Abstract
The coronavirus disease 2019 (COVID-19) pandemic has spread rapidly, becoming a major threat to global health. In addition to having required the adaptation of healthcare workers for almost 2 years, it has been much talked about, both in the media and among the scientific community. Beyond lung damage and respiratory symptoms, the involvement of the cardiovascular system largely explains COVID-19 morbimortality. In this review, we emphasize that cardiovascular involvement is common and is associated with a worse prognosis, and that earlier detection by physicians should lead to better management. First, direct cardiac involvement will be discussed, in the form of COVID-19 myocarditis, then secondary cardiac involvement, such as myocardial injury, myocardial infarction and arrhythmias, will be considered. Finally, worsening of previous cardiovascular disease as a result of COVID-19 will be examined, as well as long-term COVID-19 effects and cardiovascular complications of COVID-19 vaccines.
Keywords: COVID-19, Cardiovascular system
Abbreviations: ACE2, angiotensin-converting enzyme 2; AF, atrial fibrillation; CHF, chronic heart failure; CMR, cardiovascular magnetic resonance; COVID-19, coronavirus disease 2019; LV, left ventricular; MI, myocardial infarction; mRNA, messenger ribonucleic acid; OHCA, out-of-hospital cardiac arrest; PE, pulmonary embolism; RV, right ventricular; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STEMI, ST-segment elevation myocardial infarction
1. Introduction
Since the end of 2019, the world has been facing a pandemic of unprecedented magnitude, caused by a new membrane-enveloped positive-sense single-stranded ribonucleic acid virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). From the Chinese epicenter, the pandemic has spread rapidly, and is now evolving in successive waves of varying intensity, becoming a major threat to global health [1,2]. By the end of 2021, an estimated five million people will have died from this pandemic, including about 115,000 healthcare workers worldwide [2].
Coronavirus disease 2019 (COVID-19) usually manifests as respiratory symptoms of varying gravity, ranging from totally asymptomatic forms or “flu-like syndrome” with anosmia, to acute respiratory distress syndrome, requiring admission to an intensive care unit and mechanical ventilation [3]. Despite the pulmonary tropism of the virus, many studies have emphasized the complex interplay between the cardiovascular system and SARS-CoV-2. First, descriptive works have shown that cardiac symptoms, such as chest pain or palpitations, are frequently at the forefront of the clinical presentation [1,4,5]. Second, COVID-19 is potentially the cause of a wide range of cardiovascular complications with different inflammatory and thrombotic pathomechanisms [6], [7], [8]. Finally, previous cardiac comorbidities and cardiovascular risk factors are frequent in patients hospitalized with COVID-19, and negatively impact the prognosis [9], [10], [11].
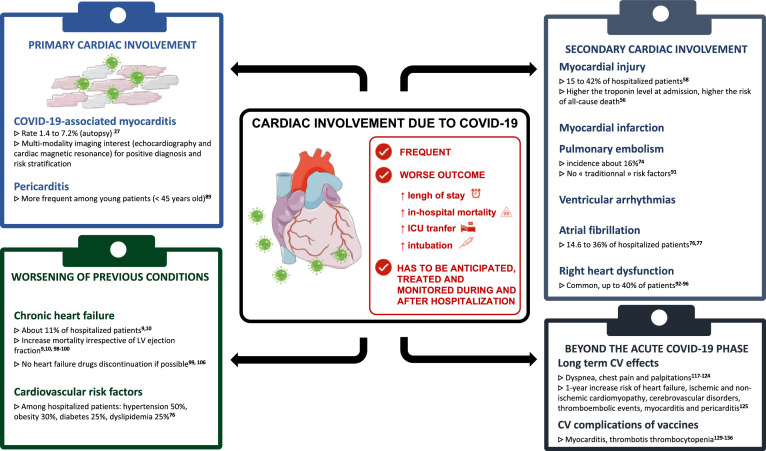
Thus, our aim was to review the available data on the impact of COVID-19 on the cardiovascular system, to alert physicians to its importance in explaining the morbimortality of the disease and to extract key messages for daily clinical practice. According to pathophysiology, the acute cardiovascular manifestations can be divided into three categories: (1) primary cardiac involvement; (2) secondary cardiac involvement; and (3) worsening of previous cardiovascular diseases. Finally, we address long-term cardiovascular complications, as well as vaccine-related consequences of COVID-19. We also provide tables summarizing the prognostic and/or diagnostic value of cardiac multimodality imaging and biomarkers in this context (Tables 1 and 2 , Fig. 1).
Table 1.
Cardiovascular manifestations secondary to COVID-19: The value of multimodality imaging.
| Echocardiography | CMR | Chest tomography | CCTA | General considerations | |
|---|---|---|---|---|---|
| Myocarditis | If clinical suspicion; assess LV function and kinetic abnormalitiesa | Positive diagnosis (Lake Louise Criteria)a | c | Rule out coronary artery diseaseb | Endomyocardial biopsy should not be performed routinely |
| Myocardial injury | c | c | c | c | The diagnosis does not require cardiac imaging, only troponin concentration |
| MI | Assess biventricular function; search for acute complications (i.e. mitral regurgitation, ventricular septal defect)a | c | c | c | The central issue is the timing of revascularization while minimizing infectious risks for the healthcare team |
| Pulmonary embolism | Not performed routinely in COVID-19 context; in case of hemodynamic instability, to rule out cardiogenic shockb | c | Certainty of diagnosis: CTPAa | c | CTPA is not always recommended for patients with COVID-19, but should be performed in case of suspicion or in the presence of risk factors |
| Right heart failure | Prognostic value of RV dysfunction and dilatationb | c | If pulmonary embolism is suspectedb | c | Right heart failure should not be routinely considered by physicians; however, if echocardiography has been requested for another reason, its presence is associated with worse outcome |
| Chronic heart failure decompensation | Certainty of diagnosis; etiology of acute heart failurea | Should not be performed routinelyb | c | c | All four exams may be of value, but not in the COVID-19 context |
Under normal circumstances, each exam may be of value, but in the COVID-19 pandemic, physicians have to deal with diagnostic efficiency and minimize the infectious risk for the healthcare team. CCTA: coronary computed tomography angiography; CMR: cardiac magnetic resonance; COVID-19: coronavirus disease 2019; CTPA: chest tomography pulmonary angiogram; LV: left ventricular; MI: myocardial infarction; RV: right ventricular.
