Abstract
Aims
Available upper reference levels (URLs) in older adults for N‐terminal pro brain natriuretic peptide (NT‐proBNP), an established biomarker for heart failure, are mainly based on small samples. We aimed to identify NT‐proBNP URL in a population‐based reference sample of individuals aged ≥65 years.
Methods and results
We analysed established NT‐proBNP predictors using quantile regression among 2459 participants of two‐independent population‐based cohorts located in Germany, the Activity and Function in the Elderly Study (ActiFE, n = 1450) and the Study of Health in Pomerania (SHIP‐TREND‐0, n = 1009). Based on predictors a reference population of 441 subjects (ActiFE, n = 227; SHIP‐TREND‐0, n = 214) without history of diabetes, cardiovascular, or pulmonary diseases and with systolic blood pressure (BP) <140 mmHg, diastolic BP ≥60 and ≤90 mmHg, haemoglobin in men ≥14 and ≤18 g/dL and in women ≥12 and ≤16 g/dL, GFR ≥60 mL/min/1.73 m2, CRP <5 mg/L, BMI ≥18 and ≤33 kg/m2, and hs‐cTnI <40 ng/L were built with NT‐proBNP median levels and 97.5% quantiles reported stratified by sex and age. In a secondary analysis the URL among 97 SHIP‐TREND‐0 participants with a left ventricular ejection fraction (LVEF) ≥50 and no diastolic dysfunction were estimated. The median age in the identified reference sample was 70 years, with 41.9% and 40.2% male participants in ActiFE and SHIP‐TREND‐0, respectively. We observed an age‐dependent increment of NT‐proBNP levels with higher values in women compared to men. Notably, NT‐proBNP levels were >125 ng/L in 165 participants (37.4%), with NT‐proBNP URL (97.5% quantiles) equal to 663, 824, 592, and 697 ng/L in men, and 343, 463, 2641, 1276 ng/L in women for ages 65–69, 70–74, 75–79, and 80+ years, respectively. In the secondary analysis with a LVEF ≥50 and no diastolic dysfunction (35 men and 62 women) NT‐proBNP levels >125 ng/L were still observed in 38 (39.2%) participants.
Conclusions
This reference sample of apparently healthy asymptomatic older adults showed an age‐related increment of NT‐proBNP levels with URL markedly higher than the European Society of Cardiology recommended cut‐off of 125 ng/L for the diagnosis of heart failure in ambulatory settings. Identifying URL in those ≥80 years remains complex. Our results attempt to provide a frame for the further investigation of age‐specific NT‐proBNP cut‐offs in older adults. Considering the demographic changes, further evaluation of NT‐proBNP URL in larger samples of older adults followed by the validation of age‐specific cut‐off values for the identification of heart failure in those 65 years or older are urgently needed.
Keywords: NT‐proBNP, Reference levels, Older adults
Introduction
As a clinical syndrome the diagnosis of heart failure (HF) remains a challenge in the ambulatory setting. N‐terminal pro brain natriuretic peptide (NT‐proBNP) is an established biomarker for HF, a condition present in more than 10% of people >70 years of age, 1 and is recognized as an independent predictor of HF with reduced and preserved ejection fraction, 2 as well as of cardiovascular 3 and total mortality. 4 Concentrations of natriuretic peptides represent a pivotal diagnostic criterion for HF in addition to symptoms/signs and echocardiography and are especially valuable in persons with HF with preserved or mid‐range ejection fraction (HFpEF, HFmrEF) or in those settings, where echocardiography is not immediately available as recommended by the ESC Guidelines. However, there is some controversy about the screening for HF in asymptomatic patients 5 as well as about the optimal cut‐off value for the identification of patients with decompensated HF in the ambulatory setting, 6 so that the correct interpretation of NT‐proBNP concentrations still remains a challenge in the process of clinical decision making. Increasing age is recognized at the same time as one of the non‐cardiac causes for elevated natriuretic peptide levels. 7 Unfortunately, currently available upper reference levels (URLs) are mainly based on small sample sizes of older adults, which are usually not well enough characterized with respect to NT‐proBNP predictors. In addition, the ageing process is quite heterogeneous making the identification of good reference sample a challenge. The World Health Organization defines healthy ageing as the process of developing and maintaining the functional ability that enables wellbeing in older age, 8 allowing the presence of well‐controlled comorbidities. In this context, being free of disease is not necessarily a requirement for healthy ageing or rather for reference populations of older adults.
So far, nonparametric methods such as empirical quantiles and quantile regression are recommended for the estimation of reference values. 9 NT‐proBNP reference values are mainly based on empirical quantiles, which tend to be less stable at the margins of the distribution, especially in the presence of outliers, and in small sample sizes. In contrast quantile regression allows the estimation of any predictor's effect for different NT‐proBNP quantiles, providing a more comprehensive picture of the associations. The aim of the present analyses was the estimation of NT‐proBNP URL stratified by sex and age for community‐dwelling older adults (≥65 years) based on data from two independent well‐characterized German population‐based cohorts using empirical quantiles and quantile regression.
Methods
Study populations
To get a sizeable sample of well characterized community‐dwelling older adults (≥65 years) for the estimation of age and sex‐stratified NT‐proBNP URL, concomitant baseline data of two German observational cohorts, the ActiFE Ulm (Activity and Function in the Elderly in Ulm) study and the Study of Health in Pomerania (SHIP) were merged.
The ActiFE Ulm study is a population‐based cohort study in community‐dwelling older adults (≥65 years) randomly selected in Ulm (Southern Germany) and adjacent regions. ActiFE baseline assessments took place between March 2009 and April 2010 with 1506 participants (initial response rate 20%). The study cohort design has been previously described. 4 , 10 SHIP is a population‐based cohort study conducted in Northeastern Germany, which comprises two independent cohorts (baseline examinations: SHIP‐0 and SHIP‐TREND‐0). 11 , 12 For these analyses, SHIP‐TREND‐0 data were used, which had its assessments between September 2008 and September 2012. For the purpose of this analysis, those participants ≥65 years were considered (n = 1098). 13 Participants with missing data for identified covariables were excluded. In total, 2459 participants (ActiFE n = 1450, SHIP‐TREND‐0; n = 1009) build the study population.
The investigation conforms with the principles outlined in the Declaration of Helsinki. 14 The ethical committees of and the University of Greifswald approved the ActiFE study (application no. 318/08) and the SHIP‐TREND‐0 study (application no. BB318/08 and BB39/08). All subjects gave written informed consent to participate in the study.
