Abstract
Acute myocarditis following mRNA COVID‐19 vaccination was reported by the European Medicine Agency safety committee as a rare adverse event. We present a case series of three young male patients with suspected acute myocarditis following BNT162b2 mRNA COVID‐19 vaccination including results of endomyocardial biopsies (EMB). Additionally, we analysed EMB of another 21 patients with clinically suspected acute myocarditis following vaccination to determine the pathohistological pattern. Overall, EMB revealed acute lymphocytic myocarditis in 5 (20.8%), chronic lymphocytic myocarditis in 6 (25%), cardiac sarcoidosis in 1 (4.2%), healed myocarditis in 6 (25%), and other diagnoses with cardiac damage of unclear aetiology in 6 (25%) cases. Our findings support the necessity of EMB in patients with suspected acute myocarditis following mRNA COVID‐19 vaccination presenting with reduced EF to establish a correct and definite diagnosis. Concerns of these rare severe adverse events after COVID‐19 immunization should not undermine its value for the global community.
Keywords: Myocarditis, COVID‐19, Vaccination, Endomyocardial biopsy
Introduction
The ongoing COVID‐19 pandemic has challenged health care systems worldwide. Immunization with COVID‐19 vaccines has substantially contributed to control the disease. From the beginning, COVID‐19 vaccines have received intensive safety monitoring. Recently, acute myocarditis following mRNA COVID‐19 vaccination was reported by the European Medicine Agency safety committee as a severe but very rare adverse event. 1 Vaccine‐related myocarditis was mainly reported in young individuals which underlines the need for a substantiated risk–benefit evaluation.
We present three cases of acute myocarditis in young patients following BNT162b2 mRNA COVID‐19 vaccination including results of left ventricular endomyocardial biopsies (EMB), which has not been reported yet. In addition, we analysed EMB of another 21 patients with clinically suspected acute myocarditis following mRNA COVID‐19 vaccination to establish a definite diagnosis.
Case reports
Case 1
An 18‐year‐old male patient was admitted because of chest pain and fever (37.9°C) 3 days after his first dose of BNT162b2 mRNA COVID‐19 vaccination. Two days prior to the vaccination, the patient had self‐limiting acute diarrhoea. Baseline laboratory results showed leukocytosis with elevated monocytes, serum C‐reactive protein (7.2 mg/dL) and hs‐troponin T levels (peak 1386 ng/L) (Table 1 ). SARS‐CoV‐2 PCR (nasopharyngeal swap) was negative. Echocardiography demonstrated severely reduced left ventricular ejection fraction (EF 33%) and myocardial oedema with patchy intramural late gadolinium enhancement (LGE) was described on cardiac magnetic resonance imaging (MRI) (Figure 1 ). Transradial left ventricular (LV) EMB revealed lymphocytic myocarditis (CD3 + T‐lymphocytes 50/mm2, CD68 + macrophages >100/mm2) (Figure 2 ). Nested (RT‐)PCR for the detection of enteroviruses (including coxsackieviruses of Groups A and B, echoviruses), parvovirus B19 (PVB19), human herpesvirus 6 (HHV6), Epstein–Barr virus, adenoviruses, human cytomegalovirus, herpes simplex virus type 1 and 2, human herpesvirus 7 (HHV7), varicella zoster virus, Toxoplasma gondii and Borrelia spp. was negative in the myocardium and EDTA blood. Microbiological stool analysis on enteroviruses remained negative but revealed Campylobacter spp. The patient received heart failure therapy, non‐steroidal anti‐inflammatory drugs (NSAID) and antibiotic treatment. A life vest was prescribed for rhythm monitoring. At follow‐up after 1 month, the patient had recovered well with an EF of 60%.
Table 1.