Remains a priority in patients with COVID-19.
Not always a priority in patients with COVID-19.
Little value in patients with COVID-19.
Table 2.
Cardiovascular manifestations secondary to COVID-19: The role of biological findings.
| Natriuretic peptides (BNP or NT-proBNP) |
Troponin |
|||
|---|---|---|---|---|
| Prognostic value | Positive diagnosis | Prognostic value | Positive diagnosis | |
| Myocarditis | X | X | ||
| Myocardial injury | X | X | ||
| Myocardial infarction | X | X | X | |
| Pulmonary embolism | X | X | ||
| Right heart failure | X | X | ||
| Chronic heart failure decompensation | X | X | ||
BNP: brain natriuretic peptide; COVID-19: coronavirus disease 2019; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Fig. 1.
Central Illustration. Cardiovascular manifestations secondary to COVID-19.Abbreviations: ICU: intensive cardiac unit, LV: left ventricular, CV: cardiovascular.
2. Primary cardiac involvement
Cardiac involvement in COVID-19 is directly connected to SARS-CoV-2 pathophysiology. Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound enzyme that degrades angiotensin II to angiotensin-(1-7), resulting in vasodilator, antifibrotic and antiapoptotic effects [12,13]. This key receptor is not only expressed on the surface of lung alveolar epithelial cells (representing the main entry site for the virus), but also in a wide variety of tissues, such as heart (e.g. arterial smooth muscle cells and endothelial cells), kidney and small intestine [12], [13], [14]. This wide range of expression of ACE2 probably explains, at least in part, the pathophysiology of COVID-19 myocarditis.
Indeed, from the beginning of the pandemic, several case series have reported SARS-CoV-2-induced myocarditis with varying degrees of severity, sometimes leading to cardiogenic shock [15], [16], [17], [18], [19], [20], [21]. Most of these cases were only based on the association of a clinical context (i.e. confirmed SARS-CoV-2 infection and/or the presence of typical chest pain and/or dyspnea), troponin concentration increase and updated cardiac magnetic resonance (CMR) recommendations (Lake Louise Criteria) [22].
However, according to the position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases, the gold standard for the diagnosis of definite myocarditis is endomyocardial biopsy [23], so direct evidence of SARS-CoV-2 damage to cardiomyocytes remains limited. In 39 consecutive autopsies from Germany, Lindner et al. demonstrated that 16 of 39 cases (41%) had more than 1000 copy numbers of the virus (1000 viral copies) into the myocardium, but without myocarditis as defined by the Dallas Criteria [23,24]. Another autopsy case study showed that the virus can enter directly into endothelial heart cells, suggesting another potential mechanism for primary cardiac involvement [25]. In a systematic review, Kawakami et al. showed that myocarditis was uncommon in autopsies or endomyocardial biopsies; indeed, histologic evidence of myocarditis caused by SARS-CoV-2 was only found in about 4.5% of cases [26]. Finally, a larger autopsy cohort, including 277 subjects so far, has shown that COVID-19 related myocarditis occurs at a rate between 1.4 and 7.2% [27]. Finally, facing these scarce data, it remains difficult to state that myocarditis observed among COVID-19 patients is primarily related to a direct viral effect on the myocardium rather than a systemic inflammatory response and cytokines storm. Thus, it may be more appropriate to refer to “COVID-19-associated myocarditis” rather than “myocarditis related to COVID-19”.
In the clinical cases and cohorts mentioned previously, there were no clinical, biological or multimodality imaging specificities that allowed a diagnosis of COVID-19-associated myocarditis rather than myocarditis with another cause. Although myocarditis can present with varying degrees of clinical severity, ranging from mild symptoms to cardiac arrest as a result of ventricular arrhythmia and/or cardiogenic shock, the typical presentation combines acute chest pain with ST/T wave changes on the electrocardiogram (i.e. ST-segment elevation or depression or T wave inversions), with an increased cardiac troponin concentration, in the absence of angiographic evidence of coronary artery disease [23].
Regarding the use of endomyocardial biopsy to confirm the diagnosis, although specific recommendations in the context of COVID-19 have not been issued, it seems unreasonable to suggest this at-risk procedure for routine practice. An endomyocardial biopsy involves risks, albeit low (i.e. 0.03% risk of death, 1.2% risk of heart perforation) [28], its diagnostic yield is low and in the majority of cases it will not change management, as there are no proven effective treatments [26]. COVID-19-associated myocarditis mainly seems to concern young patients [29]. With methodological caution secondary to the absence of endomyocardial biopsy, we have recently found a significantly increased prevalence of myocarditis in patients aged 18–45 years among consecutive patients hospitalized in medical wards for COVID-19 [30].
By comparison, multimodality imaging is of value in this situation, to confirm the diagnostic suspicion and to stratify patient risk (e.g. of development of heart failure or life-threatening ventricular arrythmias) [31]. As mentioned in the American Society of Echocardiography/European Association for Cardiovascular Imaging recommendations in the COVID-19 context, echocardiography remains the first-line examination to be used – not in routine clinical practice, but only in case of diagnostic suspicion [32,33]; it depicts several ranges of left ventricular (LV) systolic dysfunction and kinetic abnormalities [34]. Then, CMR allows more precise tissue characterization, enabling a positive diagnosis with, typically, LV dysfunction, myocardial edema and subepicardial left gadolinium enhancement in case of fibrotic scar [16,34].
Pioneering work from Puntmann et al. alerted the cardiovascular community to the possible high prevalence of myocarditis during COVID-19 [35]. One hundred patients, recently recovered from COVID-19 in one center in Germany, were studied by CMR, and were compared with 50 age- and sex-matched healthy volunteers. The huge proportions of 78% of patients with cardiac involvement and 60% with myocardial inflammation highlighted the need for systematic investigation of the long-term cardiovascular consequences of COVID-19. However, these results should be interpreted with caution, given the definitions of cardiac abnormalities used in this study, their uncertain clinical significance and some statistical issues [36]. More recently, a robust study compared 74 patients with COVID-19, 6 months after infection, with 75 age-, sex- and ethnicity-matched control subjects, and did not find any differences in cardiac structure, function or tissue characterization by CMR [37].
