Abstract
Heart failure (HF) is a major cause of mortality, hospitalizations, and reduced quality of life and a major burden for the healthcare system. The number of patients that progress to an advanced stage of HF is growing. Only a limited proportion of these patients can undergo heart transplantation or mechanical circulatory support. The purpose of this review is to summarize medical management of patients with advanced HF. First, evidence‐based oral treatment must be implemented although it is often not tolerated. New therapeutic options may soon become possible for these patients. The second goal is to lessen the symptomatic burden through both decongestion and haemodynamic improvement. Some new treatments acting on cardiac function may fulfil both these needs. Inotropic agents acting through an increase in intracellular calcium have often increased risk of death. However, in the recent Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC‐HF) trial, omecamtiv mecarbil was safe and effective in the reduction of the primary outcome of cardiovascular death or HF event compared with placebo (hazard ratio, 0.92; 95% confidence interval, 0.86–0.99; P = 0.03) and its effects were larger in those patients with more severe left ventricular dysfunction. Patients with severe HF who received omecamtiv mecarbil experienced a significant treatment benefit, whereas patients without severe HF did not (P = 0.005 for interaction). Lastly, clinicians should take care of the end of life with an appropriate multidisciplinary approach. Medical treatment of advanced HF therefore remains a major challenge and a wide open area for further research.
Keywords: Advanced heart failure, Heart failure with reduced ejection fraction, Medical management, Diuretic therapy, Inotropes, Omecamtiv mecarbil, Palliative care
Introduction
Heart failure (HF) is a major cause of mortality, hospitalizations, and reduced quality of life and a major burden for the healthcare system. The increasing prevalence and the improved survival of HF, as well as the ageing of the population, have led to an increase of the number of patients that progress to an advanced stage of HF. 1 This poses a challenge to treating clinicians, as such patients usually experience severe symptoms and markedly impaired quality of life, become less responsive or cannot tolerate evidence‐based therapies, and are at high risk of short‐term hospitalizations and death. 2 Outcomes remain poor in patients not suitable for long‐term mechanical circulatory support (MCS) or heart transplantation 3 ; however, only a limited proportion of advanced HF patients need to be selected for advanced therapies. 2 , 4
The aim of the present review is to describe the medical management of patients with advanced HF, focusing on those with reduced ejection fraction (HFrEF) (Figure 1 ). Guideline‐directed medical therapy (GDMT) remains effective in patients with advanced HF. However, patients with advanced HF are less likely to tolerate it because of hypotension, low cardiac output, and severe kidney dysfunction. Physicians should be aware that the proper use of GDMT is associated with a better prognosis and its implementation is of central importance. Furthermore, new therapeutic options that may allow symptoms' improvement and a better clinical course of HF are now available, representing a potential for further research.
Figure 1.
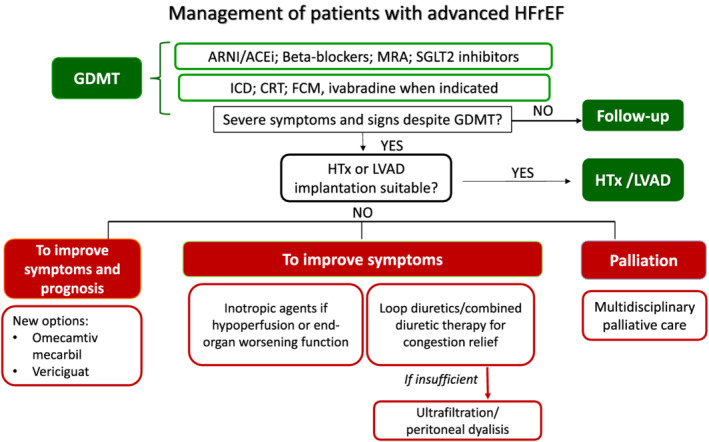
Management of advanced HFrEF patients. ACEi, angiotensin‐converting enzyme inhibitors; ARNI, angiotensin receptor neprilysin inhibitor; CRT, cardiac resynchronization therapy; FCM, ferric carboxymaltose; GDMT, guideline‐directed medical therapy; HFrEF, heart failure with reduced ejection fraction; HTx, heart transplantation; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; MRA, mineralocorticoid receptor antagonists; SGLT2, sodium‐glucose co‐transporter 2.
Definition and epidemiology
Advanced HF can be defined as a clinical syndrome characterized by persistence of severe signs and symptoms of HF, despite optimal evidence‐based treatment. It represents the stage of the syndrome when conventional therapies are no longer effective or insufficient to control patients' symptoms, requiring advanced therapeutic strategies including heart transplantation, MCS implantation, intermittent inotropes, and, sometimes, end‐of‐life (EOL) cares. 2 ‘Refractory’ HF may also be used as an interchangeable term although it implies a lack of response to treatment and a lack of reversibility of the impaired cardiac function and haemodynamic impairment and these conditions are not necessarily mandatory for advanced HF. Several classification systems could be applied to define patients with advanced HF, including New York Heart Association (NYHA) functional class IV referring to patients with symptoms at rest, or American College of Cardiology (ACC)/American Heart Association (AHA) stage D referring to patients who have refractory symptoms despite optimal medical therapy and require specialized interventions. 1 , 5 The first position statement defining advanced HF was published in 2007 by the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and a more updated version was published in 2018, providing new criteria for defining advanced HF. 2 , 6 In the most recently published ESC guidelines, advanced HF has been defined as the presence of all the following criteria: (i) severe and persistent symptoms of HF [NYHA class III (advanced) or IV]; (ii) severe cardiac dysfunction [left ventricular ejection fraction (LVEF) ≤ 30% in the setting of HFrEF]; (iii) episodes of pulmonary or systemic congestion requiring high‐dose intravenous diuretics (or diuretic combinations) or episodes of low output requiring inotropes or vasoactive drugs or malignant arrhythmias causing >1 unplanned visit or hospitalization in the last 12 months; and (iv) severe impairment of exercise capacity with inability to exercise or low 6 min walking test distance (<300 m) or pVO2 < 12 mL/kg/min or <50% predicted value, estimated to be of cardiac origin. 7
Epidemiological data are still scarce, although it is estimated that 1–10% of the HF population has advanced HF. 2 In a study conducted in Minnesota, among a random sample of Olmsted County residents aged ≥45 years old, the prevalence of advanced HF (stage D according to the ACC/AHA HF staging criteria) was 0.2% of the overall population, corresponding to 10% of the HF population. 8 , 9 Most importantly, advanced HF patients are burdened with a dramatic reduction in survival. In the same cohort of patients with stage D HF, mortality at 5 year was 80%. 10 In a more recent study, of 6836 adults with HF, 936 (13.7%) met ESC diagnostic criteria for advanced HF. 9 The median (interquartile range) time from advanced HF diagnosis to death was 12.2 months (3.7–29.9 months). 9 Similarly, other studies reported high mortality rates. In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, enrolling end‐stage HF patients ineligible for heart transplantation, the rates of mortality were 75% at 1 year and 92% at 2 years in the medical therapy group [vs. 48% and 77%, respectively, in those receiving left ventricular assist device (LVAD)]. 11 Of note, the ineligibility to heart transplantation might have selected a population at higher risk.
Treatment to improve outcome
Evidence‐based therapy for heart failure with reduced ejection fraction
Neurohormonal antagonists, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, beta‐blockers, and mineralocorticoid receptor antagonists, and sodium‐glucose co‐transporter 2 (SGLT2) inhibitors, are the mainstay of HFrEF treatment, improving the clinical course of HF. 7 , 12 , 13 , 14 , 15 , 16 Adherence to GDMT is associated with improved outcome. 17 , 18 , 19 , 20 , 21 , 22 Data from population‐based studies reported a decline in HF‐related hospitalizations and mortality over the last two decades. However, no further improvement was reported in the most recent years because of the lack of positive trials until 2019. 16 , 23 Implementation of GDMT remains a cornerstone of treatment of also the patients with advanced HF and reduced LVEF. Indeed, many trials enrolling patients with severe HF, NYHA class III–IV, and severely impaired LVEF, namely, Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II), Carvedilol Prospective Randomized Cumulative Survival Study (COPERNICUS), and the Randomized Aldactone Evaluation Study (RALES), consistently showed clinical benefits of these drugs among a population with a more advanced stage of the disease. 24 , 25 , 26 , 27 However, patients with advanced HF often do not tolerate neurohormonal modulators because of hypotension, low cardiac output, and severe kidney dysfunction. The development of circulatory haemodynamic limitations to ACEi identifies patients with severe HF and with mortality over 50% at 1 year. 28 Recently, new drugs have demonstrated benefits in patients with HFrEF, namely, SGLT2 inhibitors, vericiguat and omecamtiv mecarbil. These drugs may be more tolerated as they do not decrease systolic blood pressure meaningfully and have neutral or favourable (SGLT2 inhibitors) long‐term effects on the progression of kidney dysfunction. 29
Table 1 shows the proportion of patients with advanced HF enrolled in the most recent trials and treatment interaction. In the Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial, <1% of patients had an NYHA functional class IV. Pre‐specified subgroup analyses showed a significant interaction between NYHA class at randomization and the effect of treatment on the primary endpoint, with major benefits in the subgroup of patients with NYHA class I or II vs. NYHA class III or IV. 30 , 31 This interaction was not observed considering cardiovascular (CV) death. Thus, the benefit of sacubitril/valsartan in more severe patients remained uncertain. The rationale of the LIFE (LCZ696 in Hospitalized Advanced Heart Failure) study was to assess the feasibility, efficacy, and safety of such treatment in the most advanced phases of the disease. This 24 week prospective, multicentre trial compared the use of sacubitril/valsartan vs. valsartan alone in NYHA class IV patients with an LVEF ≤ 35% and elevated levels of N‐terminal pro b‐type natriuretic peptide (NT‐proBNP). The primary endpoint was the proportional change from baseline in the area under the curve for NT‐proBNP levels, while secondary and tertiary endpoints consisted of an assessment of clinical outcome, safety, and tolerability. 32 The study was prematurely stopped due to the Coronavirus Disease 2019 (COVID‐19) pandemic, but 335 patients were enrolled and results have been recently presented. 33 Neither treatment with sacubitril/valsartan nor valsartan decreased the median NT‐proBNP levels below baseline through 24 weeks. Sacubitril/valsartan did not improve the clinical composite of number of days alive, out of hospital, and free from HF events and did not decrease the risk of death from CV causes or HF hospitalization, nor all‐cause death, compared with valsartan. Results may be influenced by the sample size and the study duration, which was shorter than previous studies. Furthermore, the study was not powered to detect changes in CV death and/or HF hospitalizations. Importantly, there was no safety concern even if 72 eligible patients (18%) were not able to tolerate sacubitril/valsartan during the short run‐in period, and 49 patients (29%) discontinued sacubitril/valsartan during the 24 weeks of the trial. A recent real‐life study investigated the administration of sacubitril/valsartan in a real‐world cohort of more advanced HFrEF patients, with a worse clinical status than those enrolled in the PARADIGM‐HF trial. During the 6 month follow‐up, the rates of hospitalizations, NT‐proBNP levels, and the need for ambulatory levosimendan decreased and a reverse cardiac remodelling was observed in patients treated with sacubitril/valsartan. No major adverse effects were reported. 34 Martens et al. showed that patients receiving sacubitril/valsartan in clinical practice, compared with those in PARADIGM‐HF, were burdened by more severe disease. In this advanced population, sacubitril/valsartan significantly improved the NYHA class, despite a higher risk of systolic blood pressure drop compared with that reported in PARADIGM‐HF. 35 In the most recently published ESC guideline, sacubitril/valsartan (if tolerated) is recommended as a replacement for an ACEi in patients with HFrEF to reduce the risk of HF hospitalization and death. 7 On the other hand, patients with advanced HF may become intolerant to sacubitril/valsartan and this may be a reason of de‐escalation.
