Abstract
Concomitant treatment with veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) and Impella may improve outcomes in patients with cardiogenic shock compared with VA‐ECMO alone. Here, we explain a new method to introduce Impella and ECMO through the same arterial access site and jugular venous cannulation to accomplish a mobile patient concept.
Keywords: Ecpella, Impella, ECMO, Mechanical circulatory support, Mobility
Introduction
Utilization of Impella to unload the left ventricle simultaneously with veno‐arterial extracorporeal membrane oxygenation (ECMO) may improve outcomes of the refractory cardiogenic shock patients. 1 , 2 In current practice when ECMO and Impella are used together (Ecpella), multiple arterial access sites will be intervened, as such the groin interventions will limit mobilization of the patient. Here, we explain a new method to introduce Impella and ECMO through the same arterial access site and jugular venous cannulation to accomplish a mobile patient concept.
Case report
A 49‐year‐old man with non‐ischaemic cardiomyopathy was admitted to our clinic in refractory cardiogenic shock. Transthoracic echocardiography revealed severe left ventricular dysfunction (20%). He was on three inotropic agents and balloon pump support on admission. Ecpella intervention was planned due to biventricular failure, refractory cardiogenic shock, pulmonary artery diastolic pressures of 40 mmHg, and hypotension despite increased vasopressor agents.
Operative details
Under general anaesthesia, a right midclavicular incision was performed to explore the axillary artery. An 8 mm woven Vascutec graft was modified to a Y graft. The graft was sewn onto the axillary artery with a running 5/0 polypropylene suture with a longer arteriotomy not to compromise arterial flow during ECMO support (Figure 1 , Video S1). Under fluoroscopic guidance, an Impella 5.5 device was introduced into the left ventricular cavity. Despite his Impella 5.5 support, central venous pressures remained 30 mmHg with pulmonary artery pulsatility index of 0.6 (Table 1 ). Followed that from the other Y arm, a 19F arterial cannula was introduced and secured to the base of the axillary graft (Figure 2 ). Another 23F venous cannula was introduced through the right internal jugular vein and advanced into the vena cava inferior under fluoroscopic guidance. VA‐ECMO was initiated with 3.5 lt flow. The axillary wound was closed, tunnelling his Y graft under his skin.
Figure 1.
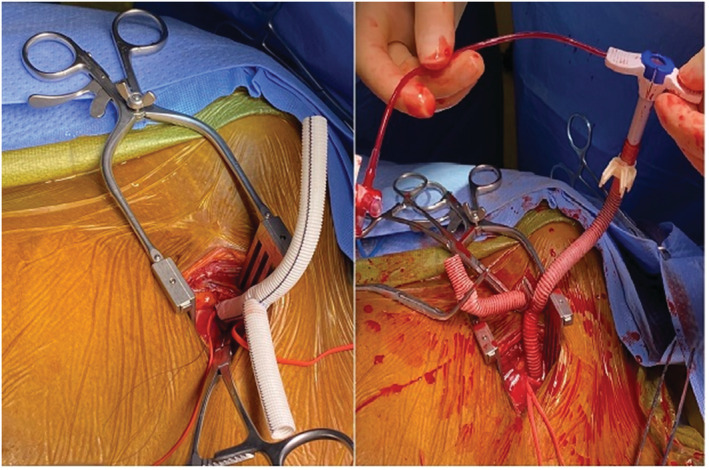
Y graft anastomosis on axillary artery.
Table 1.
Haemodynamic parameters before and after mechanical circulatory support
| Preoperative | Before Impella | After Impella | After Ecpella (10 h) |
|---|---|---|---|
| Blood pressure (mmHg) | 91/65 | 90/73 | |
| Mean arterial BP (mmHg) | 74 | 76 | 79 |
| Heart rate | 82 | 75 | |
| PA diastolic | 40 | 31 | 21 |
| CVP | 35 | 30 | 11 |
| PAPI | 0.4 | 0.5 | 2 |
| SVO2 | 52 | 77 | |
| Cardiac index | 1.9 | 2.4 | |
| Epinephrine (mcq/kg/min) | 0.04 | 0.04 | |
| Milrinone (mcq/kg/min) | 0.15 | — | |
| Norepinephrine (mcq/kg/min) | 0.1 | — |
BP, blood pressure; CVP, central venous pressure; PA, pulmonary artery; PAPI, pulmonary artery pulsatility index.
Figure 2.
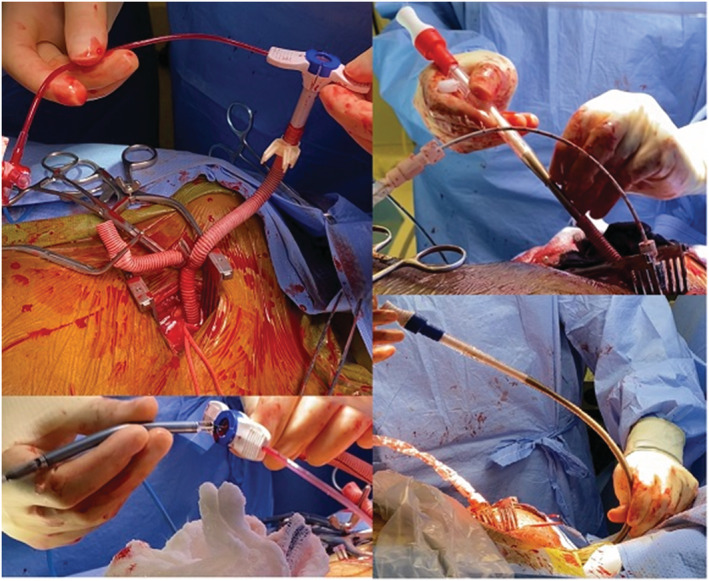
Axillary artery cannulation, Impella insertion, and cannulation of the jugular vein.
