Highlights
-
•
FujiLAM is feasible to implement at point-of-care using existing infrastructure.
-
•
FujiLAM can be performed in laboratory and consultation or other spaces.
-
•
FujiLAM adequately performed by any health worker including lay health workers.
-
•
FujiLAM well accepted by users and managers, perceived as easy to perform.
-
•
Selection of users should consider expected test demand and existing user workload.
Keywords: Tuberculosis, Diagnostic tests, FujiLAM, Feasibility studies, Low income settings
Abstract
Background
The novel urine-based FujiLAM test identifies tuberculosis in HIV-positive patients but may be challenging to use at point-of-care (POC).
Objectives
We assessed the feasibility and acceptability of using the FujiLAM test at point of care in outpatient settings.
Methods
We conducted a mixed methods study in four outpatient settings in Kenya, Mozambique, South Africa, and Uganda between November 2020 and September 2021. The test was performed at POC in existing clinic laboratories and consultation spaces. We performed direct observations in the four health facilities, individual questionnaires, proficiency testing evaluations, and individual interviews among healthcare workers performing the FujiLAM test (healthcare workers), and group discussions with programme managers.
Results
Overall, 18/19 (95%) healthcare workers and 14/14 (100%) managers agreed to participate in the study. Most assessed healthcare workers, including lay health workers (10/11; 91%), met the minimum required theoretical knowledge and practical skill in performing the FujiLAM test. Most healthcare workers (17/18; 94%) found the FujiLAM test overall “Easy/Very easy” to perform. Some challenges were mentioned: many timed steps (5/18; 28%); ensuring correct incubation period (5/18; 28%); test result readability (4/18; 22%); and difficulties with cartridge buttons (3/18; 17%). Half of the healthcare workers regularly performing the test (4/7; 57%) found it “Easy” to integrate into routine activities. Most healthcare workers and managers believed that any healthcare worker could perform the test after adequate training.
Conclusions
Implementing the FujiLAM test in outpatient POC settings is feasible and acceptable to healthcare workers and managers. This test can be performed in various clinic locations by any healthcare worker. The timed, multi-step test procedure is challenging and may affect the workload in resource-constrained health facilities.
1. Introduction
Long-standing challenges diagnosing Tuberculosis (TB) in low-resource settings represent a significant barrier to meeting global TB control targets. Sputum microscopy is often available at peripheral level, but has a poor sensitivity [1], [2], [3]. The Xpert MTB/RIF sputum assay (Cepheid, Sunnyvale, CA, USA) has a very good diagnostic performance but is not always available at primary health care level. Furthermore, TB is particularly difficult to diagnose in HIV-positive patients and sputum-based methods have limitations as some patients may have difficulty producing sputum [2].
For these reasons, urine-based tests (detecting the mycobacterial lipoarabinomannan [LAM] antigen) could radically improve the diagnosis of TB among HIV-positive patients. The Abbott TB-LAM (Determine TB LAM Ag, Abbott, Chicago, IL, USA, formerly Alere) is currently recommended by the World Health Organization (WHO) for TB diagnosis in HIV-positive patients [4]. It produces results in less than 25 min and has been shown to be feasible at point-of-care (POC) in low-resourced settings [5], [6]. The test, however, has limited sensitivity, particularly among patients with CD4 values > 200 cells/μl [7]. The newer FujiLAM (Fujifilm SILVAMP TB LAM Fujifilm, Tokyo, Japan) promises greater diagnostic sensitivity compared to the Abbott TB-LAM in HIV-positive patients [8] and may have a diagnostic value in HIV-negative patients [9]. However, it involves more steps, and has a longer results turnaround time (TAT) of one hour.
Despite 2015 WHO recommendations to use the Abbott TB-LAM, its well documented diagnostic value, ease-of-use, and affordability [5], [6], [10], [11], [12], [13], uptake at POC in resource-limited settings has been very low mainly due to budget limitations and lack of local operational research studies to inform implementation [14], [15]. Furthermore, a rapid diagnostic test which is easy to perform is not necessarily easy to implement, as observed for other rapid tests (rapid malaria test)[16]. Successful implementation of new rapid diagnostic tests may depend on various factors that are context specific. Thus, it is important to explore and understand operational characteristics at the level of intended use.
This study aimed to assess the feasibility of implementing the FujiLAM test at POC in out-patient health facilities and the acceptability of the test among healthcare workers.
2. Methods
2.1. Study design
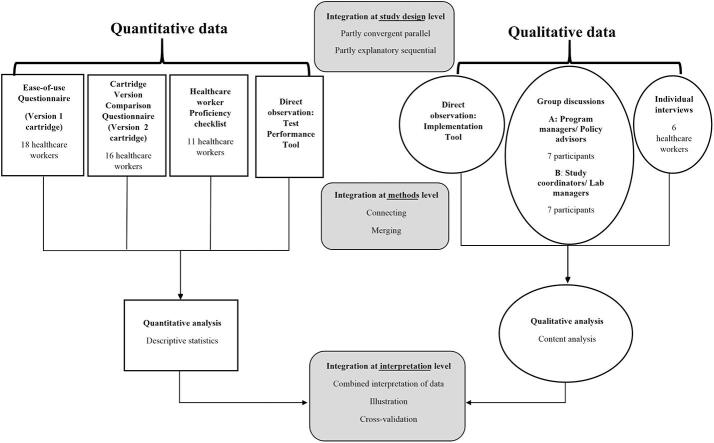
This was a mixed methods study following a pragmatic approach [17]. Quantitative and qualitative elements were used to gain a holistic understanding of the factors to consider when implementing the FujiLAM test. It employed a partly convergent parallel and partly explanatory sequential design, [18], Fig. 1.
