Abstract
Aims
Timely detection of subclinical left ventricular diastolic dysfunction (LVDDF) is of importance for precise risk stratification of asymptomatic subjects. Here, we evaluated the prevalence of LVDDF and its prognostic significance in the general population using two grading approaches: the 2016 ASE/EACVI recommendations and population‐derived, age‐specific criteria.
Methods and results
We randomly recruited 1407 community‐dwelling participants (mean age, 51.2 years; 51.1% women; 53.5% with cardiovascular risk factors). We measured left heart dimensions, strain, tricuspid regurgitation, transmitral blood flow, and mitral annular tissue velocities using conventional echocardiography and Doppler imaging. We utilized these measurements to grade of LVDDF according to the 2016 recommendations and population‐derived, age‐specific approach. According to the 2016 recommendations, 26 subjects (1.85%) were classified as having the advanced stage (Grade 2), whereas in 109 participants (7.75%) diastolic function was indeterminate. When applying the population‐derived criteria, the prevalence of advanced LVDDF was 17.9% (n = 252). During the follow‐up period (8.4 years), 100 participants experienced adverse cardiac events. After full adjustment, we did not observe any significant differences in the risk of events between subjects with indeterminate or any grade of LVDDF and subjects with normal diastolic function when classified according to the 2016 recommendation (P ≥ 0.25). In contrast, the adjusted risks of adverse cardiac events (HR = 1.28; P = 0.0045) were significantly elevated in participants with LVDDF when classified according to the population‐derived criteria.
Conclusions
Our study underscored the importance of considering age‐ and population‐derived thresholds in LVDDF grading in subjects at high cardiovascular risk which led to a better risk stratification and outcome prediction.
Keywords: Population, Echocardiography, Diastolic dysfunction, Grading approaches, Prognosis
Introduction
Diastolic dysfunction is characterized by impaired left ventricular (LV) relaxation and increased LV stiffness. 1 LV diastolic dysfunction (LVDDF) develops years to decades before the onset of heart failure (HF) symptoms. Therefore, timely detection of subclinical LVDDF is of great importance for more precise risk stratification of asymptomatic subjects. 2
Echocardiographic techniques such as pulsed‐wave Doppler and Tissue Doppler Imaging (TDI) are used to measure intra‐cardiac blood flows and myocardial velocities, thereby providing valuable information about LV diastolic function (LVDF) profiles. 3 For instance, impaired LV relaxation is characterized by decreased transmitral early (E) and enhanced atrial (A) LV filling as well as less vigorous mitral annulus motion during early diastole (e′). Moreover, combining early transmitral flow velocity with mitral annular velocity (E/e′ ratio) reflects in some degree increasing of LV filling pressure, a major consequence of increased LV stiffness related to diastolic dysfunction. It should be emphasized that LVDF assessment requires the measurement and complex interpretation of a panel of echocardiographic measures. 4
Over time, different recommendations and approaches have been utilized to grade LVDF using echocardiographic indexes in patients or populations. 5 However, due to this plethora of definitions for diastolic dysfunction used and differences in clinical characteristics of the study populations, the reported prevalence of LV diastolic dysfunction varies widely from 12% to 84%. 5 To improve diagnostic assessment and standardize interpretation of echocardiographic data in patients with symptomatic HF in clinical practice, the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) updated their recommendations for the evaluation of LVDDF. 6 Of note, when applying this updated ASE/EACVI algorithm in a middle‐to‐old‐aged asymptomatic cohort recruited from the general population, advanced LVDDF (Grade 2) was detected only in 1.4%. 7 As such, the criteria included in the updated ASE/EACVI approach seemed to detect only the most advanced cases of LVDDF in symptomatic HF patients with significantly elevated LV filling pressure and therefore might be less useful for identification of asymptomatic subjects who might have only mild increase in LV stiffness but nevertheless are at higher risk for adverse cardiovascular (CV) outcome. However, the actual prognostic value of LVDDF grades according to the 2016 ASE/EACVI recommendations has not yet been explored in asymptomatic subjects at high risk from a community‐based population sample. Previously, we have focused on developing age‐specific criteria for LVDF based on thresholds derived from the healthy reference cohort. 8 , 9 We, therefore, compared the prevalence of LVDDF and its prognostic significance in the general population using two grading approaches: the 2016 ASE/EACVI recommendations and population‐based age‐specific criteria.
Methods
The Ethics Committee of the University of Leuven approved the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO) and the subjects gave informed consent.
Study population
From August 1985 to December 2005, we randomly recruited a family‐based population sample from a geographically defined area in northern Belgium as described before. 8 , 9 Between 2005 and 2014, we re‐invited 1851 subjects for a technical examination including echocardiography. We obtained written informed consent in 1447 subjects (participation rate, 78.2%). We excluded 40 subjects from statistical analysis because of the presence of atrial fibrillation (n = 10) or a cardiac pacemaker (n = 6) or because echocardiographic parameters reflecting LVDF (Doppler velocities of transmitral flow and mitral annulus) were missing or could not be reliably measured at baseline (n = 24). In total, we thus included 1407 participants in present analyses.
