Abstract
Since most of the reports of BRONJ onset are adults, in order to clarify the current situation of BRONJ onset in children, it is necessary to search for articles and report on the current status and actual conditions of surgical treatment of children with BP preparations who are being followed up in our clinic.
In previous reports both inside and outside Japan, there was no mention of jaw bone necrosis during tooth extraction or surgery in children who were receiving or had a history of BP administration.
There were 15 children with a history of BP administration who manage the oral cavity in our clinic. No unpleasant events in the extraction of deciduous teeth were confirmed in medical records. It is necessary to intervene early on oral management of pediatric BP-administered children, especially BP-and steroid-administered children, obtain plaque control to keep the oral cavity cleaner, respond early to infectious diseases, and manage to prevent inflammation from spreading to the jawbone. When surgical treatment is unavoidable, it is important to consider reducing the invasion as much as possible and to cooperate with the medical department such as administration of antibiotics to prevent infection.
Keywords: BRONJ, Bisphosphonate, Osteogenesis imperfecta, Osteoporosis, Children, Tooth extraction
1. Introduction
In the field of pediatric dentistry, the frequency of encountering children who have received bisphosphonate (BP) preparations is not high in clinical practice. Surgical treatment, such as tooth extraction, is required in some children, but impaired tooth eruption accounts for the greater portion [1]. Desiring spontaneous replacement if possible, we have confirmed a position paper, and managed individual conditions in consultation with attending pediatricians if necessary.
BP potently inhibits bone resorption by suppressing osteoclasts. It is routinely used in cancer patients with bone metastasis or osteoporosis patients. In 2003, a study indicated the development of BP-related osteonecrosis of the jaw (BRONJ) as an adverse reaction in the sockets of BP-treated patients [2]. Thereafter, many patients with BRONJ were also reported in Japan [1], [3], [4], [5]. BRONJ induces serious symptoms, such as infectious exposure of the jaw, severe persistent pain, gingival swelling, and purulent discharge. It was reported that cutaneous fistula formation or jaw fracture occurred with the exacerbation of infection even when BRONJ was asymptomatic in the absence of pain [6]. Since the first report was published, the accumulation/analysis of many patients have deepened the understanding of BRONJ, contributing to preventive strategies. As a new drug, denosumab, was used. This drug is a human IgG2 monoclonal antibody preparation against RANKL, which suppresses bone resorption by osteoclasts like BP. But it was expected that osteonecrosis of the jaw (ONJ) would not occur because of its short half-life, no deposition or residue on bone, and no induction of apoptosis in osteoclasts [7].
Contrary to this expectation, ONJ (DRONJ, denosumab-related ONJ) developed at a similar rate [8]; Although the mechanism of action is different, both are drugs that suppress bone resorption by osteoclasts and are involved in the onset of clinically similar ONJ, so BRONJ and DRONJ has been comprehensively termed the name anti-resorptive agent-related ONJ (ARONJ). In addition, the American Association of Oral and Maxillofacial Surgery (AAOMS) has proposed the name medication-related ONJ (MRONJ) because the delivery rate of BRONJ and DRONJ increases due to the administration of drugs used in combination with anticancer drugs in cancer treatment [9].
In Japan, the incidences of BRONJ after the use of injection and an oral preparation were estimated to be 1–2% and 0.01–0.02%, respectively. Tooth extraction increases the risk of BRONJ, being the most important risk factor [4]. A study reported that the incidence of ONJ in cancer patients was higher than in patients with osteoporosis [8].
In the jaw, infection-prone environmental factors are present in comparison with other bones: the presence of the gap between the epithelium and teeth, which may be an oral source of infection, a direct approach to the jaw through the root canal, presence of oral bacteria as a source of infection, and inflammation of the jaw mediated by infectious diseases, such as caries and periodontal disease. These factors may be involved in the onset of BRONJ [10].
Most case reports on BRONJ involve adults, especially elderly patients. To clarify the current status of BRONJ in children, we report the actual status of surgical treatment in BP-preparation-treated children during follow-up in our department, and review the literature.
