Abstract
Aims
This study aims to investigate the role of echocardiographically determined left ventricular output indices on sacubitril/valsartan titration in a cohort of outpatients with heart failure and reduced ejection fraction (HFrEF).
Methods and results
We analysed 106 HFrEF patients who underwent echocardiography examination up to 1 week before starting treatment with sacubitril/valsartan. For each patient, a comprehensive list of clinical and laboratory parameters was collected, and stroke volume index (SVi), cardiac index, and flow rate were calculated. The primary endpoint was the occurrence of complete titration of sacubitril/valsartan. The secondary endpoint was the incidence of adverse events (hypotension and renal adverse events). Univariate and multivariate logistic regression were used to identify variables associated with the primary and secondary endpoints. Mean age of patients was 73.7 ± 10.4 years, 72 patients (71.7%) had ischaemic aetiology of HF, and mean ejection fraction was 29.4 ± 5.9%. At multivariate analysis, SVi [odds ratio (OR) 1.43 per 5 mL/m2 increase, 95% confidence interval (CI) 1.03–1.97; P = 0.028], serum sodium (OR 1.18, 95% CI 1.02–1.37; P = 0.022), and haemoglobin (OR 1.73, 95% CI 1.25–2.40; P = 0.001) were found to be independent predictors of titration during follow‐up. Multivariate analysis for the secondary endpoint showed SVi (OR 0.63 per 5 mL/m2 increase, 95% CI 0.44–0.90; P = 0.012) and New York Heart Association Class III (OR 2.65, 95% CI 1.07–6.5; P = 0.034) to be associated with hypotension.
Conclusions
Stroke volume index is positively associated with complete titration of sacubitril/valsartan. Patients with low SVi are more prone to experience hypotension during titration
Keywords: Sacubitril, Valsartan, Entresto, HFrEF, Titration
Background
Randomized clinical trials (RCTs) showed that sacubitril/valsartan exerts greater benefit than enalapril in patients with heart failure and reduced ejection fraction (HFrEF). 1 , 2 , 3 These two drugs have a comparable adverse event profile except for hypotension that occurs more frequently with sacubitril/valsartan and could explain its underuse in recent RCTs and registries. 4 , 5 , 6 In clinical practice, matching between arterial blood pressure (BP) values and symptomatic hypotension is not straightforward and patients with normal/low BP at baseline are often able to tolerate target doses of sacubitril/valsartan. BP is the result of interaction between left ventricular output (LVO), arterial elastance, and peripheral resistance. 7 , 8 We hypothesized that LVO is the main factor driving symptoms of hypotension and could predict sacubitril/valsartan titration better than BP.
Aims
The aim of this study was to assess the association between left ventricular output indices (LVOIs) assessed by echocardiography at baseline and the occurrence of complete titration of sacubitril/valsartan during follow‐up. The association between LVOIs and adverse events was also evaluated.
Methods
Data sources and study population
We prospectively collected data of all patients prescribed sacubitril/valsartan for the first time, from 30 October 2019 to 18 September 2020. As per internal protocol, patients prescribed with sacubitril/valsartan were followed up at our outpatient clinic with scheduled clinical visits until full titration or dose stabilization. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and without any external funding. Patients were informed that their participation was voluntary, and all gave informed consent.
Outcomes
The primary outcome was the occurrence of complete titration of sacubitril/valsartan (97/103 mg twice daily) during follow‐up. Secondary outcomes were (i) patient‐reported symptoms of hypotension (defined as dizziness, weakness, or syncope with systolic BP lower than 90 mmHg) and (ii) renal adverse events [potassium levels greater than 5.5 meq/L or any drop of estimated glomerular filtration rate (eGFR) more than 50%].
Echocardiographic examination
A comprehensive echocardiographic examination was performed using a GE Vivid S60 echocardiographic scanner (GE Healthcare, Milwaukee, WI, USA) equipped with a 3.5 MHz transducer. One expert physician performed all echocardiographic measures. Cardiac chamber dimension, left ventricular filling pressure and stroke volume, were calculated following the guidelines of American Society of Echocardiography and European Association of Cardiovascular Imaging. 9 , 10 , 11 Among LVOIs we focused on stroke volume index (SVi), cardiac index, and flow rate.