Fig. 1.
Mixed methods design, study population, data collection tools, and analysis summary.
2.2. Setting
The study was conducted between November 2020 and September 2021 as part of a multicentric diagnostic prospective study to investigate the diagnostic accuracy of the FujiLAM test in ambulatory HIV-positive patients in four countries [19]. Patient perspectives on urine sampling for urine-based TB diagnostic testing were also assessed and findings are reported separately (manuscript in review)[20]. Patients were recruited from hospital outpatient clinics in Kenya (HIV and TB clinics of Homa Bay Teaching and Referral Hospital), South Africa (Outpatients Department of Eshowe Hospital), Uganda (HIV Clinic attached to the Mbarara Regional Reference Hospital); and from primary health care clinics in Mozambique (Alto Mae Health Centre in Maputo), and South Africa (Eshowe Gateway Clinic), Table S1. Patients were asked to provide a urine specimen at the first consultation for FujiLAM testing. The manufacturer’s five-step test procedure was followed, Fig. 2.
Fig. 2.
Fujifilm SILVAMP TB LAM Version 1 test cartridge and procedure. Adapted from “Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study” by T. Broger, 2019, Lancet Infect Dis, hrrps://https://doi.org/10.1016/S1473-3099(19)30001–5.[8]. Briefly: Step 1: A volume of approximately 200 μL urine was added to the reagent tube up to the indicator line and mixed. Step 2: The urine was then incubated for 40 min at room temperature. Step 3: After mixing the incubated sample again, two drops of urine were then added to the test strip and button 2 pressed. Step 4: Once the Go-Next colour indicator mark turned orange (within 3–10 min), button 3 was pressed. Step 5: The result was read within 10 min. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The FujiLAM was performed at POC in the out-patients clinic laboratories in Kenya, Mozambique and Uganda; and in consultation rooms or other clinic spaces in Kenya and South Africa. FujiLAM test results were not used to make decisions on the patients’ management. Training of healthcare workers performing the FujiLAM test (referred from now on as “healthcare workers”) was conducted over two to four hours depending on the site. The FujiLAM test cartridge used throughout the diagnostic study was the Version 1 cartridge. A newer cartridge version, the Version 2 cartridge, was under development by the manufacturer at the time of the feasibility study and the healthcare workers’ acceptability of the Version 2 cartridge was also assessed using a prototype cartridge provided by the manufacturer. In comparison to the Version 1 cartridge, the Version 2 cartridge was smaller in size, had a narrower sample port, and a narrower results window, Figure S1. The Version 2 cartridge required only one drop of incubated urine sample whilst the Version 1 cartridge required 2 drops.
2.3. Study population
All healthcare workers from all study sites were invited to participate and were included after consenting. Purposive sampling was used to select healthcare workers for individual interviews, ensuring representation of different professional categories and study sites. Additionally, healthcare workers in management roles (study coordinators) in the health facilities conducting the diagnostic study as well as others involved in TB programme management and policy decision making (referred from now on as “managers”), were purposively selected for two group discussions.
2.4. Study procedures
We used seven study procedures: four quantitative (direct observations using the Test Performance Tool, healthcare worker proficiency testing evaluation, individual questionnaires on the test Version 1 cartridge and individual questionnaires on the test Version 2 cartridge); and three qualitative (direct observations using the Implementation Tool, individual interviews with healthcare workers, and groups discussions with managers), Fig. 1.
We conducted direct observations using two specially-developed tools: Implementation Tool and Test Performance Tool. The Implementation Tool captured data on aspects related to the implementation of the test such as health facility organisation (space and infrastructure), patient flow, human and material resources, and logistical requirements. The Test Performance Tool captured data on healthcare worker performance of the test (routine practices, processing of tests, TAT of different steps), and healthcare worker interactions with patients. These processes were observed for 40 patients (ten per study site).
We used an adapted standard proficiency testing evaluation checklist developed by FIND (Foundation for Innovative Diagnostics; Geneva Switzerland) to objectively evaluate the proficiency of a sub-group of the healthcare workers. They were judged proficient when the following testing performance targets were met: scores ≥ 80% for Part A (practical performance), ≥ 4 points for Part B (confidence in performing the test), ≥ 80% for Part C (theoretical knowledge), and ≥ 90% for Part D (interpretation of results).
We used a standardized questionnaire (Ease-of-use Questionnaire) to assess the ease-of-use of the Version 1 cartridge. It included closed and open-ended questions related to important aspects of the training, healthcare workers’ experiences and opinions of the test procedure steps, and integration of FujiLAM into their daily workload. A second questionnaire (Cartridge Version Comparison Questionnaire) was completed by healthcare workers on the ease-of-use of the prototype Version 2 cartridge.