Assessments of analyses related variables
The following variables were assessed in both cohorts: age, sex, education, smoking, and comorbidities were ascertained by interview‐based self‐report. The highest completed school education level was binary categorized (≤10 or >10 years). Smoking was categorized into never, former and current smoker. Comorbidities were assessed by answering the question: ‘Have you ever been diagnosed with any of the following diseases?: hypertension, myocardial infarction (MI), HF, stroke, or diabetes’. While SHIP addressed the presence of chronic lung disease, ActiFE differentiated between chronic obstructive pulmonary disease (COPD) and asthma. Body height and weight were measured to calculate body mass index (BMI) in kg/m2. BMI was additionally categorized in underweight (<18), normal weight (≥18 and <25), overweight (≥25 and <30), and obesity (≥30). Systolic and diastolic blood pressures (SBP and DBP) were measured three times, and the averages of the last two measurements were used for the analyses. Mortality status and date of death were obtained from the local registration offices for all participants with a median follow‐up of 9.1 and 8.3 years in ActiFE and SHIP‐TREND‐0, respectively.
In both studies, venous blood samples were drawn, immediately centrifuged, processed, and stored at −80°C until analysis. 15 , 16 NT‐proBNP measurements were performed in ActiFE one year after collection using an electro‐chemiluminescence‐immunoassay (ECLIA) [Elecsys NT‐proBNP II Test, 2010; Roche Diagnostics, Mannheim, Germany; lower limit of detection (LOD) 5 ng/L, measuring range 5–35000 ng/L, intra assay coefficient of variation (intra assay CV) 2.4%, inter assay CV 5.6–5.9%]. In SHIP NT‐proBNP was measured two years after collection by immunoassay on a Siemens Dimension VISTA (Siemens Healthcare Diagnostics, Eschborn, Germany; measuring range 5–35 000 ng/L, CV 2.3–3.6%). According to Collin‐Chavagnac et al., there are no significant differences between these two assays, showing almost perfect agreement. 17 Differences in the storage time by −80°C prior to NT‐proBNP measurement should not affect the stability of the biomarker over time. 18
Further laboratory measurements in ActiFE were as follows: Immunonephelometric assays measured Cystatin C (N Latex Cystatin C, 2010; Siemens, Eschborn, Germany; LOD 0.05 mg/L, measuring range 0.05–7.25 mg/L, intra assay CV 2.3%, inter assay CV 2.9–3.2%), and serum high‐sensitive C‐reactive protein (hs‐CRP) (N Cardio Phase TM hsCRP, 2010, Behring Nephelometer II, Fa. Siemens, Eschborn, Germany; LOD 0.16 mg/L, measuring range 0.17–1100 mg/L, intra assay CV 3.6%, inter assay CV 5.1–6.7%). A chemiflex‐microparticle‐enzyme‐immunoassay (CMIA) was used to measure high‐sensitive troponin I (hs‐TnI) (Abbott Architect i1000, Fa. Abbott, 2013; LOD 2.0 ng/L, measuring range 10–50 000 ng/L, a reported within‐run and within‐laboratory CV of 5.5%). Haemoglobin (Hb) from EDTA blood was measured by routine methods. In SHIP‐TREND‐0 immunonephelometric assays on a Dimension VISTA were used to measure serum Cystatin C (measuring range 0.023–0.8 mg/dL, CV 2.9–3.0%), and serum hs‐CRP (measuring range 0.016–0.95 mg/dL, CV 3.6–3.8%). Serum cardiac Troponin I (cTnI) was measured by immunoassay on a Siemens Dimension VISTA (Siemens Healthcare Diagnostics, Eschborn, Germany; measuring range 0.015–40 ng/mL, CV 3.6–11.4%). Hb from EDTA blood was measured using SLS‐haemoglobin [XE 5000 (Sysmex Corporation, Kobe, Japan); CV 1.8–2.7%]. Estimated glomerular filtration rate (eGFR) was calculated for both cohorts using the CKD‐EPI formula for Cystatin C. 19
Echocardiography was only available in SHIP‐TREND‐0, where two‐dimensional, M‐Mode and Doppler echocardiography were performed (Vingmed CFM 800A system, GE Medical Systems, Waukesha, WI, USA) as described previously. 20 All measurements were performed offline according to the guidelines of the American Society of Echocardiography. Certification examinations for inter‐observer variations revealed an agreement of >90%. Descriptive statistics on the following echocardiographic parameters were available for some of the SHIP‐TREND‐0 participants: left atrial diameter, interventricular septum, left ventricular ejection fraction (LVEF), E/e′, septal e′ velocity, lateral e′ velocity. Diastolic dysfunction was categorized into mild [(E/A‐ratio <0,75) and (E/E′ ratio <10)], moderate [(E/A‐ratio ≥0,75) and (MV‐Decel time ≥0,140) and (E/E′ ratio ≥10)], severe [(E/A‐ratio >2) and (MV‐Decel time <0,140) and (E/E' ratio ≥10)] according to Redfield definition. 21
Statistical analyses
Initially, descriptive statistics were performed for both study populations. Continuous variables are described as median with minimum, maximum, and interquartile range due to their skewed distributions, and categorical variables as numbers with percentages. We evaluated the effects of well‐established NT‐proBNP predictors using the nonparametric approach of quantile regression with a multiple adjusted model. 22 , 23
In a second step, we defined a reference population with an optimal profile for NT‐proBNP according to the available reference values for the established predictors. Participants of the sub‐sample were defined as having an eGFR ≥60 mL/min/1.73 m2, hsCRP <5 mg/L, haemoglobin (Hb) in men ≥14 and ≤18 g/dL and in women ≥12 and ≤16 g/dL, SBP <140 mmHg, DBP ≥60 and ≤90 mmHg, BMI ≥18 and ≤33 kg/m2 and no past medical history of diabetes or cardiovascular diseases (MI, HF, or stroke). In addition, those with a history of chronic lung disease or COPD and/or elevated cTnI (hs‐TnI ≥40 ng/L in ActiFE, cTnI ≥40 ng/L in SHIP‐TREND‐0), which might indicate the presence of cardiopulmonary disease, were excluded. At the end, 441 older people remained (ActiFE n = 227, SHIP‐TREND‐0 n = 214).