He was extubated postoperative second day and physically active afterward, including exercising on a treadmill. He stayed on Ecpella support for 19 days without any complication. His anticoagulation was IV systemic bivalirudin and bivalirudin through Impella 5.5 purge, which was monitored with aPTT. He underwent heart transplantation as status 1 with his current ECMO and Impella support, during which both devices were removed, and he was discharged 7 days later.
Discussion
Extracorporeal membrane oxygenation has many advantages for early metabolic recovery by providing improved circulation, supporting all failing organs. 3 However, left ventricular distension is observed in almost 40% of the patients, and this may lead to pulmonary edema, ventricular arrhythmias, and left ventricular thrombus formation. 3 Impella would unload the left ventricle by venting through a coapting aortic valve. Also, utilization of Impella combined with ECMO (Ecpella) decreases central venous pressure, pulmonary pressures, and preserves right ventricle function and avoids stasis in cardiac chambers and ascending aorta. In an international, multicentre cohort study, left ventricular unloading was associated with lower mortality in patients with cardiogenic shock treated with VA‐ECMO, despite higher complication rates, supporting use of left ventricular unloading in patients with cardiogenic shock treated with VA‐ECMO. 4
Bilateral femoral arteries or a combination of axillary and femoral arteries are used for cannulation in general. 1 , 5 Our ECPELLA strategy not only requires one arterial site intervention, which would decrease arterial side complications and bleeding, but also arterial and venous access above the diaphragm helps to mobilize the patient afterward. We would recommend this approach on anyone with 7 mm axillary artery diameter or higher with longer arteriotomy because Impella chord would occupy some area in the arterial opening site and in smaller axillary arteries this may cause resistance to inflow. Ecpella configurations utilizing the groin either for ECMO arterial inflow or Impella CP, or groin venous cannulation did not facilitate true mobility of the patients; hence, our patient was mobilized daily until his heart transplantation.
A recent case report describes single arterial access for Impella and ECMO placement through axillary artery on two patients. 6 First patient was placed Impella 5.0 support but later on cannulated with a purse string on the same graft with 15F ECMO cannula. Second patient was placed on central ECMO for postcardiotomy shock and later had a Y graft like our patient on the axillary artery to continue ECMO and place Impella 5.5. However, a true mobility neither achieved nor was the goal for those patients. Both patients had groin venous cannulation, and Ecpella support was only kept for 3 days, which did not endure long enough to test their innovative approach. Hence, our strategy was well planned at once for better Ecpella configuration to achieve full mobility and transplant the patient without any complications, and endurance of the system was well proved keeping Ecpella support for 19 days.
Single arterial Ecpella configuration and upper body based dual‐device haemodynamic support allowed full mobility of the advanced heart failure patient for an extended period until the next corrective therapy was available.
Conflict of interest
There is no conflict of interest to declare.
Funding
There is no funding in this study.
Supporting information
Video S1. ECMO and Impella intervention.
Bitargil, M. , Pham, S. , Haddad, O. , and Sareyyupoglu, B. (2022) Single arterial access for Ecpella and jugular venous cannulation provides full mobility on a status 1 heart transplant recipient. ESC Heart Failure, 9: 2003–2006. 10.1002/ehf2.13862.
References
- 1. Nakamura M, Imamura T. Practical management of ECPELLA. Int Heart J 2020; 61: 1094–1096. [DOI] [PubMed] [Google Scholar]
- 2. Tschope C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV, Schmidt G, Burkhoff D, Pieske B, Spillmann F. Mechanical unloading by fulminant myocarditis: LV‐IMPELLA, ECMELLA, BIPELLA, and PROPELLA concepts. J Cardiovasc Transl Res 2019; 12: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel SM, Lipinski J, Al‐Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, Medalion B, Deo S, Elgudin Y, Costa MA, Osman MN, Attizzani GF, Oliveira GH, Sareyyupoglu B, Bezerra HG. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J 2019. Jan; 65: 21–28. [DOI] [PubMed] [Google Scholar]
- 4. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, Eden M, Eitel I, Frank D, Frey N, Funamoto M, Goßling A, Graf T, Hagl C, Kirchhof P, Kupka D, Landmesser U, Lipinski J, Lopes M, Majunke N, Maniuc O, McGrath D, Möbius‐Winkler S, Morrow DA, Mourad M, Noel C, Nordbeck P, Orban M, Pappalardo F, Patel SM, Pauschinger M, Pazzanese V, Reichenspurner H, Sandri M, Schulze PC, H G Schwinger R, Sinning JM, Aksoy A, Skurk C, Szczanowicz L, Thiele H, Tietz F, Varshney A, Wechsler L, Westermann D. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international. Multicenter Cohort Study Circ 2020; 142: 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hysi I, Renaut C, Fabre O. Direct axillary artery cannulation in cardiac surgery: clinical outcomes. Asian Cardiovasc Thorac Ann 2017; 25: 502–503. [DOI] [PubMed] [Google Scholar]
- 6. Eulert‐Grehn JJ, Starck C, Kempfert J, Falk V, Potapov E. ECMELLA 2.0: single arterial access technique for a staged approach in cardiogenic shock. Ann Thorac Surg 2021; 111: e135–e137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. ECMO and Impella intervention.