We conducted individual interviews with healthcare workers to explore their experiences (perceptions of the test and its processes). We used semi-structured interview guides addressing themes organised around a predefined framework developed to answer the research questions. Interviews were conducted by three researchers either face-to-face or using WhatsApp phone audio function (Facebook, Inc); each lasted 40–90 min.
We organised group discussions with managers to explore and compare experiences and perceptions of strategic planning and implementation. Group discussions were conducted using Zoom (Zoom Video Communications, Inc, San Jose, California), with video features enabled, lasting between 90 and 120 min. Three researchers, all epidemiologists, conducted the group discussions: a lead moderator with a social science background; a medical doctor with expertise in TB; and a nurse with public health background. The interviews and group discussions were recorded and transcribed verbatim.
Further in-depth descriptions of the procedures and data tools are presented in Table S2.
2.5. Data collection and data analysis
Quantitative data was collected using paper-based tools and analysed using Microsoft Excel® (Microsoft, Redmond, WA, USA). Continuous variables were summarised as median and inter-quartile ranges (IQR) and categorical variables as numbers and percentages. The FujiLAM test TAT was defined as the time from a patient submitting urine to the time the result was available.
Qualitative data was transcribed and analysed with pre-determined and open codes using content analysis [21], [22], using Atlas TI software 9 (ATLAS.ti Scientific Software Development GmbH, Berlin Germany), and focused on the manifest content of the data. Several quotations were selected for illustrative purposes and each participant was assigned a unique identification number and their role; example Nurse #1.
To integrate the findings, we compared the results of the quantitative and qualitative components of the study in an analysis as proposed by Fetters MD et al [23]. Responses to the Ease-of-use Questionnaire helped in the selection of participants for individual interviews. During analysis, we corroborated the data from the quantitative and qualitative elements to produce a combined summary and conclusions of our findings, Fig. 1.
3. Results
3.1. Study participants
In total, 18/19 (95%) healthcare workers agreed to participate in the study: two clinicians, seven nurses, seven laboratory technicians, and two community health workers (CHWs); Table 1 presents their characteristics. The 18 healthcare workers answered the questionnaire on ease-of-use of the Version 1 cartridge (Ease-of-use Questionnaire). Six of the healthcare workers participated in individual interviews [one clinician (Kenya), one nurse (Kenya), two laboratory technicians (Mozambique and Uganda), and two community health workers (South Africa)]. The 14/14 (100%) managers agreed to participate in the study: four laboratory managers and three study coordinators participated in one group discussion, whilst four TB programme managers and three TB policy advisors participated in the other.
Table 1.
Demographic characteristics of the healthcare workers participating in the study.
| Characteristic | Kenya (N = 6) |
Mozambique (N = 3) |
South Africa (N = 5) |
Uganda (N = 4) |
Total, n (%) (N = 18) |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 4 | 0 | 3 | 0 | 7 (39) |
| Male | 2 | 3 | 2 | 4 | 11 (61) |
| Age (median, [IQR]) | 35 [29–38] | 26 [26–34] | 34 [31–38] | 35 [33–38]) | 34 [30–38] |
| Years worked in profession (median, [IQR]) | 12 [7–13] | 7 [6–12] | 7 [5–10] | 10 [9–13] | 10 [5–13] |
| Profession | |||||
| Community Health Worker | 0 | 0 | 2 | 0 | 2 (11) |
| Nurse | 3 | 0 | 2 | 2 | 7 (39) |
| Clinician | 1 | 0 | 1 | 0 | 2 (11) |
| Laboratory technician | 2 | 3 | 0 | 2 | 7 (39) |
3.2. FujiLAM testing proficiency evaluation checklist using the Version 1 cartridge
The proficiency evaluation checklist was performed for 11/18 healthcare workers (four study coordinators excluded as they were performing the evaluations, and two healthcare workers were no longer available at time of assessment). Ten out of eleven (91%) healthcare workers achieved the target score in all the four areas. The minimum score was achieved by all 11 healthcare workers in three areas (Parts A to C: practical performance, confidence in performing the test, and theoretical knowledge respectively); in Part D (interpretation of the FujiLAM results), one nurse did not achieve the minimum score required, Fig. 3.
Fig. 3.
Individual healthcare worker proficiency in conducting the FujiLAM test using the Version 1 cartridge. The diagram illustrates the individual scores achieved as well as the minimum score expected for each of the four areas (A to D) assessed.
3.3. Perceptions on the ease-of-use of the Version 1 cartridge
Almost all healthcare workers (17/18; 94%) found that overall the FujiLAM test was “Easy/Very easy” to perform, and the majority found it “Easy/Very easy” to interpret the result (15/18; 83%). Participants were asked about the ease-of-use of each step of the test procedure, Fig. 4. Some challenges in test performance were mentioned: too many timed steps in the procedure (5/18; 28%); difficulties ensuring the incubation period of 40 min (5/18; 28%); problems with results readability (very faint control or patient lines) (4/18; 22%); invalid test results (no control line) (4/18; 22%), and difficulties with the test cartridge buttons (pressing in wrong order, buttons sometimes too stiff) (3/18; 17%). Table 2 presents the challenges faced and examples of explanations from the healthcare workers.