2. Presentations and articles in Japan and other countries
We searched for articles with keywords, such as BP, children, and osteonecrosis of the jaw using the Central Medical Journal (Japanese) and PubMed, but few reports and reviews were identified. Initially, as a domestic report, there was a case report presented as a poster at a meeting held by the Japanese Society of Pediatric Dentistry in 2009 [11]. An 8-year-and-6-month-old boy with systemic juvenile idiopathic arthritis had received a steroid over a long period. As a BP preparation, Alendronate had been administered for 10 months before tooth extraction. At the time of treatment, an antimicrobial drug was prophylactically administered in cooperation with the Department of Pediatrics, and a primary tooth remaining in the late phase was extracted. After treatment, there were no abnormal findings. The authors suggested that the risk of BRONJ is low in young patients, but concluded that it was necessary to manage BP-treated patients in cooperation with the Department of Pediatrics or Department of Internal Medicine while maintaining a favorable oral hygiene state. One case report was found internationally. In this report, a 6 years 6 months year child, who was receiving bisphosphonate therapy for osteogenesis imperfecta, had numerous teeth restored and multiple primary molar extractions in the operation room under general anesthesia. The patient received prophylactic antibiotics intraoperatively, so demonstrated no clinical signs of BP-associated osteonecrosis when seen at follow-up [12]. The authors concluded that consent for extraction should include the risk of bone necrosis and careful post-operative observation to monitor would healing for receiving multiple extraction on the child with BP- treated osteogenesis imperfecta.
As many children with osteogenesis imperfecta are treated with BP preparations, a survey involving 26 children was conducted in 2014, and the results of analysis based on chart information on 67 primary teeth extracted due to exchange-phase disturbance in 13 children were reported [13]. The authors suggested that there are no problems regarding exchange-phase primary tooth extraction even in BP-treated children, because such tooth extraction was not problematic.
Internationally, in 2008, Schwartz et al. [14], Malmgren et al. [15], and Maines et al. [16] investigated the relationship between BP administration and tooth extraction/surgical treatment in children with osteogenesis imperfecta, and reported that there was no ONJ. Brown et al. [17] and Chahine et al. [18] reported tooth extraction or surgical treatment in BP-treated children with osteogenesis imperfecta or those with other diseases, respectively, and indicated that there were no abnormal findings, such as osteonecrosis of the jaw. Furthermore, Chriostou et al. [19] indicated that adulthood BRONJ had been reported in detail, whereas there had been no similar case in children. In addition, they reviewed the background of BP, indication of prescriptions for children, adverse reactions, especially BRONJ, and a protocol available for dental management guidance. As there was no public recommendation with respect to oral management for BP-treated children, Bhatt et al. proposed recommendations for dental management in BP-treated children [20]: a dental management decision pathway and risk-factor-based preoperative administration to high-risk children.
Thus, no study has reported osteonecrosis of the jaw related to tooth extraction or surgical treatment in children receiving BP or those who had received it.
3. Current/actual status of surgical treatment in BP-treated children during follow-up in our clinic
There were 15 BP-treated children in whom our department was responsible for oral management (Table 1). One-third of these (case10–15 in Table 1) had osteogenesis imperfecta, followed by Langerhans cell histiocytosis (case 1–3 in Table 1). Two children (case 6, 8 in Table 1) had received long-term steroid therapy. Eight children had experienced tooth extraction; exchange-phase primary teeth were extracted in most children. In 2 of these (case 7, 8 in Table 1), tooth extraction was performed during BP administration. There was no description of uncomfortable events related to primary tooth extraction in any medical records. As our department was responsible for oral management, there was no tooth requiring tooth extraction in the presence of inflammation. Furthermore, primary teeth with advanced root resorption were extracted in many children, and most children were followed-up in the Department of Pediatrics in our hospital; therefore, the risk of ONJ may have been low because of cooperation with the Department of Pediatrics.
Table 1.