Table 1.
The effects of interventions on outcome in patients with heart failure with reduced ejection fraction according to New York Heart Association class: recent trials
| Clinical trial | Intervention | No. of patients | Key inclusion criteria | Mean follow‐up (years) | Primary outcome | Overall treatment effect | NYHA class subgroups | Treatment effect in NYHA class | P for interaction | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| ARNI | ||||||||||
| PARADIGM‐HF 30 , 31 | Sacubitril/valsartan vs. enalapril | 8442 | LVEF ≤ 40%; NYHA II–IV (<1% NYHA IV) | 2.3 | CV death or a first HF hospitalization | 0.80 (0.73–0.87) |
I or II (n = 6308) |
Major benefit in NYHA class I–II vs. NYHA class III–IV | 0.03 | |
|
III or IV (n = 2078) |
||||||||||
| LIFE 32 , 33 | Sacubitril/valsartan vs. valsartan | 335 | Advanced HF; LVEF ≤ 35%; NYHA IV | 0.5 | Change from baseline in the area under the curve for NT‐proBNP levels |
Sacubitril/valasartan was not superior to valsartan with respect to lowering NT‐proBNP levels HR for CV death or HF hospitalization 1.32 (0.86–2.03) HR for HF hospitalizations 1.24 (0.80–1.93) |
IV (n = 335) | — | — | |
| Soluble guanylate cyclase stimulator | ||||||||||
| VICTORIA 47 , 48 | Vericiguat vs. placebo | 5050 | LVEF ≤ 45%, NYHA II–IV, recent hospitalization | 0.9 | CV death or HF hospitalization | 0.90 (0.82–0.98) | I or II (n = 2977) | 0.91 (0.80–1.04) | NS | |
| III or IV (n = 2069) | 0.87 (0.77–0.99) | |||||||||
| SGLT2i | ||||||||||
| DAPA‐HF 41 | Dapagliflozin vs. placebo | 4744 | LVEF ≤ 40%, NYHA II–IV | 1.5 | CV death or worsening HF | 0.74 (0.65–0.85) | II (n = 3203) | 0.63 (0.52–0.75) | NS | |
| III or IV (n = 1541) | 0.90 (0.74–1.09) | |||||||||
| EMPEROR‐Reduced 44 | Empagliflozin vs. placebo | 3730 | LVEF ≤ 40%, NYHA II–IV | 1.3 | CV death or worsening HF | 0.75 (0.65–0.86) | II (n = 2800) | 0.71 (0.59–0.84) | NS | |
| III or IV (n = 930) | 0.83 (0.66–1.04) | |||||||||
| Cardiac myosin activator | ||||||||||
| GALACTIC‐HF 50 , 100 , 101 | Omecamtiv mecarbil vs. placebo | 8256 | Inpatients and outpatients with NYHA II–IV; LVEF ≤ 35% | 1.8 | CV death or first HF event | 0.92 (0.86–0.99) | II (n = 4368) | 0.97 (0.87–1.08) | NS |
P for interaction for those with or without severe HF a = 0.005 P for interaction for LVEF by quartiles = 0.013 |
| III or IV (n = 3864) | 0.88 (0.80–0.97) | |||||||||
ARNI, angiotensin receptor neprilysin inhibitor; CV, cardiovascular; DAPA‐HF, Dapagliflozin and Prevention of Adverse outcomes in Heart Failure (trial); EMPEROR‐Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (trial); GALACTIC‐HF, Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (trial); HF, heart failure; HR, hazard ratio; LIFE, LCZ696 in Hospitalized Advanced Heart Failure; LVEF, left ventricular ejection fraction; No., number of patients; NS, not significant; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; NYHA, New York Heart Association; PARADIGM‐HF, Prospective Comparison of ARNI with ACE‐I to Determine Impact on Global Mortality and Morbidity in Heart Failure (trial); SGLT2i, sodium‐glucose co‐transporter 2 inhibitors; VICTORIA, Vericiguat Global Study in Patients with Heart Failure with Reduced Ejection Fraction (trial).
Severe HF was defined as the presence of all of the following criteria: New York Heart Association symptom class III to IV, left ventricular ejection fraction of 30% or less, and hospitalization for HF within the previous 6 months.
Sodium‐glucose co‐transporter 2 inhibitors act on new therapeutic pathways, different from those on which neurohormonal agents are active. 36 , 37 , 38 , 39 Beyond the diuretic and haemodynamic effects, SGLT2 inhibitors could also have an impact on myocardial metabolism, ion transporters, fibrosis, adipokines, and vascular function. 40 In the DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, dapagliflozin reduced the risk of the primary composite endpoint of CV death or worsening HF, compared with placebo, in patients with HFrEF, regardless of diabetes' history [hazard ratio (HR), 0.74; 95% confidence interval (CI), 0.65–0.85; P < 0.001]. 41 Dapagliflozin also improved physical function and the quality of life, measured through Kansas City Cardiomyopathy Questionnaire (KCCQ). 42 Importantly, dapagliflozin was safe and well tolerated, even in patients with a baseline systolic blood pressure < 110 mmHg, and the absolute benefit of the drug was large in those with the lowest systolic blood pressure, opening future perspective for the treatment of advanced HFrEF patients. 43 The more recent Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced) trial confirmed and extended the benefits of SGLT2 inhibitors in stable, more advanced HF population, with a reduction in the risk of CV death or HF hospitalization compared with placebo (HR, 0.75; 95% CI, 0.65–0.86; P < 0.001). 44 SGLT2 inhibitors also showed a slower decline in the estimated glomerular filtration rate. Given their early benefits, safety profile, and tolerability, an early upfront initiation of SGLT2 inhibitors has been supported by HF experts. 45 Adverse effects usually associated with the use of neurohormonal antagonist (hypotension, bradycardia, and hyperkalaemia) were not described with SGLT2 inhibitors, an aspect that may represent a particular advantage of SGLT2 inhibitors among fragile patients with advanced HF.
Advanced symptomatic HFrEF patients who had recently been hospitalized or had received intravenous diuretic therapy were enrolled in the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial. The recruitment of sicker patients with higher NT‐proBNP levels than those in previous HF trials was a purpose of the study and resulted in a higher risk of events. 46 The MAGGIC (Meta‐Analysis Global Group in Chronic Heart Failure) risk score in VICTORIA was higher as compared with the MAGGIC risk score in PARADIGM‐HF. Similarly, the proportion of NYHA class III or IV patients was 41% in the VICTORIA trial, compared with 25% in the PARADIGM‐HF and EMPEROR‐Reduced and 32% in the DAPA‐HF. 46 In this high‐risk population, the novel oral soluble guanylate cyclase stimulator, vericiguat, reduced the composite endpoint of CV death or HF hospitalization compared with placebo (HR, 0.90; 95% CI, 0.82–0.98; P = 0.02). 47 However, subgroup analysis showed an interaction between treatment and the primary outcome according to pre‐specified quartiles of NT‐proBNP with the benefits of vericiguat shown only in patients with NT‐proBNP levels up to 8000 pg/mL. 48 Patients who may benefit from vericiguat should be better defined, especially in the light of a possible individualized approach. 49
The positive results of the GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure) trial, comparing omecamtiv mecarbil with placebo, will be discussed in the following chapter on inotropes. 50 Importantly, this is the first inotrope that showed benefits on clinical outcome in patients with chronic HF.
Treatment to improve symptoms
Management of congestion
Each HF‐related hospitalization increases the risk for subsequent events. Worsening of congestion, with symptoms and signs of fluid overload and/or fluid redistribution, remains the major cause of hospitalization for acute HF or unplanned visits requiring intravenous diuretic treatment and this is increasingly frequent in advanced stages. 51 Moreover, persistently elevated left ventricular filling pressure is common in advanced HF and has prognostic significance. 52 Remote monitoring of congestion and pulmonary artery pressure‐guided pharmacotherapy may represent a helpful tool to reduce HF hospitalization in outpatients with advanced HF. 53 , 54 , 55 , 56 Assessment and management of congestion in patients with advanced HF has been recently reviewed. 57
The standard treatment for congestion is represented by loop diuretics, with furosemide being first choice. 58 , 59 However, in patients with advanced HF, the management of congestion can sometimes be difficult due to the high prevalence of cardiorenal syndrome. Chronically decreased perfusion and venous congestion compromise renal function. 60 Prolonged diuretic treatment leads to nephron remodelling, one of the main mechanisms behind diuretic resistance: hypertrophy and hyperplasia of the distal convoluted tubule cells, principal cells, and intercalated cells generates a gain of function, with an increased reabsorption capacity of the distal nephron. 61 When diuresis is insufficient, uptitration of oral loop diuretics should represent the first therapeutic option. Planned ambulatory intravenous administration of loop diuretics may help maintaining fluid balance and, in cases of inadequate responses, home administration of intravenous loop diuretics is suggested. Thiazide‐like drugs or metolazone are commonly used as an adjuvant therapy, along with loop diuretics, both in refractory outpatients and in acute decompensated HF. 58 , 62 However, evidence is still limited and the risk of worsening renal function or electrolyte disorders, namely, hypokalaemia and hyponatraemia, must be considered. In a propensity analysis of 13 898 hospitalized patients with acute HF, metolazone was associated with increased mortality (adjusted HR, 1.20; 95% CI, 1.04–1.39; P = 0.01). 63 As advanced stages of HF are characterized by inappropriately high levels of arginine vasopressin, leading to plasma expansion and dilutional hyponatraemia, the selective V2 receptor antagonist tolvaptan may be considered as a further decongestive strategy. In pre‐clinical HF models, the novel dual acting vasopressin V1a/V2 receptor antagonist pecavaptan showed a better haemodynamic effect compared with tolvaptan, including increase in cardiac output and cardiac index and decrease in total peripheral resistance, and the first clinical results will be available soon. 64 , 65
When the previous therapies have failed, ultrafiltration (UF) should be considered. 66 Despite safety issues, UF is associated with greater weight reduction and volume depletion and with shorter hospitalizations when patients are admitted to the hospital. UF rates superior to 250 mL/h are not recommended, and patients with right HF could only take lower rates. 67 Haematocrit and patient's weight must be closely monitored during the UF, so that treatment can be eventually stopped and resumed safely. Finally, peritoneal dialysis might be an at‐home option for patients not responding to conventional diuretic therapy. It offers many advantages, including a preserved renal function, haemodynamic stability, and less inflammation compared with haemodialysis. This strategy can lead to weight loss and a better NYHA classification and quality of life, reducing the length of in‐hospital stay. 68 However, further large, controlled, randomized studies are needed to better evaluate and define this strategy. 2
Inotropic agents: rationale and classification
Patients with end‐stage HF, who are otherwise in good health, without significant non‐cardiac comorbidities, should be referred for heart transplantation. Heart transplantation represents the gold standard treatment in such patients, with 1 year survival of almost 90% and a median survival of 12.5 years. 69 Nonetheless, it represents a limited therapeutic option, due to the disproportion between donors and possible candidates needing the transplant. Long‐term MCS is a valid alternative in patients non‐eligible to heart transplantation or in those deteriorating while waiting for transplantation. 11 MCS implantation is burdened by high costs and adverse events, limiting its use and requiring restrictive clinical criteria as well. 1 Thus, in patients with low cardiac output with end‐organ hypoperfusion, ineligible to heart transplantation or LVAD implantation, inotropes may represent a rescue strategy to improve haemodynamics. Inotrope use aims to maintain an adequate cardiac output and reduce filling pressures by enhancing cardiac contractility and, for some inotropes, also by vasodilatation and may represent a potentially useful strategy also in the chronic treatment of advanced HF, besides their role as short‐term therapies.