Fig. 4.
Healthcare workers’ self-assessment of the ease of performing the different steps in the test procedures using the Version 1 cartridge. Possible responses were “Very easy”, “Easy”, “Neutral”, “Difficult”, or “Very difficult”.
Table 2.
Challenges encountered by some healthcare workers during FujiLAM testing procedure using the Version 1 cartridge.
| Challenge | Description of challenge | Examples quotes |
|---|---|---|
| Number of steps | Too many steps and the need to time the different steps is time consuming and difficult |
“Easy, but the test has too many procedures for a quick test.” (Laboratory technician #3) “There are lot of steps and the time is too long.” (Laboratory technician #5) |
| Long incubation time | 40 min is too long to wait |
“It's hard to wait for the 40 min especially when you are working on more than one sample. The time is too long.” (Nurse #6) “The timing is very long, for when you’re waiting for the first 40 min to wait with the patient. The patient needs to go but we need to wait for these 40 min.”(CHW #1) |
| Pipetting | Difficult to collect the desired amount of urine using a pipette | “Well, where the mistake can come in is down to where you have the pipette, putting the sample in the pipette and putting in that the small nozzle container before the incubation. There is a risk of putting in more urine sample past the recommended level.” (Clinician #2) |
| Pressing buttons 2 and 3 | Risk of pressing the buttons in the wrong order, or too late Some pressure has to be exerted to press both buttons |
“They don’t know how, they don’t know when to click the first and the second buttons. Sometimes they click the second button (first), that is the problem.” (Laboratory technician #4) “This requires to push hard and allow denting the bottom.” (Laboratory technician #7) |
| Test result readability a | Very faint control or patient lines which causes uncertainty in the final result interpretation White patient line (instead of the expected light grey colour) Go-Next line in the same space as the control line b |
“Sometimes the lines are very faint, like they are not completely dark and they are not completely grey. So, like it really requires the second reader also to come in to give their views, most of the time those lines are very faint. So, you really need to be keen to interpret the results.” (Study coordinator #1) ”On the test window when they pressed button three there’s a control line that appears but then where you read the test line, it looks white rather than grey, so there’s a clear line which is white but it’s not grey or dark coloured. So, we’re not sure what’s gone wrong there.” (Study Coordinator # 2) |
| Invalid results a | No control line after the test cartridge was dropped accidentally Sometimes no control line when blood stained or cloudy urine was used |
“The wind blew out some of the equipment that was on the table and this test was also moved and dropped. So, when I picked it again, I noticed that it gave out an invalid result”. (Clinician # 1) ”It seems to be giving us invalid from samples that tend to have blood. I think there is an interference between haemoglobin and the test.” (Laboratory technician #6) |
a: Examples of how the FujiLAM test result look like if real life are provided in Figure S2.
b: This was an observation made by the feasibility study researchers whilst reviewing pictures of used FujiLAM test cartridges send from the field which had been interpreted as Invalid.
The important points identified by healthcare workers to emphasise during training are summarised in Figure S3. Overall, healthcare workers reported that they were comfortable to independently perform the test after two supervised test procedures (IQR 1–3).
3.4. Assessment of the Version 2 FujiLAM test cartridge
Sixteen healthcare workers trialled the Version 2 cartridge. All (16/16; 100%) responded that their evaluation of the ease-of-use of the FujiLAM test would “not change” or would “remain similar with only slight changes” when comparing the Version 2 and the Version 1 cartridges. Most healthcare workers (11/16, 69%) found ease-of-use of the Version 2 cartridge equal to that of the Version 1, and one third (5/16, 31%) found the Version 2 easier to perform (due to only one drop of urine required instead of two, along with better readability of control and test lines).
3.5. FujiLAM test result TAT and logistic aspects related to the use of the Version 1 cartridge
The median FujiLAM result TAT was 70 min (IQR 62–81), Table S3. The main reasons for delays in testing were: attending to other patients’ care and being busy with other tests.
Additional equipment required to perform the test included urine sample collection bottles and timing devices (stopwatches and personal mobile phones with alarms). The FujiLAM test was stored and performed using the existing infrastructure with no modifications to any of the testing areas.
3.6. Perceptions regarding the potential integration of the FujiLAM test into the model of care
Both healthcare workers and managers suggested that the FujiLAM was suitable for a POC test in decentralised outpatient facilities even in peripheral health care centres. Opinions differed on where in the facility the test should be performed. Some participants supported laboratory-based testing arguing that the many timed steps would add to already high workloads for nurses and clinicians. Supporters of testing in consultation rooms or other clinic spaces argued that the FujiLAM was an easy test to perform and that the real-life workload associated with the test was not likely to be high based on the demand for the test (few tests needed per day).