Hence, we proceed to estimate NT‐proBNP 50% (median) and 97.5% quantiles, representing the URL, using both empirical quantiles stratified by sex and age (65–69, 70–74, 75–79, ≥80 years old), and quantile regression with sex and age (continuous) as covariates. In a secondary analysis the URL among those with an LVEF ≥50 and without diastolic dysfunction (n = 97) were estimated.
The level of significance in the analyses was 5%. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria; www.R‐project.org). The quantile regression analyses were performed with R Software and the quantreg package (version 5.55) with default options.
Results
Study population and N‐terminal pro brain natriuretic peptide predictors
Participant's characteristics of ActiFE and SHIP‐TREND‐0 study populations are described in Table 1 . Far more older adults were obese in SHIP‐TREND‐0 compared with ActiFE and considerably less had normal weight. Furthermore, in SHIP‐TREND‐0 markedly more subjects had a history of hypertension and diabetes, and more were taking ≥5 medications. In contrast, ActiFE participants had higher SBP and DBP, slightly higher NT‐proBNP levels and a higher prevalence of self‐reported HF. Overall, 57.6% and 52.7% of the participants were noted to have NT‐proBNP levels >125 ng/L, and even 18.9% and 14.8% with NT‐proBNP levels >450 ng/L in ActiFE and SHIP‐TREND‐0, respectively.
Table 1.
Characteristics of study population (N = 2459).
| ActiFE N= 1450 | SHIP‐TREND‐0 N = 1009 | |
|---|---|---|
| Age (years), median (min, q1, q3, max) | 74 (65, 70, 81, 91) | 71 (65, 68, 75, 84) |
| Male, n (%) | 828 (57.1) | 526 (52.1) |
| Education level a , n (%) | ||
| ≤10 years | 1131 (78.7) | 876 (80.2) |
| >10 years | 306 (21.3) | 216 (19.8) |
| Smoker, n (%) | ||
| Never | 723 (49.9) | 465 (46.1) |
| Former | 621 (42.8) | 473 (46.9) |
| Current | 106 (7.3) | 71 (7.0) |
| Body mass index (kg/m2), n (%) | ||
| [18, 25) | 370 (25.5) | 133 (13.2) |
| [25, 30) | 723 (49.9) | 467 (46.3) |
| ≥30 | 357 (24.6) | 409 (40.5) |
| Systolic blood pressure (mmHg), median (min, q1, q3, max) | 144.5 (90.5, 135.0, 150.0, 193.0) | 135.5 (75.5, 124.5, 147.5, 222.5) |
| Diastolic blood pressure (mmHg), median (min, q1, q3, max) | 78.5 (49.5, 71.0, 86.5, 124.0) | 75.5 (46.0, 69.0, 82.5, 130.0) |
| Comorbidities, n (%) | ||
| Hypertension | 779 (53.7) | 706 (70.0) |
| Myocardial infarction | 125 (8.6) | 75 (7.4) |
| Heart failure | 211 (14.6) | 62 (6.1) |
| Stroke | 77 (5.3) | 48 (4.8) |
| Diabetes | 194 (13.4) | 218 (21.6) |
| Chronic obstructive pulmonary disease / chronic lung disease a | 31 (2.2) | 55 (6.0) |
| Number medications, n (%) | ||
| 0 | 236 (16.3) | 100 (9.9) |
| 1 | 192 (13.2) | 76 (7.5) |
| 2 | 211 (14.6) | 117 (11.6) |
| 3 | 195 (13.5) | 134 (13.3) |
| 4 | 172 (11.9) | 123 (12.2) |
| ≥5 | 444 (30.6) | 459 (45.5) |
| NT‐proBNP (ng/L), median (min, q1, q3, max) | 153 (2.5, 82, 318, 12384) | 133 (12, 71, 266, 13460) |
| NT‐proBNP >125 ng/L, n (%) | 839 (57.9) | 525 (52.0) |
| Estimated glomerular filtration rate | 84.7 (6.0, 67.6, 97.8, 123.9) | 91.0 (11.8, 74.4, 100.5, 139.2) |
| (mL/min/1.73 m2), median (min, q1, q3, max) | ||
| Haemoglobin (g/dL), median (min, q1, q3, max) | 14.3 (9.3, 13.6, 15.1, 21.3) | 13.7 (7.7, 13.0, 14.5, 17.7) |
| High sensitive C‐reactive protein (mg/L), median (min, q1, q3, max) | 1.7 (0.1, 0.9, 3.7, 173.0) | 1.6 (0.1, 0.8, 3.2, 69.5) |
| Troponin I (ng/L) ranges a , n (%) | ||
| <40 | 1419 (97.9) | 978 (97.1) |
| ≥40 | 30 (2.1) | 29 (2.9) |
| Mortality | ||
| Deaths, n (%) | 428 (29.5) | 187 (18.5) |
| Survival time (years), median (min, q1, q3, max) | 9.1 (0.1, 8.3, 9.4, 9.9) | 8.4 (0.2, 7.4, 9.3, 10.5) |
| Mortality rate (per 1000 person‐years) [95% CI] | 36.2 [32.9, 39.8] | 23.0 [20.0, 26.6] |
CI, confidence interval; NT‐proBNP, N‐terminal pro brain natriuretic peptide.
For the following descriptive variables, missings were noted: education level [ActiFE (n = 13), SHIP‐TREND‐0 (n = 6)], COPD/chronic lung disease [ActiFE (n = 16), SHIP‐TREND‐0 (n = 94)], Troponin I [ActiFE (n = 1), SHIP‐TREND‐0 (n = 2)].
Figure 1 shows results of multiple adjusted quantile regression for the identification of NT‐proBNP predictors. Covariables positively associated with NT‐proBNP for most of the conditional quantiles were age, history of MI, and history of HF, SBP, and hsCRP. Inverse associations were seen with BMI, eGFR, and Hb. Sex became significant only in the upper part of the distribution (i.e. for high quantiles above 90%). The corresponding regression coefficients for the 50% and 97.5% quantiles are reported in Table S1 .
Figure 1.