As far as we know, no studies have compared the prognosis of COVID-19-associated myocarditis with that of other myocarditis. Interestingly, Huang et al. showed that more than half of patients who recovered from COVID-19 had abnormal CMR findings, such as myocardial edema and fibrosis, although suspicion of myocarditis was not raised [4]. Further studies with prospective follow-up will be needed to answer this question. Nevertheless, in the majority of cases, under appropriate medical therapy (i.e. beta-blockers and angiotensin-converting enzyme inhibitors), progressive LV systolic recovery is achieved [34].
3. Secondary cardiac involvement
Probably much more frequent than primary cardiac involvement, secondary cardiac involvement is related to indirect heart damage occurring during COVID-19. This entity can take on many forms, including myocardial injury, type I or II myocardial infarction (MI), sudden cardiac death, atrial fibrillation (AF), inflammatory myocarditis, acute pulmonary embolism or right ventricular (RV) failure.
3.1. Myocardial injury
According to the most recent universal definition of MI, the term myocardial injury should be used when there is evidence of elevated cardiac troponin values, at least above the 99th percentile of the upper reference limit [38]. This complication was described at the beginning of the pandemic in several studies of COVID-19 with very different levels of severity [1,3,4,[39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]]. In a large cohort study, including 2736 patients hospitalized with COVID-19 with a troponin measurement within the first 24 h of admission, Lala et al. showed that myocardial injury was common (in about 36% of patients). Furthermore, the higher the troponin concentration at admission, the higher the risk of all-cause death, including for patients without previous cardiovascular disease and/or risk factors [46]. Sandoval et al. showed that the frequency of myocardial injury increases with the severity of illness, ranging from 1% in COVID-19 survivors to 100% among the most severe forms. Moreover, patients with myocardial injury have a higher risk of acute respiratory distress syndrome, ventricular fibrillation and tachycardia [51]. A meta-analysis including more than 6000 patients found a prevalence of myocardial injury in COVID-19 ranging from 15% to 42% according to age and disease severity [52]; myocardial injury was again associated with all-cause mortality.
All of these elements highlight the value of troponin measurement for stratifying the risk of progression to a severe COVID-19 form, helping physicians with decision making when efficient triage is crucial (Table 2). Several mechanisms can explain myocardial injury, including inflammation and cytokine storm, prothrombotic and procoagulant state, imbalance between supply and dioxygen demand (i.e. critical illness, sepsis, acute respiratory failure) and direct viral toxicity [46,50]. It is important to emphasize that patients with myocardial injury do not require specific treatment except for their underlying conditions. Finally, even if prognostic significance of troponin increase has been highlighted, troponin on its own is not always relevant. Indeed, it must be always interpreted considering cardiovascular risk factors, comorbidities and above all clinical data.
3.2. MI and acute coronary syndrome
When there is clinical evidence of acute ischemic myocardial injury, the term MI should be used [38]. Type 1 MI (i.e. secondary to acute atherosclerosis plaque disruption) must be separated from type 2 MI, related to imbalance between oxygen demand and supply with no involvement of coronary atherothrombosis [38]. In a retrospective Italian cohort, Stefanini et al. showed that ST-segment elevation MI (STEMI) could be the first manifestation of COVID-19. In their study, 24 of 28 patients did not have a positive COVID-19 test before angiography [53]. In another study, Rodriguez-Leor et al. highlighted that patients with STEMI secondary to COVID-19 more frequently had acute heart failure at admission (P < 0.001) and increased in-hospital mortality (odds ratio 4.85, 95% confidence interval 2.04–11.51; P < 0.001) even after adjustment [54].
We should also mention that the first lockdown had a major impact on the incidence of MI. Indeed, several English, Italian and French studies emphasized a significant decrease in hospital admissions for both STEMI and non-STEMI, ranging from 12% to 40%, followed by an increase in out-of-hospital sudden cardiac death and long-term complications, such as heart failure [55], [56], [57], [58], [59]. This led to the writing of a position statement from the Society for Cardiovascular Angiography and Interventions, the American College of Cardiology and the American College of Emergency Physicians concerning the management of acute MI during the COVID-19 pandemic [60].
Finally, several cases of Takotsubo syndrome have been reported in the COVID-19 context [20,61,62]. This entity is classed as an MI with non-obstructive coronary arteries (MINOCA), implemented in the European guidelines in 2020 [63]. Takotsubo syndrome is acute reversible heart failure caused by acute severe stress, leading to sudden surges of catecholamines, which result in myocardial sideration and infarction. Although the prognosis of Takotsubo syndrome is generally good, with ad integrum recovery of LV function, there are no specific studies on Takotsubo prognosis in the context of COVID-19.
3.3. Arrhythmias
A direct consequence of both myocardial injury and MI, and the drop in in-hospital admissions for MI during the COVID-19 pandemic, was the increase in malignant arrhythmias (ventricular tachycardia and/or fibrillation) leading to sudden cardiac death. This is all the more true if there is an underlying arrhythmogenic substrate, such as ischemic cardiomyopathy [64], associated with a cytokine storm that acts as a trigger, and added to this a sympathetic hyperactivation, as can be encountered in the COVID-19 context, thus meeting the definition of the “Coumel triangle”, which is necessary for the genesis of an arrythmia.
Several studies have warned public health authorities about the significant increase in out-of-hospital cardiac arrests (OHCAs) during the COVID-19 pandemic and lockdown. Marijon et al. showed that the maximum weekly rate of OHCAs in Paris was twice as high as during the same period before the pandemic (13.42 to 26.64% per million inhabitants), and Lai et al. found that the rate was three times higher in New York City [65,66]. These two studies found a significant increase in OHCAs at home, with less bystander cardiopulmonary resuscitation and shockable rhythm, longer delays to intervention and lower survival rates at hospital admission. Although sudden cardiac deaths are related not only to COVID-19 infection, but also to underlying conditions (i.e. cardiovascular risk factors, ischemic and non-ischemic cardiomyopathy) coupled with the indirect effect of lockdown, Baldi et al., from Northern Italy, showed that incidence of OHCA was associated with the cumulative incidence of COVID-19 [67]. Turagam et al. showed that malignant arrythmia was not common (5%) among patients hospitalized with COVID-19, but was significantly more frequent among patients who died from COVID-19 [68].