Among those who performed the test regularly during the study (seven healthcare workers); 57% (4/7) found the integration of the FujiLAM test into their daily activities “Easy” (one nurse, two laboratory technicians, and one CHW); 29% (2/7) expressed that it was “Difficult” (two laboratory technicians); and 14% (1/7) gave their response as “Neutral”. Of those who performed the test occasionally (11 users), 45% (5/11) gave their response as “Neutral” (two nurses, one laboratory technician, one clinician, and one CHW); 36% (4/11) expressed that it was “Difficult/Very difficult” (three nurses and one clinician); and 18% (2/11) found it “Easy” (two laboratory technicians). Details of the other activities performed by healthcare workers are presented in Table S4. Difficulties in integrating the test into daily routines were: multiple timed steps making it difficult for healthcare workers to multi-task; and 40 min incubation period which users viewed as being too long. These concerns were even raised by some of the healthcare workers who thought integrating the test was “Easy”.
Despite these concerns, the majority of interviewed healthcare workers (4/6; 67%) reported that they could continue with other activities during the incubation time (Table S4), and made use of alarms in their timing devices to help them keep track of the incubation period and other timed steps. In Kenya, the healthcare workers sometimes performed the tests in batches towards the end of the day when they did not expect any more patients, since they were not giving the FujiLAM test results to the patients, nor using them for clinical decision making.
From a technical perspective, most healthcare workers and managers believed that any qualified or lay health worker could perform the FujiLAM test after adequate training. Two healthcare workers, however, expressed reservations about using lay health workers such as CHWs to perform the test based on the wide spectrum of their capabilities as the selection criteria for these posts were not standardised. From a management perspective, the criteria for who should perform the test was more related to expected extra workload, available healthcare workers, and individual healthcare worker motivation.
Regarding integration into a TB diagnostic algorithm, the FujiLAM test was generally viewed more favourably compared to the Abbott TB-LAM test given its superior diagnostic sensitivity. However, some concerns were raised on the eventual uptake of the FujiLAM test due to lack of funding, and the potential increase in workload among healthcare workers.
Table S5 presents quotes from healthcare workers and managers on integrating the FujiLAM test into their workflow.
4. Discussion
Our study is the first, to our knowledge, to evaluate the feasibility and acceptability of the novel urine-based FujiLAM test for TB. We found that implementing the FujiLAM test at POC in outpatient settings was feasible and well accepted by healthcare workers, programme managers, and policy makers; it was perceived as “Very easy” or “Easy” to perform, and its potential integration into the model of care was viewed favourably. The test was performed using the existing infrastructure in clinic laboratories and in consultation rooms and other clinic spaces. The test was adequately performed by the majority of the nurses, clinicians, laboratory technicians and lay health workers. This suggests that most healthcare workers, no matter their health training background, would be technically capable of competently performing the test. Overall, the simple infrastructure requirements and positive acceptability by healthcare workers are similar to those described for the Abbott TB-LAM test (formerly Alere), which is also a urine-based rapid TB diagnostic test [6], [10]. The main challenges faced were the multiple timed steps and the relatively long incubation period.
Questions of where and who will perform the test are critical considerations for programme managers and policy makers and may have an impact on the successful implementation of the test. In outpatient clinics with existing laboratories the test was easily integrated among other tests. In Mozambique and Uganda, laboratory technicians performed the tests in laboratories located within the out-patient clinics, and could easily incorporate them into their daily routines with minimal delays.
In clinics with no laboratories, a consultation room or separate space could be considered where clinical personnel or lay workers could perform the tests. However, in this type of setting the multiple timed steps may interfere with general patient flow. The long waiting time for incubation could be problematic although healthcare workers reported that they could effectively utilise the 40 min to carry out other activities. Assigning specific healthcare workers each day to be in charge of testing could help to reduce disturbances in the patient flow. This would however be heavily reliant on adequate staffing levels at the health facility, and could be, as mentioned in another study, ineffective in settings with high healthcare worker turnover as it would require frequent retraining to perform the test [10].
A critical aspect is the number of patients in need of FujiLAM, which depends on the size of the clinic and on the criteria used for patients’ eligibility. The additional workload may not be an issue if only a few patients per day are in need of testing. In our settings, all HIV-positive patients with symptoms of TB and all patients with advanced HIV and no symptoms of TB were eligible for the diagnostic study. The average number of patients eligible for the test using these criteria varied from three tests/week in the clinics of Mozambique and South Africa to six in Kenya and 10 in Uganda. These numbers seem manageable and are probably similar to those in outpatient health facilities throughout Africa. However, any additional work may be difficult to integrate in facilities already suffering from human resources shortages [24], [25]; and even though the test is overall perceived as technically easy to perform, its introduction could further increase the complexity of the existing daily routines[16]. It is thus important to thoroughly evaluate the expected demand for the test and the available human resources and infrastructure in each health facility before implementation.