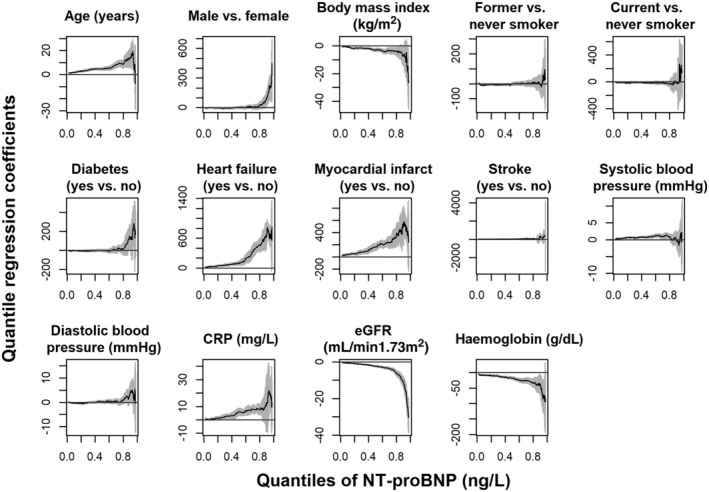
Each plot shows the quantile regression coefficients with 95% confidence band (on the y‐axis) of a specific covariable in dependence of different quantiles (on the x‐axis), that is, different parts of the conditional distribution of the target variable NT‐proBNP (ng/L), among participants ≥65 years old and BMI ≥18 of ActiFE and SHIP‐TREND‐0 (N = 2459).
Reference population and N‐terminal pro brain natriuretic peptide reference values
The identified reference population included 227 in ActiFE (15.1%) and 214 in SHIP‐TREND‐0 (19.5%). Compared with the total study populations they were noted to be overall younger, with a higher proportion of women as well as individuals with a school education >10 years, but a lower proportion of participants with obesity and hypertension (Table 2 ). The median NT‐proBNP levels were notably lower in these subpopulations as compared with the respective source study populations: ActiFE 93.0 vs. 153 ng/L and SHIP‐TREND‐0 103.5 vs. 136 ng/L. With a median follow‐up time of 9.3 and 8.8 years, the ActiFE and the SHIP‐TREND‐0 reference samples showed a mortality rate of 10.2 [95% confidence interval (CI) 6.6, 15.6] and 8.2 [95% CI 4.9, 13.6] per 1000 person‐years (Table 2 ), markedly lower than the mortality rates observed in the source study samples (ActiFE: 36.3 [95% CI 33.1, 39.9], SHIP‐TREND‐0: 24.3 [95% CI 21.3, 27.8]) (Table 1 ).
Table 2.
Characteristics of the identified reference participants ≥65 years old of ActiFE and SHIP‐TREND‐0 (n = 441).
| ActiFE n = 227 | SHIP‐TREND‐0 n = 214 | |
|---|---|---|
| Age (years), median (min, q1, q3, max) | 70 (65, 68, 74, 89) | 70 (65, 67, 73, 82) |
| Male, n (%) | 95 (41.9) | 86 (40.2) |
| Education level, n (%) | [2 missing values] | |
| ≤10 years | 170 (75.6) | 159 (74.3) |
| >10 years | 55 (24.4) | 55 (25.7) |
| Smoker, n (%) | ||
| Never | 112 (49.3) | 121 (56.5) |
| Former | 95 (41.9) | 78 (36.5) |
| Current | 20 (8.8) | 15 (7.0) |
| Body mass index (kg/m2), n (%) | ||
| [18, 25) | 87 (38.3) | 46 (21.5) |
| [25, 30) | 112 (49.3) | 117 (54.7) |
| [30, 33] | 28 (12.3) | 51 (23.8) |
| Systolic blood pressure (mmHg), median (min, q1, q3, max) | 131.5 (97.0, 123.5, 135.5, 139.5) | 127.5 (88.5, 119.5, 132.5, 139.5) |
| Diastolic blood pressure (mmHg), median (min, q1, q3, max) | 74.0 (60.0, 70.0, 79.5, 90.0) | 73.3 (60.0, 68.5, 78.0, 89.0) |
| Hypertension, n (%) | 63 (27.8) | 124 (57.9) |
| Number medications, n (%) | ||
| 0 | 70 (30.8) | 37 (17.3) |
| 1 | 48 (21.2) | 32 (15.0) |
| 2 | 39 (17.2) | 31 (14.5) |
| 3 | 32 (14.1) | 35 (16.4) |
| 4 | 20 (8.8) | 27 (12.6) |
| ≥5 | 18 (7.9) | 52 (24.3) |
| NT‐proBNP (ng/L), median (min, q1, q3, max) | 93.0 (2.5, 57.9, 149.0, 2641.0) | 103.5 (14.0, 62.0, 196.0, 2311.0) |
| NT‐proBNP > 125 ng/L, n (%) | 75 (33.0) | 90 (42.1) |
| Estimated glomerular filtration rate (mL/min/1.73 m2), | 94.5 (61.0, 79.5, 101.7, 118.3) | 95.7 (60.2, 84.7, 102.6, 139.2) |
| median (min, q1, q3, max) | ||
| Haemoglobin (g/dL), median (min, q1, q3, max) | 14.5 (12.0, 13.7, 15.1, 17.4) | 14.0 (12.1, 13.2, 14.8, 17.7) |
| High sensitive C‐reactive protein (mg/L), median (min, q1, q3, max) | 1.2 (0.1, 0.6, 2.0, 5.0) | 1.1 (0.1, 0.7, 1.9, 4.9) |
| Mortality | ||
| Deaths, n (%) | 21 (9.3) | 15 (7.0) |
| Survival time (years), median (min, q1, q3, max) | 9.3 (1.9, 9.0, 9.6, 9.9) | 8.8 (1.2, 8.0, 9.6, 10.4) |
| Mortality rate (per 1000 person‐years) [95% CI] | 10.2 [6.6, 15.6] | 8.2 [4.9, 13.6] |
CI, confidence interval; NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Empirical quantiles and the URL for the reference population are presented in Table 3 stratified by sex and age group, where the URL for older adults ≥65 years old were 663 ng/L in men and 744 ng/mL in women. A relevant proportion on men (n = 49, 27.1%) and women (n = 116, 44.6%) showed NT‐proBNP levels >125 ng/L. The corresponding age‐specific and sex‐specific histograms and URL levels for each cohort can be found in Figures S1 and S2 , and Table S2 , respectively.
Table 3.