Patients' characteristics with suspected acute myocarditis following mRNA COVID‐19 vaccination (n = 3) including laboratory, cardiac MRI imaging and EMB results, and therapy
| Case | #1 | #2 | #3 | |
|---|---|---|---|---|
| Age (years) | 18 | 22 | 38 | |
| Sex | M | M | M | |
| COVID‐19 mRNA vaccination | first dose | second dose | second dose | |
| Symptoms onset after vaccination (days) | 3 | 1 | 4 | |
| Chest pain | ++ | ++ | ++ | |
| Shortness of breath | − | − | − | |
| Fatigue | + | + | + | |
| Fever | + | − | − | |
| Non cardiac comorbidities | campylobacter enteritis | none | immune checkpoint inhibitor therapy for malign melanoma |
| Laboratory results | ||||
|---|---|---|---|---|
| Peak hs Troponin T (ng/L) | 1386 | 1104 | (0–14 ng/L) | |
| Peak hs Troponin I (ng/L) | 38 735 | (2–45 ng/L) | ||
| Peak CK (U/L) | 830 | 1378 | 729 | (0–190 U/L) |
| NT‐proBNP (ng/L) | 991 | 528 | 1330 | (0–86 ng/L) |
| C‐reactive protein (mg/dL) | 6.5 | 11.3 | 11.4 | (0–0.5 mg/dL) |
| Procalcitonin (ngl/mL) | 0.3 | 0.1 | 0.1 | (0–0.5 ng/L) |
| Interleucin‐6 (pg/mL) | 5.8 | 15.3 | (0–7 pg/mL) | |
| Leucocytes (G/L) | 10.3 | 8.2 | 9.2 | (3.9–8.8 G/L) |
| Neutrophiles (G/L) | 6.5 | 5.1 | 6.0 | (1.82–7.42 G/L) |
| Lymphocytes (G/L) | 1.6 | 1.6 | 1.7 | (1.0–4.0 G/L) |
| Monocytes (G/L) | 1.1 | 1.1 | 0.98 | (0.19–0.77 G/L) |
| Eosinophiles (G/L) | 0.01 | 0.15 | 0.5 | (0–0.5 G/L) |
| Basophiles (G/L) | 0.03 | 0.05 | 0.03 | (0–0.2 G/L) |
| Haemoglobin (g/dL) | 14.8 | 15.8 | 14.0 | (14.0–17.0) |
| Thrombocytes (G/L) | 184 | 166 | 198 | (151–400 G/L) |
| Creatinine | 0.97 | 0.9 | 0.89 | (0.7–1.2 mg/dL) |
| SARS‐CoV‐2 PCR | Negative | Negative | Negative | |
| Cardiac MRI | ||||
|---|---|---|---|---|
| LVEF (%) | 33 | 40 | 48 | (56–65%) |
| Myocarditis | ++ | ++ | ++ | |
| T1 mapping | ++ | n/a | ++ | |
| T2 mapping/T2 db fs | ++ | + | ++ | |
| Late gadolinum enhancement | ++ | ++ | ++ | |
| Pericarditis | + | + | + | |
| Endomyocardial biopsy analysis | ||||
|---|---|---|---|---|
| CD3 + T‐lymphocytes | 50 ↑ | 5 | 30 ↑ | cells/mm2 |
| CD68 + macrophages | >100 ↑ | 14 | 34 ↑ | cells/mm2 |
| CD20 + B‐lymphocytes | 3 | 0 | 0 | cells/mm2 |
| Eosinophiles | 2 | 0 | 0 | cells/mm2 |
| Giant cells | 0 | 0 | 0 | cells/mm2 |
| SARS‐CoV‐2 PCR | Negative | Negative | Negative | |
| Nested viral (RT‐)PCR | Negative | HHV6B | Negative | |
| Therapy | ||||
|---|---|---|---|---|
| Heart failure therapy | + | + | + | |
| NSAID | + | + | + | |
| Colchicine | − | + | − | |
| Immunosuppressive therapy | − | − | prednisolone | |
M, male; ++, strongly positive; +, positive; −, negative; magnetic resonance imaging; n/a, not available; LVEF, left ventricular ejection fraction; PCR, polymerase chain reaction; NSAID, non‐steroidal anti‐inflammatory drugs.
Figure 1.
