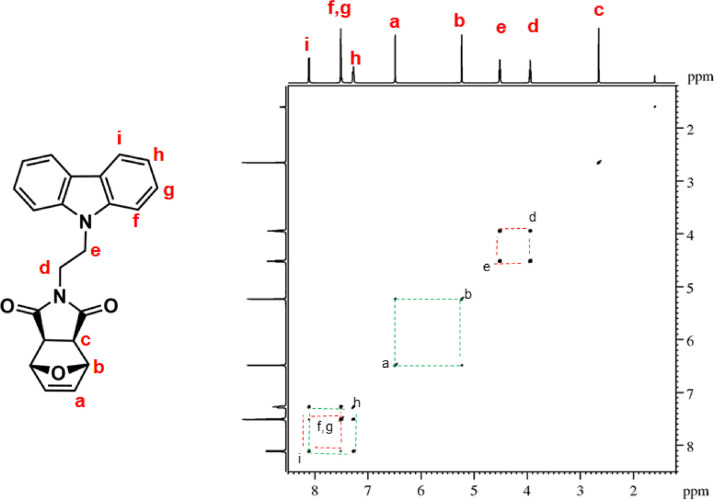
1H–1H COSY NMR spectrum of ONDI-XyCAR (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Fig. 18.
13C NMR spectrum of NDI-EtCOU (CDCl3, 150 MHz).
Fig. 19.
1H–1H COSY NMR spectrum of NDI-EtCOU (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Fig. 22.
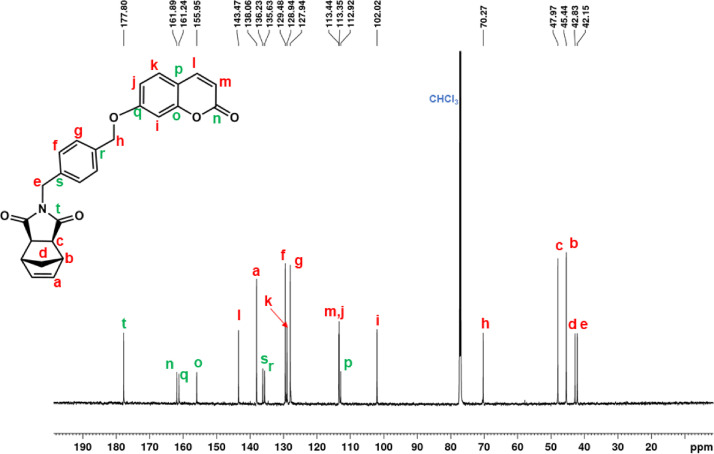
13C NMR spectrum of NDI-XyCOU (CDCl3, 150 MHz).
Fig. 23.
1H–1H COSY NMR spectrum of NDI-XyCOU (CDCl3, 600 MHz). Diagonal peaks refer to the resonances in the 1H NMR spectrum, while the cross peaks indicate the coupled protons defining a spin system.
Table 1.
1H and 13C NMR data of NDI and ONDI compounds in CDCl3 at 25 °C.