Echocardiography
A complete echocardiographic protocol is provided in the Data Supplement. Briefly, two experienced observers did the ultrasound examination using a Vivid7 Pro (GE Vingmed, Horten, Norway) interfaced with a 2.5 to 3.5 MHz phased‐array probe in accordance to recommendations. 10 , 11 All recordings included at least five cardiac cycles and were digitally stored for off‐line analysis.
One experienced observer (T. K.) analysed the echocardiograms blinded to the participants' characteristics on a workstation running EchoPac software (GE Vingmed). LV mass and LV and left atrial (LA) volumes were indexed to body surface area. LV hypertrophy was defined as a LV mass index exceeding 115 g/m2 (men) or 95 g/m2 (women).
Transmitral blood flow Doppler signals were used to measure peak early (E) and late (A) diastolic velocities, their ratio (E/A) and A flow duration. From the PV flow signal, we measured the duration of PV reversal time during atrial systole (AR). On tricuspid continuous Doppler recordings (if detectable), we determined the peak velocity of the TR jet at the modal frequency. From pulsed‐wave TDI recordings, we measured the early (e′) and late (a′) diastolic peak velocities of the mitral annulus displacement at four walls. We calculated the E/e′ ratio by dividing transmitral E peak by e′ averaged from the septal and lateral acquisition sites or four sites. The methodology of other echocardiographic indexes is provided in the Supporting information .
We reported the intra‐observer reproducibility of the LV structural and diastolic indexes measured by the experienced observer (T. K.) in more detail previously. 12
Classification of left ventricular diastolic function according to the 2016 recommendations
The 2016 ASE/EACVI grading Algorithm A 6 utilizes four criteria to evaluate LVDF in subjects with normal EF and without myocardial disease: (i) Average E/e′ >14; (ii) Septal e′ velocity <7 cm/s or lateral e′ velocity <10 cm/s; (iii) tricuspid valve regurgitation peak >2.8 m/s; and (iv) LA volume index (LAVi) >34 mL/m2. LVDF was considered normal if less than two criteria were met and indeterminate if exactly half (two criteria) of the key criteria were fulfilled. A subject was labelled as having LVDDF if at least three out of four ASE/EACVI criteria were fulfilled. Moreover, we applied the Algorithm B for LVDDF grading 6 in 362 participants with previous history of cardiac disease including ischaemic heart disease, and/or structural abnormalities (including moderate valvular abnormalities), and/or in patients with diabetes mellitus, and/or LV hypertrophy.
Assessment of outcome
To study the incidence of cardiac mortality and morbidity in relation to baseline LVDDF grades, we collected outcome data on average 8.4 years after the echocardiographic examination. We ascertained vital status of participants until 17 January 2018. We applied the International Classification of Disease codes for the immediate and underlying cause of mortality and morbidity. 9 We collected information on the incidence of non‐fatal events via a follow‐up visit or a telephone interview and via the medical records of the general practitioners and regional hospitals. In all participants, we adjudicated and ascertained the self‐reported disease information against the medical records of the general practitioners and regional hospitals. 9 , 13 Adverse cardiac events included fatal and non‐fatal coronary events (myocardial infarction, ischaemic heart diseases including angina and coronary revascularization), fatal and non‐fatal HF, new‐onset atrial fibrillation, life‐threatening arrhythmias, and valvular heart disease including cardiac surgery. Only the first event within every category was considered in the outcome analysis.
We provided a detailed information on other measurements and selection of the healthy reference group in the Supporting information .
Statistical analysis
We used SAS software version 9.4 (SAS Institute, Cary, NC, USA) for database management and statistical analysis. We compared means and proportions using a large sample z test and χ 2 test, respectively. Statistical significance was a two‐sided P value below 0.05. In the healthy reference group, we determined age‐specific percentiles of the LV diastolic indexes. We additionally calculated the 95% confidence intervals of the 2.5% (E/A ratio and e′ peaks) or 97.5% (E/A, E/e′, LA strain, and LAVi) thresholds from their bootstrap distribution 14 obtained from 1000 random samples from the study population, using the PROC SURVEYSELECT procedure implemented in SAS software. We used frailty Cox regression models to calculate multivariable‐adjusted hazard ratios expressing the risk for cardiac events in each LVDDF category with normal diastolic function as reference group. Baseline characteristics considered as covariables in Cox regression were age, sex, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, history of cardiac disease, and LV mass index.
Results
Characteristics of participants at baseline
The 1407 participants (51.1% women) included 611 (43.4%) hypertensive subjects of whom 347 (56.8%) were on antihypertensive drug treatment. The mean age was 51.2 ± 15.7 years. Tables 1 and 2 list the clinical and echocardiographic characteristics of participants in the entire study population and in the healthy reference group.
TABLE 1.