One alternative would be to train other clinic healthcare workers, not necessarily medical ones, to conduct the tests. Task shifting from medical to non-medically trained healthcare workers or lay health workers has been implemented successfully and less successfully for other POC rapid diagnostic tests [16], [26], [27]. In our study, CHWs successfully performed the test and described it as easy to use despite its complexity. Other non-medically trained healthcare workers such as nurse assistants or laboratory assistants, could potentially be trained to perform the test. However, as observed during the implementation of other rapid diagnostic tests, non-medically trained healthcare workers such as CHWs may require more frequent training and supervision even after initial implementation [16], [28], [29], and this should be taken into account in terms of time required as well as human, and material resources. Overall, considering some challenges faced during the testing procedure such as result readability, with one healthcare worker failing to correctly interpret the results during the proficiency evaluation, it may be necessary to offer more training and supervision for all healthcare workers regardless of medical training background during implementation to ensure correct result interpretation. Difficulties to read faint result lines were also experienced in a study using FujiLAM to test cerebrospinal fluid [30]. The proficiency evaluation tool we used, developed by FIND, would be very useful to identify individuals requiring more support as well as the specific support required.
Since the FujiLAM test was implemented at POC, the TAT was low and results were available on the same day. Although the TAT was shorter than Xpert MTB/RIF, [6], [31], healthcare workers reported that the test procedure was longer than they expected of a rapid test, comparing it to the Abbott TB-LAM which had a shorter TAT [6]. The FujiLAM test could be performed in batches to increase efficiency, although this would also increase the TAT and may be inconvenient for patients, thereby losing one of its main advantages. Clinic organisation should ensure same day results within a reasonable timeframe to enable commencement of TB treatment on the consultation day. Same day results were an important characteristic of an acceptable TB diagnostic test from a patient perspective [20].
A strength of our study is that we conducted it in four different countries with different implementation models. The diversity of the study settings strengthens the generalisability of our findings; planners in other settings can identify and adapt the most suitable model for them. Secondly, the mixed methods design enabled us to compare and contrast the quantitative and qualitative findings and to gain better understanding of the different aspects of implementing the FujiLAM test.
There were some limitations to our study. First, some participants might have felt that their employment would be negatively affected by expressing certain views. We reduced the risk of response bias by ensuring that all individual interviews and group discussions were conducted by researchers external to the implementation sites, who had no hierarchical working relationship with the study respondents, by emphasising that participation was voluntary, and by ensuring their confidentiality by removing their names from specific comments. Second, individual healthcare worker proficiency testing evaluations were conducted by persons well known to the healthcare worker being assessed, thus a potential for bias. However, the assessors were in management positions and had a direct line of management over the assessed healthcare worker. Our assumption was that the assessments were conducted with the same objectivity as would be applied in real life daily supervision of work. Third, the FujiLAM test was conducted within study settings where extra effort had been put in place to ensure adequate human resources thereby limiting the generalisability of introducing the test into busy settings. However, in two of the four study sites, very little additions were necessary to existing healthcare workers and overall, we believe that our findings are relevant even in real-life settings as they highlight possible barriers and solutions.
5. Conclusions
Implementing the FujiLAM test at POC in outpatient settings is feasible using existing infrastructure. The test can be performed in clinic laboratories, consultation rooms or other clinic spaces, and by healthcare workers with diverse backgrounds (including lay health workers). The FujiLAM test is perceived as easy to perform and is well accepted as a POC test by healthcare workers and managers despite the drawbacks such as several timed steps in the test procedure and concerns regarding integration of the test into daily routines. These drawbacks may be addressed by ensuring adequate training and supervision as well as identifying the best clinic spaces and healthcare workers to perform the test according to the context of the health facility. Findings from this study and others, including those on diagnostic accuracy, patient perspectives, and cost effectiveness would provide a holistic view on the value of the FujiLAM test for programme managers and policy makers.
6. Ethics and consent
Permission to conduct the study was obtained from the Médecins Sans Frontières Ethics Review Board (ERB:1985) and from the national ethics review committees in Kenya (Kenya Medical Research Institute: 687), Mozambique (Comité Nacional de Bioética para Saude: 172/CNBS/20), Uganda (Uganda National Council for Science and Technology: HS2764), and South Africa (KwaZulu-Natal University Biomedical Research Ethics Committee: BREC/1623/2020). All participants provided signed consent to participate in the study; they were assigned unique identifiers and transcripts were anonymised.
7. Paper context
Slow uptake of the easy-to-use urine based test for TB diagnosis (Abbott TB-LAM) raises concerns about the future implementation of a newer, more sensitive but relatively complex test (FujiLAM). We found that the FujiLAM test was acceptable to healthcare workers despite some challenges faced; and technically could be competently performed by healthcare workers with different backgrounds including lay-workers. Implementation of the FujiLAM test is feasible in outpatient settings. Further evaluations on the FujiLAM routine use will be needed after it is approved for wider implementation.
Funding
This work was funded by the Agence Nationale de Recherche sur le SIDA (ANRS | Maladies infectiouses emergentes 20314) and by Médecins Sans Frontières (MSF).