Statistical analysis
Baseline characteristics were summarized as means and standard deviations, medians, and interquartile ranges, or percentages. The effect of baseline characteristic on the occurrence of the primary endpoint was estimated using univariate logistic regression. Clinically relevant variables with P‐value < 0.10 were added to the multivariate regression model. Furthermore, patients were divided by SVi tertiles, and the occurrence of primary and secondary outcomes was analysed accordingly. All the analyses were conducted using STATA Version 16.1 (College Station, TX, USA).
Results
Patients' characteristics
Patients' characteristics are reported in Table 1 . The final study population consisted of 106 patients with a mean age of 73.7 ± 10.4 years. Seventy‐two patients (71.7%) had ischaemic aetiology of HF with a mean ejection fraction of 29.4 ± 5.9%. The median visit number was two (interquartile range 2–3), and 93.4% of patients achieved stable dose of sacubitril/valsartan within four visits.
Table 1.
Baseline characteristics of the overall population and according to the achieving of complete titration
| Variable | Overall | Incomplete titration | Complete titration | P‐value |
|---|---|---|---|---|
| Patients, no. | 106 | 44 | 62 | |
| Age, years (SD) | 73.7 (10.4) | 76.4 (9.4) | 71.6 (10.7) | 0.025 |
| Male sex, no. (%) | 87 (82.1) | 36 (81.8) | 51 (82.3) | 0.95 |
| CV risk factors | ||||
| Hypertension, no. (%) | 89 (84.0) | 36 (81.8) | 53 (85.5) | 0.61 |
| Diabetes, no. (%) | 38 (35.8) | 15 (34.1) | 23 (37.1) | 0.75 |
| Physical features (SD) | ||||
| Systolic BP, mmHg (SD) | 125.4 (14.7) | 124 (14.9) | 126.4 (14.5) | 0.42 |
| Diastolic BP, mmHg (SD) | 74.7 (9.3) | 73.2 (8.6) | 75.7 (9.7) | 0.16 |
| Pulse pressure, mmHg (SD) | 50.7 (10.6) | 50.8 (9.6) | 50.6 (11.4) | 0.91 |
| Heart rate, b.p.m. (SD) | 67.9 (11.7) | 68.8 (13.6) | 67.2 (10.3) | 0.51 |
| BMI, kg/m2 (SD) | 27.5 (4.6) | 27.0 (4.3) | 27.8 (4.9) | 0.44 |
| Aetiology of HF | 0.52 | |||
| Ischaemic aetiology, no. (%) | 76 (71.7) | 33 (75.0) | 43 (69.4) | |
| Non‐ischaemic aetiology, no. (%) | 30 (28.3) | 11 (25.0) | 19 (30.6) | |
| Comorbidities | ||||
| Chronic renal failure (eGFR < 60 mL/min/1.72 m2), no. (%) | 66 (62.3) | 33 (75.0) | 33 (53.2) | 0.023 |
| Baseline laboratory analysis | ||||
| Serum sodium, mg/mL (SD) | 140.5 (3.4) | 139.7 (4.1) | 141.0 (2.6) | 0.075 |
| Serum potassium, mg/mL (SD) | 4.4 (0.6) | 4.5 (0.5) | 4.4 (0.6) | 0.24 |
| eGFR, mL/min/1.72 m2 a (SD) | 54.0 (20.3) | 49.0 (21.7) | 57.6 (18.5) | 0.032 |
| Haemoglobin, g/dL (SD) | 13.2 (1.6) | 12.5 (1.4) | 13.6 (1.6) | <0.001 |
| Median BNP values, pg/mL (IQR) | 412 (190–806) | 603 (191–1019) | 371 (156–553) | 0.11 |
| NYHA class, no. (%) | 0.18 | |||
| I | 5 (4.7) | 2 (4.5) | 3 (4.8) | |
| II | 68 (64.2) | 24 (54.5) | 44 (71.0) | |
| III | 33 (31.1) | 18 (40.9) | 15 (24.0) | |
| Heart rhythm at baseline ECG, no. (%) | 0.13 | |||
| Sinus rhythm | 50 (47.2) | 19 (43.2) | 31 (50.0) | |
| Atrial fibrillation/flutter | 28 (26.4) | 9 (20.5) | 19 (30.6) | |
| Paced rhythm | 28 (26.4) | 16 (36.4) | 11 (18.4) | |
| Echocardiogram | ||||
| LVEDVi, mL/m2 (SD) | 111.0 (42.5) | 103.9 (36.1) | 114.9 (45.6) | 0.25 |