Clinical characteristics of all participants and healthy references
| Characteristic | Entire population (n = 1407) | Healthy reference (n = 568) |
|---|---|---|
| Anthropometrics | ||
| Age, years | 51.2 ± 15.7 | 41.7 ± 13.3 |
| Female, n (%) | 719 (51.1) | 295 (51.8) |
| Body height, cm | 169.4 ± 9.5 | 170.9 ± 9.2 |
| Body weight, kg | 76.1 ± 14.9 | 69.9 ± 11.4 |
| Body mass index, kg/m2 | 26.5 ± 4.4 | 23.8 ± 2.7 |
| Waist circumference, cm | 90.7 ± 12.5 | 82.8 ± 9.1 |
| Systolic BP, mmHg | 130.4 ± 17.5 | 118.7 ± 9.7 |
| Diastolic BP, mmHg | 80.7 ± 9.7 | 76.0 ± 7.4 |
| Pulse pressure, mmHg | 49.7 ± 14.7 | 42.7 ± 8.4 |
| MAP, mmHg | 97.2 ± 10.8 | 90.3 ± 7.2 |
| Heart rate, bpm | 63.8 ± 9.3 | 63.1 ± 8.6 |
| Questionnaire data | ||
| Current smoking, n (%) | 585 (38.0) | 142 (24.9) |
| Drinking alcohol, n (%) | 560 (39.8) | 259 (45.4) |
| Hypertensive, n (%) | 611 (43.4) | / |
| Treated for HT, n (%) | 347 (24.4) | / |
| History of DM | 61 (4.3) | / |
| History of cardiac disease | 80 (5.7) | / |
| Biochemical data | ||
| Total cholesterol, mmol/L | 5.09 ± 0.96 | 4.96 ± 0.93 |
| HDL cholesterol, mmol/L | 1.46 ± 0.38 | 1.54 ± 0.39 |
| Serum creatinine, μmol/L | 79.7 ± 16.4 | 77.9 ± 13.8 |
| Blood sugar, mmol/L | 4.82 ± 0.73 | 4.66 ± 0.43 |
| Insulin, μmol/L | 5.13 (2.00 to 12.0) | 4.07 (2.00 to 8.71) |
BP, blood pressure; bpm, beats per minutes; CV, cardiovascular; DM, diabetes mellitus; HT, hypertension; MAP, mean arterial pressure.
Values are mean (±SD), number of subjects (%) or geometric mean (10–90% percentile interval).
TABLE 2.
Echocardiographic characteristics of all participants and healthy references
| Characteristic | Entire population (n = 1407) | Healthy reference (n = 568) |
|---|---|---|
| Left ventricular | ||
| Internal diameter, cm | 5.03 ± 0.47 | 4.97 ± 0.43 |
| Septal wall thickness, cm | 0.97 ± 0.16 | 0.89 ± 0.13 |
| Posterior wall thickness, cm | 0.90 ± 0.13 | 0.83 ± 0.12 |
| Mass index, g/m2 | 90.9 ± 21.3 | 82.8 ± 17.0 |
| Ejection fraction, % | 60.9 ± 6.0 | 61.5 ± 5.6 |
| Strain, % | 19.3 ± 2.3 | 19.6 ± 2.0 |
| Left atrium | ||
| Volume index, ml/m2 | 31.0 ± 9.2 | 28.2 ± 7.0 |
| Strain, % | 31.8 ± 9.5 | 37.2 ± 8.4 |
| Transmitral Doppler data | ||
| E peak, cm/s | 73.1 ± 16.4 | 78.6 ± 14.9 |
| A peak, cm/s | 61.5 ± 17.2 | 52.3 ± 13.2 |
| E/A ratio | 1.30 ± 0.51 | 1.60 ± 0.52 |
| Tissue Doppler data | ||
| e′ septal, cm/s | 9.19 ± 2.93 | 11.1 ± 2.63 |
| e′ lateral, cm/s | 12.4 ± 4.19 | 15.2 ± 3.68 |
| 4‐site e′ average, cm/s | 11.1 ± 3.64 | 13.6 ± 3.12 |
| Septal‐lateral E/e′ ratio | 7.23 ± 2.32 | 6.14 ± 1.26 |
| 4‐site E/e′ ratio | 7.13 ± 2.37 | 5.95 ± 1.26 |
| LV diastolic function by ASE/EACVI guidelines | ||
| Normal | 996 (70.8) | 521 (91.4) |
| Indeterminate | 109 (7.75) | 10 (1.75) |
| Diastolic dysfunction | ||
| Grade 1 | 276 (19.6) | 37 (6.5) |
| Grade 2 | 26 (1.85) | 2 (0.35) |
LV, left ventricular.
Values are mean (±SD) or number of subjects (%).
Incidence of events
In our community‐based sample, the median follow‐up period was 8.4 years (5th to 95th percentile, 4.0 to 13.2). During 11 884 person‐years of follow‐up, 100 subjects had at least one cardiac event (8.4 events per 1000 person‐years). Table S1 lists the cause‐specific fatal and non‐fatal events of the study cohort.
Left ventricular diastolic dysfunction by the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging criteria
In the entire cohort, 499 (35.5%) had septal e′ peak <7 cm/s and/or lateral e′ peak <10 m/s (2nd criterion) and 434 (30.9%) subjects had a LAVi >34 mL/m2 (4th criterion). In contrast, only 21 (1.5%) and 13 (0.9%) subjects fulfilled the 1st (E/e′ ratio >14) and the 3rd (TR peak >2.8 m/s) criteria, respectively (Figure S1 ). As a result of the 2016 ASE/EACVI criteria prevalence, 26 subjects (1.85%) were classified as having Grade 2 LVDDF (suggestive of significantly elevated LV filling pressure as defined in the Algorithms A and B), whereas in 109 participants (7.75%), LVDF was indeterminate. Among 362 participants with normal EF and myocardial disease in whom we used Algorithm B to define LVDF grade, 276 subjects were classified as having Grade 1 mainly based on transmitral profile.