AF is the most frequent arrythmia encountered nowadays [69]. In the COVID-19 context, AF was also highlighted as one of the most frequent comorbidities among hospitalized patients, ranging from 14.6% in the Critical COVID-19 France study [70] to 36% in a cohort from Northern Italy [71]. Musikantow et al. found a new-onset AF incidence rate of 4%, leading to a debate about whether AF should be considered as a “simple bystander” or a real risk factor for worse prognosis [72,73]. In a meta-analysis of 31 studies, Romiti et al. showed that AF during COVID-19 was associated with an increased risk of all-cause mortality (odds ratio 3.97, 95% confidence interval 2.76–5.71), which was consistent among patients with new-onset AF [74].
3.4. Venous thromboembolic complications
Another form of secondary cardiovascular involvement in the COVID-19 context is represented by venous thromboembolic complications and pulmonary embolism (PE). At the very beginning of the pandemic, and driven by the possibility of a proinflammatory state, with abnormal hemostasis, endothelial dysfunction, disseminated intravascular coagulation and a proinflammatory response caused by COVID-19 [75], [76], [77], several reports indicated a higher PE prevalence in the COVID-19 context than is usually encountered in non-infected critically ill patients [78], [79], [80], [81], [82], [83]. In a meta-analysis, Suh et al. showed that, among 27 seven studies that included 3342 patients with COVID-19, the incidence rate of PE was 16.5% and of deep vein thrombosis was 14.8% [84]. The Critical COVID-19 France study identified independent risk factors associated with the occurrence of PE, which did not include traditional thromboembolic risk factors, but rather a proinflammatory and coagulopathy state in the COVID-19 context, such as male sex, elevated C-reactive protein and time from symptom onset to hospitalization. By comparison, anticoagulation with a therapeutic dose before admission or anticoagulation with a prophylactic dose during hospitalization were PE protective factors [85]. PE pathophysiology in the COVID-19 context, as well as the possibility of chronic thromboembolic pulmonary hypertension as a result of COVID-19 will be addressed in another review.
3.5. Right heart involvement
As a direct consequence of both pulmonary vascular disease (e.g. pulmonary embolism, endothelial dysfunction) and parenchymal lung involvement, right heart failure also appears to be a major form of secondary cardiac involvement in the COVID-19 context. This reflects a maladaptation between the right heart and the pulmonary vascular tree, which can be worse if RV dysfunction was present before the infection, secondary to left heart disease and/or pulmonary hypertension. Several echocardiographic studies have demonstrated that right heart dysfunction is common among patients with COVID-19. Szekely et al. showed that RV dilatation and dysfunction was present in 39% of those with COVID-19 [86], which is in line with other studies [87], [88], [89], [90]. Further, RV dilatation and dysfunction have also been identified as independent prognostic factors among patients with COVID-19 [88,90]. Thus, and even if the echocardiography is not be performed systematically [91], in addition to the frequently used clinical and/or biological prognostic variables mentioned below, physicians must be aware that RV dysfunction and/or dilatation can also be useful to identify patients at high risk (i.e. of death and/or transfer to an intensive care unit).
4. Worsening of previous cardiovascular diseases
4.1. Chronic heart failure
Chronic heart failure (CHF) has rapidly emerged as one of the most important comorbidities among patients with COVID-19. Panagides et al [10]., from The Critical COVID-19 France study, found that 317 of 2809 hospitalized patients (11.3%) had a history of CHF, which is consistent with the study by Alvarez-Garcia et al [9].. Among more than 30,000 patients with a pre-existing CHF diagnosis, Rumery et al. showed that a positive COVID-19 reverse transcription polymerase chain reaction (RT-PCR) was associated with a 30-day hospital admission rate of 27% and a 30-day mortality rate of 37%, irrespective of age, sex, cardiovascular risk factors and comorbidities [92]; this was also found in other studies [9,10,93,94]. Another interesting finding from these studies was that survival in patients with COVID-19 was not driven by LV ejection fraction [9,10], which should prompt physicians to consider CHF history as being associated with death, regardless of the ejection fraction. This lack of LV ejection fraction effect may be explained by the fact that heart failure with preserved ejection fraction is, by nature, associated with several comorbidities and cardiovascular risk factors, such as hypertension and diabetes, which are also implicated in COVID-19 prognosis. In addition, CHF was also associated with significant two- and three-fold increases in the risk of intensive care unit transfer and intubation, respectively [9]. Experience with concentrations of natriuretic peptides (brain natriuretic peptide and N-terminal prohormone of brain-natriuretic peptide) in the COVID-19 context remains limited, yet the levels of these biomarkers seem to be correlated with disease severity and mortality, leading the European Society of Cardiology to recommend their assessment whenever heart failure is suspected [95].
Tomasoni et al. showed that CHF had a severe impact on clinical course among patients hospitalized with COVID-19, as it was significantly associated with an increased risk of acute heart failure, sepsis, acute kidney injury and multiorgan failure compared with controls, yet significantly less pulmonary embolism, probably related to a higher curative anticoagulation rate at admission [96].
At the start of the pandemic, several studies warned public health authorities about the interaction between angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers and COVID-19 severity [13,97,98]. Indeed, knowing that SARS-CoV-2 enters cells (primarily type II pneumocytes) by binding to its functional receptor, ACE2, it was hypothesized that inhibition of angiotensin-converting enzyme would upregulate ACE2, increasing SARS-CoV-2 entry into the organism and thus lung damage. Therefore, it was unclear whether to suspend these treatments in case of COVID-19. The BRACE-CORONA randomized control trial answered this question, showing that there were no statistical differences concerning length of hospital stay and all-cause mortality [99]. Nevertheless, very few patients with CHF were enrolled in this study (<2%), but Rey et al, in a retrospective study, showed that discontinuation of heart failure drugs (i.e. beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptors blockers, mineralocorticoid receptor antagonists) was associated with a higher rate of all-cause mortality [94]. Like other cardiovascular manifestations mentioned above, the first COVID-19 wave was associated with a decrease in hospitalization for acute heart failure, ranging from –30% to –60%, followed by a significant increase in mortality (+60%) following the first lockdown [100,101].