Descriptive quantiles separated by sex and age group among apparently healthy participants ≥65 years old of ActiFE and SHIP‐TREND‐0.
| Quantile | All apparently healthy people (N = 441) |
|---|---|
| 97.5% | 663 |
| 50% (median) | 98 |
| Men (n = 181) | Women (n = 260) | |
|---|---|---|
| 97.5% | 663 | 744 |
| 50% (median) | 81 | 116 |
| NT‐proBNP >125 ng/L, n (%) | 49 (27.1%) | 116 (44.6%) |
| Age group | Age group | |||||||
|---|---|---|---|---|---|---|---|---|
| [65,70) (n = 75) | [70,75) (n = 64) | [75,80) (n = 27) | 80+ (n = 15) | [65,70) (n = 121) | [70,75) (n = 92) | [75,80) (n = 33) | 80+ (n = 14) | |
| 97.5% | 663 | 824 | 592 | 697 | 343 | 463 | 2641 | 1276 |
| 50% (median) | 58 | 89 | 116 | 140 | 100 | 128 | 152 | 203 |
NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Based on quantile regression with covariables age and sex, the median NT‐proBNP values increased by 5.6 ng/L for 1 year increment in age [95% CI 3.5, 8.3], whereas in the 97.5% quantile the increment equals 46.8 ng/L [95% CI 23.6, 116.1] (Figure 2 ). In contrast to the results obtained with the study population the sex effect comparing men vs. women observed in the reference population for median NT‐proBNP values (−37.9 ng/L [95% CI −51.3, −22.3]) became insignificant for quantiles above 90% of the conditional NT‐proBNP distribution (97.5% quantile: 41.4 ng/L [95% CI −526.4, 501.8]). A graphical comparison of the empirical quantiles and results of quantile regression with the reference values (97.5% quantiles) for both approaches stratified by sex and age is shown in Figure S3 .
Figure 2.
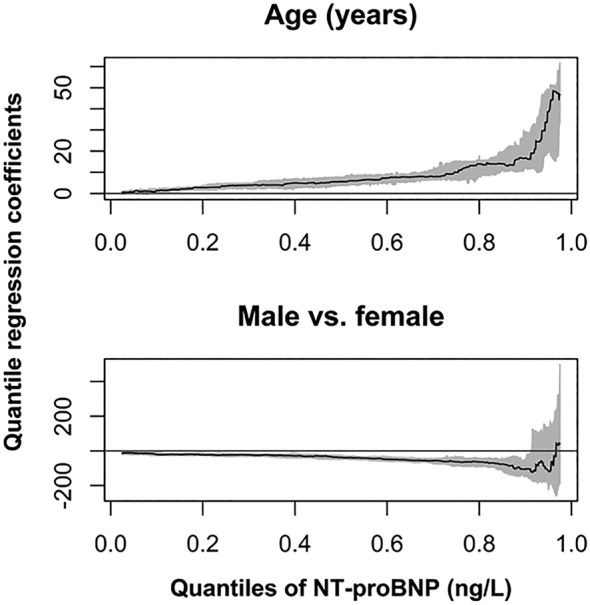
Plots of regression coefficients of linear quantile regression with 95% confidence band (on the y‐axis) for NT‐proBNP (ng/L) as outcome, and age and sex as covariables in dependence of a grid of quantiles (on the x‐axis) among apparently healthy participants ≥65 years old of ActiFE and SHIP‐TREND‐0 (n = 441).
Secondary analysis
The exploratory analysis using the echocardiographic data available only for SHIP‐TREND‐0 participants showed statistically significant differences between those included in the reference SHIP‐subsample and the remaining subjects (Tables S3 and S4 ). In a subgroup with an LVEF ≥50% and no diastolic dysfunction of 97 subjects (35 men and 62 women) NT‐proBNP levels >125 ng/L were still observed in 38 (39.2%) participants. Based on empirical quantiles, the URLs for older adults ≥65 years old were 592 ng/L in men and 744 ng/mL in women (Table S5 ). Figure 3 shows the NT‐proBNP distribution (A) in the source populations, (B) in the reference populations as well as (C) in the subsample with LVEF ≥50 and none diastolic dysfunction.
Figure 3.
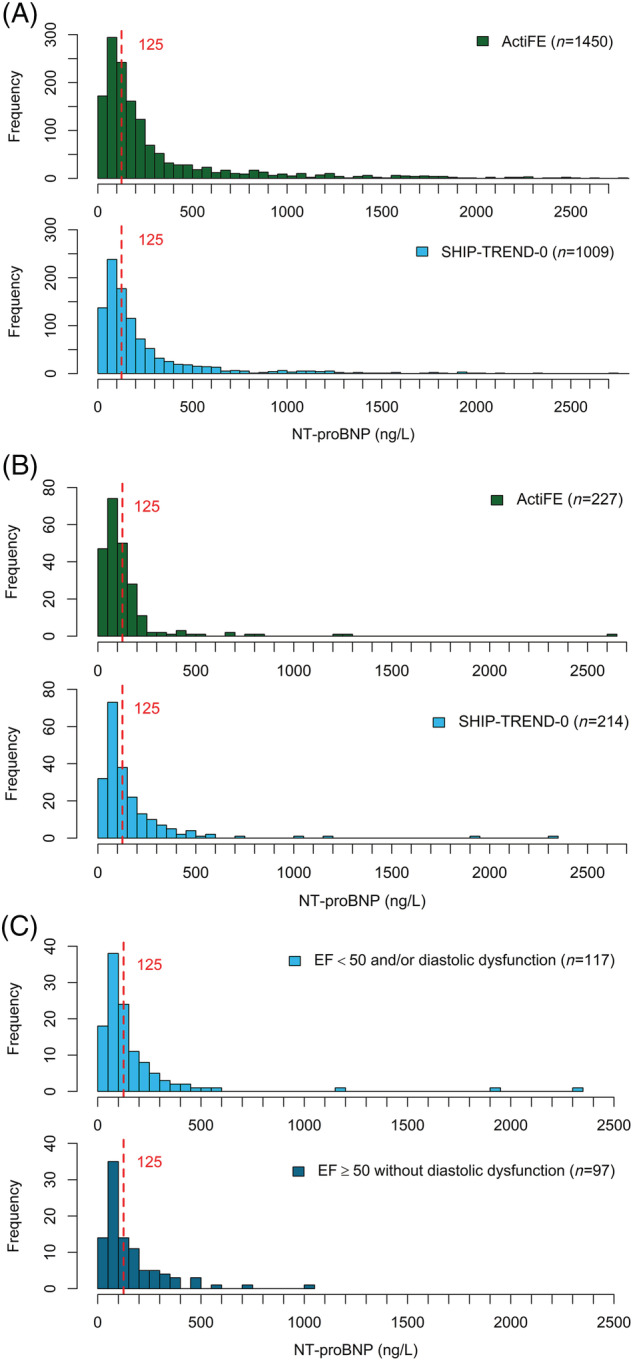
(A) Overall distribution of NT‐proBNP in both cohorts. (B) Distribution of NT‐proBNP in the reference populations. (C) Distribution of NT‐proBNP in the SHIP‐TREND‐0 reference population stratified according to echocardiographic data.