Children with experience of BP administration who are managing the oral cavity in our clinic.
| NO | Diagnosis | date at start | sex | first visit to our clinic | BP | Administration period | invasive dental procedore | |
|---|---|---|---|---|---|---|---|---|
| 1 | langerhans cell histiocytosis | 2009.6.8 | 3y1m | m | 2009.7.1 | zoledronic acid | 2009.6–12 | 2012.7 upper right primary central incisor, lower right primary lateral and central incisor, lower left primary central incisor extraction (root resorption)2014.2 lower left primary lateral incisor extraction |
| 2 | langerhans cell histiocytosis | 2005.10.12 | 2y1m | f | 2005.11.14 | zoledronic acid | 2009.6–2011.8 | 2011.12 upper right primary central and lateral incisor, upper left primary central and lateral incisor extraction (Almost no roots)2013.2 lower left primary canine extraction |
| 3 | langerhans cell histiocytosis | 2007.5.31 | 1y6m | m | 2007.5.31 | zoledronic acid | 2009.4–12 | 2010.1 lower right second primary molar, lower left first and second primary molar extraction2014.8 upper left primary lateral incisor extraction |
| 4 | neuroblastoma | 2008.2.14 | 2y1m | f | 2008.2.14 | zoledronic acid | 2009.2–2010.6 | 2014.1 lower right and left second primary molar hemi section+convenient tooth extraction |
| 5 | juvenile dermatomyositis | 2014.2.21 | 9y2m | f | 2014.3.5 | risedronate sodium | 2014.3–2015.8 | |
| 6 | systemic onset juvenile idiopathic arthritis | 2010.8.12 | 5y8m | m | 2010.11.22 | risedronate sodium | 2015.4- | |
| 7 | fibrous bone atypicality symptom | 2003.5.12 | 9y0m | m | 2007.12.13 | alendronate sodium hydrate | 2004.4–2013.8 | 2009.11 upper left first primary molar extraction (root resorption) |
| 8 | early-onset sarcoidosis | 1991.1.11 | 0y9m | f | 2004.6.25 | alendronate sodium hydrate | 2001.11–2014.2 | 2004.7 upper left second primary molar extraction 2004.9 lower right second molar fenestration2005.5 lower right third molar extraction 2006 3 lower right second molar extraction |
| 9 | chronic myeloid leukemia | 2005.1 | 6y8m | m | 2012.5.2 | alendronate sodium hydrate | 2009.6–8 | 2012.7 upper right and left second primary molar extraction2016.8 supernumerary tooth extraction |
| pamidronate disodium | 2009.9–2010.8 | |||||||
| 10 | osteogenesis imperfectaⅢ | 2009.1.19 | f | 2010.9.22 | pamidronate disodium | 2011.1- | ||
| 11 | osteogenesis imperfectaⅢ | 2006.1.8 | f | 2008.9.9 | pamidronate disodium | 2006,1–2016.1 | ||
| 12 | osteogenesis imperfectaⅢ | 2012.7.4 | f | 2015.12.25 | pamidronate disodium | under administration | ||
| 13 | osteogenesis imperfectaⅢb | f | 2008.12.16 | pamidronate disodium | under administration at the referral visit | |||
| 14 | osteogenesis imperfectaⅣb | 2013.1 | 6y1m | f | 2009.11.6 | pamidronate disodium | 2013.8–2015.12 | |
| 15 | osteogenesis imperfectaⅤ Pierre Robin syn | 2011.7.29 | 4y0m | m | 2014.1.7 | pamidronate disodium | 2012.1–2014.10 | 2015.1 upper right primary central and lateral incisor, upper left primary central incisor extraction |
4. A patient with a poor long-term outcome
Of the children presented in Table 1, one in whom delayed healing after cystectomy involving a tooth. (case 8 in Table 1).
The patient is a girl with the initial consultation at the age of 14 years and 3 months.
Her chief complaint was caries treatment.