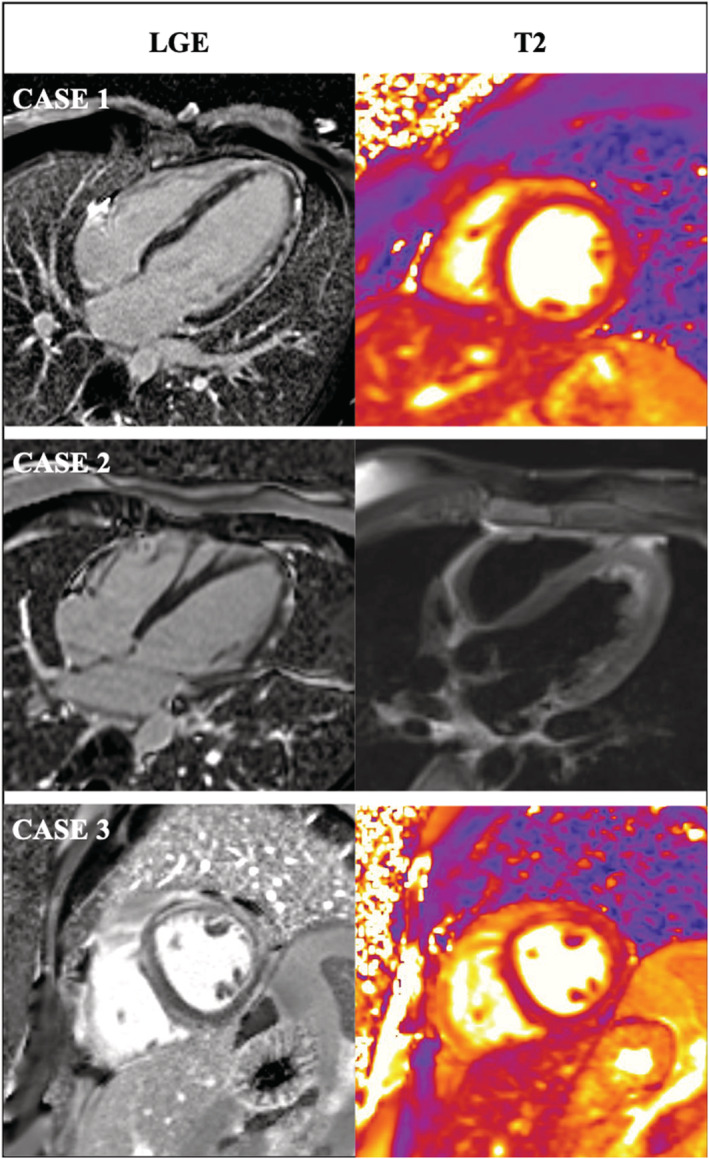
Cardiac magnetic resonance imaging demonstrated acute myocarditis with patchy intramural to subepicardial late gadolinum enhancement (LGE) with myocardial oedema in T2‐weighted imaging in Cases 1, 2, and 3.
Figure 2.
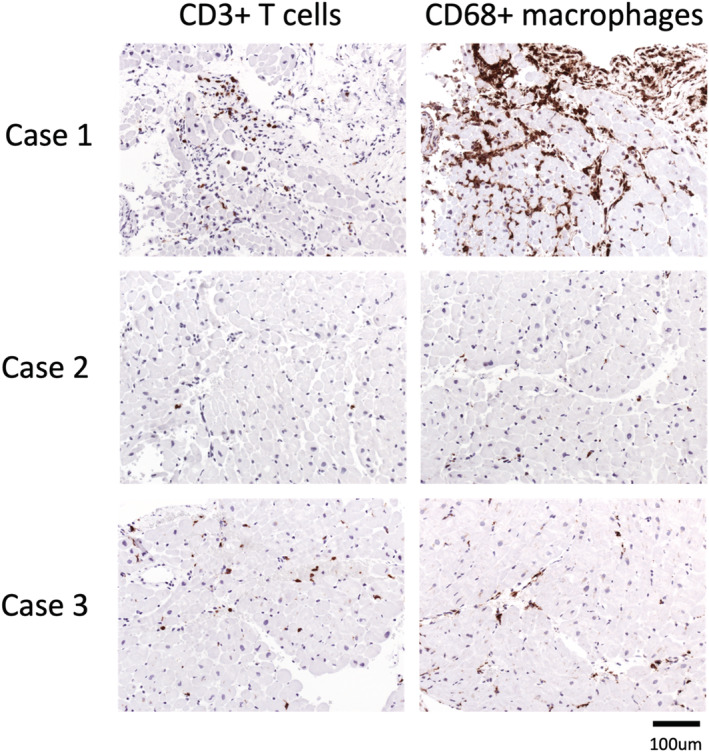
EMB analysis of patients with acute myocarditis following COVID‐19 mRNA vaccination revealing acute lymphocytic myocarditis with elevated CD3 + T cells and CD68 + macrophages in Cases 1 and 3. No lymphocytic, eosinophilic, or giant cell myocarditis was detected in Case 2. (RT‐)PCR revealed HHV6B DNA in the myocardium and HHV7 persistence in the EDTA blood in Case 2.