The age‐ and sex‐standardized incidence rates for adverse cardiac events increased across these groups: from 5.0/1000 person‐years in the subjects who defined as having normal LVDF (number of events, n = 33), 13.2/1000 person‐years (n = 20) in the indeterminate group, 14.4/1000 person‐years (n = 41) in subjects with Grade 1, and to 27.2/1000 person‐years in subjects with Grade 2 LVDDF (n = 6).
Detection of left ventricular diastolic function profiles by population‐based age‐specific Doppler criteria
Figure 1 show the age‐specific percentiles of the transmitral E/A ratio, Tissue Doppler Imaging (TDI) e′ peaks, LA strain, LA volume index (LAVi), and E/e′ ratios in reference participants free from any CV disease or risk factors selected from the entire study population (n = 568). Table S2 shows the 2.5th and 97.5th age‐group specific percentiles for the transmitral E/A ratio, the 2.5th percentile for the e′ peaks and the 97.5th percentiles for the E/e′ ratio, LA strain, and LAVi in the reference subjects. Using the bootstrap approach, we calculated the confidence intervals of the 2.5th or 97.5th percentiles in the reference group by age categories. Next, we rounded these age‐specific percentiles to the closest integer value (Table S2 ).
Figure 1.
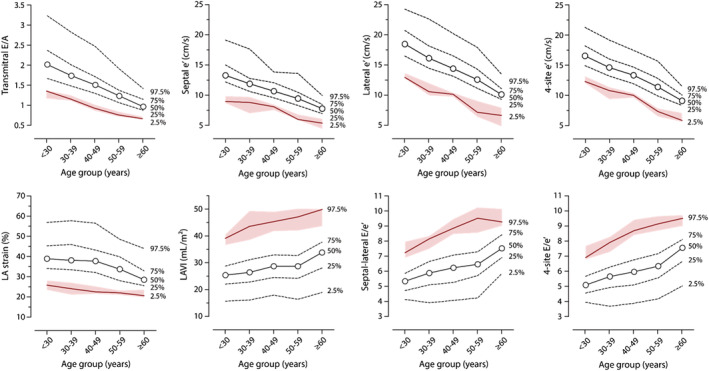
Age‐specific percentiles of E/A ratio, e′ peaks, left atrial (LA) strain and volume index and E/e′ ratio for the healthy reference sample (n = 568). Shaded area represents 95% confidence intervals of the 2.5% or 97.5% thresholds (red line) as derived from their bootstrap distributions.
Using these population‐derived age‐specific cut‐offs, we combined these echocardiographic parameters to identify the profiles of LVDF in our cohort. Table 3 shows the prevalence of each LVDF profiles as well as crude and age‐ and sex‐standardized incidence rates of adverse cardiac events in these groups. The first identified group (n = 127; 9.0%) included subjects with normal E/e′ ratio (≤8.5) and an abnormally low age‐specific e′ velocity or transmitral E/A ratio (less than 2.5th percentile of the reference subgroup). The event rates in this group were comparable to subjects with normal LVDF (low‐risk group; Table 3 ). On the other hand, subjects with normal E/e′ but with both low age‐specific E/A and TDI e′ velocity (n = 47; 3.3%) demonstrated higher incidence rates of cardiac events as compared with the group with normal LVDF (24.0/1000 person/years vs 4.8/1000 person/years; Table 3 ).
TABLE 3.
Incidence rates (95% CI) of adverse cardiac events per diastolic function profile as defined by age‐specific reference limits for E/A, e′ peak and normal/grey‐zone/high E/e′
| Profile | N | CV risk factors (%) | N of cardiac events | Cardiac events per 1000 person‐years | |
|---|---|---|---|---|---|
| Crude | Standardized | ||||
| No LV diastolic dysfunction | |||||
| Normal E/A, e′, and E/e′ | 1028 (73.1%) | 433 (42.1%) | 36 | 4.1 | 4.8 (3.3 to 6.4) |
| Low risk LV diastolic dysfunction | |||||
| Normal E/A + low e′ + normal E/e′ | 49 (3.5%) | 37 (75.5%) | 0 | — | — |
| Low E/A + normal e′ + normal E/e′ | 78 (5.5%) | 53 (68.0%) | 8 | 12.0 | 7.3 (2.2 to 12.3) |
| All | 127 (9.0%) | 90 (70.9%) | 8 | 7.7 | 6.8 (2.1 to 11.5) |
| High risk LV diastolic dysfunction | |||||
| Low E/A + low e′ + normal E/e′ | 47 (3.3%) | 43 (91.5%) | 8 | 24.0 | 21.0 (5.2 to 36.8) |
| Normal E/A + normal e′ + high E/e′ a | 127 (9.0%) | 113 (89.0%) | 32 | 30.2 | 18.8 (11.9 to 25.7) |
| Normal E/A + low e′ + high E/e′ a | 42 (3.0%) | 40 (95.2%) | 4 | 13.4 | 9.0 (1.9 to 17.9) |
| Low E/A + low e′ + high E/e′ a | 36 (2.6%) | 33 (91.7%) | 12 | 46.7 | 41.1 (14.4 to 67.8) |
| All | 252 (17.9%) | 229 (90.9%) | 56 | 28.7 | 21.7 (15.3 to 28.1) |
Incidence rates were calculated as number of events per 1000 subject‐years. Standardized event rates accounted for sex and age (age groups: <50 years; ≥50 years). The cardiovascular (CV) risk factors included hypertension, obesity, renal failure, a history of diabetes mellitus, and/or a history of cardiac disease.