| Ejection fraction, no. (%) | 29.4 (5.9) | 28.0 (6.5) | 30.4 (5.3) | 0.044 |
| SV, mL (SD) | 60.0 (17.0) | 55.6 (14.5) | 63.3 (18.3) | 0.02 |
| SVi, mL/m2 (SD) | 31.0 (7.9) | 28.7 (7.0) | 32.6 (8.1) | 0.011 |
| Cardiac index, L/min/m2 (SD) | 2.1 (0.7) | 2.0 (0.6) | 2.2 (0.7) | 0.12 |
| Flow rate, mL/min (SD) | 215.4 (58.9) | 203.8 (49.7) | 223.6 (63.8) | 0.10 |
| PAPs, mmHg (SD) | 43.7 (10.1) | 45.3 (9.6) | 42.4 (10.4) | 0.34 |
| Diastolic dysfunction b , no. (%) | 0.12 | |||
| Grade I | 24 (22.6) | 8 (18.2) | 16 (25.8) | |
| Grade II | 22 (20.8) | 14 (31.8) | 8 (12.9) | |
| Grade III | 30 (28.3) | 14 (31.8) | 16 (25.8) | |
| Undetermined | 30 (28.3) | 8 (18.2) | 22 (35.5) | |
| Heart failure treatments | ||||
| Beta‐blocker, no. (%) | 96 (90.1) | 40 (91.0) | 56 (90.3) | 0.92 |
| MRAs, no. (%) | 89 (84.0) | 38 (86.4) | 51 (82.3) | 0.57 |
| Loop diuretics, no. (%) | 97 (91.5) | 42 (95.5) | 55 (88.7) | 0.22 |
| Median loop diuretics dose, mg/day c (IQR) | 50 (25–75) | 50 (25–100) | 50 (25–75) | 0.055 |
| Loop diuretics preventive reduction, no. (%) | 32 (30.2) | 11 (25.0) | 21 (33.9) | 0.33 |
| ICD/CRT‐D, no. (%) | 38 (35.8) | 19 (43.2) | 19 (30.6) | 0.18 |
| CRT‐P/CRT‐D, no. (%) | 17 (16.0) | 8 (18.2) | 9 (14.5) | 0.61 |
BMI, body mass index; BP, blood pressure; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; CV, cardiovascular; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEDVi, left ventricular end‐diastolic volume index; MRAs, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; PAPs, pulmonary artery pressures; SBP, systolic blood pressure; SV, stroke volume; SVi, stroke volume index.
Values are reported as mean and standard deviation (SD), number and percentage, or median and interquartile range (IQR). P‐values < 0.05 are reported in bold.
The Modification of Diet in Renal Disease formula has been used.
Loop diuretic doses are reported as furosemide equivalents.
Patients with undermined status were excluded from this analysis.
Primary outcome
The baseline characteristics of patients according to the occurrence of primary endpoint are shown in Table 1 . At the univariate analysis, SV [odds ratio (OR) 1.15 per 5 mL increase, 95% confidence interval (CI) 1.01–1.31; P = 0.025], SVi (OR 1.38 per 5 mL/m2 increase, 95% CI 1.05–1.81; P = 0.018), left ventricular ejection fraction (OR 1.07, 95% CI 0.99–1.14; P = 0.051), eGFR (OR 1.02, 95% CI 1.00–1.05; P = 0.036), haemoglobin (OR 1.62, 95% CI 1.22–2.16; P = 0.001), and age (OR 0.95, 95% CI 0.91–0.99; P = 0.019) were predictors of complete titration (Table 2 ). At the multivariate analysis, SVi (OR 1.43 per 5 mL/m2 increase, 95% CI 1.03–1.97; P = 0.028), haemoglobin level (OR 1.73, 95% CI 1.25–2.40; P = 0.001), and baseline serum sodium (OR 1.18, 95% CI 1.02–1.37; P = 0.022) remained independent predictors of outcome (Table 3 ; Figure 1 A ). We further analysed the occurrence of the primary outcome according to tertiles of SVi. The first SVi tertile ranged from 15.13 to 26.9 mL/m2, the second tertile from 27.00 to 33.4 mL/m2, and the third tertile from 33.7 to 59.9 mL/m2. The primary outcome occurred in 16 (44.4%) patients in the first tertile, 22 (62.9%) in the second tertile, and 24 (68.6%) in the third tertile (Figure 2 ), P‐value for trend = 0.04.