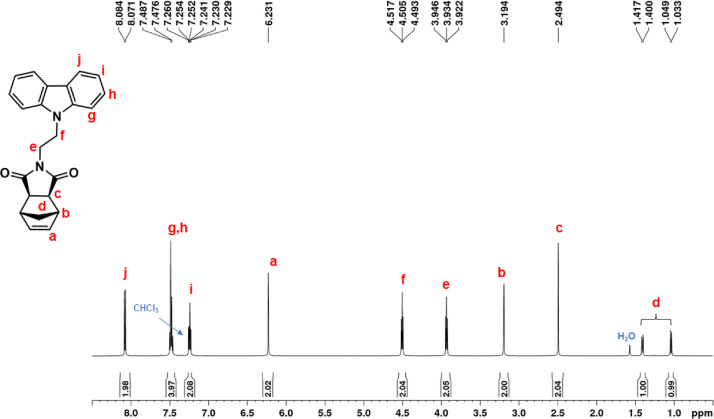
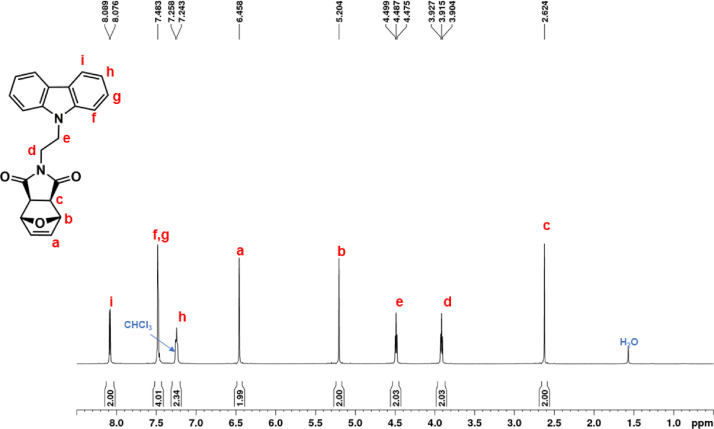
| NDI-EtCAR | 1H NMR (600 MHz, CDCl3, Fig. 1): δ = 1.04 (d, 1H, d, J = 9.9 Hz), 1.41 (d, 1H, d, J = 9.9 Hz), 2.49 (c, 2H, s), 3.19 (b, 2H, s), 3.93 (e, 2H, t, J = 7.2 Hz), 4.50 (f, 2H, t, J = 7.2 Hz), 6.23 (a, 2H, s), 7.24 (i, 2H, t, J = 7.6 Hz), 7.48 (g + h, 4H, overlapping peaks), 8.08 (j, 2H, d, J = 7.7 Hz). 13C NMR (150 MHz, CDCl3, Fig. 2): δ = 36.55 (e), 39.16 (f), 42.93 (d), 45.12 (b), 47.97 (c), 108.54 (g), 119.52 (i), 120.60 (j), 123.19 (k), 126.04 (h), 137.83 (a), 140.31 (l), 177.82 (m). |
| ONDI-EtCAR |
1H NMR (600 MHz, CDCl3, Fig. 5): δ = 2.62 (c, 2H, s), 3.91 (d, 2H t, J = 7.2 Hz), 4.49 (e, 2H, t, J = 7.2 Hz), 5.20 (b, 2H, s), 6.46 (a, 2H, s), 7.25 (h, 2H, br m), 7.48 (f + g, 4H, overlapping peaks), 8.08 (i, 2H, d, J = 7.7 Hz). 13C NMR (150 MHz, CDCl3, Fig. 6): δ = 36.80 (d), 39.81 (e), 47.55 (c), 80.92 (b), 108.49 (f), 119.51 (h), 120.62 (i), 123.22 (j), 126.03 (g), 136.61 (a), 140.34 (k), 175.92 (l). |
| NDI-XyCAR |
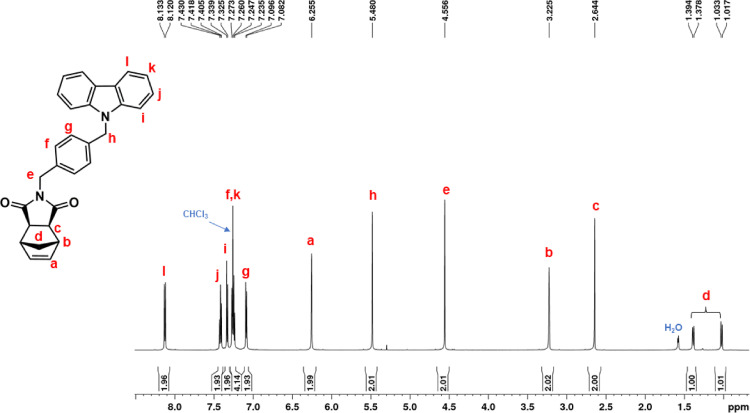
1H NMR (600 MHz, CDCl3, Fig. 9): δ = 1.02 (d, 1H, d, J = 9.9 Hz), 1.39 (d, 1H, d, J = 9.9 Hz), 2.64 (c, 2H, s), 3.22 (b, 2H, s), 4.56 (e, 2H, s), 5.48 (h, 2H, s), 6.25 (a, 2H, s), 7.09 (g, 2H, d, J = 7.9 Hz), 7.25 (f + k, 4H, overlapping peaks), 7.33 (i, 2H, d, J = 7.9 Hz), 7.42 (j, 2H, t, J = 7.6 Hz), 8.13 (l, 2H, d, J = 7.7 Hz). 13C NMR (150 MHz, CDCl3, Fig. 10): δ = 42.03 8 (e), 42.81 (d), 45.39 (b), 46.41 (h) 47.92 (c), 108.96 (i), 119.38 (k), 120.53 (l), 123.16 (m), 125.97 (g), 126.91 (j), 129.40 (f), 135.37 (p), 137.08 (o), 138.04 (a), 140.70 (n), 177.75 (q). |
| ONDI-XyCAR |