High E/e′ was defined as E/e′ ≥9.5 or as E/e′ between 8.5 and 9.5 in combination with any of following echocardiographic abnormalities: low left atrial (LA) strain (<23%), LA enlargement (LAVi ≥45 mL/m2), tricuspid regurgitation (intense flow with peak TR velocity >2.5 m/s), and prolonged reverse atrial flow (i.e. mitral atrial flow ≤ reverse pulmonary vein flow—10 ms).
Another identified profile included subjects with elevated E/e′, which was defined as E/e′ ≥9.5 or as borderline E/e′ between 8.5 and 9.5 in combination with any of following echocardiographic abnormalities: low LA strain (<23%), LA enlargement (LAVi ≥45 mL/m2), tricuspid regurgitation (intense flow with peak TR velocity >2.5 m/s), and prolonged reverse atrial flow (i.e. mitral atrial flow ≤ reverse pulmonary vein flow—10 ms). In this group, we identified subjects who had both normal age‐specific E/A and TDI e′ (n = 127; 9.0%) or those who had normal E/A but an abnormally low age‐specific e′ velocity (n = 42; 3.0%) or had both low age‐specific E/A and e′ velocity (n = 36; 2.6%). The standardized incidence rates per 1000 person‐years in these subgroups were 18.8, 9.0, and 41.1 for cardiac events (Table 3 ).
Figure 2 illustrates the flowchart for identification of subclinical LVDDF profile based on the population‐derived age‐specific cut‐off values of echocardiographic parameters reflecting diastolic function. The clinical and echocardiographic characteristics of subjects by group of LVDF (normal, low, and high risk) appear in Table S3 .
Figure 2.
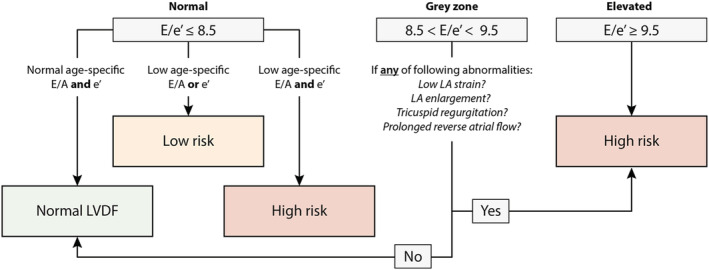
Flowchart for identification of early left ventricular diastolic dysfunction (LVDDF) in the general population. Echocardiographic abnormalities include: low LA strain (<23%), LA enlargement (LAVi ≥45 mL/m2), tricuspid regurgitation (intense flow with peak TR velocity >2.5 m/s) and prolonged reverse atrial flow (i.e. mitral atrial flow ≤ reverse pulmonary vein flow—10 ms). LA, left atrium; LV, left ventricle.
Risk associated with left ventricular diastolic dysfunction
Figure 3 demonstrates the Kaplan–Meier cumulative incidence of adverse cardiac events per LVDF group as defined by the 2016 ASE/EACVI recommendations and population‐derived age‐specific criteria. Table 4 shows the multivariable‐adjusted hazard ratios (HRs) expressing the risk for future cardiac events per LVDDF profile as compared with normal LVDF. After full adjustments, subjects with an indeterminate LVDF (HR:1.07; P = 0.41) or with LVDDF Grade 1 (HR:1.17; P = 0.25) or Grade 2 (HR:1.20; P = 0.28) by the 2016 algorithm did not exhibit significantly higher risk for events as compared with subjects who defined as having normal LVDF (Table 4 ). The risks of adverse cardiac events (HR = 1.28; P = 0.0045) were significantly elevated in participants with advanced stages of LVDDF as compared with subjects with normal LVDF when classified according to the population‐derived criteria (Table 4 ). Similar results were eventually observed for incident HF and atrial fibrillation (Table 4 ).
Figure 3.