Table 2.
Univariate logistic regression for predictors of primary outcome
| Variable | OR (95% CI) | P‐value |
|---|---|---|
| Patients, no. | 106 | |
| Age, years | 0.95 (0.91–0.99) | 0.019 |
| Male sex, no. (%) | 1.03 (0.38–2.82) | 0.95 |
| CV risk factors | ||
| Hypertension | 1.31 (0.46–3.71) | 0.61 |
| Diabetes | 1.14 (0.51–2.56) | 0.75 |
| Physical features | ||
| Systolic BP (mmHg) | 1.01 (0.98–1.04) | 0.41 |
| Diastolic BP (mmHg) | 1.03 (0.99–1.08) | 0.16 |
| Pulse pressure (mmHg) | 1.00 (0.96–1.04) | 0.91 |
| Heart rate (b.p.m.) | 0.98 (0.94–1.02) | 0.25 |
| BMI (kg/m2) | 0.99 (0.96–1.02) | 0.49 |
| Aetiology of HF | ||
| Ischaemic aetiology | 0.75 (0.32–1.80) | 0.53 |
| Baseline laboratory analysis | ||
| Serum sodium (mg/mL) | 1.12 (0.99–1.28) | 0.081 |
| Serum potassium (mg/mL) | 0.64 (0.30–1.35) | 0.24 |
| eGFR (mL/min/1.72 m2) | 1.02 (1.00–1.05) | 0.036 |
| Haemoglobin (g/dL) | 1.62 (1.22–2.16) | 0.001 |
| BNP (pg/mL) | 0.71 (0.46–1.11) | 0.14 |
| NYHA Class III | 0.46 (0.20–1.06) | 0.069 |
| Atrial fibrillation/flutter at baseline ECG | 1.72 (0.69–4.27) | 0.244 |
| Paced rhythm at baseline ECG | 0.42 (0.17–1.01) | 0.053 |
| Echocardiogram | ||
| LVEDVi (mL/m2) | 1.01 (1.00–1.02) | 0.25 |
| Ejection fraction (%) | 1.07 (1.00–1.15) | 0.051 |
| SV (per 5 mL) | 1.15 (1.01–1.31) | 0.025 |
| SVi (per 5 mL/m2) | 1.38 (1.05–1.81) | 0.018 |
| Cardiac index (mL/min/m2) | 1.00 (1.00–1.00) | 0.13 |
| Flow rate (mL/min) | 1.01 (1.00–1.01) | 0.11 |
| PAPs (mmHg) | 0.97 (0.92–1.03) | 0.33 |
| Diastolic dysfunction (Grade III) | 1.05 (0.42–2.63) | 0.92 |
| Loop diuretics preventive reduction | 1.54 (0.65–3.64) | 0.33 |
BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; CI, confidence interval; CV, cardiovascular; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEDVi, left ventricular end‐diastolic volume index; NYHA, New York Heart Association; OR, odds ratio; PAPs, pulmonary artery pressures; SV, stroke volume; SVi, stroke volume index. P‐values < 0.05 are reported in bold.
Table 3.