Psotka et al. have recently classified inotropes in calcitropes, which modulate calcium signalling, myotropes, acting on the sarcomere through a calcium‐independent mechanism, and mitotropes, which exert their action on mitochondrial energy production. 70 Calcitropes include the traditional inotropes: catecholamines, phosphodiesterase (PDE)‐3 inhibitors, and cardiac glycoside (i.e. digitalis). Among catecholamines, which act on the β‐adrenoceptor‐adenylyl cyclase system, epinephrine and norepinephrine are vasopressors mainly used in an acute cardiogenic shock, as a bridge to haemodynamic stability. 71 Dobutamine mainly acts on β1 cardiac receptors rather than α1 and β2 vascular receptors, increasing stroke volume and without causing peripheral vasoconstriction. PDE‐3 inhibitors, including amrinone, milrinone, and enoximone, inhibit the enzyme PDE‐3 with consequently increased concentrations of available cAMP and intracellular calcium. Milrinone is currently the most widely used drug within this class, followed by enoximone. Both drugs were associated with an improvement in haemodynamics and functional capacity in patients with advanced HF. 72 Levosimendan is a PDE inhibitor with additive properties, making it able to increase calcium sensitivity during systole without impairing diastolic relaxation. This action leads to increased cardiac output, reduced wedge pressures, peripheral vasodilation, and symptoms relief. 73 , 74 Istaroxime exerts a dual function: on one hand, it stimulates sarcoplasmatic reticulum Ca2+‐ATPase SERCA2a; on the other hand, it inhibits the Na‐K pump, resulting in both an inotropic and a lusitropic effect. 75 Omecamtiv mecarbil—the first drug of the myotrope class—is a direct activator of cardiac myosin in a calcium‐independent manner. It increases the contractile force by enforcing the interaction between myosin and actin. 76 , 77 Results from non‐clinical studies and a randomized, phase 2a trial investigating another cardiac myosin activator, danicamtiv, were recently published. 78 Mitotropes are currently under study in HFrEF patients, with promising results in small clinical studies, but there is a lack of randomized trials providing more consistent results. 70
Inotropic agents: negative results from randomized controlled clinical trials
Results from clinical trials investigating the efficacy of inotropes in the treatment of advanced HF patients are summarized in Table 2 .
Table 2.
Summary of trials investigating inotropic agents in advanced heart failure
| Drug | Classification/mechanism of action | Trials | Summary of results |
|---|---|---|---|
| Dobutamine 79 | Calcitrope/β‐adrenergic receptor agonist | FIRST (post hoc analysis) | Increased mortality, worsened HF, myocardial infarction, and cardiac arrest with dobutamine. |
| Milrinone 81 , 82 | Calcitrope/phosphodiesterase‐3 inhibitor | PROMISE; OPTIME‐CHF | Higher incidence of hypotension, arrhythmia, and mortality with milrinone vs. placebo. |
| Enoximone 72 , 83 | Calcitrope/phosphodiesterase‐3 inhibitor | Enoximone Multicenter Trial Group; ESSENTIAL trials | No difference in mortality with enoximone vs. placebo. |
| Levosimendan 84 , 85 , 86 , 87 , 88 | Calcitrope/phosphodiesterase‐3 inhibitor and additive properties | LIDO; REVIVE; SURVIVE; PERSIST; LION‐HEART | Improved haemodynamics, increased symptomatic relief, lower natriuretic peptide levels, and lower HF hospitalizations with levosimendan vs. dobutamine and placebo; no improvement in survival with levosimendan vs. placebo. |
| Omecamtiv mecarbil 50 , 99 , 100 , 101 | Myotrope/direct myosin activator | COSMIC‐HF; GALACTIC‐HF | Increased cardiac output, positive cardiac remodelling, and reduced composite endpoint of CV mortality or HF events with omecamtiv mecarbil vs. placebo (greater benefit in those with lower LVEF and severe HF). |
COSMIC‐HF, Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure; CV, cardiovascular; ESSENTIAL, Studies of Oral Enoximone Therapy in Advanced HF; FIRST, Flolan International Randomized Survival Trial; GALACTIC‐HF, Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure; HF, heart failure; LIDO, Levosimendan Infusion versus DObutamine; LION‐HEART, Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients; LVEF, left ventricular ejection fraction; OPTIME‐CHF, Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure; PERSIST, Effects of Peroral Levosimendan in the Prevention of Further Hospitalisations in Patients with Chronic Heart Failure; PROMISE, Prospective Randomized Milrinone Survival Evaluation; REVIVE (I, II), Randomized EValuation of Intravenous LeVosimendan Efficacy; SURVIVE, Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support.
Despite its theoretical potential benefit among patients with worsening, advanced HF, there is a lack of dedicated studies evaluating dobutamine in this particular setting. In a post hoc analysis of the Flolan International Randomized Survival Trial (FIRST), a study investigating the use of epoprosteonol, treatment with intravenous continuous dobutamine was associated with higher 6 month mortality rate in patients with advanced HF (70.5% vs. 37.1% in controls; P < 0.001). Also, the occurrence of first event, including worsening HF, need for vasoactive medications, resuscitated cardiac arrest, and myocardial infarction, was higher in the dobutamine group. 79 Although results were confirmed after adjustment for baseline characteristics, the study was not designed to compare dobutamine with placebo and the selection of sicker patients requiring inotropes might have influenced the results. In a retrospective single‐centre study, continuous intravenous home dobutamine was associated with improvement in symptomatic status and HF hospitalizations in 21 end‐stage HF patients. 80
Among 1088 ambulatory patients with severe chronic HF, oral milrinone was associated with an increase in all‐cause and CV mortality and this effect was also more evident in those with the most severe symptoms (NYHA IV). 81 In the OPTIME‐CHF (Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure) trial, 951 patients admitted with an exacerbation of chronic HF not requiring intravenous inotropic support were randomized to receive a 48 h infusion of either milrinone (0.5 μg/kg/min) or saline placebo. Results showed no difference in incidence of death or readmission; however, milrinone was associated with a higher incidence of sustained hypotension requiring intervention (10.7% vs. 3.2% in the placebo group; P < .001) and new atrial arrhythmias (4.6% vs. 1.5%; P = 0.004). 82 Similarly, enoximone did not improve survival in the Studies of Oral Enoximone Therapy in Advanced HF (ESSENTIAL) programme. 83
Levosimendan Infusion versus DObutamine (LIDO) Study showed that levosimendan improved haemodynamic performance more effectively than dobutamine in patients with severe, low‐output HF. 84 The Randomized EValuation of Intravenous LeVosimendan Efficacy (REVIVE) and Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trials examined safety and efficacy of levosimendan in patients with acute decompensated HF, compared with placebo and dobutamine, respectively. 85 , 86 In the SURVIVE trial, 180 day mortality (the primary endpoint) was not different between dobutamine and levosimendan. 86 In the REVIVE trial, levosimendan was associated with more frequent hypotension and cardiac arrhythmias during the infusion period compared with placebo, and a non‐significant risk of death. 85 Both trials showed benefits in terms of symptomatic relief and decrease in natriuretic peptide levels. The PERSIST trial (Effects of Peroral Levosimendan in the Prevention of Further Hospitalisations in Patients with Chronic Heart Failure) firstly showed improvement in quality of life and decrease in NT‐proBNP levels in patients with severe chronic HF (NYHA class IIIB–IV and LVEF < 30%) treated with oral levosimendan compared with placebo. 87 More recently, in the small, multicentre, randomized, placebo‐controlled LION‐HEART trial (Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients), intermittent levosimendan decreased NT‐proBNP levels and reduced HF rehospitalizations (HR, 0.25; 95% CI, 0.11–0.56; P = 0.001). 88 Similar results were reported in other small studies. 89 , 90 , 91 , 92 The ongoing LeoDOR study (NCT03437226) will assess the efficacy and safety of repetitive levosimendan given for 12 weeks in advanced HF patients. 93
Randomized controlled trials with drugs acting through an increase in intracellular calcium failed to prove benefits in terms of outcome, with an increase in mortality in some cases. The reasons behind this failure may be multiple. 94 First, haemodynamic improvement and symptomatic relief do not necessarily translate into an improvement in outcome. 95 Increasing contractility in a failing heart may induce short‐term benefits in terms of symptoms and also increase myocardial work and oxygen consumption with long‐term deterioration of myocardial function. Second, benefits of chronic inotropic use might be limited to specific HF phenotypes (i.e. ischaemic vs. non‐ischaemic). 96 Third, the effects may be dose dependent and mainly due to an increase in sudden death so that administration of lower doses and concomitant beta‐blocker and implantable cardioverter defibrillator treatment may prevent untoward effects. 83 , 94 , 97
A recent meta‐analysis including 66 studies showed that in patients receiving ambulatory inotrope infusions, there was a greater improvement in NYHA functional class than in controls, without a significant effect on mortality risk (pooled risk ratio, 0.68; 95% CI, 0.40–1.17; P = 0.16; 9 trials). 98 Improvement in quality of life and functional capacity, with neutral impact on survival, should represent the ideal target of chronic inotropic use in the setting of advanced HF. Ahmad et al. suggest that, besides a careful selection of patients with advanced HF (not still in an early phase of the disease but also not too advanced), future trials testing inotropes should include patients who are already on maximally tolerated medical therapy (including beta‐blockers) and already have received implantable cardioverter defibrillator (if indicated). Cardiorenal biomarkers may be used with safety purpose and a run‐in phase might be useful to exclude patients with cardiac injury or severe adverse effects. 94
Inotropic agents: positive trials and future perspectives
Omecamtiv mecarbil, a first‐in‐class selective cardiac myosin activator, has been evaluated in chronic HFrEF patients in the phase 2 COSMIC‐HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure) trial. 99 After 20 weeks of oral treatment, omecamtiv mecarbil increased left ventricular systolic ejection time and stroke volume and decreased the left ventricular end‐systolic and end‐diastolic dimensions, suggesting favourable reverse cardiac remodelling. Recently, the results of the GALACTIC‐HF trial have been published. In this phase 3 trial, 8256 patients with symptomatic chronic HF and LVEF ≤ 35% were randomized to receive omecamtiv mecarbil (using pharmacokinetic‐guided doses of 25, 37.5, or 50 mg twice daily) or placebo, in addition to standard HF therapy. Chronic oral use of omecamtiv mecarbil reduced the composite endpoint of an HF event or death from CV causes compared with placebo (HR, 0.92; 95% CI, 0.86–0.99; P = 0.03). The benefit was consistent in the subgroup of patients with NYHA class III or IV (HR, 0.88; 95% CI, 0.80–0.97) 50 (Table 1 ). Moreover, among the pre‐specified subgroups, LVEF was the strongest modifier of the treatment effect of omecamtiv mecarbil. Patients with baseline LVEF ≤ 22% (lowest quartile) had the greatest relative risk reduction for the composite endpoint (HR, 0.83; 95% CI, 0.73–0.95), compared with patients with LVEF ≥ 33% (HR, 0.99; 95% CI, 0.84–1.16; interaction as LVEF by quartiles, P = 0.013). 100 These findings are somehow expected because omecamtiv mecarbil improves cardiac performance with a selectivity for improving systolic function. In a post hoc analysis, among 8232 patients enrolled in the clinical trial, 2258 patients (27%) met the specified criteria for severe HF (NYHA III‐IV, LVEF ≤ 30%, and hospitalization for HF within the previous 6 months). Patients with severe HF treated with omecamtiv mecarbil experienced a significant reduction of the primary endpoint (HR, 0.80; 95% CI, 0.71–0.90). On the contrary, patients without severe HF had no significant treatment benefit (HR, 0.99; 95% CI, 0.91–1.08; P = 0.005 for interaction). Results were similar also for CV death. 101 Given these meaningful results, omecamtiv mecarbil has been strongly suggested as an important component of advanced HFrEF treatment. 102 Importantly, safety outcomes, namely, ventricular arrhythmias, major cardiac ischaemic events including myocardial infarction, hospitalization for unstable angina, and coronary revascularization, occurred at a similar rate in the two treatment groups. In addition, no adverse effects on blood pressure, serum potassium levels, or renal function were reported, with a slight decrease in heart rate probably reflecting the reduced sympathomimetic activation. As regards biomarkers, at Week 24, a small increase in troponin was observed (median cardiac troponin I level was 4 ng/L higher), without increasing the risk of clinical adverse effects. On the other hand, NT‐proBNP level was 10% lower in the omecamtiv mecarbil group than in the placebo group. 50 These findings were in line with previous randomized clinical trials, both in chronic and in acute settings and even when omecamtiv mecarbil was administered intravenously, as in ATOMIC‐AHF. 99 , 103 The ongoing METEORIC‐HF (NCT03759392) will assess efficacy of omecamtiv mecarbil in improvement of exercise capacity in subjects with HFrEF and decreased exercise tolerance.