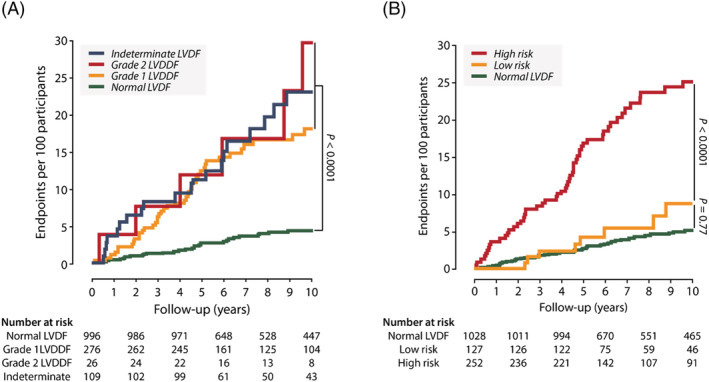
Cumulative incidence estimates (1‐Kaplan–Meier survival estimates) for fatal and non‐fatal cardiac events per left ventricular (LV) diastolic (dys)function group as defined by the 2016 ASE/EACVI recommendations (A) and age‐specific criteria (B). P values are for the differences between groups by the log‐rank test. LVD(D)F, LV diastolic (dys)function. (A) ASE/EACVI guidelines; (B) age‐specific criteria.
TABLE 4.
Adjusted hazard ratios for adverse cardiac events associated with LVDDF groups as defined by the 2016 ASE/EACVI recommendations or population‐derived age‐specific criteria
| Fatal and non‐fatal cardiac events | Fatal and non‐fatal HF and AF events | ||||||
|---|---|---|---|---|---|---|---|
| Algorithm | Total N | N of events | HR (95% Cl) | P value | N of events | HR (95% Cl) | P value |
| ASE/EACVI 2016 | |||||||
| Normal LV diastolic function | 996 | 33 | Reference | / | 10 | Reference | / |
| Indeterminate | 109 | 20 | 1.07 (0.91 to 1.25) | 0.41 | 10 | 1.16 (0.91 to 1.48) | 0.23 |
| Grade 1 LVDDF | 276 | 41 | 1.17 (0.89 to 1.54) | 0.25 | 23 | 1.48 (0.96 to 2.28) | 0.075 |
| Grade 2 LVDDF | 26 | 6 | 1.20 (0.86 to 1.66) | 0.28 | 4 | 1.35 (0.86 to 2.13) | 0.19 |
| Population age‐specific criteria | |||||||
| Normal LV diastolic function | 1028 | 36 | Reference | / | 13 | Reference | / |
| Low risk LVDDF | 127 | 8 | 1.13 (0.76 to 1.68) | 0.53 | 4 | 1.18 (0.66 to 2.10) | 0.58 |
| High risk LVDDF | 252 | 56 | 1.28 (1.08 to 1.51) | 0.0045 | 30 | 1.50 (1.00 to 1.69) | 0.047 |
CI, confidence interval; LV, left ventricular; LVDDF, LV diastolic dysfunction.
Hazard ratios (HRs) express the adjusted risk in each group of LVDDF at baseline compared with the risk in the subjects with normal LVDF. All HRs were adjusted for sex, age, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, history of cardiac disease, and left ventricular mass index.
Discussion
In this study, we explored prognostic significance of LVDDF in the general population defining by two grading approaches: the 2016 ASE/EACVI recommendations and population‐derived, age‐specific criteria. When applying the 2016 ASE/EACVI recommendations, the advanced grade of LVDDF (Grade 2) was identified in 1.85% of the general population cohort. Following the Algorithm B of the 2016 recommendation, the Grade 1 (19.6%) can be classified mainly in preselected participants with myocardial disease including LV hypertrophy based on transmitral inflow (E/A ratio). Of note, the participants with any grade of LVDDF and even with indeterminate LVDF according to the 2016 recommendations (7.75%) demonstrated higher incidence rate for cardiac events compared with those who was defined as having normal LVDF. However, after adjustment for important CV risk factors and LV mass index, this association lost statistical significance. When applying the population‐based age‐specific echocardiographic criteria, the prevalence of any of abnormal diastolic profiles was 26.5%. In these participants, we identified LVDDF profiles at lower and higher risk for adverse cardiac events. Even after full adjustment, the risk for future events was significantly elevated in participants with advanced grade of LVDDF than in subjects with normal function as defined by population‐based criteria.
The process of impairment of LV relaxation and LV stiffening characterizing LVDDF begins years to decades before symptoms of HF or ischaemic heart disease are present. Therefore, timely identification of early LVDDF even in asymptomatic subjects at high risk of atherosclerotic events and HF might be of importance for a better risk stratification. 2 Within this context, Doppler velocities of transmitral blood flow and mitral annulus are widely used to evaluate LVDF profiles as a non‐invasive alternative to an invasive LV pressure–volume measurements. 15 , 16 Previous studies in general population already demonstrated the independent prognostic value of different TDI indexes in particularly TDI e′ for CV mortality and morbidity. 9
Over the years, numerous approaches of echocardiographic indexes combination used to grade LVDDF in clinical and community‐based studies. 5 In attempt to standardize and simplify LVDF assessment and thus increase its clinical utility, the ASE and EACVI updated their recommendations for the evaluation of LVDF. 6 The recommendations were mainly designed to detect clinically significant increase in LV filling pressure in symptomatic HF patients. 17 , 18 , 19 , 20 This algorithm has been tested in clinical studies that used invasive filling pressure as reference method in HF patients. 17 , 18 , 19 However, usefulness of this algorithm in detection of early stages of LVDDF (when LV stiffness only mildly elevated) in asymptomatic patients at high CV risk such as patients with hypertension or diabetes should be further investigated. Indeed, in 1000 EPIPorto subjects (≥45 years) recruited from the general population, the prevalence of LVDDF dropped from 38.1% to 1.4% when shifting from the 2009 to the 2016 ASE/EACVI criteria. 7 Similarly, only 20 of 1485 STANISLAS participants (1.3%) had LVDDF according to the 2016 ASE/EACVI criteria, whereas prevalence of diastolic dysfunction ranged from 5.7% to 8.8% when using previous expert recommendations. 21 However, these studies did not utilize the Algorithm B in subjects with myocardial disease for instance with LV hypertrophy 21 or defined LVDDF according to the Algorithm A only. 7
In our community‐based sample including 1407 subjects between 18 and 90 years old, prevalence of the advanced stage of LVDDF (Grade 2) was 1.85% when applying the 2016 ASE/EACVI approach utilizing both algorithms. The Grade 1 was assigned to the most of pre‐selected subjects with myocardial disease (i.e. at high CV risk) based entirely on transmitral inflow profile without applying any age‐specific cut‐offs.