Multivariate logistic regression for predictors of primary outcome
| Variable | OR (95% CI) | P‐value |
|---|---|---|
| Age (years) | 0.94 (10.88–1.01) | 0.075 |
| Serum sodium (mg/mL) | 1.18 (1.02–1.37) | 0.022 |
| eGFR (mL/min/1.72 m2) | 1.03 (0.99–1.06) | 0.14 |
| Haemoglobin (g/dL) | 1.73 (1.25–2.40) | 0.001 |
| NYHA Class III | 0.45 (0.14–1.45) | 0.18 |
| Paced rhythm at baseline ECG | 0.41 (0.11–1.48) | 0.17 |
| Ejection fraction (1‐point %) | 1.04 (0.94–1.16) | 0.47 |
| SVi (per 5 mL/m2) | 1.43 (1.03–1.97) | 0.028 |
CI, confidence interval; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; OR, odds ratio; SVi, stroke volume index. P‐values < 0.05 are reported in bold.
Figure 1.
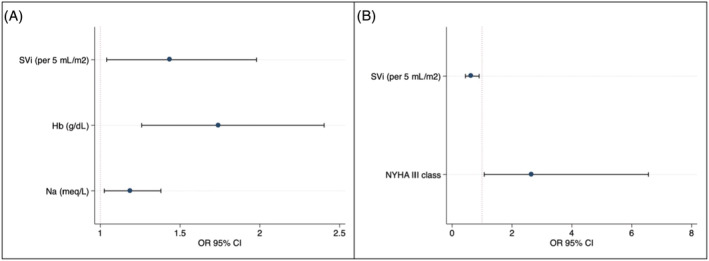
Odds ratio for primary outcome and hypotension at multivariate logistic regression. (A) Sacubitril/valsartan complete titration. (B) Occurrence of hypotension. CI, confidence interval; Hb, haemoglobin; NYHA, New York Heart Association; OR, odds ratio; SVi, stroke volume index.
Figure 2.
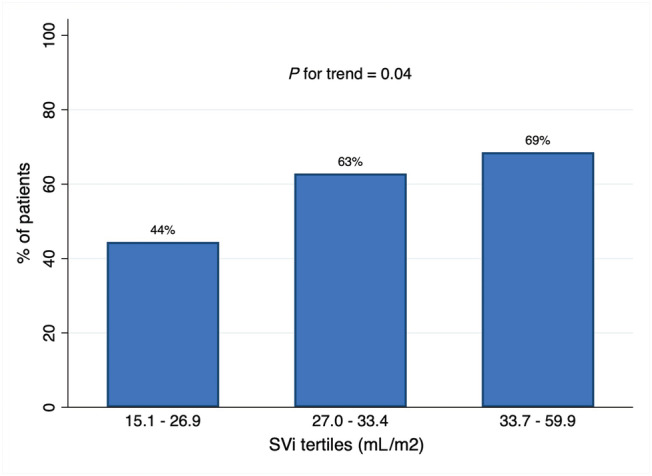
Primary outcome according to SVi tertiles. SVi, stroke volume index.
Secondary outcome
Forty (37.7%) patients experienced at least one adverse event. Twenty‐six patients (25.2%) experienced symptomatic hypotension, 16 (17.5%) experienced an acute decrease of eGFR, and 18 (17.5%) experienced hyperkalaemia (Table 4 ). In a multivariate logistic regression model, SVi (OR 0.63 per 5 mL/m2 increase, 95% CI 0.44–0.90; P = 0.012) and New York Heart Association Class III (OR 2.65, 95% CI 1.07–6.5; P = 0.034) were independent predictors of symptomatic hypotension (Table 5 ; Figure 1 B ). None of the LVOIs was a predictor of renal outcomes at univariate analysis.
Table 4.
Secondary endpoints according to tertiles of stroke volume index
|
Adverse events N (%) |
Overall | First tertile (n = 36) | Second tertile (n = 35) | Third tertile (n = 35) | P‐value |
|---|---|---|---|---|---|
| Symptomatic hypotension | 26 (25.2) | 14 (39.9) | 5 (14.3) | 7 (20.0) | 0.041 |
| Acute decrease of eGFR | 16 (17.5) | 6 (16.7) | 6 (17.1) | 4 (11.4) | 0.76 |
| Hyperkalaemia | 18 (17.5) | 7 (19.4) | 7 (20.4) | 4 (11.4) | 0.56 |
eGFR, estimated glomerular filtration rate.