Discussion
Applying quantile regression in a study sample of 2459 community‐dwelling older adults we identified age, sex, BMI, systolic blood pressure, eGFR, haemoglobin and history of HF and MI as significant predictors of NT‐proBNP levels. Furthermore, the contribution of identified predictors such as age and sex varied along the NT‐proBNP quantiles. Using well‐established predictors, we defined a reference subsample, and estimated NT‐proBNP URL (97.5% quantile) with empirical quantiles and with quantile regression for men and women, stratified by age observing an age‐dependent increment of NT‐proBNP, with higher levels in women compared with men. Of note, even in a subsample of subjects with an LVEF ≥50% and without diastolic dysfunction, the URLs were higher than European Society of Cardiology recommended cut‐off of 125 ng/L for the diagnosis of HF in ambulatory settings across all strata. 7 Our results point out the complexity behind the identification of reference values in older populations and can give an explanation for the difficulties of their interpretation and the use of available cut‐points in the clinical context.
Although it is well recognized that NT‐proBNP values increase with age, available reference values mainly based on not so well‐characterized small sample sizes and/or not stratified by age and sex. Alehagen et al. studied 218 healthy individuals aged >65 years from southeastern Sweden reporting a URL of 540 ng/L. 24 Hess et al. reported a NT‐proBNP 97.5% quantile of 278 and 303 ng/L for adults 60–69 years old based on 125 men and 33 women, respectively. 25 However, the identification of healthy subjects based on the guidelines for the identification of healthy blood donors was only based on self‐report excluding those with severe cardiovascular or neurological diseases, coagulation disorders, recurrent syncope or epilepsy, neoplasms or, with other reported severe active or chronic diseases, but not taking into account chronic kidney disease, BMI or anaemia, all established NT‐proBNP predictors. 26 Fradley et al. estimated NT‐proBNP URL in participants of the Framingham Heart Study aged 20–59 years using empirical and quantile regression approaches with a high variability in the range of number of participants in the 5 years age strata (between 16 and 297). 27 They also identified BMI and blood pressure as predictors as well as an age‐dependent NT‐proBNP increment for both genders, with substantially higher levels in women compared with men at any age. The same trend was observed in our reference subsample of older adults (Figure S4 ). This trend, however, could also be explained by lower eGFR in women as compared with men, especially when using creatinine‐based equations, due to the fact that creatinine is linked to lower muscle mass in women. Our data showed that eGFR and haemoglobin also play an important role as predictors for NT‐proBNP. These results gain on significance considering the pathophysiological changes such as decreased kidney function or increased prevalence of anaemia observed during ageing.
With respect to NT‐proBNP in the identification of acute HF, the European Society of Cardiology recommends the use of an URL of 125 ng/L in non‐acute settings and of 300 ng/L in acute settings to rule out HF across all ages. 28 In this context, data coming from the BELFRAIL cohort have shown a high sensitivity for the detection of HF when using the NT‐proBNP cut‐off ≥125 pg/mL in patients 80+. However, the specificity was as low as 34%. Among 259 patients with elevated NT‐proBNP who underwent echocardiogram, HF was present in only 68, so that as high as 73.7% of the referrals for echocardiogram could be considered as not necessary. 29 The 97.5% quantile levels identified in our sample of asymptomatic older adults with an optimal profile with respect to NT‐proBNP predictors for those ≥70 were all above the recommended cut‐offs. Recent data coming from the ICON‐RELOADED Study, which aimed to validate the age‐specific NT‐proBNP cut‐offs for rule‐in of 450, 900, and 1800 ng/L for ages <50, 50 to 75, and 75 years, showed the least best performance with respect to sensitivity, specificity, positive and negative predictive value, and positive and negative likelihood ratio precisely among those with 75 years and older. 30 In our study sample, still 4.2% of the asymptomatic subjects ≤75 years had NT‐proBNP levels >900 ng/L, while 5.4% of asymptomatic subjects >75 years had NT‐proBNP levels >1800 ng/L (Table S6 ). As NT‐proBNP levels are a composite of different factors leading to haemodynamic stress/strain, not necessarily representing HF, our results may underscore the need to refrain from a single cut‐point in older populations in order to capture the heterogeneity of ageing. Perhaps using quantile regression equations based on even larger study samples could help to predict the individual expected URL taking into account the observed individual variability of identified NT‐proBNP predictors at the time of presentation at the doctor's office. A similar approach has also been suggested by Cleland et al. in the settings of the recent debate about the struggle towards a universal definition of HF. 31 Following such an algorithm would support the efficient utilization of further tests such as echocardiograms, 32 a diagnostic procedure, which is unfortunately not routinely available worldwide.
Strengths and limitations
Regarding the required sample size for the estimation of URL using nonparametric methods, a minimum of 120 individuals per strata are recommended in the reference population as a standard for general practice by the NCCLS committee. 9 With our reference population, we were able to fulfil this requirement in just one stratum. A larger source population with at least 600 participants per stratum would have been needed in order to build a representative sample of older adults for reliable sex‐stratified and age‐stratified reference values. However, if it is not possible to get apparently healthy individuals in sufficient numbers for each defined category, the nonparametric method is still the recommended approach. In one of the source populations (ActiFE), we lack baseline information on atrial fibrillation or echocardiography, making it impossible to assure the absence of any unknown structural heart disease. However, in the setting of an observational study, participants were asymptomatic at the time of the examination, and both studies add important value by allowing an excellent multidimensional characterization of the older population, including relevant NT‐proBNP predictors such as kidney function and anaemia.