1H NMR (400 MHz, CDCl3, Fig. 13): δ = 2.80 (c, 2H, s,), 4.58 (d, 2H, s), 5.25 (b, 2H, s), 5.47 (g, 2H, s), 6.48 (a, 2H, s), 7.08 (f, 2H, d, J = 7.4 Hz), 7.23 (e + j, 4H, overlapping peaks), 7.33 (h, 2H, d, J = 8.1 Hz), 7.42 (i, 2H, t, J = 7.5 Hz), 8.12 (k, 2H, d, J = 7.7). 13C NMR (100 MHz, CDCl3, Fig. 14): δ = 42.15 (d), 46.34 (g), 47.61 (c), 81.02 (b), 108.97 (h), 119.35 (j), 120.51 (k), 123.12 (l), 125.97(f), 126.86 (i), 128.61 (e), 134.80 (o), 136.64 (a), 136.90 (n), 140.68 (m), 175.93 (p). |
| NDI-EtCOU |
1H NMR (600 MHz, CDCl3, Fig. 17): δ = 1.27 (d, 1H, d, J = 9.9 Hz), 1.47 (d, 1H, d, J = 9.9 Hz), 2.72 (c, 2H, s), 3.28 (b, 2H, s), 3.94 (e, 2H, t, J = 5.6 Hz), 4.21 (f, 2H, t, J = 5.6 Hz), 6.25 (k, dd, 2H, J = 9.5 Hz, J = 1.4 Hz), 6.28 (a, 2H, s), 6.76 (g, 2H, s), 6.77 (h, 2H, d, J = 9.0 Hz), 7.35 (i, 2H, d, J = 8.2 Hz), 7.61 (j, 2H, d, J = 9.5 Hz). 13C NMR (150 MHz, CDCl3, Fig. 18): δ = 37.64 (e), 42.73 (d), 45.43 (b), 47.98 (c), 64.41 (f), 101.80 (g), 112.76 (h), 113.11 (n), 135.65 (k), 128.99 (i), 137.93 (a), 143.37 (j), 155.86 (m), 161.12 (o), 161.32 (l), 177.97 (p). |
| NDI-XyCOU |
1H NMR (600 MHz, CDCl3, Fig. 21): δ = 1.06 (d, 1H, d, J = 9.8 Hz), 1.42 (d, 1H, d, J = 9.8 Hz), 2.69 (c, 2H, s), 3.25 (b, 2H, s), 4.63 (e, 2H, s), 5.09 (h, 2H, s), 6.24 (m, 2H, d, J = 9.5 Hz), 6.27 (a, 2H, s), 6.85 (i, 2H, s), 6.89 (j, 2H, d, J = 9.0 Hz), 7.37 (g + k, 3H, overlapping peaks), 7.41 (f, 2H, d, J = 7.8 Hz), 7.62 (l, d, 2H, J = 9.5 Hz). 13C NMR (150 MHz, CDCl3, Fig. 22): δ = 42.15 (e), 42.83 (d), 45.44 (b), 47.97 (c), 70.27 (h), 102.02 (i), 112.92 (p), 113.35 (m), 113.44 (j), 127.94 (g), 128.94 (k), 129.48 (f), 135.63 (r), 136.22 (s), 138.06 (a), 143.47 (l), 155.95 (o), 161.24 (q), 161.89 (n), 177.80 (t). |
Fig. 1.
1H NMR spectrum of NDI-EtCAR (CDCl3, 600 MHz).
Fig. 4.
1H–13C HSQC NMR spectrum of NDI-EtCAR (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonded.
Fig. 5.