The medical history is described below. The diagnosis of sarcoidosis was made at the age of 10 months. Since then, steroid and immunosuppressive therapies had been continued.
Later, she was diagnosed with Cushing’s syndrome related to long-term steroid therapy.
In particular, steroid-induced osteoporosis was observed, but withdrawal from steroids was impossible due to cardiac sarcoidosis, and oral administration was continued.
Steroid-induced osteoporosis led to compression fracture of the 3rd lumbar vertebra at 11 years of age. To achieve bone mass recovery, the use of a BP (Alendronate) preparation was started, and this treatment is being continued.
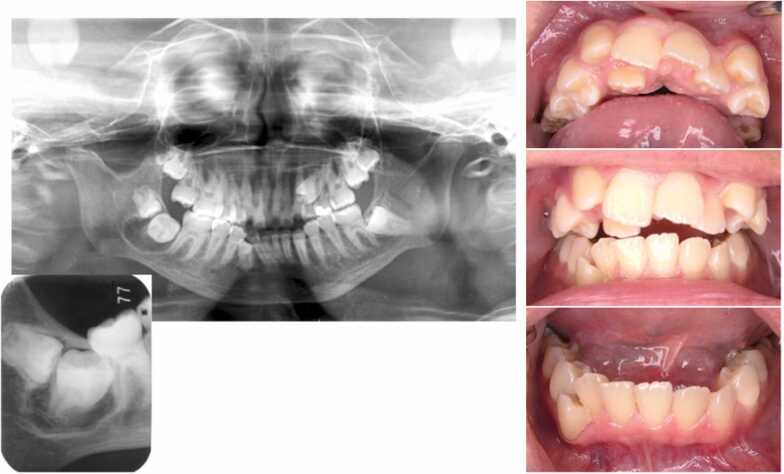
As the physical examination, it was found that lower left first molar was dentin caries, upper left second primary molar prolonged retention, upper left second premolar was malposition/impaction, and lower right second and third molar tooth was containing cyst (Fig. 1).
Fig. 1.
An intraoral photograph and X-ray at the first visit out clinic (14 years and 3 months). It is indicated lower left first molar dentin caries, upper left second primary molar prolonged retention, upper left second premolar malposition and impaction and lower right third molar and second molar follicular dental cyst.
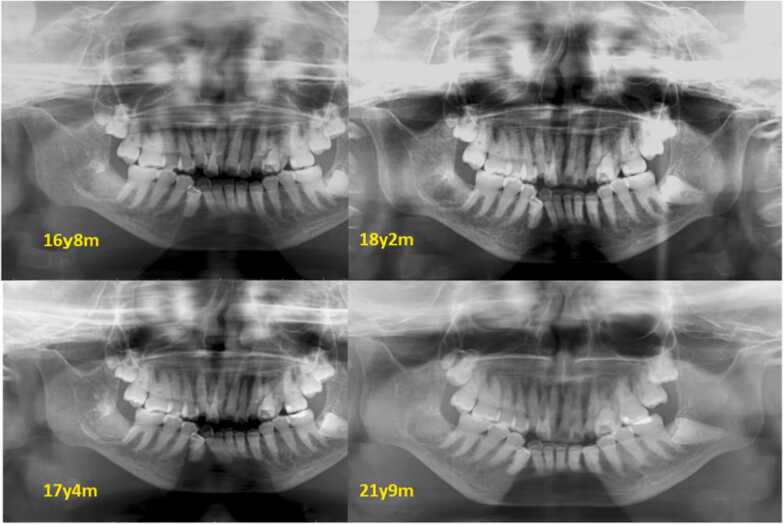
Treatment and course was CR repair of lower left first molar, the extraction of upper left second primary molar (at 14 years and 4 months of age), lower right second and third molar fenestration at the tooth-containing cyst site (at 14 years and 6 months of age), lower right third molar tooth extraction (at 15 years and 5 months of age), and lower right second molar tooth extraction (at 16 years and 2 months of age) Fig. 2, Fig. 3.
Fig. 2.