Conclusions
Our study demonstrated an age‐dependent increment of NT‐proBNP in a sample of asymptomatic healthy community‐dwelling older adults, with higher levels in women compared with men, and with URLs >125 ng/L, the highest cut‐off recommended for the identification of patients with acute HF in ambulatory settings. 7 These results emphasize the importance of larger reference samples of well‐characterized older adults for the estimation of stable and reliable reference limits stratified by age and sex, as well as the need of novel statistical approaches and clinical algorithms for the identification of individualized URLs to increase precision of this valuable clinical parameter in the identification of patients at risk optimizing the diagnostic procedure. Our results attempt to provide a frame for the further investigation of age‐specific NT‐proBNP cut‐offs in older adults for the identification of HF in the ambulatory setting, helping to close the gaps in evidence with respect to the diagnosis of this condition as pointed out by the current ESC guidelines on HF. 7
Conflict of interest
WK reports personal fees from AstraZeneca, Novartis, Pfizer, the Medicines Company, DalCor, Kowa, Amgen, Corvidia, Daiichi‐Sankyo, Berlin‐Chemie, Sanofi, Bristol‐Myers Squibb, Novo Nordisk, OMEICOS, LIB Therapeutics and Genentech, and grants and non‐financial support from Abbott, Roche Diagnostics, Beckmann, and Singulex, all outside the submitted work. MD reports honoraria for lectures and/or consulting from Bayer, Daiichi‐Sankyo, Hexal and Novartis, all not related to this manuscript. All other authors did not declare any conflicts of interest.
Funding
ActiFE was supported by a grant from the Ministry of Science, Research and Arts, state of Baden‐Wuerttemberg, Germany, as part of the Geriatric Competence Center, Ulm University. Study was also funded in part by the German Research Foundation (DFG, Grant agreement RO2602/14‐1 and DE2674/1‐1).
SHIP is part of the Community Medicine Research Network of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg—West‐Pomerania. Open Access funding enabled and organized by Projekt DEAL.
Supporting information
Table S1. Regression coefficients [95% CI] of quantile regression for NT‐proBNP (ng/L) and for different quantiles among participants 65 years old and BMI 18 of ActiFE and SHIP‐TREND‐0 (N = 2459). Bold numbers show significant coefficients.
Table S2. Descriptive quantiles stratified by sex and cohort.
Figure S1. Sex‐specific distribution of NT‐proBNP in the reference population.
Figure S2. Age‐ and sex‐specific distribution of NT‐proBNP in the reference population.
Figure S3. The 97.5% and 50% descriptive quantiles (horizontal lines) and quantiles of quantile regression (regression lines) for NT‐proBNP (ng/L) dependent of age and separated by sex among reference participants 65 years old of the merged cohort of ActiFE and SHIP‐TREND‐0 (N = 441).
Table S3. Descriptive Statistics for echocardiographic parameters in SHIP‐Trend‐0.
Table S4. Descriptive Statistics for echocardiographic parameters in SHIP‐Trend‐0, complete case analysis.
Table S5. Descriptive quantiles stratified by sex among SHIP‐TREND‐0 reference subsample in contrast to those from the reference population with EF ≥50% and without diastolic dysfunction.
Figure S4. 97.5% and 50% quantiles of NT‐proBNP adjusted by age and sex using quantile regression. Data for the age groups 20–59 years are extracted from the publication of Fradley et al (27) where they used 5‐years age groups in their quantile regression. Data for the ages 65–90 years resulted from the present analyses where age is used as continuous variable in quantile regression.
Table S6. Proportion of asymptomatic subjects with NT‐proBNP levels > 900 ng/L (among those ≤75 years), and > 1800 ng/L (among those > 75 years).
Braisch, U. , Koenig, W. , Rothenbacher, D. , Denkinger, M. , Friedrich, N. , Felix, S. B. , Ittermann, T. , Dörr, M. , and Dallmeier, D. (2022) N‐terminal pro brain natriuretic peptide reference values in community‐dwelling older adults. ESC Heart Failure, 9: 1703–1712. 10.1002/ehf2.13834.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL Jr, Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG, Ho JE. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol 2018; 3: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linssen GC, Bakker SJ, Voors AA, Gansevoort RT, Hillege HL, de Jong PE, van Veldhuisen DJ, Gans RO, de Zeeuw D. N‐terminal pro‐B‐type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J 2010; 31: 120–127. [DOI] [PubMed] [Google Scholar]
- 4. Dallmeier D, Denkinger M, Peter R, Rapp K, Jaffe AS, Koenig W, Rothenbacher D, Acti FESG. Sex‐specific associations of established and emerging cardiac biomarkers with all‐cause mortality in older adults: The ActiFE study. Clin Chem 2015; 61: 389–399. [DOI] [PubMed] [Google Scholar]
- 5. Salzano A, D'Assante R, Israr MZ, Eltayeb M, D'Agostino A, Bernieh D, De Luca M, Rega S, Ranieri B, Mauro C, Bossone E, Squire IB, Suzuki T, Marra AM. Biomarkers in heart failure: clinical insights. Heart Fail Clin 2021; 17: 223–243. [DOI] [PubMed] [Google Scholar]
- 6. McCullough PA, Kluger AY. Interpreting the wide range of NT‐proBNP concentrations in clinical decision making. J Am Coll Cardiol 2018; 71: 1201–1203. [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, E.S.C.S.D. Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Diehr P, Williamson J, Burke GL, Psaty BM. The aging and dying processes and the health of older adults. J Clin Epidemiol 2002; 55: 269–278. [DOI] [PubMed] [Google Scholar]
- 9. Horowitz GL. Reference intervals: practical aspects. EJIFCC 2008; 19: 95–105. [PMC free article] [PubMed] [Google Scholar]
- 10. Denkinger MD, Franke S, Rapp K, Weinmayr G, Duran‐Tauleria E, Nikolaus T, Peter R, Acti FEUSG. Accelerometer‐based physical activity in a large observational cohort—study protocol and design of the activity and function of the elderly in Ulm (ActiFE Ulm) study. BMC Geriatr 2010; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed 2001; 46: 186–194. [DOI] [PubMed] [Google Scholar]
- 12. Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Junger M, Mayerle J, Kraft M, Lerch MM, Dorr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Glaser S, Ewert R, Fietze I, Penzel T, Doren M, Rathmann W, Haerting J, Hannemann M, Ropcke J, Schminke U, Jurgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kuhn JP, Kuhn J, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Volker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W. Cohort profile: the study of health in Pomerania. Int J Epidemiol 2011; 40: 294–307. [DOI] [PubMed] [Google Scholar]