1H NMR spectrum of ONDI-EtCAR (CDCl3, 600 MHz).
Fig. 8.
1H–13C HSQC NMR spectrum of ONDI-EtCAR (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonde.
Fig. 9.
1H NMR spectrum of NDI-XyCAR (CDCl3, 600 MHz).
Fig. 12.
1H–13C HSQC NMR spectrum of NDI-XyCAR (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonded.
Fig. 13.
1H NMR spectrum of ONDI-XyCAR (CDCl3, 400 MHz).
Fig. 16.
1H–13C HSQC NMR spectrum of ONDI-XyCAR (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonded.
Fig. 17.
1H NMR spectrum of NDI-EtCOU (CDCl3, 600 MHz).
Fig. 20.
1H–13C HSQC NMR spectrum of NDI-EtCOU (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonded.
Fig. 21.
1H NMR spectrum of NDI-XyCOU (CDCl3, 600 MHz).
Fig. 24.
1H–13C HSQC NMR spectrum of NDI-XyCOU (CDCl3, 600 MHz). The presence of cross peaks in the spectrum indicates protons and carbons that are directly bonded.
The raw 1H, 13C, 1H–1H COSY, and 1H–13C HSQC data obtained from NMR instrument were in the form of FID files. They were processed and plotted using BRUKER TOPSPIN software to obtain images shown in Figs. 1–24. The raw NMR data are shared a Mendeley Data repository in the form of text files (.txt), JCAMP-DX files and in Microsoft Excel Worksheet format for 1D spectra [2].
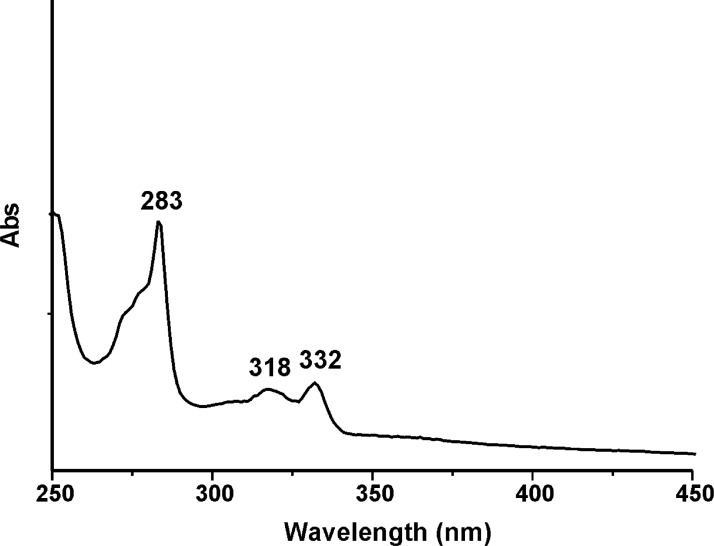
UV–Vis and fluorescence spectroscopy were used to determine the fluorescent properties of all compounds. Figs. 25–28 show the UV-Visible absorption spectra of carbazole derivatives NDI-EtCAR, ONDI-ETCAR, NDI-XyCAR and ONDI-XyCAR. All the spectra present typical peak maxima (in the range 318–343 nm) derived from π–π* absorption of the carbazole group [3].
Fig. 26.
UV-Visible absorption spectrum of ONDI-EtCAR (10−4 M in THF).
Fig. 27.
UV-Visible absorption spectrum of NDI-XyCAR (10−4 M in THF).
Fig. 25.
UV-Visible absorption spectrum of NDI-EtCAR (10−4 M in THF).
Fig. 28.
UV-Visible absorption spectrum of ONDI-XyCAR (10−4 M in THF).