Thus, in line with previous general population studies, 7 , 21 our findings suggest that by applying the 2016 ASE/EACVI algorithm, we might underestimate the prevalence of early stages of diastolic dysfunction in the asymptomatic participants at risk. The low sensitivity of the 2016 ASE/EACVI algorithm to detect high‐risk profile of LVDDF when applied in the community might be explained by the implementation of age‐unspecific threshold values, inclusion of the TR peak velocity and high threshold for the E/e′ ratio. First, although age is an important determinant of transmitral and myocardial Doppler velocities even in healthy reference subjects 8 , 22 , 23 and this fact was appreciated in the expert recommendations, LVDF cut‐off values have not been standardized for age. 3 , 6 , 17 Second, as a measure of pulmonary hypertension in patients with symptomatic HF, 24 assessment of TR peak velocity was included in the newest ASE/EACVI recommendations. However, in line with Almeida et al., 7 we observed a TR velocity >2.8 m/s only in 13 subjects (0.9%) of the general population. A TR velocity of more than 2.8 m/s might rather indicate clinically relevant pulmonary hypertension in advanced HF patients 24 than be an indicator of early diastolic dysfunction in asymptomatic subjects at high CV risk.
Of note, after full adjustment, we did not observe any significant differences in the risk of cardiac events or incident HF and atrial fibrillation between subjects with any grades of LVDDF or indeterminate diastolic function, and subjects with normal LVDF when classified by the ASE/EACVI recommendations. On the other hand, the risks of all cardiac events were significantly elevated in participants with advanced LVDDF (i.e. with abnormally high E/e′ and other indexes) as compared with subjects with normal LVDF when classified according to population‐based age‐specific criteria after more than 8 years of follow‐up. This finding remains similar to what we reported on predictive value of LVDDF for a 5 year follow‐up period. 9 It should be noted that 57 out of 100 cardiac events were related to coronary heart disease (myocardial infarction, unstable angina and revascularization). Indeed, myocardial ischaemia even in its asymptomatic early stage slows ventricular relaxation, impairs ventricular distensibility, and in consequence, can trigger diastolic dysfunction. 25 Using the population‐derived criteria of LVDDF, we might improve risk stratification in the subjects at risk for clinically overt coronary heart disease and therefore modify the management and treatment strategy in these patients.
We previously demonstrated that in addition to diastolic dysfunction, subclinical systolic dysfunction (low LV longitudinal strain) along with LV hypertrophy are important prognostic markers in the community. 13 Therefore, the comprehensive assessment of cardiac function and structure in which LV diastolic dysfunction plays a major role would be important for risk stratification.
Study limitations
Our findings must be interpreted in the context of the limitations and strengths of our study. First, the assessment of echocardiographic indexes is prone to measurements errors. In the present study, however, only two experienced observers recorded all images using a highly standardized protocol. Moreover, all digitally stored images were centrally post‐processed by a single observer with good reproducibility. Second, we included in our analysis 80 (5.7%) participants with a previous history of cardiac diseases from whom 28 experienced a recurrent event during follow‐up. However, we applied adjustment for previous cardiac disease. Third, the gold standard for assessing diastolic function remains the pressure–volume relationship, but this requires an invasive approach, which is difficult and impractical to implement in subjects at CV risk recruited from the general population. On the other hand, Doppler techniques along with strain assessment open up the possibility of evaluating non‐invasively cardiac function. These techniques have been previously validated in numerous invasive studies. Finally, information on biomarkers such as NT‐proBNP might further improve identification of subjects with early subclinical stages of HF. However, in this study, we mainly focus on the prognostic role of echocardiographic criteria reflecting LVDF for CV risk stratification in asymptomatic subjects.
Conclusion
In the general population, application of the population‐based age‐specific criteria for diastolic function grading improved risk stratification in asymptomatic subjects. Our study underscored importance of considering age‐ and population‐derived thresholds in the grading approaches of early LVDDF especially in patients at CV risk.
Funding
The Research Unit Hypertension and Cardiovascular Epidemiology (Leuven, Belgium) currently receives support from the Research Foundation Flanders (FWO‐Flanders; grants G.0880.13, 1225021N, 1S07421N, and G0C5319N).