- 13. Bahls M, Atzler D, Markus MRP, Friedrich N, Boger RH, Volzke H, Felix SB, Schwedhelm E, Dorr M. Low‐circulating homoarginine is associated with dilatation and decreased function of the left ventricle in the general population. Biomolecules 2018; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Human experimentation: code of ethics of the world medical association. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dallmeier D, Klenk J, Peter RS, Denkinger M, Peter R, Rapp K, Koenig W, Rothenbacher D. A prospective assessment of cardiac biomarkers for hemodynamic stress and necrosis and the risk of falls among older people: the ActiFE study. Eur J Epidemiol 2016; 31: 427–435. [DOI] [PubMed] [Google Scholar]
- 16. Masuch A, Pietzner M, Bahls M, Budde K, Kastenmuller G, Zylla S, Artati A, Adamski J, Volzke H, Dorr M, Felix SB, Nauck M, Friedrich N. Metabolomic profiling implicates adiponectin as mediator of a favorable lipoprotein profile associated with NT‐proBNP. Cardiovasc Diabetol 2018; 17: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collin‐Chavagnac D, Dehoux M, Schellenberg F, Cauliez B, Maupas‐Schwalm F, Lefevre G, G. Societe Francaise de Biologie Clinique Cardiac Markers Working . Head‐to‐head comparison of 10 natriuretic peptide assays. Clin Chem Lab Med 2015; 53: 1825–1837. [DOI] [PubMed] [Google Scholar]
- 18. Dallmeier D, Pencina MJ, Rajman I, Koenig W, Rothenbacher D, Brenner H. Serial measurements of N‐terminal pro‐brain natriuretic peptide in patients with coronary heart disease. PLoS ONE 2015; 10: e0117143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, Investigators C‐E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volzke H, Haring R, Lorbeer R, Wallaschofski H, Reffelmann T, Empen K, Rettig R, John U, Felix SB, Dorr M. Heart valve sclerosis predicts all‐cause and cardiovascular mortality. Atherosclerosis; 209: 606–610. [DOI] [PubMed] [Google Scholar]
- 21. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 22. Davino C, Furno M, Vistocco D. Quantile Regression—Theory and Applications, Wiley Series in Probability and Statistics. Wiley; 2013. [Google Scholar]
- 23. Koenker R. Quantile Regression, Econometric Society Monographs. Cambridge University Press; 2010. [Google Scholar]
- 24. Alehagen U, Goetze JP, Dahlstrom U. Reference intervals and decision limits for B‐type natriuretic peptide (BNP) and its precursor (Nt‐proBNP) in the elderly. Clin Chim Acta 2007; 382: 8–14. [DOI] [PubMed] [Google Scholar]
- 25. Hess G, Runkel S, Zdunek D, Hitzler WE. Reference interval determination for N‐terminal‐B‐type natriuretic peptide (NT‐proBNP): a study in blood donors. Clin Chim Acta 2005; 360: 187–193. [DOI] [PubMed] [Google Scholar]
- 26. Brandstädter WHW, Bein G et al. Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie) vom Juli 2000—Guidelines for the collection of blood and blood components and for the use of blood products (hemotherapy) from July 2000. Dtsch Arztebl 2000; 98: A‐3074/B‐2610/C‐2418. [Google Scholar]
- 27. Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, Levy D, Vasan RS, Wang TJ. Reference limits for N‐terminal‐pro‐B‐type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 2011; 108: 1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, M. Authors/Task Force, and R. Document . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 29. Smeets M, Degryse J, Janssens S, Mathei C, Wallemacq P, Vanoverschelde JL, Aertgeerts B, Vaes B. Diagnostic rules and algorithms for the diagnosis of non‐acute heart failure in patients 80 years of age and older: a diagnostic accuracy and validation study. BMJ Open 2016; 6: e012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Januzzi JL Jr, Chen‐Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Rivers EJ, Walters EL, Gaggin HK, I.‐R. Investigators . N‐terminal pro‐B‐type natriuretic peptide in the emergency department: the ICON‐RELOADED study. J Am Coll Cardiol 2018; 71: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 31. Cleland JGF, Pfeffer MA, Clark AL, Januzzi JL, McMurray JJV, Mueller C, Pellicori P, Richards M, Teerlink JR, Zannad F, Bauersachs J. The struggle towards a universal definition of heart failure—how to proceed? Eur Heart J 2021; 42: 2331–2343. [DOI] [PubMed] [Google Scholar]
- 32. Modin D, Andersen DM, Biering‐Sorensen T. Echo and heart failure: when do people need an echo, and when do they need natriuretic peptides? Echo Res Pract 2018; 5: R65–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Regression coefficients [95% CI] of quantile regression for NT‐proBNP (ng/L) and for different quantiles among participants 65 years old and BMI 18 of ActiFE and SHIP‐TREND‐0 (N = 2459). Bold numbers show significant coefficients.
Table S2. Descriptive quantiles stratified by sex and cohort.
Figure S1. Sex‐specific distribution of NT‐proBNP in the reference population.
Figure S2. Age‐ and sex‐specific distribution of NT‐proBNP in the reference population.
Figure S3. The 97.5% and 50% descriptive quantiles (horizontal lines) and quantiles of quantile regression (regression lines) for NT‐proBNP (ng/L) dependent of age and separated by sex among reference participants 65 years old of the merged cohort of ActiFE and SHIP‐TREND‐0 (N = 441).
Table S3. Descriptive Statistics for echocardiographic parameters in SHIP‐Trend‐0.
Table S4. Descriptive Statistics for echocardiographic parameters in SHIP‐Trend‐0, complete case analysis.
Table S5. Descriptive quantiles stratified by sex among SHIP‐TREND‐0 reference subsample in contrast to those from the reference population with EF ≥50% and without diastolic dysfunction.
Figure S4. 97.5% and 50% quantiles of NT‐proBNP adjusted by age and sex using quantile regression. Data for the age groups 20–59 years are extracted from the publication of Fradley et al (27) where they used 5‐years age groups in their quantile regression. Data for the ages 65–90 years resulted from the present analyses where age is used as continuous variable in quantile regression.
Table S6. Proportion of asymptomatic subjects with NT‐proBNP levels > 900 ng/L (among those ≤75 years), and > 1800 ng/L (among those > 75 years).