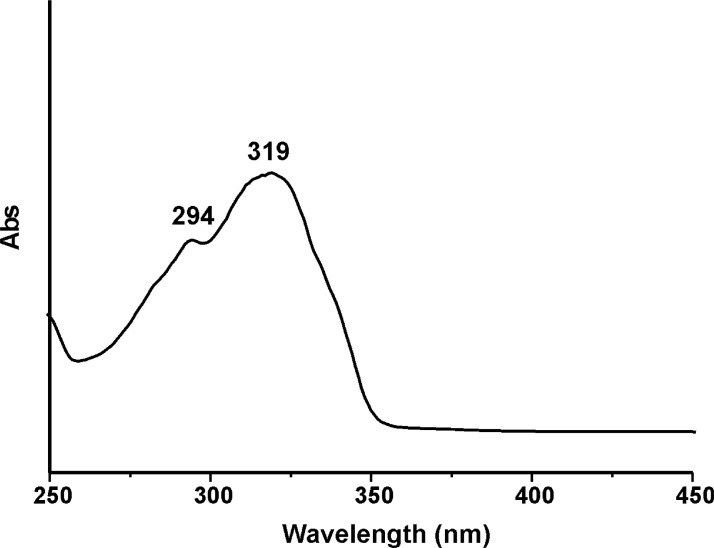
Figs. 29 and 30 display the UV-Visible absorption spectra of coumarin derivatives NDI-EtCOU and NDI-XyCOU. In these spectra, the two bands at around 294 and 319 nm are due to π–π* transitions attributed to electrons of benzene nucleus and pyrone one, respectively [4].
Fig. 29.
UV-Visible absorption spectrum of NDI-EtCOU (10−6 M in CHCl3).
Fig. 30.
UV-Visible absorption spectrum of NDI-XyCOU (10−6 M in CHCl3).
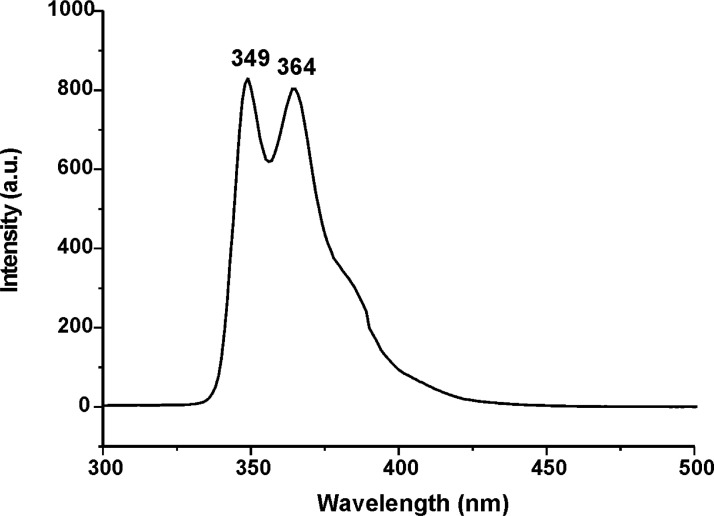
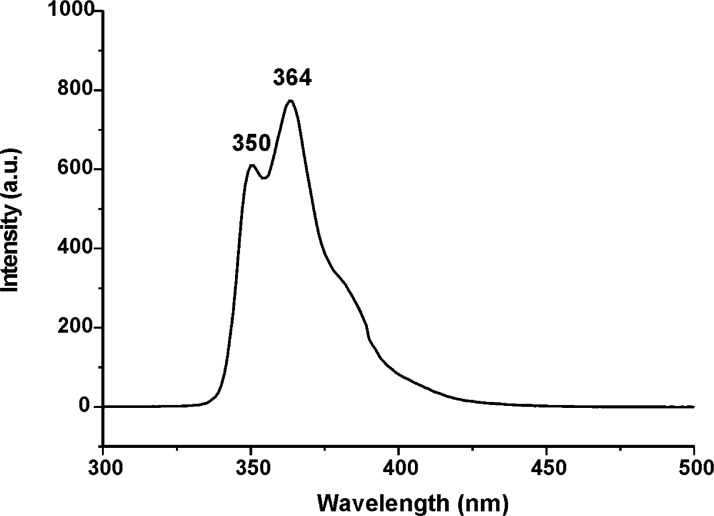
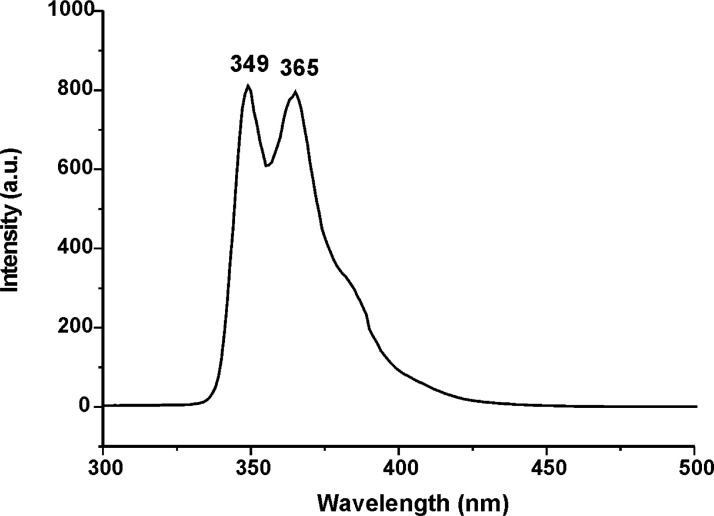
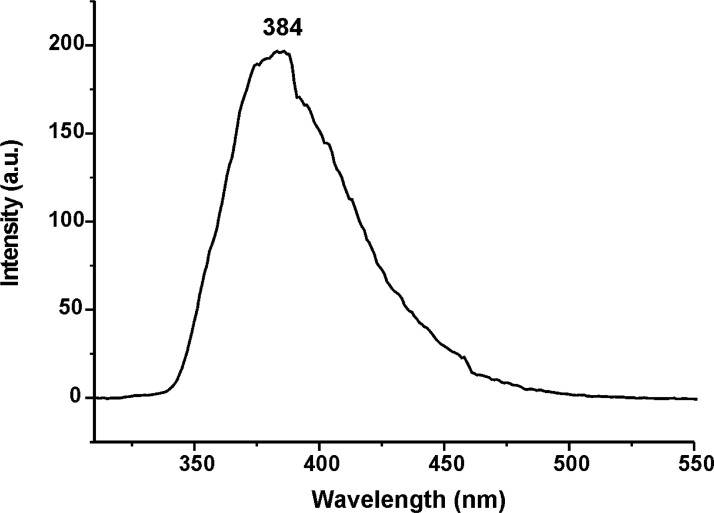
Figs. 31–34 depict the fluorescence spectra of carbazole derivatives NDI-EtCAR, ONDI-ETCAR, NDI-XyCAR and ONDI-XyCAR with bands at 349 and 364 typical of carbazole group emission [5]. Figs. 35 and 36 show the fluorescence spectra of coumarin derivatives NDI-EtCOU and NDI-XyCOU, where is observed a broad band peaked at 384 nm characteristic of coumarin group emission [6]. The raw UV–Vis and fluorescence data are shared as a Mendeley Data repository in Microsoft Excel file formats [2].
Fig. 32.