The DIGIT‐HF (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure) trial has been designed to demonstrate the role of digitoxin on the top of standard care in improving mortality and morbidity in advanced HFrEF. 104
Palliative care
The assessment of the quality of life is a key point in the management of patients with advanced HF. Several scales and questionnaires may help physicians in the assessment of quality of life: the Minnesota Living with Heart Failure Questionnaire ranges from 0 to 105 points, with higher scores associated to a poorer quality of life; the KCCQ and the EQ 5D visual analogue scale range from 0 to 100, with higher scores related to a better quality of life. 105 Defining the need for palliative care through patient‐reported outcome measures, up to a quarter of patients hospitalized with HF may need palliative care. 106
The HFA of the ESC has recently published a position paper providing a day‐to‐day practical clinical guidance on palliation strategies. 107 EOL care includes not only relief of congestion and improvement of end‐organ perfusion (see chapters above) but also psychosocial support, treatment of anxiety and depression, and, in the final stages, relief of dyspnoea with opioids or benzodiazepines as second line‐treatment. 108
The PAL‐HF (Palliative Care in Heart Failure) trial enrolled 150 patients with end‐stage HF. Seventy‐five patients were treated with a multidisciplinary palliative approach, while the others received standard care. The palliative care arm showed a better quality of life, less anxiety and depression, and a better spiritual well‐being compared with the standard care cohort. 109 A meta‐analysis of randomized controlled trials comparing palliative care interventions to usual care in patients with advanced HF showed that palliative care interventions were associated with a significant reduction in hospitalizations and modest improvement in quality of life and symptomatic burden. 110
Patients with advanced HF might overestimate their life expectancy. It is important that advanced care planning is engaged at an early phase of the disease, before heart transplantation or LVAD implantation. 111 Such process aims to enhance patients' autonomy in decision‐making regarding their EOL, basing on values and beliefs of each person. A team of expert physicians should guide the patient and their family during decision‐making, providing them information regarding the prognosis and the treatment options.
Conclusion
Advanced HF patients present a poor prognosis. Heart transplantation and LVAD implantation have higher mortality benefit than medical therapy alone. However, only few patients can undergo advanced therapies and recent landmark clinical trials have offered new therapeutic options in these high‐risk patients, namely, the direct cardiac myosin activator, omecamtiv mecarbil. This drug may become the foundation in the treatment of advanced HFrEF patients, but the way seems still long to go and further research is urgently needed.
Conflict of interest
F.G. reports consulting fees from Abbott, Pfizer, Bayer, Ionis, Alnylam, and Boehringer‐Ingelheim and speakers fees from Novartis, AstraZeneca, and Orion Pharma.
M.M. reports consulting fees from Actelion, Amgen, AstraZeneca, Abbott vascular, Bayer, Servier, Edwards Therapeutics, Livanova, Vifor Pharma, and WindTree Therapeutics, as member of Trials' Committees or Advisory Boards or for speeches at sponsored meetings in the last 3 years.
Other authors declare that they have no conflict of interest.
Funding
No external funding was used in the preparation of this manuscript.
Tomasoni, D. , Vishram‐Nielsen, J. K. K. , Pagnesi, M. , Adamo, M. , Lombardi, C. M. , Gustafsson, F. , and Metra, M. (2022) Advanced heart failure: guideline‐directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Failure, 9: 1507–1523. 10.1002/ehf2.13859.
References
- 1. Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail 2020; 8: 523–536. [DOI] [PubMed] [Google Scholar]
- 2. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, de Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 3. Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde‐McLean RC, DeVore AD, Pamboukian SV, Thibodeau JT, Teuteberg JJ, Cadaret L, Xie R, Taddei‐Peters W, Naftel DC, Kirklin JK, Stevenson LW, Stewart GC. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) registry. J Heart Lung Transplant 2019; 38: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baudry G, Nesseler N, Flecher E, Vincentelli A, Goeminne C, Delmas C, Porterie J, Nubret K, Pernot M, Kindo M, Hoang Minh T, Rouvière P, Gaudard P, Michel M, Senage T, Boignard A, Chavanon O, Para M, Verdonk C, Pelcé E, Gariboldi V, Anselme F, Litzler PY, Blanchart K, Babatasi G, Bielefeld M, Bouchot O, Hamon D, Lellouche N, Bailleul X, Genet T, Eschalier R, d'Ostrevy N, Bories MC, Akar RA, Blangy H, Vanhuyse F, Obadia JF, Galand V, Pozzi M. Characteristics and outcome of ambulatory heart failure patients receiving a left ventricular assist device. ESC Heart Fail 2021; 8: 5159–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC Jr, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) , International Society for Heart and Lung Transplantation , Heart Failure Society of America . ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation 2001; 104: 2996–3007. [DOI] [PubMed] [Google Scholar]
- 6. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M, on behalf of the Heart Failure Association of the European Society of Cardiology . Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9: 684–694. [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 9. Dunlay SM, Roger VL, Killian JM, Weston SA, Schulte PJ, Subramaniam AV, Blecker SB, Redfield MM. Advanced heart failure epidemiology and outcomes: a population‐based study. JACC Heart Fail 2021; 9: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant 2008; 27: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 11. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL, Meier P. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 12. Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Pannaux M, Swedberg K. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Eur J Heart Fail 2018; 20: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 13. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS, on behalf of the QUALIFY Investigators . Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 14. Carubelli V, Lombardi C, Specchia C, Peveri G, Oriecuia C, Tomasoni D, di Pasquale M, Inciardi R, Garrafa E, Metra M. Adherence and optimization of angiotensin converting enzyme inhibitor/angiotensin II receptors blockers and beta‐blockers in patients hospitalized for acute heart failure. ESC Heart Fail 2021; 8: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, Fonarow GC, Vaduganathan M, Coats AJS. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail 2021; 23: 1499–1511. [DOI] [PubMed] [Google Scholar]
- 16. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail 2016; 18: 503–511. [DOI] [PubMed] [Google Scholar]
- 17. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L, on behalf of the Heart Failure Association of the ESC (HFA) . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 18. Rossignol P, Lainscak M, Crespo‐Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH, Heart Failure Long‐Term Registry Investigators Group , Christopher Peter Gale GB, Branko Beleslin RS, Andrzej Budaj PL, Ovidiu Chioncel RO, Nikolaos Dagres DE, Nicolas Danchin FR, David Erlinge SE, Jonathan Emberson GB, Michael Glikson IL, Alastair Gray GB, Meral Kayikcioglu TR, Aldo Maggioni IT, Klaudia Vivien Nagy HU, Aleksandr Nedoshivin RU, Anna‐Sonia Petronio IT, Jolien Roos‐Hesselink NL, Lars Wallentin SE, Uwe Zeymer DE, Crespo‐Leiro M, Anker S, Mebazaa A, Coats A, Filippatos G, Ferrari R, Maggioni AP, Piepoli MF, Goda A, Diez M, Fernandez A, Fruhwald F, Fazlibegovic E, Gatzov P, Kurlianskaya A, Hullin R, Christodoulides T, Hradec J, Nielsen OW, Nedjar R, Uuetoa T, Hassanein M, Jimenez JFD, Harjola VP, Logeart D, Chumburidze V, Tousoulis D, Milicic D, Merkely B, O'Donoghue E, Amir O, Shotan A, Shafie D, Metra M, Matsumori A, Mirrakhimov E, Kavoliuniene A, Erglis A, Vataman E, Otljanska M, Kostovska ES, DeMarco DC, Drozdz J, Fonseca C, Chioncel O, Dekleva M, Shkolnik E, Dahlstrom U, Lainscak M, Goncalvesova E, Temizhan A, Estrago V, Bajraktari G, Auer J, Ablasser K, Fruhwald F, Dolze T, Brandner K, Gstrein S, Poelzl G, Moertl D, Reiter S, Podczeck‐Schweighofer A, Muslibegovic A, Vasilj M, Fazlibegovic E, Cesko M, Zelenika D, Palic B, Pravdic D, Cuk D, Vitlianova K, Katova T, Velikov T, Kurteva T, Gatzov P, Kamenova D, Antova M, Sirakova V, Krejci J, Mikolaskova M, Spinar J, Krupicka J, Malek F, Hegarova M, Lazarova M, Monhart Z, Hassanein M, Sobhy M, el Messiry F, el Shazly AH, Elrakshy Y, Youssef A, Moneim AA, Noamany M, Reda A, Dayem TKA, Farag N, Halawa SI, Hamid MA, Said K, Saleh A, Ebeid H, Hanna R, Aziz R, Louis O, Enen MA, Ibrahim BS, Nasr G, Elbahry A, Sobhy H, Ashmawy M, Gouda M, Aboleineen W, Bernard Y, Luporsi P, Meneveau N, Pillot M, Morel M, Seronde MF, Schiele F, Briand F, Delahaye F, Damy T, Eicher JC, Groote P, Fertin M, Lamblin N, Isnard R, Lefol C, Thevenin S, Hagege A, Jondeau G, Logeart D, le Marcis V, Ly JF, Coisne