Case 2
A 22‐year‐old male patient, presented with chest pain and fatigue with an onset 1 day after his second dose of BNT162b2 mRNA COVID‐19 vaccination. Baseline hs‐troponin I levels were markedly elevated (peak 38 735 ng/L). Cardiac MRI demonstrated typical signs of an acute myocarditis with moderately reduced EF (EF 40%) with myocardial oedema and patchy intramural and pericardial LGE (Figure 1 ). LV EMB revealed mild interstitial fibrosis without evidence of lymphocytic or eosinophilic myocarditis (Figure 2 ). In the myocardium HHV6B and in the EDTA blood, HHV7 DNA was detected by (RT‐)PCR. The patient recovered on heart failure therapy, NSAID, and colchicine within 1 month.
Case 3
A 38‐year‐old male patient was admitted because of fatigue and chest pain 4 days after his second mRNA BNT162b2 COVID‐19 vaccination. The patient had received an immune checkpoint inhibitor therapy (ipilimumab and nivolumab) for malignant melanoma 7 days before symptom onset. Laboratory tests revealed elevated C‐reactive protein and hs‐troponin T levels (peak 1104 ng/L). Cardiac MRI showed a mildly reduced EF with 48% and myocardial oedema with patchy intramural to subepicardial LGE (Figure 1 ). Acute lymphocytic myocarditis with elevated CD3 + positive T‐lymphocytes (30/mm2) and CD68 + macrophages (34 cells/mm2) was diagnosed by LV EMB (Figure 2 ). No nucleic acids of pathogens described above were found by (RT‐)PCR in the myocardium. Medical heart failure therapy and an immunosuppressive therapy (prednisolone) was initiated. The patient recovered well, and EF normalized at follow‐up after 1 month.
Discussion
In this case series, we describe three patients with suspected acute myocarditis that occurred within 1 to 4 days after the first or second dose of BNT162b2 mRNA COVID 19 vaccination. All patients presented with chest pain and fatigue, had a mildly to severely reduced EF, and were haemodynamically and respiratory stable without arrhythmias. They recovered completely with heart failure treatment and NSAID within 1 month. One patient required prednisolone therapy because there was a potential risk of immune checkpoint inhibitor‐related myocarditis (Case 3). A campylobacter enteritis was diagnosed in another patient (Case 1). EMB revealed acute lymphocytic myocarditis in these two cases without detection of cardiotropic agents. One patient showed only mild fibrosis without signs of lymphocytic, eosinophilic, or giant cell myocarditis but was positive for HHV6B DNA in the myocardium (Case 2). This patient was diagnosed as acute peri‐myocarditis according to current Lake Louise criteria with myocardial oedema on T2 mapping and patchy intramural and pericardial LGE. Sampling error might have contributed to the inconclusive EMB result.
The first report of an association between mRNA COVID‐19 vaccination and acute myocarditis and pericarditis was presented by Israel's Health Ministry in June 2021. 2 Meanwhile several case series showing a younger aged male predominance were published. 3 , 4 , 5 Diagnosis of vaccine‐related myocarditis was based on the clinical presentation and non‐invasive imaging techniques (echocardiography and cardiac MRI) in most patients. Except for smallpox vaccine, immunizations are rarely associated with myocarditis and pericarditis. 6 This has encouraged debate whether the temporal connection indicates causation. EMB, which is regarded as the gold standard for the diagnosis of definite myocarditis, was not reported. 7 However, a careful diagnostic work‐up including EMB is of utmost importance to avoid erroneous conclusions.
Little is known about the underlying mechanisms of vaccine‐related myocarditis. Expression of cytokines by induction of anti‐idiotype cross‐reactive antibodies, molecular mimicry mechanism between viral proteins and cardiomyocyte proteins and RNA vaccines itself as an immunogen potent bystander are discussed. 8 However, all these mechanisms remain speculative. Even less is known about the pathohistological pattern in the myocardium.