All of these data should encourage physicians to detect patients with heart failure early during COVID-19 hospitalizations, not to discontinue CHF drugs and to readdress patients to their attending cardiologist upon discharge.
4.2. Cardiovascular risk factors
Another main characteristic of patients hospitalized with COVID-19 is a high prevalence of cardiovascular risk factors. In a multicenter French study, Bonnet et al [70]. found that hypertension was found in half of patients (50.8%) and obesity in 30% of cases, whereas diabetes and dyslipidemia were present in about one quarter of patients (23.7% and 28%, respectively), and 13.5% of the cohort were smokers, which is consistent with other reports [1,4,[102], [103], [104]]. Diabetes, obesity and hypertension seem to be associated with worse COVID-19 forms and outcomes (i.e. in-hospital death, intensive care unit transfer and/or need for mechanical ventilation) [105], [106], [107], [108], [109], [110]. However, contradictory data exist concerning smoking habits [111], [112], [113]. We recently showed that young patients aged 18–45 years were less likely to present the composite of in-hospital death or intensive care unit transfer during COVID-19 compared with older patients [30], concordant with previous reports observing that old age is a strong risk factor for worse prognosis [114]. However, our analysis found a particular effect of COVID-19 on cardiovascular outcomes in young patients, with an increased prevalence of myocarditis and pericarditis, although thrombotic complications occurred with a similar incidence to that in older patients. Finally, Weizman et al. showed that although female sex was associated with a lower risk of intensive care unit transfer, the presence of pre-existing cardiovascular risk factors and disease among women was associated with high morbimortality [115].
It is therefore difficult to evaluate the impact of COVID-19 on the decompensation of these diseases because, to our knowledge, no study has specifically answered this question (unlike for heart failure and MI). Nevertheless, physicians must be attentive to treatment after hospitalization discharge, and encourage patients to resume specialized care to avoid a post-pandemic recrudescence of cardiovascular fatal events (i.e. acute heart failure, MI, stroke and sudden cardiac death).
5. Beyond the acute phase of COVID-19
5.1. Long-term effects of SARS-CoV-2 infection
Viral infections are known to have the potential to cause chronic cardiovascular effects. Previous data on severe acute respiratory syndrome coronavirus 1 suggested a worse cardiovascular prognosis after infection, and altered lipid metabolism [116]. Similarly, emergency department visits for influenza-like illness were correlated with cardiovascular disease mortality [117]. After the acute phase of COVID-19, a constellation of persistent symptoms, including cardiovascular symptoms, could persist [118,119]. Whereas dyspnea is the most frequent persistent symptom of a probable multifactorial etiology [118,120,121], chest pain is frequently reported long term after the acute phase of COVID-19; its prevalence is approximately 20% at 60 days after a moderate-to-severe form of the disease [118,122], and 5% at 6 months [120]. This frequent symptom echoes the different CMR abnormalities observed after the acute COVID-19 phase, as mentioned previously. However, the determinants and mechanisms of persistent chest pain remain incompletely understood, and future investigations will be required. Palpitations were also common, with a prevalence of 14% at 60 days [122] and 9% at 6 months [120]. In parallel, electrocardiographic changes and arrhythmias were observed via systematic screening in approximately one third of patients after a non-severe form of COVID-19 [123]. Finally, COVID-19 infection may have a long-term impact, with: (1) possible long-term sequalae of acute cardiovascular complications, such as pulmonary embolism, acute MI or myocarditis; (2) the disruption of routine follow-up of chronic cardiac conditions induced by the pandemic; and (3) persistent aforementioned symptoms, the mechanisms, prognosis and psychological impact of which are not well known.
Recently, Xie et al, reported the long-term cardiovascular outcomes of COVID-19 [124]. Studying a population of 153,760 COVID-19 patients (non-hospitalized, hospitalized and admitted to intensive care unit) compared to two sets of control patients including more than 10 millions of individuals, they found that: i) there was a significant 12-months increased of ischemic and non-ischemic heart disease, heart failure, thromboembolic events, cerebrovascular disorders, pericarditis and myocarditis, ii) this risk was consistent among non-hospitalized patients and higher according to the care setting during the acute COVID-19 phase. These results highlight the importance of close cardiovascular follow-up at least during the year following a COVID-19 but also of preventions messages that physicians should spread more widely to the population.
5.2. Cardiovascular complications of COVID-19 vaccines
Several cardiovascular complications have been associated with COVID-19 vaccination. The issue of myocarditis or pericarditis after a COVID-19 vaccine has been raised in several case reports after messenger ribonucleic acid (mRNA) vaccines (Pfizer-BioNTech and Moderna) [125], [126], [127]. Although it is difficult to estimate its precise prevalence, this complication seems to be uncommon [128,129], and is associated with favorable outcomes and recovery [130]. The most important study to date was based on surveillance data from the nationwide vaccination campaign in Israel, involving more than five million individuals [131]. The authors observed 136 cases of definite or probable myocarditis occurring in temporal proximity to two doses of the BNT162b2 mRNA vaccine (Pfizer-BioNTech). The risk of myocarditis seems to be higher among those aged 16–19 years, with a prevalence of approximately 1 in 6637 of this population. In another large study derived from an Israeli population with at least one dose of the BNT162b2 mRNA vaccine, the estimated incidence of myocarditis was 2.13 cases per 100,000 persons, with a higher prevalence among males aged 16–29 years, and a majority of cases of mild or moderate severity [129]. The mechanism of vaccine-induced myocarditis is not completely understood, but may be related to the immune response after vaccination, to the active component of the vaccine or to the mRNA sequence coding for the SARS-CoV-2 spike protein.