CRediT authorship contribution statement
Sekai Chenai Mathabire Rücker: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Pascale Lissouba: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing. Milcah Akinyi: Investigation, Project administration. Alex Vicent Lubega: Investigation, Project administration. Rosanna Stewart: Investigation, Project administration, Writing – review & editing. Natalia Tamayo Antabak: Investigation, Project administration, Writing – review & editing. Ivan Taremwa Mugisha: Investigation, Project administration. Liesbet Ohler: Investigation, Project administration, Writing – review & editing. Hélder Macuácua: Investigation. May Atieno: Investigation. Winnie Muyindike: Supervision, Writing – review & editing. Stavia Turyahabwe: Writing – review & editing. Gordon Odhiambo Okomo: Writing – review & editing. Aleny Mahomed Couto: Writing – review & editing. Mohammed Musoke: Resources, Supervision, Writing – review & editing. Claire Bossard: Investigation. Catherine Hewison: Writing – review & editing. Zibusiso Ndlovu: Methodology, Writing – review & editing. Helena Huerga: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all the participants who took part in this assessment. Many thanks also to the Ministry of Health from each site, as well as MSF and Epicentre teams on the ground for the support rendered. Special thanks to Tony Reid who supported us with language editing for the final draft of the manuscript. This work was funded by the Agence Nationale de Recherche sur le SIDA (ANRS | Maladies infectiouses emergentes 20314) and by Médecins Sans Frontières (MSF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2022.100316.
Contributor Information
Sekai Chenai Mathabire Rücker, Email: Chenai.MATHABIRE@epicentre.msf.org.
Pascale Lissouba, Email: Pascale.lissouba@epicentre.msf.org.
Milcah Akinyi, Email: Milcah.akinyi@epicentre.msf.org.
Alex Vicent Lubega, Email: Alex.lubega@epicentre.msf.org.
Natalia Tamayo Antabak, Email: MSFCH-Mozambique-HoM@geneva.msf.org.
Ivan Taremwa Mugisha, Email: Ivan.MUGISHA@epicentre.msf.org.
Liesbet Ohler, Email: MSFOCB-Eshowe-DeputyCoord@brussels.msf.org.
May Atieno, Email: msff-homa-bay-labmentor@paris.msf.org.
Mohammed Musoke, Email: msff-nairobi-medco@paris.msf.org.
Claire Bossard, Email: Claire.bossard@epicentre.msf.org.
Catherine Hewison, Email: Cathy.Hewison@paris.msf.org.
Zibusiso Ndlovu, Email: zee.ndlovu@joburg.msf.org.
Helena Huerga, Email: helena.huerga@epicentre.msf.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Alfred N., Lovette L., Aliyu G., Olusegun O., Meshak P., Jilang T., et al. Optimising Mycobacterium tuberculosis detection in resource limited settings. BMJ Open. 2014;4(3):e004093. doi: 10.1136/bmjopen-2013-004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter J.G., Theron G., van Zyl-Smit R., Haripersad A., Mottay L., Kraus S., et al. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;40(5):1211–1220. doi: 10.1183/09031936.00201711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn S.D., Wood R. Tuberculosis in Antiretroviral Treatment Services in Resource-Limited Settings: Addressing the Challenges of Screening and Diagnosis. J Infect Dis. 2011;204 doi: 10.1093/infdis/jir411. suppl_4:S1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV - Policy update (2019). 2019;:27. https://www.who.int/tb/publications/2019/diagnose_tb_hiv/en/. Accessed 9 Mar 2021.
- 5.Lawn S.D., Kerkhoff A.D., Vogt M., Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: A descriptive study. Lancet Infect Dis. 2012;12(3):201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathabire Rucker S.C., Cossa L., Harrison R.E., Mpunga J., Lobo S., Kisaka Kimupelenge P., et al. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: a multisite study. Glob Health Action. 2019;12(1):1672366. doi: 10.1080/16549716.2019.1672366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjerrum S., Schiller I., Dendukuri N., Kohli M., Nathavitharana R.R., Zwerling A.A., et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019;2019(10) doi: 10.1002/14651858.CD011420.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broger T., Sossen B., du Toit E., Kerkhoff A.D., Schutz C., Ivanova Reipold E., et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis. 2019;19(8):852–861. doi: 10.1016/S1473-3099(19)30001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broger T., Nicol M.P., Sigal G.B., Gotuzzo E., Zimmer A.J., Surtie S., et al. Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest. 2020;130(11):5756–5764. doi: 10.1172/JCI140461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwaura M., Engel N. Constructing confidence: User perspectives on AlereLAM testing for tuberculosis. Int J Infect Dis. 2021;112:237–242. doi: 10.1016/j.ijid.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Huerga H., Mathabire Rucker S.C., Cossa L., Bastard M., Amoros I., Manhiça I., et al. Diagnostic value of the urine lipoarabinomannan assay in HIV-positive, ambulatory patients with CD4 below 200 cells/μl in 2 low-resource settings: A prospective observational study. PLOS Med. 2019;16(4):e1002792. doi: 10.1371/journal.pmed.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huerga H., Ferlazzo G., Bevilacqua P., Kirubi B., Ardizzoni E., Wanjala S., et al. Incremental yield of including determine-TB LAM assay in diagnostic algorithms for hospitalized and ambulatory HIV-positive patients in Kenya. PLoS ONE. 2017;12(1):e0170976. doi: 10.1371/journal.pone.0170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M., Hanrahan C., Wang Z.Y., Dendukuri N., Lawn S.D., Denkinger C.M., et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews. 2016 doi: 10.1002/14651858.CD011420.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Médecins Sans Frontières STP. Out of step 2017: TB policies in 29 countries—a survey of prevention, testing and treatment policies and practices. 2017. https://reliefweb.int/sites/reliefweb.int/files/resources/out_of_step_report_3rd_ed_july_2017.pdf. Accessed 3 Apr 2022.