Secular change of the extraction area (Panoramic X-ray photograph).
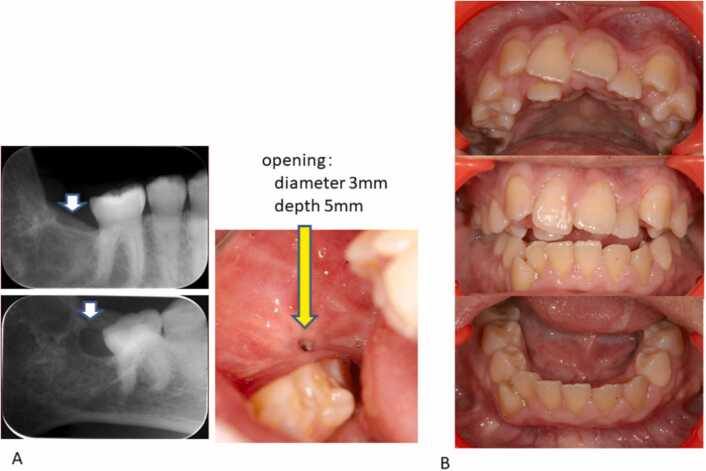
Fig. 3.
Dental X-ray photograph and the intraoral photograph. A dental X-ray and intraoral photogarph of lower right second molar equivalency area. B intraoral photograph (22 year and 6 moths old).
After that, tooth-extracted sockets were covered with granulation tissue. Although the gingival orifice was reduced, bone recovery was not achieved. At 21 years of age, It subsided at 22 years and 9 months of age through frequent lavage and gauze exchanges.
Childhood osteoporosis is classified into two types: primary and secondary osteoporosis, as described for adults. Primary osteoporosis involves genetic diseases, such as osteogenesis imperfecta. Secondary osteoporosis is related to various diseases or drug administration, such as steroid administration. Risk factors include malnutrition, a reduction in physical activity, chronic inflammation, and endocrine dysfunction. If childhood secondary osteoporosis is mild, it may be spontaneously reduced in the process of skeletal development by overcoming risk factors. However, this girl had received a steroid and BP preparation over a long period based on the general condition. Due to her general condition, steroid and BP medications could not be temporarily discontinued for oral surgical procedures. Patients taking steroids are considered to be susceptible to infection because they show considerable delayed wound healing and immunosuppression. Furthermore, her age at the time of cyst treatment was 14–15 years. Childhood bone-mass acquisition reaches a peak in puberty, but decreases with aging [21]. In this girl, the development of osteoporosis was consistent with such timing, and this may have led to delayed healing after lower third molar and second molar extraction treatment. We expected to reduce the size of the cyst and eruption of the second molar by performing fenestration in cooperation with our oral surgery department, however unfortunately it was necessary to remove both cyst and teeth, which would eventually take a long time to heal [22]. It is thought that the healing was delayed due to long- term use of steroid. In patients requiring long-term steroid therapy for a systemic disease and receiving BP therapy over a long period to reduce osteoporosis, such as the above patient, jaw/surgical treatment should be avoided if possible. If necessary, treatment and subsequent continuous management until healing must be performed in cooperation with the Department of Pediatrics or Department of Internal Medicine.
5. Pathogenesis of BRONJ
For fracture prevention/treatment in patients with bone diseases characterized by bone fragility, such as osteoporosis, BP preparations, which are highly useful, are used. However, osteonecrosis of the jaw, as an adverse reaction, in adults has been reported, although the number of patients is small. To clarify the pathogenesis of BRONJ, animal experiments have been conducted.