In addition to myocarditis, previous studies have suggested that SARS-CoV-2 vaccines with an adenovirus vector, including the ChAdOx1 nCov-19 vaccine (AstraZeneca), may cause thrombotic thrombocytopenia and subsequent thromboembolic complications [132,133]. A previous study showed a 1.97-fold increased risk of thromboembolic events among individuals who received the AstraZeneca vaccine compared with the general population [134]. The pathophysiology of this complication includes, at least in part, the generation of anti-PF4 antibodies, which activate platelets via specific receptors, an established mechanism also observed in heparin-induced thrombocytopenia [135]. Vaccine-induced immune thrombotic thrombocytopenia consists of venous or arterial thrombosis in atypical sites, such as cerebral sinus venous thrombosis, associated with mild-to-severe thrombocytopenia occurring a few days after the ChAdOx1 nCoV-19 vaccine.
Other COVID-19 vaccine side effects have been suspected, with an unclear causal relationship or very rare incidence: arrhythmia [136]; MI [137,138]; Takotsubo cardiomyopathy [139,140]; and systemic hypertension [141,142].
6. Conclusion
In this review, we have depicted the cardiovascular consequences of COVID-19; whether they are related to primary myocardial involvement, secondary myocardial involvement or decompensation of a pre-existing cardiovascular disease, they are always associated with a worse prognosis (Graphical abstract). Physicians need to be aware that beyond pulmonary damage, cardiovascular involvement must be sought out and treated. This pandemic continues to progress in successive waves of varying intensity with different variants. Further studies are required to fully understand the cardiovascular impact of COVID-19 during the acute phase and long term, and to describe the potential specific cardiovascular impact of some variants.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest concerning this article. Dr Fauvel reports grants from Pfizer and consulting fees from Janssen, outside the submitted work. Prof Cohen acknowledges the following without any relationship to the current manuscript: research grant from RESICARD (research nurses); and consultant and lecture fees from the companies Amgen, AstraZeneca, Bayer Pharma, Alliance BMS- Pfizer, Novartis, and Sanofi-Aventis.
References
- 1.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Corona- virus disease (COVID-19) outbreak (https://www.who.int).
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan I.H., Zahra S.A., Zaim S., Harky A. At the heart of COVID-19. J. Card. Surg. 2020;35(6):1287–1294. doi: 10.1111/jocs.14596. [DOI] [PubMed] [Google Scholar]
- 8.Schultheiss H.P., Baumeier C., Pietsch H., Bock C.T., Poller W., Escher F. Cardiovascular consequences of viral infections: from COVID to other viral diseases. Cardiovasc. Res. 2021;117(13):2610–2623. doi: 10.1093/cvr/cvab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Garcia J., Lee S., Gupta A., Cagliostro M., Joshi A.A., Rivas-Lasarte M., et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J. Am. Coll. Cardiol. 2020;76(20):2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagides V., Vincent F., Weizman O., Jonveaux M., Trimaille A., Pommier T., et al. History of heart failure in patients with coronavirus disease 2019: insights from a French registry. Arch. Cardiovasc. Dis. 2021;114(5):415–425. doi: 10.1016/j.acvd.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 15.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet N. Am. Ed. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garot J., Amour J., Pezel T., Dermoch F., Messadaa K., Felten M.L., et al. SARS-CoV-2 fulminant myocarditis. JACC Case Rep. 2020;2(9):1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luetkens J.A., Isaak A., Zimmer S., Nattermann J., Sprinkart A.M., Boesecke C., et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging. 2020;13(5) doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 19.Paul J.F., Charles P., Richaud C., Caussin C., Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur. Heart J. Cardiovasc. Imaging. 2020;21(7):776. doi: 10.1093/ehjci/jeaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala S., Peretto G., Gramegna M., Palmisano A., Villatore A., Vignale D., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J. Am. Coll. Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 23.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2013;34(33):2648a–2649d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 24.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox S.E., Li G., Akmatbekov A., Harbert J.L., Lameira F.S., Brown J.Q., et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142(11):1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami R., Sakamoto A., Kawai K., Gianatti A., Pellegrini D., Nasr A., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J. Am. Coll. Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halushka M.K., Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U., et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American heart association, the American college of cardiology, and the European society of cardiology endorsed by the heart failure society of America and the heart failure association of the European society of cardiology. Eur. Heart J. 2007;28(24):3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 29.Ukimura A., Satomi H., Ooi Y., Kanzaki Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res. Treat. 2012;2012 doi: 10.1155/2012/351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trimaille A., Ribeyrolles S., Fauvel C., Chaumont C., Weizman O., Pommier T., et al. Cardiovascular characteristics and outcomes of young patients with COVID-19. J. Cardiovasc. Dev. Dis. 2021;8(12):165. doi: 10.3390/jcdd8120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudski L., Januzzi J.L., Rigolin V.H., Bohula E.A., Blankstein R., Patel A.R., et al. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19: JACC scientific expert panel. J. Am. Coll. Cardiol. 2020;76(11):1345–1357. doi: 10.1016/j.jacc.2020.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020;33(6):648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., Salvo G.D., Donal E., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging. 2020;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agricola E., Beneduce A., Esposito A., Ingallina G., Palumbo D., Palmisano A., et al. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc. Imaging. 2020;13(8):1792–1808. doi: 10.1016/j.jcmg.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagel E., Puntmann V.O. Errors in statistical numbers and data in study of cardiovascular magnetic resonance imaging in patients recently recovered from COVID-19. JAMA Cardiol. 2020;5(11):1307–1308. doi: 10.1001/jamacardio.2020.4661. [DOI] [PubMed] [Google Scholar]