To determine the pathohistological pattern, we analysed EMB of another 21 patients. In total, our study cohort consisted of 24 patients with a median age 30 years (IQR 19–38 years, 22 males) with clinically suspected acute myocarditis following mRNA COVID‐19 vaccination with a median EF of 48% (IQR 40–60%). Acute myocarditis was diagnosed by elevated troponin levels, pathological echocardiography findings and cardiac MRI (12 before EMB, 2 after EMB). EMB was performed at the discretion of the treating physician. Sixteen EMB (66.7%) were performed in the LV and 8 (33.3%) in the RV. EMB revealed acute lymphocytic myocarditis in 5 (20.8%), chronic lymphocytic myocarditis in 6 (25%), cardiac sarcoidosis in 1 (4.2%), healed myocarditis in 6 (25%), and other diagnoses with cardiac damage of unclear aetiology in 6 (25%) cases. Viral PCR detected HHV6B in 3 (12.5%), PVB19 in 2 (8.4%), and EBV in 1 (4.2%) patient(s), respectively and 18 (75%) patients remained viral negative. Results are presented in Table 2 . Sampling errors cannot be ruled out completely, especially in right ventricular EMB. However, this finding stresses the importance of EMB in suspected vaccine‐related myocarditis to establish a correct diagnosis.
Table 2.
Clinical characteristics and results of EMB of patients with clinically suspected acute myocarditis following mRNA COVID‐19 vaccination (n = 24)
| ID | EMB localization | Sex | Age | EF (%) | Cardiac histology | Viral PCR | Cardiac MRI |
|---|---|---|---|---|---|---|---|
| Acute myocarditis | |||||||
| #1 (case 1) | LV | M | 18 | 33 | Acute lymphocytic myocarditis | Negative | Patchy intramural LGE |
| #2 | RV | M | 35 | 42 | Acute lymphocytic myocarditis | EBV | Inferolateral LGE |
| #3 (case 3) | LV | M | 38 | 48 | Acute lymphocytic myocarditis | Negative | Patchy intramural LGE |
| #4 | LV | M | 28 | 48 | Acute eosinophilic endomyocarditis (Löffler endomyocardits) | Negative | n/a |
| #5 | LV | M | 40 | 43 | Acute focal lymphocytic myocarditis | Negative | n/a |
| Chronic myocarditis | |||||||
| #6 | LV | M | 20 | 60 | Mild healing/chronic lmphocytic myocarditis | Negative | Apical lateral LGE |
| #7 | LV | M | 68 | 30 | Mild healing/chronic lmphocytic myocarditis | Negative | Diffuse focal LGE |
| #8 | LV | M | 19 | n/a | Mild healing/chronic lmphocytic myocarditis | Negative | n/a |
| #9 | RV | M | 17 | 50 | Mild healing/chronic lmphocytic myocarditis | PVB19 (377 copies/μg DNA) | Planned after EMB |
| #10 | RV | M | 16 | 65 | Mild healing/chronic lmphocytic myocarditis | Negative | Planned after EMB |
| #11 | LV | M | 20 | 40 | Mild healing/chronic lmphocytic myocarditis | Negative | LV free wall LGE |
| Cardiac sarcoidosis | |||||||
| #12 | LV | M | 26 | 49 | Focal granulomatous inflammation with non‐necrotizing epithelioid cells granulomas with multi‐nucleated giant cells | Negative | n/a |
| Healed myocarditis | |||||||
| #13 (case 2) | LV | M | 22 | 40 | Healed myocarditis | HHV6B | Patchy intramural and pericardial LGE |
| #14 | LV | M | 16 | 75 | Healed myocarditis | Negative | Inferior LGE |
| #15 | RV | F | 45 | n/a | Healed myocarditis | Negative | Subepicardial midventricular LGE |
| #16 | RV | M | 21 | 60 | Healed myocarditis | PVB19 (678 copies//μg DNA) | Lateral basal and apical subepicardial LGE |
| #17 | RV | n/a | 36 | 35–40 | Healed myocarditis | HHV6B | n/a |
| #18 | LV | M | 32 | 60 | Healed myocarditis | HHV6B | n/a |
| Other diagnoses (cardiac damage with unclear aetiology) | |||||||
| #19 | LV | M | 39 | 20 | Chronic myocardial damage with moderate perivascular accentuated fibrosis and microangiopathy | Negative | n/a |
| #20 | LV | M | 33 | 65 | Chronic myocardial damage with interstitial fibrosis | Negative | lateral LGE |
| #21 | LV | M | 35 | 60 | Mild chronic myocardial damage | Negative | n/a |
| #22 | RV | M | 38 | 15 | Chronic myocardial damage with interstitial fibrosis | Negative | n/a |
| #23 | LV | M | 43 | 48 | Moderate chronic myocardial damage | Negative | n/a |
| #24 | RV | M | 17 | 50 | Mild chronic myocardial damage | Negative | focal LGE |
EMB revealed acute myocarditis in 5 (20.8%), chronic myocarditis in 6 (25%), cardiac sarcoidosis in 1 (4.2%), healed myocarditis in 6 (25%) and other diagnoses with cardiac damage of unclear aetiology in 6 (25%) patients.