Fujita et al. examined the pathogenesis of steroid-induced osteoporosis of the growth-period jaw and BP treatment using rats [23]. Steroid-induced osteoporosis may occur through inhibition of bone formation related to the steroid-associated suppression of osteoblast proliferation/differentiation, enhancement of apoptosis, and functional disorder [24]. They prepared a steroid-induced osteoporosis model by administering a steroid to growth-period rats, and indicated that a reduction in the cortical bone quality rather than the cavernous bone in the mandible was an important factor for a reduction in the bone strength, and that the pathogenesis of osteoporosis of the mandible differed from that of the long bones. After BP is absorbed in the digestive tract, it is deposited in the bone resorption cavity within a few hours, and ingested by osteoclasts through phagocytosis. Subsequently, apoptosis is induced through enzyme inhibition. The above model was treated with risedronate. Although there was no recovery from delayed growth of the fibula, recovery from delayed mandibular growth, especially mandibular length recovery, was confirmed. An improvement in the bone strength, which had reduced in the two bones, was achieved. There are several hypotheses on the pathogenesis of BRONJ, [25], [26], [27], [28] but, as a factor for the jaw-specific disease that is frequent in adults, a study reported that high-concentration BP enhanced oral bacterial growth [29], and some studies indicated that histological findings of osteonecrosis of the jaw included inflammation in all cases [30], [31]. These studies suggest the involvement of the oral flora, such as periodontal disease bacteria.
Kikuiri et al. prepared a mouse model using immunocompromised mice from the viewpoint that steroid-therapy-related immunosuppression plays an important role in the onset of BRONJ, and reported the pathogenesis of BRONJ [32]. BP and steroid administration to immunocompromised mice (beige nu/nu Xid (III)) did not lead to closure of the epithelium of a tooth-extracted socket, facilitating examination of necrotic bone exposure. Pathologically, the infiltration of inflammatory cells and extensive loss of osteocytes were observed. As immune-response-related effects were eliminated due to mouse characteristics, they investigated changes in the status of the tooth-extracted socket using T cells, compared the size of an open wound with that after the infusion of T-cell groups (complete T-cell population (PanT), Treg cells alone, and PanT or Treg cell-removed population), and found that the presence or absence of Treg cells was closely involved in the presence or absence of medication-related osteonecrosis of the jaw (MRONJ). Concerning the pathogenesis of BRONJ, it is suggested that the immune responses of Th cells to Treg-cell hypofunction persist through surgical invasion related to tooth extraction in the bone tissue in which the kinetic balance is suppressed through the actions of BP, enlarging the extent of jaw injury and contributing to the onset of BRONJ [33].
Thus, ONJ models have been prepared by extracting teeth after BP or RANKL inhibitor administration using animals [28], [31], [34], [35]. The condition/pathogenesis of BRONJ or ARONJ may be clarified by utilizing these, and research for new drug development may be promoted.
6. Conclusion
We reviewed the current status based on the contents of a presentation regarding BRONJ in children at the 28th symposium of the Japanese Society of Pediatric Oral Maxillofacial Surgery.
As many adults with BRONJ or ARONJ have been reported, close attention should be paid when performing surgical treatment, such as tooth extraction, in BP-treated children. However, fortunately, no study has reported childhood BRONJ or ARONJ. In a position paper, it was also stated that there has been no report on BRONJ or DRONJ development in BP-treated children with osteogenesis imperfecta [1]. It has not yet been clarified whether children using absorption inhibitors such as BP and denosumab are at risk of developing ONJ after surgical procedures such as tooth extraction. It is stated that it may be involved in the susceptibility to BRONJ when becoming an adult using BP continuously for a long period of time from childhood because of reduction of bone turnover over years [36]. In addition, it was stated that the onset of BRONJ is triggered by infection, and that infection prevention can be sufficiently prevented before dental treatment to reduce the occurrence of BRONJ [1]. Therefore, if it is necessary to use BP for long term, it seems that plaque control in the oral cavity on a daily basis from childhood.
It is expected that the pathogenic mechanism of BRONJ will be clarified by further case analysis and studies using model animals, and that a treatment method for osteoporosis that does not develop BRONJ will be developed. It has also been suggested that the immune system and oral bacteria are involved in the onset of BRONJ. Children are growing day by day and are changing systemically, locally in the oral cavity, morphologically and functionally. Comprehensive analysis of the bacterial flora in the oral cavity of children has revealed that it changes with growth [37]. From these facts, the age-related involvement in the onset of BRONJ may also be clarified.