Adverse events were reported as numbers and percentage. P‐values < 0.05 are reported in bold.
Table 5.
Univariate and multivariate analysis of predictor of symptomatic hypotension
| Variable |
Univariate analysis OR (95% CI), P‐value |
Multivariate analysis OR (95% CI), P‐value |
|---|---|---|
| Patients, no. | 106 | |
| Age, years | 1.02 (0.98–1.07), P = 0.33 | — |
| Male sex, no. (%) | 1.92 (0.51–7.19), P = 0.34 | — |
| CV risk factors | ||
| Hypertension | 0.74 (0.23–2.35), P = 0.61 | — |
| Diabetes | 0.74 (0.29–1.91), P = 0.54 | — |
| Physical features | ||
| Systolic BP (mmHg) | 0.99 (0.96–1.02), P = 0.36 | — |
| Diastolic BP (mmHg) | 0.96 (0.91–1.02), P = 0.16 | — |
| Pulse pressure (mmHg) | 1.00 (0.96–1.04), P = 0.96 | — |
| Heart rate (b.p.m.) | 1.00 (0.96–1.04), P = 0.84 | — |
| BMI (kg/m2) | 1.02 (0.99–1.05), P = 0.29 | — |
| Aetiology of HF | ||
| Ischaemic aetiology | 1.10 (0.41–2.96), P = 0.86 | — |
| Baseline laboratory analysis | ||
| Serum sodium (mg/mL) | 0.90 (0.79–1.04), P = 0.15 | — |
| Serum potassium (mg/mL) | 1.26 (0.56–2.84), P = 0.58 | — |
| eGFR (mL/min/1.72 m2) | 0.99 (0.97–1.02), P = 0.61 | — |
| Haemoglobin (g/dL) | 0.78 (0.59–1.04), P = 0.096 | 0.80 (0.59–1.09), P = 0.15 |
| lnBNP (pg/mL) | 1.02 (0.63–1.65), P = 0.93 | — |
| NYHA Class III | 3.00 (1.20–7.53), P = 0.019 | 2.65 (1.07–6.5), P = 0.034 |
| Atrial fibrillation/flutter at baseline ECG | 0.42 (0.13–1.36), P = 0.15 | — |
| Paced rhythm at baseline ECG | 1.70 (0.65–4.43), P = 0.28 | — |
| Echocardiogram | ||
| LVEDVi (mL/m2) | 1.01 (1.00–1.02), P = 0.28 | — |
| Ejection fraction (%) | 0.89 (0.83–0.97), P = 0.005 | 0.92 (0.85–1.00), P = 0.053 |
| SVi (per 5 mL/m2) | 0.65 (0.46–0.91), P = 0.012 | 0.63 (0.44–0.90), P = 0.012 |
| Cardiac index (mL/min/m2) | 1.00 (1.00–1.00), P = 0.033 | — |
| Flow rate (mL/min) | 0.99 (0.98–1.00), P = 0.021 | — |
| PAPs (mmHg) | 0.96 (0.90–1.03), P = 0.24 | — |
| Diastolic dysfunction (Grade III) | 0.86 (0.30–2.51), P = 0.79 | — |
| Loop diuretics preventive reduction | 1.24 (0.48–3.19), P = 0.65 | — |
BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; CI, confidence interval; CV, cardiovascular; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEDVi, left ventricular end‐diastolic volume index; NYHA, New York Heart Association; OR, odds ratio; PAPs, pulmonary artery pressures; SVi, stroke volume index. P‐values < 0.05 are reported in bold.