- 15.Singhroy D.N., MacLean E., Kohli M., Lessem E., Branigan D., England K., et al. Adoption and uptake of the lateral flow urine LAM test in countries with high tuberculosis and HIV/AIDS burden: current landscape and barriers. Gates Open Res. 2020;4:24. doi: 10.12688/gatesopenres.13112.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beisel U., Umlauf R., Hutchinson E., Chandler C.I.R. The complexities of simple technologies: re-imagining the role of rapid diagnostic tests in malaria control efforts. Malar J. 2016;15:64. doi: 10.1186/s12936-016-1083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan D.L. Paradigms Lost and Pragmatism Regained. J Mix Methods Res. 2007;1:48–76. doi: 10.1177/2345678906292462. [DOI] [Google Scholar]
- 18.Creswell JW. Research design. Qualitative, quantitative & mixed methods approaches. 4th edition. California, London, New Dehli, Singapore: Sage Publications, Inc; 2014.
- 19.Helena Huerga, Mathieu Bastard, Alex Vicent Lubega, Milcah Ariong’o, Natalia Tamayo Antabak, Rosanna Stewart, Ivan Mugisha Taremwa, Liesbet Ohler, Winnie Muyindike the FSG. Diagnostic performance of the novel FujiLAM assay to detect tuberculosis in HIV-positive patients from four African countries. In: The 52nd Union World Conference on Lung Health. 2021.
- 20.Lissouba, Pascale; Akatukwasa, Cecilia; Atieno, Lucy; Akinyi, Milcah; Lubega, Alex; Muyindike, Winnie; Musoke, Mohammed; Mathabire Rücker, Sekai Chenai; Huerga H. Perspectives and perceptions of urine sampling and urine-based TB testing among patients in Kenya and Uganda. In: The 52nd Union World Conference on Lung Health. 2021. https://wclh2021.abstractserver.com/eposter/#/viewer/340.
- 21.Elo S., Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh H.-F., Shannon S.E. Three Approaches to Qualitative Content Analysis. Qual Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 23.Fetters M.D., Curry L.A., Creswell J.W. Achieving Integration in Mixed Methods Designs-Principles and Practices. Health Serv Res. 2013;48:2134–2156. doi: 10.1111/1475-6773.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petti C.A., Polage C.R., Quinn T.C., Ronald A.R., Sande M.A. Laboratory Medicine in Africa: A Barrier to Effective Health Care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 25.Olmsted S.S., Moore M., Meili R.C., Duber H.C., Wasserman J., Sama P., et al. Strengthening Laboratory Systems in Resource-Limited Settings. Am J Clin Pathol. 2010;134(3):374–380. doi: 10.1309/AJCPDQOSB7QR5GLR. [DOI] [PubMed] [Google Scholar]
- 26.Kaindjee-Tjituka F., Sawadogo S., Mutandi G., Maher A.D., Salomo N., Mbapaha C., et al. Task-shifting point-of-care CD4+ testing to lay health workers in HIV care and treatment services in Namibia. Afr. J Lab Med. 2017;6(1) doi: 10.4102/ajlm.v6i1.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wongkanya R., Pankam T., Wolf S., Pattanachaiwit S., Jantarapakde J., Pengnongyang S., et al. HIV rapid diagnostic testing by lay providers in a key population-led health service programme in Thailand. J virus Erad. 2018;4(1):12–15. doi: 10.1016/S2055-6640(20)30235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwangala S., Moland K.M., Nkamba H.C., Musonda K.G., Monze M., Musukwa K.K., et al. Task-Shifting and Quality of HIV Testing Services: Experiences from a National Reference Hospital in Zambia. PLoS ONE. 2015;10(11):e0143075. doi: 10.1371/journal.pone.0143075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuoka J., Poudel K.C., Poudel-Tandukar K., Nguon C., Ly P., Socheat D., et al. Assessing the quality of service of village malaria workers to strengthen community-based malaria control in Cambodia. Malar J. 2010;9:109. doi: 10.1186/1475-2875-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn C.M., Kagimu E., Okirworth M., Bangdiwala A.S., Mugumya G., Ramachandran P.S., et al. Fujifilm SILVAMP TB LAM Assay on Cerebrospinal Fluid for the Detection of Tuberculous Meningitis in Adults With Human Immunodeficiency Virus. Clin Infect Dis. 2021;73(9):e3428–e3434. doi: 10.1093/cid/ciaa1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak N., Choi S.M., Lee J., Park Y.S., Lee C.-H., Lee S.-M., et al. Diagnostic Accuracy and Turnaround Time of the Xpert MTB/RIF Assay in Routine Clinical Practice. PLoS ONE. 2013;8(10):e77456. doi: 10.1371/journal.pone.0077456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.