EBV, Epstein–Barr‐Virus; EMB, endomyocardial biopsy; EF, ejection fraction; HHV6B, human herpes virus 6B; LV, left ventricle; M, male; n/a, not available; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PVB19, parvovirus B19; RV, right ventricle; W, female.
Thus, COVID‐19 immunization is not necessarily related with the induction of acute or chronic myocarditis. It is likely that other pre‐existing cardiac diseases influence possible adverse cardiac events following COVID‐19 mRNA vaccination. In all three cases described above, alternative causes (campylobacter enteritis, HHV6 infection, immune‐checkpoint inhibitor therapy) need to be considered in this context. Overall, acute lymphocytic myocarditis was diagnosed by EMB only in 5 out of 24 patients (20.8%). EMB results of our study cohort demonstrated a broad spectrum of pathohistological patterns including acute, chronic, and healed myocarditis, cardiac sarcoidosis and other diagnoses with cardiac damage of unclear aetiology. Careful patients' history, laboratory work‐up, echocardiography, and cardiac MRI must strongly be recommended in all patients with suspected acute myocarditis following mRNA COVID‐19 vaccination. EMB should strongly be considered in selected patients presenting with reduced EF and especially in those with acute heart failure and/or ventricular arrhythmias according to the recently published position statement on EMB to establish a definite diagnosis. 9 Further studies are needed to characterize patient subgroups at risk for vaccine‐related myocarditis. Concerns about these possible rare adverse events after COVID‐19 immunization should not undermine the general confidence in its value for the community.
Conflict of interest
DK, KK, MG, ST, NB, CS, and JK declare no conflict of interest.
Kiblboeck, D. , Klingel, K. , Genger, M. , Traxler, S. , Braunsteiner, N. , Steinwender, C. , and Kellermair, J. (2022) Myocarditis following mRNA COVID‐19 vaccination: call for endomyocardial biopsy. ESC Heart Failure, 9: 1996–2002. 10.1002/ehf2.13791.
References
- 1. European Medicine Agency Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis. 2021, July 9th. https://www.ema.europa.eu/en/news/comirnaty‐spikevax‐possible‐link‐very‐rare‐cases‐myocarditis‐pericarditis (4 August 2021).
- 2. Ministry of Health. Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021 (including). June 2021. https://gov.il/en/departments/news/01062021‐03 (4 August 2021).
- 3. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol 2021; 6: 1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, Flanagan MC, Khera A, Maghsoudi A, Mekonnen G, Muthukumar A, Saeed IM, Sherwood MW, Sinha SS, O'Connor CM, deFilippi CR. Myocarditis temporally associated with COVID‐19 vaccination. Circulation 2021; 144: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abu Mouch S, Roguin A, Hellou A, Ishai A, Shoshan U, Mahamid L, Zoabi M, Aisman M, Goldshmidt N, Yanay NB. Myocarditis following COVID‐19 mRNA vaccination. Vaccine 2021; 39: 2790–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mei R, Raschi E, Forcesi E, Diemberger I, de Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: Gaining insights through the vaccine adverse event reporting system. Int J Cardiol 2018; 273: 183–186. [DOI] [PubMed] [Google Scholar]
- 7. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H‐P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648. [DOI] [PubMed] [Google Scholar]
- 8. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID‐19 vaccination: what do we know so far? Children 2021; 8: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seferovic PM, Tsutsui H, McNamara DM, Ristić AD, Basso C, Bozkurt B, Cooper LT Jr, Filippatos G, Ide T, Inomata T, Klingel K. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society position statement on endomyocardial biopsy. Eur J Heart Fail 2021; 23: 854–871. [DOI] [PubMed] [Google Scholar]