D, Lequeux B, le Moal V, Mascle S, Lotton P, Behar N, Donal E, Thebault C, Ridard C, Reynaud A, Basquin A, Bauer F, Codjia R, Galinier M, Tourikis P, Stavroula M, Tousoulis D, Stefanadis C, Chrysohoou C, Kotrogiannis I, Matzaraki V, Dimitroula T, Karavidas A, Tsitsinakis G, Kapelios C, Nanas J, Kampouri H, Nana E, Kaldara E, Eugenidou A, Vardas P, Saloustros I, Patrianakos A, Tsaknakis T, Evangelou S, Nikoloulis N, Tziourganou H, Tsaroucha A, Papadopoulou A, Douras A, Polgar L, Merkely B, Kosztin A, Nyolczas N, Nagy AC, Halmosi R, Elber J, Alony I, Shotan A, Fuhrmann AV, Amir O, Romano S, Marcon S, Penco M, di Mauro M, Lemme E, Carubelli V, Rovetta R, Metra M, Bulgari M, Quinzani F, Lombardi C, Bosi S, Schiavina G, Squeri A, Barbieri A, di Tano G, Pirelli S, Ferrari R, Fucili A, Passero T, Musio S, di Biase M, Correale M, Salvemini G, Brognoli S, Zanelli E, Giordano A, Agostoni P, Italiano G, Salvioni E, Copelli S, Modena MG, Reggianini L, Valenti C, Olaru A, Bandino S, Deidda M, Mercuro G, Dessalvi CC, Marino PN, di Ruocco MV, Sartori C, Piccinino C, Parrinello G, Licata G, Torres D, Giambanco S, Busalacchi S, Arrotti S, Novo S, Inciardi RM, Pieri P, Chirco PR, Galifi MA, Teresi G, Buccheri D, Minacapelli A, Veniani M, Frisinghelli A, Priori SG, Cattaneo S, Opasich C, Gualco A, Pagliaro M, Mancone M, Fedele F, Cinque A, Vellini M, Scarfo I, Romeo F, Ferraiuolo F, Sergi D, Anselmi M, Melandri F, Leci E, Iori E, Bovolo V, Pidello S, Frea S, Bergerone S, Botta M, Canavosio FG, Gaita F, Merlo M, Cinquetti M, Sinagra G, Ramani F, Fabris E, Stolfo D, Artico J, Miani D, Fresco C, Daneluzzi C, Proclemer A, Cicoira M, Zanolla L, Marchese G, Torelli F, Vassanelli C, Voronina N, Erglis A, Tamakauskas V, Smalinskas V, Karaliute R, Petraskiene I, Kazakauskaite E, Rumbinaite E, Kavoliuniene A, Vysniauskas V, Brazyte‐Ramanauskiene R, Petraskiene D, Stankala S, Switala P, Juszczyk Z, Sinkiewicz W, Gilewski W, Pietrzak J, Orzel T, Kasztelowicz P, Kardaszewicz P, Lazorko‐Piega M, Gabryel J, Mosakowska K, Bellwon J, Rynkiewicz A, Raczak G, Lewicka E, Dabrowska‐Kugacka A, Bartkowiak R, Sosnowska‐Pasiarska B, Wozakowska‐Kaplon B, Krzeminski A, Zabojszcz M, Mirek‐Bryniarska E, Grzegorzko A, Bury K, Nessler J, Zalewski J, Furman A, Broncel M, Poliwczak A, Bala A, Zycinski P, Rudzinska M, Jankowski L, Kasprzak JD, Michalak L, Soska KW, Drozdz J, Huziuk I, Retwinski A, Flis P, Weglarz J, Bodys A, Grajek S, Kaluzna‐Oleksy M, Straburzynska‐Migaj E, Dankowski R, Szymanowska K, Grabia J, Szyszka A, Nowicka A, Samcik M, Wolniewicz L, Baczynska K, Komorowska K, Poprawa I, Komorowska E, Sajnaga D, Zolbach A, Dudzik‐Plocica A, Abdulkarim AF, Lauko‐Rachocka A, Kaminski L, Kostka A, Cichy A, Ruszkowski P, Splawski M, Fitas G, Szymczyk A, Serwicka A, Fiega A, Zysko D, Krysiak W, Szabowski S, Skorek E, Pruszczyk P, Bienias P, Ciurzynski M, Welnicki M, Mamcarz A, Folga A, Zielinski T, Rywik T, Leszek P, Sobieszczanska‐Malek M, Piotrowska M, Kozar‐Kaminska K, Komuda K, Wisniewska J, Tarnowska A, Balsam P, Marchel M, Opolski G, Kaplon‐Cieslicka A, Gil RJ, Mozenska O, Byczkowska K, Gil K, Pawlak A, Michalek A, Krzesinski P, Piotrowicz K, Uzieblo‐Zyczkowska B, Stanczyk A, Skrobowski A, Ponikowski P, Jankowska E, Rozentryt P, Polonski L, Gadula‐Gacek E, Nowalany‐Kozielska E, Kuczaj A, Kalarus Z, Szulik M, Przybylska K, Klys J, Prokop‐Lewicka G, Kleinrok A, Aguiar CT, Ventosa A, Pereira S, Faria R, Chin J, de Jesus I, Santos R, Silva P, Moreno N, Queirós C, Lourenço C, Pereira A, Castro A, Andrade A, Guimaraes TO, Martins S, Placido R, Lima G, Brito D, Francisco AR, Cardiga R, Proenca M, Araujo I, Marques F, Fonseca C, Moura B, Leite S, Campelo M, Silva‐Cardoso J, Rodrigues J, Rangel I, Martins E, Correia AS, Peres M, Marta L, Silva GF, Severino D, Durao D, Leao S, Magalhaes P, Moreira I, Cordeiro AF, Ferreira C, Araujo C, Ferreira A, Baptista A, Radoi M, Bicescu G, Vinereanu D, Sinescu CJ, Macarie C, Popescu R, Daha I, Dan GA, Stanescu C, Dan A, Craiu E, Nechita E, Aursulesei V, Christodorescu R, Otasevic P, Seferovic PM, Simeunovic D, Ristic AD, Celic V, Pavlovic‐Kleut M, Lazic JS, Stojcevski B, Pencic B, Stevanovic A, Andric A, Simić D, Ašanin M, Iric‐Cupic V, Jovic M, Davidovic G, Milanov S, Mitic V, Atanaskovic V, Antic S, Pavlovic M, Stanojevic D, Stoickov V, Ilic S, Ilic MD, Petrovic D, Stojsic S, Kecojevic S, Dodic S, Adic NC, Cankovic M, Stojiljkovic J, Mihajlovic B, Radin A, Radovanovic S, Krotin M, Klabnik A, Goncalvesova E, Pernicky M, Murin J, Kovar F, Kmec J, Semjanova H, Strasek M, Iskra MS, Ravnikar T, Suligoj NC, Komel J, Fras Z, Jug B, Glavic T, Losic R, Bombek M, Krajnc I, Krunic B, Horvat S, Kovac D, Rajtman D, Cencic V, Letonja M, Winkler R, Valentincic M, Melihen‐Bartolic C, Bartolic A, Vrckovnik MP, Kladnik M, Pusnik CS, Marolt A, Klen J, Drnovsek B, Leskovar B, Anguita MJF, Page JCG, Martinez FMS, Andres J, Bayes‐Genis A, Mirabet S, Mendez A, Garcia‐Cosio L, Roig E, Leon V, Gonzalez‐Costello J, Muntane G, Garay A, Alcade‐Martinez V, Fernandez SL, Rivera‐Lopez R, Puga‐Martinez M, Fernandez‐Alvarez M, Serrano‐Martinez JL, Crespo‐Leiro M, Grille‐Cancela Z, Marzoa‐Rivas R, Blanco‐Canosa P, Paniagua‐Martin MJ, Barge‐Caballero E, Cerdena IL, Baldomero IFH, Padron AL, Rosillo SO, Gonzalez‐Gallarza RD, Montanes OS, Manjavacas AMI, Conde AC, Araujo A, Soria T, Garcia‐Pavia P, Gomez‐Bueno M, Cobo‐Marcos M, Alonso‐Pulpon L, Cubero JS, Sayago I, Gonzalez‐Segovia A, Briceno A, Subias PE, Hernandez MV, Cano MJR, Sanchez MAG, Jimenez JFD, Garrido‐Lestache EB, Pinilla JMG, Villa BG, Sahuquillo A, Marques RB, Calvo FT, Perez‐Martinez MT, Gracia‐Rodenas MR, Garrido‐Bravo IP, Pastor‐Perez F, Pascual‐Figal DA, Molina BD, Orus J, Gonzalo FE, Bertomeu V, Valero R, Martinez‐Abellan R, Quiles J, Rodrigez‐Ortega JA, Mateo I, ElAmrani A, Fernandez‐Vivancos C, Valero DB, Almenar‐Bonet L, Sanchez‐Lazaro IJ, Marques‐Sule E, Facila‐Rubio L, Perez‐Silvestre J, Garcia‐Gonzalez P, Ridocci‐Soriano F, Garcia‐Escriva D, Pellicer‐Cabo A, Fuente Galan L, Diaz JL, Platero AR, Arias JC, Blasco‐Peiro T, Julve MS, Sanchez‐Insa E, Aured‐Guallar C, Portoles‐Ocampo A, Melin M, Hägglund E, Stenberg A, Lindahl IM, Asserlund B, Olsson L, Dahlström U, Afzelius M, Karlström P, Tengvall L, Olsson B, Kalayci S, Temizhan A, Cavusoglu Y, Gencer E, Yilmaz MB, Gunes H. Unravelling the interplay between hyperkalaemia, renin‐angiotensin‐aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC‐HFA‐EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020; 22: 1378–1389. [DOI] [PubMed] [Google Scholar]
- 19. Rosano GMC, Moura B, Metra M, Böhm M, Bauersachs J, Ben Gal T, Adamopoulos S, Abdelhamid M, Bistola V, Čelutkienė J, Chioncel O, Farmakis D, Ferrari R, Filippatos G, Hill L, Jankowska EA, Jaarsma T, Jhund P, Lainscak M, Lopatin Y, Lund LH, Milicic D, Mullens W, Pinto F, Ponikowski P, Savarese G, Thum T, Volterrani M, Anker SD, Seferovic PM, Coats AJS. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2021; 23: 872–881. [DOI] [PubMed] [Google Scholar]
- 20. Lainščak M, Milinković I, Polovina M, Crespo‐Leiro MG, Lund LH, Anker SD, Laroche C, Ferrari R, Coats AJS, McDonagh T, Filippatos G, Maggioni AP, Piepoli MF, Rosano GMC, Ruschitzka F, Simić D, Ašanin M, Eicher JC, Yilmaz MB, Seferović PM, European Society of Cardiology Heart Failure Long‐Term Registry Investigators Group , Gale CP, Chair GB, Branko Beleslin RS, Andrzej Budaj PL, Ovidiu Chioncel RO, Nikolaos Dagres DE, Nicolas Danchin FR, David Erlinge SE, Jonathan Emberson GB, Michael Glikson IL, Alastair Gray GB, Meral Kayikcioglu TR, Aldo Maggioni IT, Klaudia Vivien Nagy HU, Aleksandr Nedoshivin RU, Anna‐Sonia Petronio IT, Jolien Roos‐Hesselink NL, Lars Wallentin SE, Uwe Zeymer DE, Crespo‐Leiro M, Anker S, Mebazaa A, Coats A, Filippatos G, Ferrari R, Maggioni AP, Piepoli MF, Al AG, Ar MD, Ar AF, At FF, Fazlibegovic E, Bg PG, By AK, Ch RH, Cy TC, Cz JH, Dk OWN, Dz RN, Ee TU, Eg MH, Es JFDJ, Fi VPH, Fr DL, Ge VC, Gr DT, Hr DM, Hu BM, O'Donoghue E, Il OA, Il AS, Ir DS, It MM, Jp AM, Kg EM, Lt AK, Lv AE, Vataman E, Mk MO, Mk ESK, Mt DCDM, Pl JD, Fonseca C, Ro OC, Rs MD, Ru ES, Se UD, Si ML, Sk EG, Tr AT, Uy VE, Xk GB, Auer J, Ablasser K, Fruhwald F, Dolze T, Brandner K, Gstrein S, Poelzl G, Moertl D, Reiter S, Podczeck‐Schweighofer A, Muslibegovic A, Vasilj M, Fazlibegovic E, Cesko M, Zelenika D, Palic B, Pravdic D, Cuk D, Vitlianova K, Katova T, Velikov T, Kurteva T, Gatzov P, Kamenova D, Antova M, Sirakova V, Krejci J, Mikolaskova M, Spinar J, Krupicka J, Malek F, Hegarova M, Lazarova M, Monhart Z, Hassanein M, Sobhy M, el Messiry F, el Shazly AH, Elrakshy Y, Youssef A, Moneim AA, Noamany M, Reda A, Dayem TKA, Farag N, Halawa SI, Hamid MA, Said K, Saleh A, Ebeid H, Hanna R, Aziz R, Louis O, Enen MA, Ibrahim BS, Nasr G, Elbahry A, Sobhy H, Ashmawy M, Gouda M, Aboleineen W, Bernard Y, Luporsi P, Meneveau N, Pillot M, Morel M, Seronde MF, Schiele F, Briand F, Delahaye F, Damy T, Eicher JC, Groote P, Fertin M, Lamblin N, Isnard R, Lefol C, Thevenin S, Hagege A, Jondeau G, Logeart D, le Marcis V, Ly JF, Coisne D, Lequeux B, le Moal V, Mascle S, Lotton P, Behar N, Donal E, Thebault C, Ridard C, Reynaud A, Basquin A, Bauer F, Codjia R, Galinier M, Tourikis P, Stavroula M, Tousoulis D, Stefanadis C, Chrysohoou C, Kotrogiannis I, Matzaraki V, Dimitroula T, Karavidas A, Tsitsinakis