- 37.Joy G., Artico J., Kurdi H., Seraphim A., Lau C., Thornton G.D., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc. Imaging. 2021;14(11):2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., et al. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 39.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. COVID-19 in critically ill patients in the seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandoval Y., Januzzi J.L., Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J. Am. Coll. Cardiol. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu L., Liu X., Su Y., Ma J., Hong K. Prevalence and impact of cardiac injury on COVID-19: a systematic review and meta-analysis. Clin. Cardiol. 2021;44(2):276–283. doi: 10.1002/clc.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Leor O., Cid Alvarez A.B., Pérez de Prado A., Rossello X., Ojeda S., Serrador A., et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426–1433. doi: 10.4244/EIJ-D-20-00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Rosa S., Spaccarotella C., Basso C., Calabrò M.P., Curcio A., Filardi P.P., et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammad T.A., Parikh M., Tashtish N., Lowry C.M., Gorbey D., Forouzandeh F., et al. Impact of COVID-19 pandemic on ST-elevation myocardial infarction in a non-COVID-19 epicenter. Catheter. Cardiovasc. Interv. 2021;97(2):208–214. doi: 10.1002/ccd.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mafham M.M., Spata E., Goldacre R., Gair D., Curnow P., Bray M., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mesnier J., Cottin Y., Coste P., Ferrari E., Schiele F., Lemesle G., et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5(10):e536–e542. doi: 10.1016/S2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rangé G., Hakim R., Beygui F., Angoulvant D., Marcollet P., Godin M., et al. Incidence, delays, and outcomes of STEMI during COVID-19 outbreak: analysis from the France PCI registry. J. Am. Coll. Emerg. Phys. Open. 2020;1(6):1168–1176. doi: 10.1002/emp2.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahmud E., Dauerman H.L., Welt F.G.P., Messenger J.C., Rao S.V., Grines C., et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP) J. Am. Coll. Cardiol. 2020;76(11):1375–1384. doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer P., Degrauwe S., Van Delden C., Ghadri J.R., Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur. Heart J. 2020;41(19):1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortuno S., Jozwiak M., Mira J.P., Nguyen LS. Case report: takotsubo syndrome associated with novel coronavirus disease 2019. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.614562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collet J.P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 64.Mitacchione G., Schiavone M., Gasperetti A., Forleo GB. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur. Heart J. Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lai P.H., Lancet E.A., Weiden M.D., Webber M.P., Zeig-Owens R., Hall C.B., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5(10):1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marijon E., Karam N., Jost D., Perrot D., Frattini B., Derkenne C., et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5(8):e437–e443. doi: 10.1016/S2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baldi E., Sechi G.M., Mare C., Canevari F., Brancaglione A., Primi R., et al. Out-of-hospital cardiac arrest during the covid-19 outbreak in Italy. N. Engl. J. Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turagam M.K., Musikantow D., Goldman M.E., Bassily-Marcus A., Chu E., Shivamurthy P., et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ. Arrhythm. Electrophysiol. 2020;13(11) doi: 10.1161/CIRCEP.120.008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 70.Bonnet G., Weizman O., Trimaille A., Pommier T., Cellier J., Geneste L., et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: the critical COVID-19 France (CCF) study. Arch. Cardiovasc. Dis. 2021;114(5):352–363. doi: 10.1016/j.acvd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur. Heart J. 2020;41(32):3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchis-Gomar F., Perez-Quilis C., Lavie CJ. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID-19 patients? Eur. Heart J. 2020;41(32):3092–3093. doi: 10.1093/eurheartj/ehaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romiti G.F., Corica B., Lip G.Y.H., Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID-19: a systematic review and meta-analysis. J. Clin. Med. 2021;10(11):2490. doi: 10.3390/jcm10112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. http://www.onlinejacc.org/content/early/2020/04/15/j.jacc.2020.04.031 [Internet]Apr 17 [cited 2020 Apr 22]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.G.B. Danzi, M. Loffi, G. Galeazzi, E. Gherbesi Acute pulmonary embolism and COVID-19 pneumonia: a random association?. Eur. Heart J. 41(19):1858 [Internet]. [cited 2020 Apr 22]; Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehaa254/5813284 [DOI] [PMC free article] [PubMed]
- 79.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;23 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7146714/ [Internet] Apr 10 [cited 2020 Apr 22]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-Dimer levels. Radiology. 2020;23 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 83.Trimaille A., Curtiaud A., Marchandot B., Matsushita K., Sato C., Leonard-Lorant I., et al. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb. Res. 2020;193:166–169. doi: 10.1016/j.thromres.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suh Y.J., Hong H., Ohana M., Bompard F., Revel M.P., Valle C., et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2):E70–E80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.C. Fauvel, O. Weizman, A. Trimaille, D. Mika, T. Pommier, N. Pace, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur. Heart J. 41(32):3058-3068. [Internet]. [cited 2020 Jul 14]; Available from:https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehaa500/5870571 [DOI] [PMC free article] [PubMed]
- 86.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19) - a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J., Volodarskiy A., Sultana R., Pollie M.P., Yum B., Nambiar L., et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(17):1965–1977. doi: 10.1016/j.jacc.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Li H., Zhu S., Xie Y., Wang B., He L., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc. Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. http://www.sciencedirect.com/science/article/pii/S1936878X20303429 [Internet] Apr 28 [cited 2020 Jul 14]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soulat-Dufour L., Fauvel C., Weizman O., Barbe T., Pezel T., Mika D., et al. Prognostic value of right ventricular dilatation in patients with COVID-19: a multicentre study. Eur. Heart J. Cardiovasc. Imaging. 2021;23(4):569–577. doi: 10.1093/ehjci/jeab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jain S.S., Liu Q., Raikhelkar J., Fried J., Elias P., Poterucha T.J., et al. Indications and findings on transthoracic echocardiogram in COVID-19. J. Am. Soc. Echocardiogr. 2020 doi: 10.1016/j.echo.2020.06.009. S089473172030376X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rumery K., Seo A., Jiang L., Choudhary G., Shah N.R., Rudolph J.L., et al. Outcomes of coronavirus disease-2019 among veterans with pre-existing diagnosis of heart failure. ESC Heart Fail. 2021;8(3):2338–2344. doi: 10.1002/ehf2.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhatt A.S., Jering K.S., Vaduganathan M., Claggett B.L., Cunningham J.W., Rosenthal N., et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9(1):65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rey J.R., Caro-Codón J., Rosillo S.O., Iniesta Á.M., Castrejón-Castrejón S., Marco-Clement I., et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 2020;22(12):2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.The Task Force for the management of COVID-19 of the European Society of Cardiology. Baigent C., Windecker S., Andreini D., Arbelo E., Barbato E., et al. European society of cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1—epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2021;43(11):1033–1058. doi: 10.1093/eurheartj/ehab696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomasoni D., Inciardi R.M., Lombardi C.M., Tedino C., Agostoni P., Ameri P., et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the cardio-COVID-Italy multicentre study. Eur. J. Heart Fail. 2020;22(12):2238–2247. doi: 10.1002/ejhf.2052. [DOI] [PubMed] [Google Scholar]