Fluorescence spectrum of ONDI-EtCAR (10−4 M in THF) (Λecc = 290 nm).
Fig. 33.
Fluorescence spectrum of NDI-XyCAR (10−4 M in THF) (Λecc = 290 nm).
Fig. 31.
Fluorescence spectrum of NDI-EtCAR (10−4 M in THF) (Λecc = 290 nm).
Fig. 34.
Fluorescence spectrum of ONDI-XyCAR (10−4 M in THF) (Λecc = 290 nm).
Fig. 35.
Fluorescence spectrum of NDI-EtCOU (10−6 M in CHCl3) (Λecc = 300 nm).
Fig. 36.
Fluorescence spectrum of NDI-XyCOU (10−6 M in CHCl3) (Λecc = 300 nm).
2. Experimental Design, Materials and Methods
Materials and methods to prepare monomers NDI-EtCAR, ONDI-EtCAR, NDI-XYCAR, ONDI-XyCAR, NDI-EtCOU and NDI-XYCOU are described in ref. [1].
2.1. NMR spectroscopy
The monomer samples for NMR analysis were prepared dissolving 10 mg of each compound in 0.5 mL of deuterated chloroform (CDCl3), and tetramethylsilane (TMS) was used as the internal chemical shift reference.
1H and 13C NMR spectra were acquired on a Bruker ASCEND 600 spectrometer at 298.0 K. The 1H NMR spectra were recorded at 600 MHz and 13C NMR spectra at 150 MHz. The spectral width is 10.0138 ppm/ 6009.615 Hz for the 1H NMR and 197.21 ppm / 29,761.904 Hz for the 13C NMR.