G, Kapelios C, Nanas J, Kampouri H, Nana E, Kaldara E, Eugenidou A, Vardas P, Saloustros I, Patrianakos A, Tsaknakis T, Evangelou S, Nikoloulis N, Tziourganou H, Tsaroucha A, Papadopoulou A, Douras A, Polgar L, Merkely B, Kosztin A, Nyolczas N, Nagy AC, Halmosi R, Elber J, Alony I, Shotan A, Fuhrmann AV, Amir O, Romano S, Marcon S, Penco M, di Mauro M, Lemme E, Carubelli V, Rovetta R, Metra M, Bulgari M, Quinzani F, Lombardi C, Bosi S, Schiavina G, Squeri A, Barbieri A, di Tano G, Pirelli S, Ferrari R, Fucili A, Passero T, Musio S, di Biase M, Correale M, Salvemini G, Brognoli S, Zanelli E, Giordano A, Agostoni P, Italiano G, Salvioni E, Copelli S, Modena MG, Reggianini L, Valenti C, Olaru A, Bandino S, Deidda M, Mercuro G, Dessalvi CC, Marino PN, di Ruocco MV, Sartori C, Piccinino C, Parrinello G, Licata G, Torres D, Giambanco S, Busalacchi S, Arrotti S, Novo S, Inciardi RM, Pieri P, Chirco PR, Galifi MA, Teresi G, Buccheri D, Minacapelli A, Veniani M, Frisinghelli A, Priori SG, Cattaneo S, Opasich C, Gualco A, Pagliaro M, Mancone M, Fedele F, Cinque A, Vellini M, Scarfo I, Romeo F, Ferraiuolo F, Sergi D, Anselmi M, Melandri F, Leci E, Iori E, Bovolo V, Pidello S, Frea S, Bergerone S, Botta M, Canavosio FG, Gaita F, Merlo M, Cinquetti M, Sinagra G, Ramani F, Fabris E, Stolfo D, Artico J, Miani D, Fresco C, Daneluzzi C, Proclemer A, Cicoira M, Zanolla L, Marchese G, Torelli F, Vassanelli C, Voronina N, Erglis A, Tamakauskas V, Smalinskas V, Karaliute R, Petraskiene I, Kazakauskaite E, Rumbinaite E, Kavoliuniene A, Vysniauskas V, Brazyte‐Ramanauskiene R, Petraskiene D, Stankala S, Switala P, Juszczyk Z, Sinkiewicz W, Gilewski W, Pietrzak J, Orzel T, Kasztelowicz P, Kardaszewicz P, Lazorko‐Piega M, Gabryel J, Mosakowska K, Bellwon J, Rynkiewicz A, Raczak G, Lewicka E, Dabrowska‐Kugacka A, Bartkowiak R, Sosnowska‐Pasiarska B, Wozakowska‐Kaplon B, Krzeminski A, Zabojszcz M, Mirek‐Bryniarska E, Grzegorzko A, Bury K, Nessler J, Zalewski J, Furman A, Broncel M, Poliwczak A, Bala A, Zycinski P, Rudzinska M, Jankowski L, Kasprzak JD, Michalak L, Soska KW, Drozdz J, Huziuk I, Retwinski A, Flis P, Weglarz J, Bodys A, Grajek S, Kaluzna‐Oleksy M, Straburzynska‐Migaj E, Dankowski R, Szymanowska K, Grabia J, Szyszka A, Nowicka A, Samcik M, Wolniewicz L, Baczynska K, Komorowska K, Poprawa I, Komorowska E, Sajnaga D, Zolbach A, Dudzik‐Plocica A, Abdulkarim AF, Lauko‐Rachocka A, Kaminski L, Kostka A, Cichy A, Ruszkowski P, Splawski M, Fitas G, Szymczyk A, Serwicka A, Fiega A, Zysko D, Krysiak W, Szabowski S, Skorek E, Pruszczyk P, Bienias P, Ciurzynski M, Welnicki M, Mamcarz A, Folga A, Zielinski T, Rywik T, Leszek P, Sobieszczanska‐Malek M, Piotrowska M, Kozar‐Kaminska K, Komuda K, Wisniewska J, Tarnowska A, Balsam P, Marchel M, Opolski G, Kaplon‐Cieslicka A, Gil RJ, Mozenska O, Byczkowska K, Gil K, Pawlak A, Michalek A, Krzesinski P, Piotrowicz K, Uzieblo‐Zyczkowska B, Stanczyk A, Skrobowski A, Ponikowski P, Jankowska E, Rozentryt P, Polonski L, Gadula‐Gacek E, Nowalany‐Kozielska E, Kuczaj A, Kalarus Z, Szulik M, Przybylska K, Klys J, Prokop‐Lewicka G, Kleinrok A, Aguiar CT, Ventosa A, Pereira S, Faria R, Chin J, de Jesus I, Santos R, Silva P, Moreno N, Queirós C, Lourenço C, Pereira A, Castro A, Andrade A, Guimaraes TO, Martins S, Placido R, Lima G, Brito D, Francisco AR, Cardiga R, Proenca M, Araujo I, Marques F, Fonseca C, Moura B, Leite S, Campelo M, Silva‐Cardoso J, Rodrigues J, Rangel I, Martins E, Correia AS, Peres M, Marta L, Silva GF, Severino D, Durao D, Leao S, Magalhaes P, Moreira I, Cordeiro AF, Ferreira C, Araujo C, Ferreira A, Baptista A, Radoi M, Bicescu G, Vinereanu D, Sinescu CJ, Macarie C, Popescu R, Daha I, Dan GA, Stanescu C, Dan A, Craiu E, Nechita E, Aursulesei V, Christodorescu R, Otasevic P, Seferovic PM, Simeunovic D, Ristic AD, Celic V, Pavlovic‐Kleut M, Lazic JS, Stojcevski B, Pencic B, Stevanovic A, Andric A, Simić D, Ašanin M, Iric‐Cupic V, Jovic M, Davidovic G, Milanov S, Mitic V, Atanaskovic V, Antic S, Pavlovic M, Stanojevic D, Stoickov V, Ilic S, Ilic MD, Petrovic D, Stojsic S, Kecojevic S, Dodic S, Adic NC, Cankovic M, Stojiljkovic J, Mihajlovic B, Radin A, Radovanovic S, Krotin M, Klabnik A, Goncalvesova E, Pernicky M, Murin J, Kovar F, Kmec J, Semjanova H, Strasek M, Iskra MS, Ravnikar T, Suligoj NC, Komel J, Fras Z, Jug B, Glavic T, Losic R, Bombek M, Krajnc I, Krunic B, Horvat S, Kovac D, Rajtman D, Cencic V, Letonja M, Winkler R, Valentincic M, Melihen‐Bartolic C, Bartolic A, Vrckovnik MP, Kladnik M, Pusnik CS, Marolt A, Klen J, Drnovsek B, Leskovar B, Anguita MJF, Page JCG, Martinez FMS, Andres J, Bayes‐Genis A, Mirabet S, Mendez A, Garcia‐Cosio L, Roig E, Leon V, Gonzalez‐Costello J, Muntane G, Garay A, Alcade‐Martinez V, Fernandez SL, Rivera‐Lopez R, Puga‐Martinez M, Fernandez‐Alvarez M, Serrano‐Martinez JL, Crespo‐Leiro M, Grille‐Cancela Z, Marzoa‐Rivas R, Blanco‐Canosa P, Paniagua‐Martin MJ, Barge‐Caballero E, Cerdena IL, Baldomero IFH, Padron AL, Rosillo SO, Gonzalez‐Gallarza RD, Montanes OS, Manjavacas AMI, Conde AC, Araujo A, Soria T, Garcia‐Pavia P, Gomez‐Bueno M, Cobo‐Marcos M, Alonso‐Pulpon L, Cubero JS, Sayago I, Gonzalez‐Segovia A, Briceno A, Subias PE, Hernandez MV, Cano MJR, Sanchez MAG, Jimenez JFD, Garrido‐Lestache EB, Pinilla JMG, Villa BG, Sahuquillo A, Marques RB, Calvo FT, Perez‐Martinez MT, Gracia‐Rodenas MR, Garrido‐Bravo IP, Pastor‐Perez F, Pascual‐Figal DA, Molina BD, Orus J, Gonzalo FE, Bertomeu V, Valero R, Martinez‐Abellan R, Quiles J, Rodrigez‐Ortega JA, Mateo I, ElAmrani A, Fernandez‐Vivancos C, Valero DB, Almenar‐Bonet L, Sanchez‐Lazaro IJ, Marques‐Sule E, Facila‐Rubio L, Perez‐Silvestre J, Garcia‐Gonzalez P, Ridocci‐Soriano F, Garcia‐Escriva D, Pellicer‐Cabo A, Fuente Galan L, Diaz JL, Platero AR, Arias JC, Blasco‐Peiro T, Julve MS, Sanchez‐Insa E, Aured‐Guallar C, Portoles‐Ocampo A, Melin M, Hägglund E, Stenberg A, Lindahl IM, Asserlund B, Olsson L, Dahlström U, Afzelius M, Karlström P, Tengvall L, Wiklund PA, Olsson B, Kalayci S, Temizhan A, Cavusoglu Y, Gencer E, Yilmaz MB, Gunes H. Sex‐ and age‐related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020; 22: 92–102. [DOI] [PubMed] [Google Scholar]
- 21. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlström U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Metra M. Guideline‐directed medical therapy for heart failure does not exist: a non‐judgmental framework for describing the level of adherence to evidence‐based drug treatments for patients with a reduced ejection fraction. Eur J Heart Fail 2020; 22: 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt M, Ulrichsen SP, Pedersen L, Botker HE, Sorensen HT. Thirty‐year trends in heart failure hospitalization and mortality rates and the prognostic impact of co‐morbidity: a Danish nationwide cohort study. Eur J Heart Fail 2016; 18: 490–499. [DOI] [PubMed] [Google Scholar]
- 24. Group CTS . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 25. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999; 353: 9–13. [PubMed] [Google Scholar]
- 26. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, Staiger C, Curtin EL, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 27. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 28. Kittleson M, Hurwitz S, Shah MR, Nohria A, Lewis E, Givertz M, Fang J, Jarcho J, Mudge G, Stevenson LW. Development of circulatory‐renal limitations to angiotensin‐converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol 2003; 41: 2029–2035. [DOI] [PubMed] [Google Scholar]
- 29. Ameri P, Bertero E, Maack C, Teerlink JR, Rosano G, Metra M. Medical treatment of heart failure with reduced ejection fraction: the dawn of a new era of personalized treatment? Eur Heart J Cardiovasc Pharmacother 2021; 7: 539–546. [DOI] [PubMed] [Google Scholar]
- 30. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 31. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, on behalf of the PARADIGM‐HF Committees Investigators . Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mann DL, Greene SJ, Givertz MM, Vader JM, Starling RC, Ambrosy AP, Shah P, McNulty S, Mahr C, Gupta D, Redfield MM, Lala A, Lewis GD, Mohammed SF, Gilotra NA, DeVore A, Gorodeski EZ, Desvigne‐Nickens P, Hernandez AF, Braunwald E, LIFE Investigators . Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the LIFE trial. JACC Heart Fail 2020; 8: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mann DL, Givertz MM, Vader JM, Starling RC, Shah P, McNulty SE, Anstrom KJ, Margulies KB, Kiernan MS, Mahr C, Gupta D. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moliner‐Abós C, Rivas‐Lasarte M, Pamies Besora J, Fluvià‐Brugues P, Solé‐González E, Mirabet S, López López L, Brossa V, Pirla MJ, Mesado N, Álvarez‐García J, Roig E. Sacubitril/valsartan in real‐life practice: experience in patients with advanced heart failure and systematic review. Cardiovasc Drugs Ther 2019; 33: 307–314. [DOI] [PubMed] [Google Scholar]
- 35. Martens P, Belien H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail 2018; 5: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, Polovina MM, Komajda M, Seferović J, Sari I, Cosentino F, Ambrosio G, Metra M, Piepoli M, Chioncel O, Lund LH, Thum T, de Boer RA, Mullens W, Lopatin Y, Volterrani M, Hill L, Bauersachs J, Lyon A, Petrie MC, Anker S, Rosano GMC. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose‐lowering drugs in patients with heart failure. Eur J Heart Fail 2020; 22: 196–213. [DOI] [PubMed] [Google Scholar]