- 97.G.M. Kuster, O. Pfister, T. Burkard, Q. Zhou, R. Twerenbold, P. Haaf, et al. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. [Internet]. [cited 2020 Apr 22]; Available from: 10.1093/eurheartj/ehaa235/5810479 [DOI] [PMC free article] [PubMed]
- 98.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: What is the evidence? JAMA. 2020;323(18):1769–1770. doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 99.Lopes R.D., Macedo A.V.S., de Barros E Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bromage D.I., Cannatà A., Rind I.A., Gregorio C., Piper S., Shah A.M., et al. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur. J. Heart Fail. 2020;22(6):978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doolub G., Wong C., Hewitson L., Mohamed A., Todd F., Gogola L., et al. Impact of COVID-19 on inpatient referral of acute heart failure: a single-centre experience from the south-west of the UK. ESC Heart Fail. 2021;8(2):1691–1695. doi: 10.1002/ehf2.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. Feb 28; NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGurnaghan S.J., Weir A., Bishop J., Kennedy S., Blackbourn L.A.K., McAllister D.A., et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sutter W., Duceau B., Vignac M., Bonnet G., Carlier A., Roussel R., et al. Association of diabetes and outcomes in patients with COVID-19: propensity score-matched analyses from a French retrospective cohort. Diabetes Metab. 2021;47(4) doi: 10.1016/j.diabet.2020.101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei Z.Y., Qiao R., Chen J., Huang J., Wu H., Wang W.J., et al. The influence of pre-existing hypertension on coronavirus disease 2019 patients. Epidemiol. Infect. 2021;149:e4. doi: 10.1017/S0950268820003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J., Wu J., Sun X., Xue H., Shao J., Cai W., et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: a meta-analysis. Epidemiol. Infect. 2020;148:e106. doi: 10.1017/S095026882000117X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lutchman D. Could the smoking gun in the fight against COVID-19 be the (rh)ACE-2? Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.01560-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rossato M., Russo L., Mazzocut S., Di Vincenzo A., Fioretto P., Vettor R. Current smoking is not associated with COVID-19. Eur. Respir. J. 2020;55(6) doi: 10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weizman O., Mika D., Cellier J., Geneste L., Trimaille A., Pommier T., et al. Characteristics and impact of cardiovascular comorbidities on coronavirus disease 2019 in women: a multicentre cohort study. Arch. Cardiovasc. Dis. 2021;114(5):394–406. doi: 10.1016/j.acvd.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Q., Zhou L., Sun X., Yan Z., Hu C., Wu J., et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci. Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., Pérez-Contreras M., Fernández-Martínez N.F., Ruiz-Montero R., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(1):129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou M., Wong C.K., Un K.C., Lau Y.M., Lee J.C.Y., Tam F.C.C., et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE. 2021;16(2) doi: 10.1371/journal.pone.0246732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matta A., Kallamadi R., Matta D., Bande D. Post-mRNA COVID-19 vaccination myocarditis. Eur. J. Case Rep. Intern Med. 2021;8(8) doi: 10.12890/2021_002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tailor P.D., Feighery A.M., El-Sabawi B., Prasad A. Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine. Eur. Heart J. Case Rep. 2021;5(8):ytab319. doi: 10.1093/ehjcr/ytab319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Visclosky T., Theyyunni N., Klekowski N., Bradin S. Myocarditis following mRNA COVID-19 vaccine. Pediatr. Emerg. Care. 2021;37(11):583–584. doi: 10.1097/PEC.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 128.Hajjo R., Sabbah D.A., Bardaweel S.K., Tropsha A. Shedding the light on post-vaccine myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients. Vaccines (Basel) 2021;9(10):1186. doi: 10.3390/vaccines9101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after COVID-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shiyovich A., Witberg G., Aviv Y., Eisen A., Orvin K., Wiessman M., et al. Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur. Heart J. Cardiovasc. Imaging. 2021;00:1–8. doi: 10.1093/ehjci/jeab230. [DOI] [PubMed] [Google Scholar]
- 131.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gupta A., Sardar P., Cash M.E., Milani R.V., Lavie CJ. COVID-19 vaccine- induced thrombosis and thrombocytopenia-a commentary on an important and practical clinical dilemma. Prog. Cardiovasc. Dis. 2021;67:105–107. doi: 10.1016/j.pcad.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sadoff J., Davis K., Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination - response from the manufacturer. N. Engl. J. Med. 2021;384(20):1965–1966. doi: 10.1056/NEJMc2106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marchandot B., Carmona A., Trimaille A., Curtiaud A., Morel O. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J. Thromb. Thrombolysis. 2021;52(3):689–691. doi: 10.1007/s11239-021-02505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reddy S., Reddy S., Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus. 2021;13(5):e14837. doi: 10.7759/cureus.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chatterjee S., Ojha U.K., Vardhan B., Tiwari A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab. Syndr. 2021;15(3):1055–1056. doi: 10.1016/j.dsx.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tajstra M., Jaroszewicz J., Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc. Interv. 2021;14(9):e103–e104. doi: 10.1016/j.jcin.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crane P., Wong C., Mehta N., Barlis P. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021;14(10) doi: 10.1136/bcr-2021-246580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jani C., Leavitt J., Al Omari O., Dimaso A., Pond K., Gannon S., et al. COVID-19 vaccine-associated Takotsubo cardiomyopathy. Am. J. Ther. 2021;28(3):361–364. doi: 10.1097/MJT.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 141.Meylan S., Livio F., Foerster M., Genoud P.J., Marguet F., Wuerzner G., et al. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021;77(6):e56–e57. doi: 10.1161/HYPERTENSIONAHA.121.17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zappa M., Verdecchia P., Spanevello A., Visca D., Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur. J. Intern. Med. 2021;90:111–113. doi: 10.1016/j.ejim.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]