It is important to keep the balance between immune-response activation and suppression for maintaining cell/tissue homeostasis [38]. However, previous long-term steroid therapy may affect this balance in some cases. In children treated with steroids for a long period, the influence on susceptibility to infection or bone remodeling, which depends on the general condition, must be considered more carefully than in children treated with BP alone.
With respect to oral management in BP-treated children, early intervention should be performed, and plaque control is necessary to keep the oral cavity clean. Concerning infection, early management may be necessary to prevent inflammation affecting the jaw. When surgical treatment is required, considerations must be paid so that procedures are minimally invasive, and cooperation with the Department of Pediatrics, such as antibiotic administration for infection control, may be important.
Role of funding source
There is no funding for this review.
The research was presented at the 28th symposium of the Japanese Society of Pediatric Oral Maxillofacial Surgery on October 29, 2016.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgement
We would like to thank Medical English Service (www.med-english.com) for English language editing.
References
- 1.Jawbone necrosis review committee: Pathophysiology and management of bone resorption inhibitor-related osteonecrosis of the jaw: Jawbone Necrosis Review Committee Position Paper 2016. (Japanese) (〈https://www.jsoms.or.jp/medical/wp-content/uploads/2015/08/position_paper2016.pdf〉).
- 2.Marx R.E. Pamidronate (Aredia) and Zoledronate(Zometa) incuced avascular necrosis of the jaws: growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 3.Urade M., Tanaka N., Furusawa K., Shimada J., Shibata T., Kirita T., et al. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;11:e364–e371. doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Fact-finding survey on BRONJ treatment. JSOMS 2015. (Japanese) 〈https://www.jsoms.or.jp/medical/work/study/bronj/〉.
- 5.Shibahara T., Yago K., Imai Y. National survey on bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2018;76:2105–2112. doi: 10.1016/j.joms.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S., et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Min Metab. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 7.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Saad F., Brown J.E., Van Poznak C., Ibrahim T., Stemmer S.M., Stopeck A.T., et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 9.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Hinson A.M., Smith C.W., Siegel E.R., Stack B.C., Jr. Is bisphosphonate-related osteonecrosis of the jaw an infection? a histological and microbiological ten-year summary. Int J Dent. 2014;2014 doi: 10.1155/2014/452737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funayama H., Fukase N., Ide M., Asada Y. Dental treatment experience for juvenile idiopathic arthritis patients receiving oral bisphosphonates. Jpn j Ped Dent. 2009;47:307. (Japanese) [Google Scholar]
- 12.Milano M., Wright T., Loechner K.J. Dental implications of Osteogenesis imperfect: treatment with Ⅳ bisphosphonate: report of a case. Pedia Dent. 2011;33:348–351. [PubMed] [Google Scholar]
- 13.Saga K., Okawa R., Nomura R., Nakano K. Analysis of children with osteogenesis imperfecta who had their deciduous teeth extracted during the permanent tooth replacement period. Jpn j Ped Dent. 2015;53:144–145. (Japanese) [Google Scholar]
- 14.Schwartz S., Joseph C., Iera D., Vu D.D. Bisphosphonates, osteonecrosis, osteogenesis imperfecta and dental extractions: a case series. J Can Dent Assoc. 2008;74:537–542. [PubMed] [Google Scholar]
- 15.Malmgren Malmgren B., Aström E., Söderhäll S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008;37:196–200. doi: 10.1111/j.1600-0714.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 16.Maines E., Monti E., Doro F., Morandi G., Cavarzere P., Antoniazzi F. Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Min Metab. 2012;30:434–438. doi: 10.1007/s00774-011-0331-3. [DOI] [PubMed] [Google Scholar]