- 37. Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund LH, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC. Heart Failure Association of the European Society of Cardiology update on sodium‐glucose co‐transporter 2 inhibitors in heart failure. Eur J Heart Fail 2020; 22: 1984–1986. [DOI] [PubMed] [Google Scholar]
- 38. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 39. Tomasoni D, Adamo M, Anker MS, von Haehling S, Coats AJS, Metra M. Heart failure in the last year: progress and perspective. ESC Heart Fail 2020; 7: 3505–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2017; 2: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 41. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 42. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, Bengtsson O, Sjöstrand M, Langkilde AM, Anand IS, Chiang CE, Chopra VK, de Boer RA, Diez M, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Verma S, Docherty KF, Jhund PS, McMurray JJV. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA‐HF). Eur Heart J 2020; 41: 3402–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 45. Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJ, Filippatos G, Greene SJ, McDonagh TA, Ponikowski P, Rosano G, Seferovic P, Vaduganathan M, Voors AA, Metra M. Sodium‐glucose co‐transporter 2 inhibitors as an early, first line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pieske B, Patel MJ, Westerhout CM, Anstrom KJ, Butler J, Ezekowitz J, Hernandez AF, Koglin J, Lam CSP, Ponikowski P, Roessig L, Voors AA, O'Connor CM, Armstrong PW, on behalf of the VICTORIA Study Group , Abidin IZ, Atar D, Bahit MC, Benecke JLA, Bocchi EA, Bonderman D, Cho MC, Chiang CE, Cohen‐Solal A, Cowie M, Edelmann F, Emdin M, Escobedo J, Ezekowitz JA, Givertz MM, Kaye DM, Lanas F, Lassus J, Lewis BS, Lopatin Y, López‐Sendón J, Lund LH, McDonald K, Melenovský V, Mosterd A, Noori E, Oto MA, Palomino ALG, Piña IL, Ponikowski P, Pouleur AC, Refsgaard J, Reyes E, Saldarriaga C, Senni M, Sim D, Siu D, Sliwa‐Hähnle K, Sweitzer NK, Troughton RW, Tsutsui H, Tziakas DN, Vazquez‐Tanus JB, Zhang J. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019; 21: 1596–1604. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 48. Ezekowitz JA, O'Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, Butler J, Lam CSP, Ponikowski P, Emdin M, Patel MJ, Pieske B, Roessig L, Hernandez AF, Armstrong PW. N‐terminal pro‐B‐type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail 2020; 8: 931–939. [DOI] [PubMed] [Google Scholar]
- 49. Lombardi CM, Cimino G, Pagnesi M, Dell'Aquila A, Tomasoni D, Ravera A, Inciardi R, Carubelli V, Vizzardi E, Nodari S, Emdin M, Aimo A. Vericiguat for heart failure with reduced ejection fraction. Curr Cardiol Rep 2021; 23: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE, GALACTIC‐HF Investigators . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021; 384: 105–116. [DOI] [PubMed] [Google Scholar]
- 51. Tomasoni D, Lombardi CM, Sbolli M, Cotter G, Metra M. Acute heart failure: more questions than answers. Prog Cardiovasc Dis 2020; 63: 599–606. [DOI] [PubMed] [Google Scholar]
- 52. Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky‐Fallick C, Moriguchi J, Kobashigawa J, Walden J. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol 1990; 66: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 53. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 54. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, for the MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 55. Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, Abraham WT, CHAMPION Trial Investigators . Pulmonary artery pressure‐guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2017; 70: 1875–1886. [DOI] [PubMed] [Google Scholar]
- 56. Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, Costanzo MR, Henderson JD, Brett ME, Adamson PB, Stevenson LW, CardioMEMS Post‐Approval Study Investigators . Lower rates of heart failure and all‐cause hospitalizations during pulmonary artery pressure‐guided therapy for ambulatory heart failure: one‐year outcomes from the CardioMEMS post‐approval study. Circ Heart Fail 2020; 13: e006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lombardi CM, Cimino G, Pellicori P, Bonelli A, Inciardi RM, Pagnesi M, Tomasoni D, Ravera A, Adamo M, Carubelli V, Metra M. Congestion in patients with advanced heart failure: assessment and treatment. Heart Fail Clin 2021; 17: 575–586. [DOI] [PubMed] [Google Scholar]
- 58. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐la Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 59. Gupta AK, Tomasoni D, Sidhu K, Metra M, Ezekowitz JA. Evidence‐based management of acute heart failure. Can J Cardiol 2021; 37: 621–631. [DOI] [PubMed] [Google Scholar]
- 60. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med 2017; 377: 1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenberg J, Gustafsson F, Galatius S, Hildebrandt PR. Combination therapy with metolazone and loop diuretics in outpatients with refractory heart failure: an observational study and review of the literature. Cardiovasc Drugs Ther 2005; 19: 301–306. [DOI] [PubMed] [Google Scholar]
- 63. Brisco‐Bacik MA, ter Maaten J, Houser SR, Vedage NA, Rao V, Ahmad T, Wilson FP, Testani JM. Outcomes associated with a strategy of adjuvant metolazone or high‐dose loop diuretics in acute decompensated heart failure: a propensity analysis. J Am Heart Assoc 2018; 7: e009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mondritzki T, Mai TA, Vogel J, Pook E, Wasnaire P, Schmeck C, Hüser J, Dinh W, Truebel H, Kolkhof P. Cardiac output improvement by pecavaptan: a novel dual‐acting vasopressin V1a/V2 receptor antagonist in experimental heart failure. Eur J Heart Fail 2021; 23: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldsmith SR, Burkhoff D, Gustafsson F, Voors A, Zannad F, Kolkhof P, Staedtler G, Colorado P, Dinh W, Udelson JE. Dual vasopressin receptor antagonism to improve congestion in patients with acute heart failure: design of the AVANTI trial. J Card Fail 2021; 27: 233–241. [DOI] [PubMed] [Google Scholar]
- 66. Costanzo MR, Ronco C, Abraham WT, Agostoni P, Barasch J, Fonarow GC, Gottlieb SS, Jaski BE, Kazory A, Levin AP, Levin HR, Marenzi G, Mullens W, Negoianu D, Redfield MM, Tang WHW, Testani JM, Voors AA. Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research. J Am Coll Cardiol 2017; 69: 2428–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, Smelser JM, Kaneshige AM, Chomsky DB, Adler ED, Haas GJ, Watts JA, Nabut JL, Schollmeyer MP, Fonarow GC. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail 2016; 4: 95–105. [DOI] [PubMed] [Google Scholar]
- 68. Grossekettler L, Schmack B, Meyer K, Brockmann C, Wanninger R, Kreusser MM, Frankenstein L, Kihm LP, Zeier M, Katus HA, Remppis A, Schwenger V. Peritoneal dialysis as therapeutic option in heart failure patients. ESC Heart Fail 2019; 6: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, Sadavarte A, Singh TP, Zuckermann A, Stehlik J, International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty‐sixth adult heart transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019; 38: 1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF Jr, Rosano GMC, Lancellotti P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol 2019; 73: 2345–2353. [DOI] [PubMed] [Google Scholar]
- 71. Maack C, Eschenhagen T, Hamdani N, Heinzel FR, Lyon AR, Manstein DJ, Metzger J, Papp Z, Tocchetti CG, Yilmaz MB, Anker SD, Balligand JL, Bauersachs J, Brutsaert D, Carrier L, Chlopicki S, Cleland JG, de Boer RA, Dietl A, Fischmeister R, Harjola VP, Heymans S, Hilfiker‐Kleiner D, Holzmeister J, de Keulenaer G, Limongelli G, Linke WA, Lund LH, Masip J, Metra M, Mueller C, Pieske B, Ponikowski P, Ristić A, Ruschitzka F, Seferović PM, Skouri H, Zimmermann WH, Mebazaa A. Treatments targeting inotropy. Eur Heart J 2019; 40: 3626–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lowes BD, Shakar SF, Metra M, Feldman AM, Eichhorn E, Freytag JW, Gerber MJ, Liard JF, Hartman C, Gorczynski R, Evans G, Linseman JV, Stewart J, Robertson AD, Roecker EB, Demets DL, Bristow MR. Rationale and design of the enoximone clinical trials program. J Card Fail 2005; 11: 659–669. [DOI] [PubMed] [Google Scholar]
- 73. Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 74. Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study Investigators Circulation 2000; 102: 2222–2227. [DOI] [PubMed] [Google Scholar]
- 75. Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M. Hemodynamic properties of a new‐generation positive luso‐inotropic agent for the acute treatment of advanced heart failure. Am J Cardiol 2007; 99: 41A–46A. [DOI] [PubMed] [Google Scholar]
- 76. Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA. Dose‐dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first‐in‐man study. Lancet 2011; 378: 667–675. [DOI] [PubMed] [Google Scholar]
- 77. Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double‐blind, placebo‐controlled, crossover, dose‐ranging phase 2 trial. Lancet 2011; 378: 676–683. [DOI] [PubMed] [Google Scholar]