Conclusions
Our study tested the hypothesis that LVOIs could predict sacubitril/valsartan complete titration and tolerability. Among LVOIs, SV and SVi were the only positively associated with complete titration of sacubitril/valsartan. These findings are consistent with a recent paper showing that SVi is the best comprehensive parameter of LVO. 12 Furthermore, SVi was inversely related to the occurrence of symptomatic hypotension. Arterial BP is generated by the interaction of LVO, peripheral resistance, and great vessel elastance. HFrEF patients usually have low LVO and increased peripheral resistance, mediated by neurohormonal response. This neurohormonal response is accountable for restoring a ‘normal’ BP even in a low cardiac output state. The treatment with sacubitril/valsartan, exerting many vasodilatory effects, could unmask the low output state reducing peripheral resistance and precipitating symptoms of hypotension in those with very low SVi and BP. In contrast with previous reports, systolic BP, age, and eGFR did not predict a successful drug titration in our cohort at multivariate analysis. Alongside with SVi, baseline haemoglobin level independently predicted titration. Haemoglobin level has well‐known prognostic implication in HFrEF but, except for Pharithi et al., 13 there are no studies highlighting its association with sacubitril/valsartan titration. In this study, however, the association was not confirmed after correction for other covariates. Finally, consistent with previous findings from Dashwood et al., also, baseline sodium level resulted to be an independent predictor of outcome at our multivariate analysis (Table 2 ). 14 This finding highlights the importance of reassessing and eventually adapting diuretic therapy while starting treatment with sacubitril/valsartan, to avoid hypovolaemia. The main finding of this analysis is that patients with low SVi are more prone to develop symptomatic hypotension while treated with sacubitril/valsartan. This should not be perceived as a deterrent to initiate and titrate sacubitril/valsartan therapy but should instead encourage physicians to correct modifiable factors in patients that struggle to tolerate the treatment.
We identified some limitations in our study. This was a monocentric study based on echocardiographic examinations performed at our institution. The echocardiographic examinations were not dedicated to our aim (despite performed by a standardized protocol and expert cardiologists), and some patients were excluded because of inadequate image quality or lacking pulsed‐wave sampling at left ventricular outflow tract. Moreover, we supposed that in patients with low SVi, an increase in peripheral resistance is responsible for normal BP. In vivo assessment of peripheral resistance could support our findings but, unfortunately, we did not use this evaluation in our clinical routine.
Conflict of interest
The authors have nothing to declare.
Tolomeo, P. , Zucchetti, O. , D'Aniello, E. , Punzo, N. , Marchini, F. , Di Ienno, L. , Tonet, E. , Pavasini, R. , Rapezzi, C. , Campo, G. , and Serenelli, M. (2022) Left ventricular output indices and sacubitril/valsartan titration: role of stroke volume index. ESC Heart Failure, 9: 2037–2043. 10.1002/ehf2.13891.
References
- 1. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 3. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Al Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, TRANSITION Investigators . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 4. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 5. Peri‐Okonny PA, Mi X, Khariton Y, Patel KK, Thomas L, Fonarow GC, Sharma PP, Duffy CI, Albert NM, Butler J, Hernandez AF, McCague K, Williams FB, DeVore AD, Patterson JH, Spertus JA. Target doses of heart failure medical therapy and blood pressure: insights from the CHAMP‐HF registry. JACC: Heart Fail. 2019; 7: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD. The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail. 2019; 21: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka H, Heiss G, McCabe EL, Meyer ML, Shah AM, Mangion JR, Wu J, Solomon SD, Cheng S. Hemodynamic correlates of blood pressure in older adults: the Atherosclerosis Risk in Communities (ARIC) study. J Clin Hypertens. 2016; 18: 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003; 89: 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 10. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 11. Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984; 70: 425–431. [DOI] [PubMed] [Google Scholar]
- 12. Mele D, Pestelli G, Smarrazzo V, Dal Molin D, Luisi GA, Trevisan F, Fiorencis A, Ferrari R. Left ventricular output indices in hospitalized heart failure: when “simpler” may not mean “better.”. Int J Cardiovasc Imaging. 2021; 37: 59–68. [DOI] [PubMed] [Google Scholar]
- 13. Pharithi RB, Ferre‐Vallverdu M, Maisel AS, O'Connell E, Walshe M, Sweeney C, Barton J, McDonald K, O'Hare D, Watson C, Gallagher J, Ledwidge M, McDonald K. Sacubitril‐valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail. 2020; 7: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dashwood A, Vale C, Laher S, Chui F, Rheault H, Gan J, Wong YW. Impact of patient and model of care factors on titration and tolerability of sacubitril/valsartan: an early Australian real‐world experience. Heart Lung Circ. 2020; 29: 1688–1695. [DOI] [PubMed] [Google Scholar]