- 17.Brown J.J., Ramalingam L., Zacharin M.R. Bisphosphonate-associated osteonecrosis of the jaw: does it occur in children? Clin Endocrinol. 2008;68:863–867. doi: 10.1111/j.1365-2265.2008.03189.x. [DOI] [PubMed] [Google Scholar]
- 18.Chahine C., Cheung M.S., Head T.W., Schwartz S., Glorieux F.H., Rauch F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J Pedia. 2008;153:719–720. doi: 10.1016/j.jpeds.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Christou J., Johnson A.R., Hodgson T.A. Bisphosphonate-related osteonecrosis of the jaws and its relevance to children--a review. Int J Paediatr Dent. 2013;23:330–337. doi: 10.1111/ipd.12047. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt R.N., Hibbert S.A., Munns C.F. The use of bisphosphonates in children: review of the literature and guidelines for dental management. Aust Dent J. 2014;59:9–19. doi: 10.1111/adj.12140. [DOI] [PubMed] [Google Scholar]
- 21.Consens N.I.H. Statement: Osteoporos Diagn Ther. 2000;17:1–45. [Google Scholar]
- 22.Tsuboi A., Niizato N., Mitsuhata C., Kozai K. A case of long-term steroid and bisphosphonate patients who underwent dentate cystectomy. Jpn J Ped Dent. 2013;51:113. [Google Scholar]
- 23.Fujita Y. Mechanisms of glucocorticoid-induced osteoporosis and bisphosphonate therapy in the growing mandible. Jpn J Ped Dent. 2012;50:161–169. [Google Scholar]
- 24.O’Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 25.Favia G., Pilolli G.P., Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009;45:406–413. doi: 10.1016/j.bone.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Landesberg R., Cozin M., Cremers S., Woo V., Kousteni S., Sinha S., et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66:839–847. doi: 10.1016/j.joms.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwamoto J., Matsumoto H., Takeda T., Sato Y., Liu X., Yeh J.K. Effects of vitamin K (2) and risedronate on bone formation and resorption, osteocyte lacunar system, and porosity in the cortical bone of glucocorticoid-treated rats. Calcif Tissue Int. 2008;83:121–128. doi: 10.1007/s00223-008-9146-1. [DOI] [PubMed] [Google Scholar]
- 28.Allen M.R., Burr D.B. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi Y., Hiraga T., Ueda A., Wang L., Matsumoto-Nakano M., Hata K., et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Min Metab. 2010;28:165–175. doi: 10.1007/s00774-009-0128-9. [DOI] [PubMed] [Google Scholar]
- 30.Rizzoli R., Burlet N., Cahall D., Delmas P.D., Eriksen E.F., Felsenberg D., et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42:841–847. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Mawardi H., Giro G., Kajiya M., Ohta K., Almazrooa S., Alshwaimi E., et al. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90:1339–1345. doi: 10.1177/0022034511420430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuiri T., Kim I., Yamaza T., Akiyama K., Zhang Q., Li Y., et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Min Res. 2010;25:1668–1679. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuiri T. Role of immune cells in development of bisphosphonate-related osteonecrosis of the jaw. Jpn J Ped Dent. 2015;53:1–8. [Google Scholar]
- 34.Aghaloo T.L., Cheong S., Bezouglaia O., Kostenuik P., Atti E., Dry S.M., et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Min Res. 2014;29:843–854. doi: 10.1002/jbmr.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Molon R.S., Shimamoto H., Bezouglaia O., Pirih F.Q., Dry S.M., Kostenuik P., et al. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Min Res. 2015;30:1627–1640. doi: 10.1002/jbmr.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte N.T., Rech B.O., Martins I.G., Franco J.B., Ortega K.L. Can children be affected by bisphosphonate-related osteonecrosis of the jaw? A systematic review. Int J Oral Maxillofac Surg. 2020;49:183–191. doi: 10.1016/j.ijom.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Kado N., Mitsuhta C., Kozai K..The oral microbiome analysis of children with Down syndrome. Jpn j Ped Dent 2020: 58(abstracs of 58th annual conference of JAPD):103.
- 38.Noack M., Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory disease. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]