1H–1H COSY and 1H–13C HSQC experiments were used to complete assignments of the NMR signals. The acquisition time for the COSY was 0.170 and 0.206 s for the 1H. The spectral width of the 1H–1H COSY for the 1H is 10.0 ppm / 6009.615 Hz.
The acquisition time for the 1H–13C HSQC was 0.170 s for the 1H and 1.101 s for the 13C. The spectral width of the HSQC for the 1H is 10.0138 ppm/ 6009.615 Hz and 200.00 ppm / 30,183.881 Hz for 13C.
2.2. UV-Visible and fluorescence spectroscopy
The solutions of monomer samples containing compounds with carbazole group (NDI-EtCAR, ONDI-EtCAR,NDI-XyCAR, ONDI-XyCAR) for UV–Vis and fluorescence analysis were prepared by using Tetrahydrofuran inhibitor-free, HPLC grade, ≥99.9% (UV-absorbence ≤0.02 at 300 nm). The absorption spectra of THF solutions (1 × 10−4 M) were recorded in the range of 250–500 nm in air at room temperature, while the emission spectra were recorded in the range of 300–600 nm in air at room temperature. The excitation wavelength was selected at 290 nm.
The solutions of monomer samples containing coumarin group (NDI-EtCOU, NDI-XyCOU) for UV–vis and fluorescence analysis were prepared by using Chloroform HPLC grade, ≥99.9% (UV-absorbence ≤ 0.01 at 400 nm, 0.01 at 290 nm, 0.01 at 270 nm). The absorption spectra of CHCl3 solutions (1 × 10−6 M) were recorded in the range of 250–500 nm in air at room temperature, while the emission spectra were recorded in the range of 310–600 nm in air at room temperature. The excitation wavelength was selected at 300 nm.
CRediT authorship contribution statement
Rubina Troiano: Investigation, Data curation. Mario Carratù: Investigation, Data curation. Stefania Pragliola: Data curation, Visualization, Writing – original draft. Antonella Caterina Boccia: Data curation, Visualization, Writing – original draft. Fabia Grisi: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgments
This work was supported by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica.
Data Availability
Dataset for norbornene and oxanorbornene dicarboximides functionalized with carbazole and coumarin groups. (Original data) (Mendeley Data).
References
- 1.Troiano R., Carratù M., Pragliola S., Boccia A.C., Grisi F. ROMP of norbornene and oxanorbornene derivatives with pendant fluorophore carbazole and coumarin groups. Eur. Polym. J. 2022;167 doi: 10.1016/j.eurpolymj.2022.111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.R. Troiano, M. Carratù, S. Pragliola, A.C. Boccia, F. Grisi, Dataset for norbornene and oxanorbornene dicarboximides functionalized with carbazole and coumarin groups, Mendeley Data, v1, 2022, doi: 10.17632/h3khzrtcg7.1. [DOI] [PMC free article] [PubMed]
- 3.Gong X., Lim S.H., Ostrowski J.C., Moses D., Bardeen C.J., Bazan G.C. Phosphorescence from iridium complexes doped into polymer blends. J. Appl. Phys. 2004;95:948–953. doi: 10.1063/1.1635976. [DOI] [Google Scholar]
- 4.Kehrlösser D., Träger J., Kim H.-C., Hampp N. Synthesis and Photochemistry of Coumarin-Based Self-Assembled Monolayers on Silicon Oxide Surfaces. Langmuir. 2010;26:3878–3882. doi: 10.1021/la903433r. [DOI] [PubMed] [Google Scholar]
- 5.Berlman I.B. Academic Press; New York, NY, USA: 1971. Handbook of Fluorescence Spectra of Aromatic Molecules. [Google Scholar]
- 6.Hua C.-J., Zhang K., Xin M., Ying T., Gao J.R., Jia J.-H., Li Y.-J. High quantum yield and pH sensitive fluorescence dyes based on coumarin derivatives: fluorescence characteristics and theoretical study. RSC Adv. 2016;6:49221–49227. doi: 10.1039/C6RA05996A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset for norbornene and oxanorbornene dicarboximides functionalized with carbazole and coumarin groups. (Original data) (Mendeley Data).