- 78. Voors AA, Tamby JF, Cleland JG, Koren M, Forgosh LB, Gupta D, Lund LH, Camacho A, Karra R, Swart HP, Pellicori P, Wagner F, Hershberger RE, Prasad N, Anderson R, Anto A, Bell K, Edelberg JM, Fang L, Henze M, Kelly C, Kurio G, Li W, Wells K, Yang C, Teichman SL, Rio CL, Solomon SD. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail 2020; 22: 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Connor CM, Gattis WA, Uretsky BF, Adams KF Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J 1999; 138: 78–86. [DOI] [PubMed] [Google Scholar]
- 80. Martens P, Vercammen J, Ceyssens W, Jacobs L, Luwel E, van Aerde H, Potargent P, Renaers M, Dupont M, Mullens W. Effects of intravenous home dobutamine in palliative end‐stage heart failure on quality of life, heart failure hospitalization, and cost expenditure. ESC Heart Fail 2018; 5: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991; 325: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 82. Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M, Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) Investigators . Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002; 287: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 83. Metra M, Eichhorn E, Abraham WT, Linseman J, Bohm M, Corbalan R, DeMets D, de Marco T, Elkayam U, Gerber M, Komajda M, Liu P, Mareev V, Perrone SV, Poole‐Wilson P, Roecker E, Stewart J, Swedberg K, Tendera M, Wiens B, Bristow MR, for the ESSENTIAL Investigators . Effects of low‐dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double‐blind, placebo‐controlled, parallel group ESSENTIAL trials. Eur Heart J 2009; 30: 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study . Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet 2002; 360: 196–202. [DOI] [PubMed] [Google Scholar]
- 85. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado‐Herrera L, Salon J, Garratt C, Huang B, Sarapohja T. Effect of levosimendan on the short‐term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013; 1: 103–111. [DOI] [PubMed] [Google Scholar]
- 86. Mebazaa A, Nieminen MS, Packer M, Cohen‐Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M, SURVIVE Investigators . Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007; 297: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 87. Nieminen MS, Cleland JG, Eha J, Belenkov Y, Kivikko M, Põder P, Sarapohja T. Oral levosimendan in patients with severe chronic heart failure—the PERSIST study. Eur J Heart Fail 2008; 10: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 88. Comín‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodríguez JL, Grau M, Bruguera J, on behalf of the LION‐HEART Study Investigators . Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 20: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 89. Oliva F, Comin‐Colet J, Fedele F, Fruhwald F, Gustafsson F, Kivikko M, Borbély A, Pölzl G, Tschöpe C. Repetitive levosimendan treatment in the management of advanced heart failure. Eur Heart J Suppl 2018; 20: I11–I20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oliva F, Perna E, Marini M, Nassiacos D, Cirò A, Malfatto G, Morandi F, Caico I, Perna G, Meloni S, Vincenzi A, Villani A, Vecchi AL, Minoia C, Verde A, de Maria R, RELEVANT‐HF study group . Scheduled intermittent inotropes for Ambulatory Advanced Heart Failure. The RELEVANT‐HF multicentre collaboration. Int J Cardiol 2018; 272: 255–259. [DOI] [PubMed] [Google Scholar]
- 91. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 92. Garcia‐Gonzalez MJ, Aldea Perona A, Lara Padron A, Morales Rull JL, Martinez‐Selles M, de Mora MM, de Mora Martin M, López Díaz J, López Fernandez S, Ortiz Oficialdegui P, Jiménez Sosa A. Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: the LAICA study. ESC Heart Fail 2021; 8: 4820–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pölzl G, Allipour Birgani S, Comín‐Colet J, Delgado JF, Fedele F, García‐Gonzáles MJ, Gustafsson F, Masip J, Papp Z, Störk S, Ulmer H, Vrtovec B, Wikström G, Altenberger J. Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post‐discharge period. ESC Heart Fail 2019; 6: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ahmad T, Miller PE, McCullough M, Desai NR, Riello R, Psotka M, Böhm M, Allen LA, Teerlink JR, Rosano GMC, Lindenfeld J. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail 2019; 21: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chernomordik F, Freimark D, Arad M, Shechter M, Matetzky S, Savir Y, Shlomo N, Peled A, Goldenberg I, Peled Y. Quality of life and long‐term mortality in patients with advanced chronic heart failure treated with intermittent low‐dose intravenous inotropes in an outpatient setting. ESC Heart Fail 2017; 4: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O'Connor CM. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME‐CHF study. J Am Coll Cardiol 2003; 41: 997–1003. [DOI] [PubMed] [Google Scholar]
- 97. Mebazaa A, Nieminen MS, Filippatos GS, Cleland JG, Salon JE, Thakkar R, Padley RJ, Huang B, Cohen‐Solal A. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta‐blockers in SURVIVE. Eur J Heart Fail 2009; 11: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nizamic T, Murad MH, Allen LA, McIlvennan CK, Wordingham SE, Matlock DD, Dunlay SM. Ambulatory inotrope infusions in advanced heart failure: a systematic review and meta‐analysis. JACC Heart Fail 2018; 6: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF Jr, Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, COSMIC‐HF Investigators . Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC‐HF): a phase 2, pharmacokinetic, randomised, placebo‐controlled trial. Lancet 2016; 388: 2895–2903. [DOI] [PubMed] [Google Scholar]
- 100. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Biering‐Sørensen T, Böhm M, Bonderman D, Fang JC, Lanfear DE, Lund M, Momomura SI, O'Meara E, Ponikowski P, Spinar J, Flores‐Arredondo JH, Claggett BL, Heitner SB, Kupfer S, Abbasi SA, Malik FI, GALACTIC‐HF Investigators . Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC‐HF. J Am Coll Cardiol 2021; 78: 97–108. [DOI] [PubMed] [Google Scholar]
- 101. Felker GM, Solomon SD, Claggett B, Diaz R, McMurray JJV, Metra M, Anand I, Crespo‐Leiro MG, Dahlström U, Goncalvesova E, Howlett JG, MacDonald P, Parkhomenko A, Tomcsányi J, Abbasi SA, Heitner SB, Hucko T, Kupfer S, Malik FI, Teerlink JR. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALACTIC‐HF randomized clinical trial. JAMA Cardiol 2022; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abete R, Iacovoni A, Senni M. The myosin activator: is another step forward in heart failure therapy? Eur Heart J Suppl 2021; 23: E151–E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JGF, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, ATOMIC‐AHF Investigators . Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC‐AHF study. J Am Coll Cardiol 2016; 67: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 104. Bavendiek U, Berliner D, Dávila LA, Schwab J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber K, Hänselmann A, Oldhafer M, Schallhorn S, von der Leyen H, Schröder C, Veltmann C, Störk S, Böhm M, Koch A, Bauersachs J, DIGIT‐HF Investigators and Committees , Bavendiek U, Bauersachs J, Koch A, von der Leyen H, Veltmann C, Böhm M, Störk S, Tebbe U, von Haehling S, Haass M, Anker S, Mohacsi P, Pölzl G, Trampisch H, Dávila LA, Weber K, Zimmermann S, Neuhaus B. Rationale and design of the DIGIT‐HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double‐blind, placebo‐controlled study. Eur J Heart Fail 2019; 21: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough P, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 106. Campbell RT, Petrie MC, Jackson CE, Jhund PS, Wright A, Gardner RS, Sonecki P, Pozzi A, McSkimming P, McConnachie A, Finlay F, Davidson P, Denvir MA, Johnson MJ, Hogg KJ, McMurray JJV. Which patients with heart failure should receive specialist palliative care? Eur J Heart Fail 2018; 20: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hill L, Prager Geller T, Baruah R, Beattie JM, Boyne J, de Stoutz N, di Stolfo G, Lambrinou E, Skibelund AK, Uchmanowicz I, Rutten FH, Čelutkienė J, Piepoli MF, Jankowska EA, Chioncel O, Ben Gal T, Seferovic PM, Ruschitzka F, Coats AJS, Strömberg A, Jaarsma T. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail 2020; 22: 2327–2339. [DOI] [PubMed] [Google Scholar]
- 108. Arestedt K, Brannstrom M, Evangelista LS, Stromberg A, Alvariza A. Palliative key aspects are of importance for symptom relief during the last week of life in patients with heart failure. ESC Heart Fail 2021; 8: 2202–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, Yang H, Anstrom KJ, Dodson GC, Taylor DH Jr, Kirchner JL, Mark DB, O'Connor CM, Tulsky JA. Palliative Care in Heart Failure: the PAL‐HF randomized, controlled clinical trial. J Am Coll Cardiol 2017; 70: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sahlollbey N, Lee CKS, Shirin A, Joseph P. The impact of palliative care on clinical and patient‐centred outcomes in patients with advanced heart failure: a systematic review of randomized controlled trials. Eur J Heart Fail 2020; 22: 2340–2346. [DOI] [PubMed] [Google Scholar]
- 111. Bayoumi E, Sheikh F, Groninger H. Palliative care in cardiac transplantation: an evolving model. Heart Fail Rev 2017; 22: 605–610. [DOI] [PubMed] [Google Scholar]