Supporting information
Table S1. Fatal and non‐fatal adverse cardiac events.
Figure S1. Percentage of participants fulfilling any of the 2016 ASE/EACVI criteria. Total number of participants is 1407. LAVI indicates LA volume index; TR, tricuspid regurgitation jet velocity.
Table S2. Age‐specific percentiles for transmitral E/A, E/e′, e′ peak and left atrial volume index and strain in 568 healthy reference subjects.
Table S3. Clinical characteristics and prevalence of abnormal echocardiographic traits per diastolic function profile.
Kuznetsova, T. , Cauwenberghs, N. , Sabovčik, F. , Kobayashi, Y. , and Haddad, F. (2022) Evaluation of diastole by echocardiography for detecting early cardiac dysfunction: an outcome study. ESC Heart Failure, 9: 1775–1783. 10.1002/ehf2.13863.
References
- 1. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DEJ, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 3. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 4. Hatle L. How to diagnose diastolic heart failure a consensus statement. Eur Heart J 2007; 28: 2421–2423. [DOI] [PubMed] [Google Scholar]
- 5. Selmeryd J, Henriksen E, Leppert J, Hedberg P. Interstudy heterogeneity of definitions of diastolic dysfunction severely affects reported prevalence. Eur Heart J Cardiovasc Imaging 2016; 17: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 7. Almeida JG, Fontes‐Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, Flachskampf FA, Leite‐Moreira A, Azevedo A. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. 2018;19(4):380–386. 10.1093/ehjci/jex252 [DOI] [PubMed] [Google Scholar]
- 8. Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2009; 2: 105–112. [DOI] [PubMed] [Google Scholar]
- 9. Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, Staessen JA. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc 2014; 3: e000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 2004; 17: 1086–1119. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 12. Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, López B, Thijs L, Jin Y, Malyutina S, Stolarz‐Skrzypek K, Casiglia E, Díez J, Narkiewicz K, Kawecka‐Jaszcz K, Staessen JA. Prevalence of left ventricular diastolic dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovasc Ultrasound 2012; 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuznetsova T, Cauwenberghs N, Knez J, Yang WY, Herbots L, D'hooge J, Haddad F, Thijs L, Voigt JU, Staessen JA. Additive prognostic value of left ventricular systolic dysfunction in a population‐based cohort. Circ Cardiovasc Imaging 2016; 9: e004661. [DOI] [PubMed] [Google Scholar]
- 14. Hesterberg T, Moore D, Monaghan S, Clipson A, Epstein R. Bootstrap methods and permutation tests. In Moore D., McCabe G., Bruce A., eds. Introduction to the Practice of Statistics. New York: WH Freeman & Co; 2017. [Google Scholar]
- 15. Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol 2008; 51: 679–689. [DOI] [PubMed] [Google Scholar]
- 16. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation 2000; 102: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 17. Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, Magne J, Laginha S, Hagendorff A, Haland TF, Aaberge L, Martinez C, Rapacciuolo A, Santoro C, Ilardi F, Postolache A, Dulgheru R, Mateescu AD, Beladan CC, Deleanu D, Marchetta S, Auffret V, Schwammenthal E, Habib G, Popescu BA. Echo‐Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro‐Filling study. Eur Heart J Cardiovasc Imaging 2017; 18: 961–968. [DOI] [PubMed] [Google Scholar]
- 18. Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937–1948. [DOI] [PubMed] [Google Scholar]
- 19. Balaney B, Medvedofsky D, Mediratta A, Singh A, Ciszek B, Kruse E, Shah AP, Addetia K, Lang RM, Mor‐Avi V. Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head‐to‐head comparison with the 2009 guidelines. J Am Soc Echocardiogr 2018; 31: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanchis L, Andrea R, Falces C, Poyatos S, Vidal B, Sitges M. Differential clinical implications of current recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2018; 31: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 21. Huttin O, Fraser AG, Coiro S, Bozec E, Selton‐Suty C, Lamiral Z, Frikha Z, Rossignol P, Zannad F, Girerd N. Impact of changes in consensus diagnostic recommendations on the echocardiographic prevalence of diastolic dysfunction. J Am Coll Cardiol 2017; 69: 3119–3121. [DOI] [PubMed] [Google Scholar]
- 22. Schirmer H, Lunde P, Rasmussen K. Mitral flow derived Doppler indices of left ventricular diastolic function in a general population; the Tromso study. Eur Heart J 2000; 21: 1376–1386. [DOI] [PubMed] [Google Scholar]
- 23. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart 2006; 92: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community‐based study. J Am Coll Cardiol 2009; 53: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohara T, Little WC. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr Opin Cardiol 2010; 25: 613–621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fatal and non‐fatal adverse cardiac events.
Figure S1. Percentage of participants fulfilling any of the 2016 ASE/EACVI criteria. Total number of participants is 1407. LAVI indicates LA volume index; TR, tricuspid regurgitation jet velocity.
Table S2. Age‐specific percentiles for transmitral E/A, E/e′, e′ peak and left atrial volume index and strain in 568 healthy reference subjects.
Table S3. Clinical characteristics and prevalence of abnormal echocardiographic traits per diastolic function profile.


